A Simple and Efficient Method for Preparing High-Purity α-CaSO4·0.5H2O Whiskers with Phosphogypsum
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.3. Data Analysis
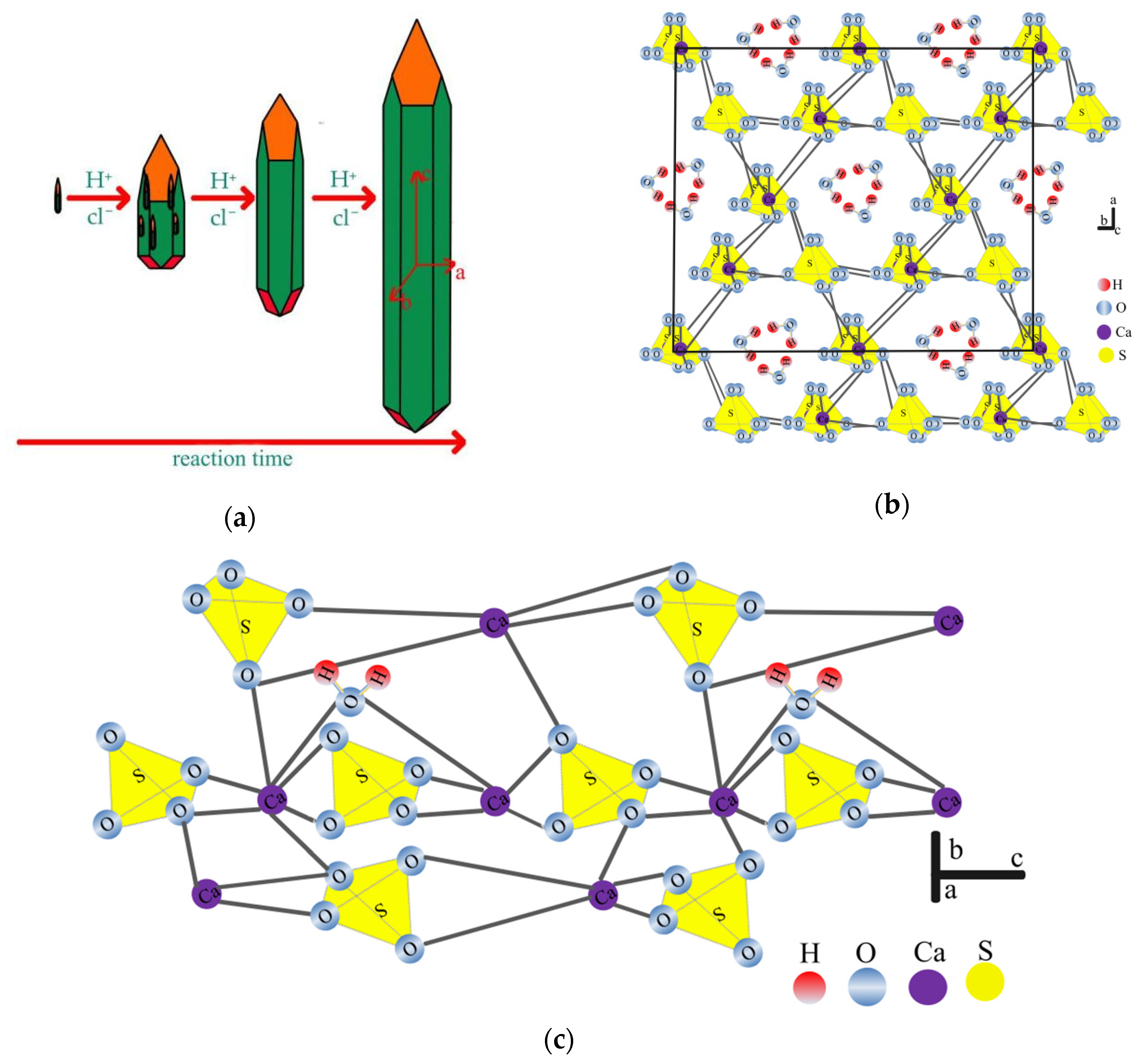
2.4. Theoretical Basis
3. Results and Discussion
3.1. CaSO4·2H2O Extraction from PG
3.1.1. Effect of the Dissolution Amount, Crystallization Amount and Extraction Rate
3.1.2. Effect of Acid-Leaching Products at Different Dissolution Cycle Numbers
3.2. Preparation of HH Whiskers
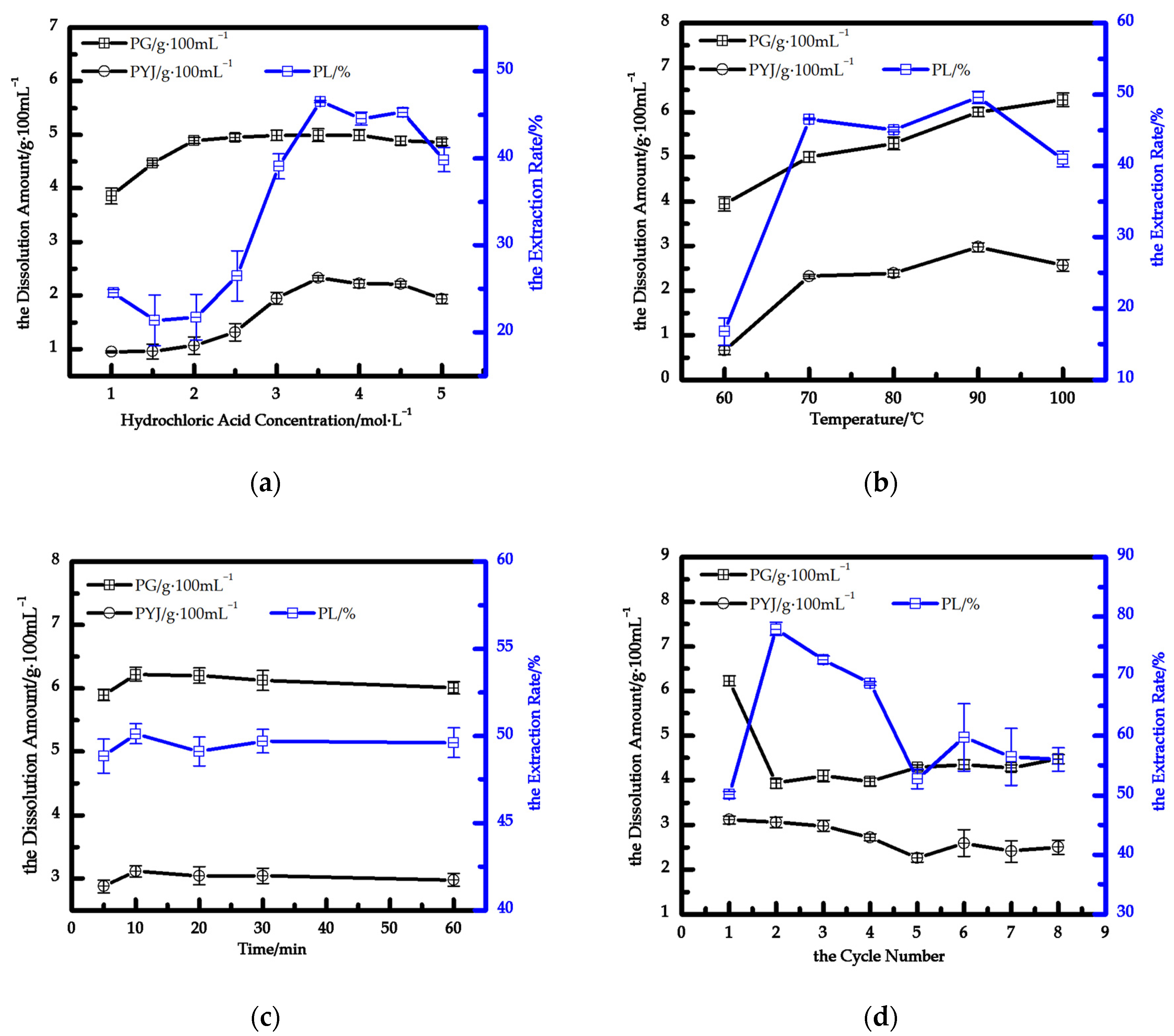
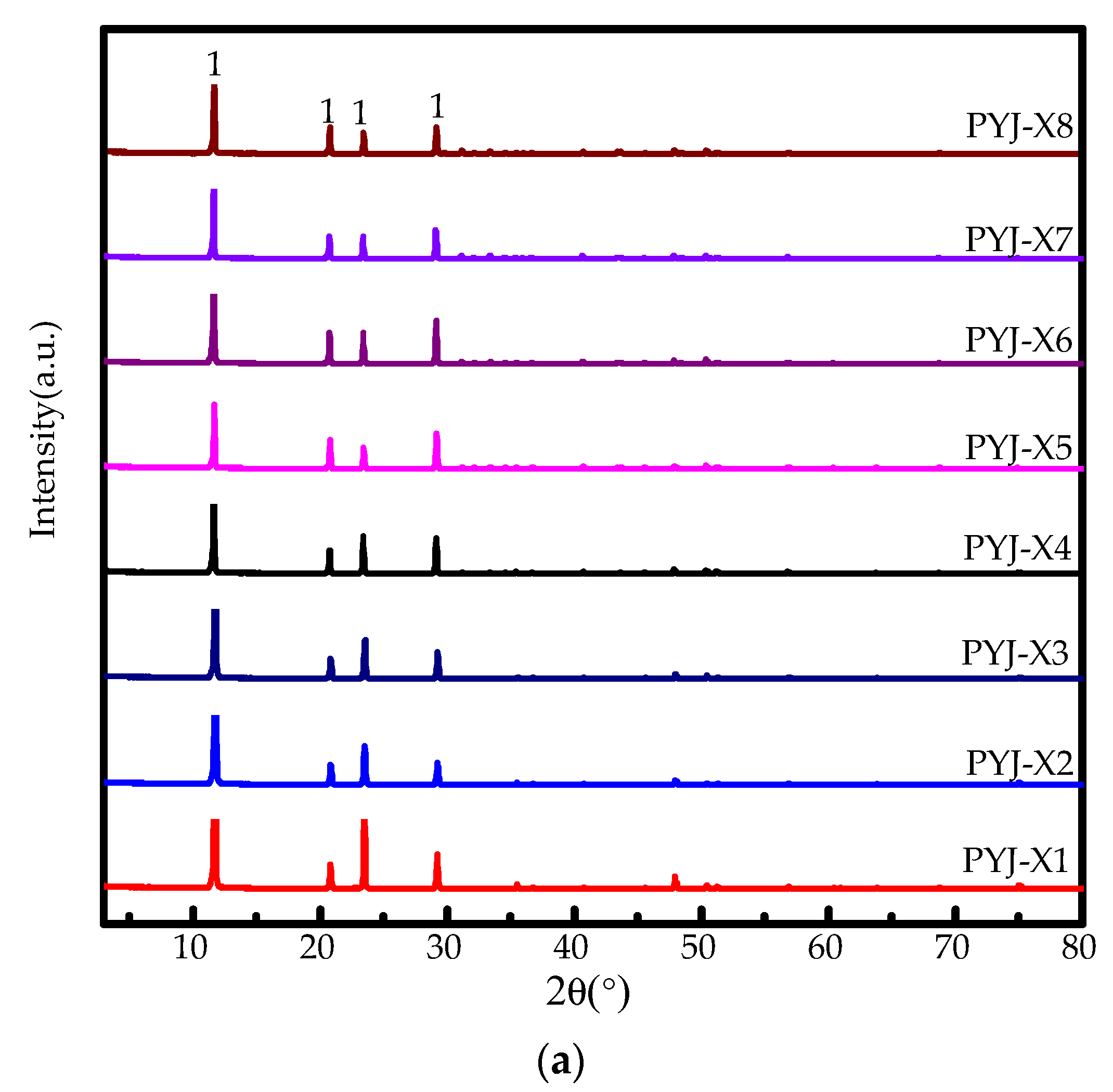
3.2.1. Effect of HCl Concentrations and Reaction Temperatures
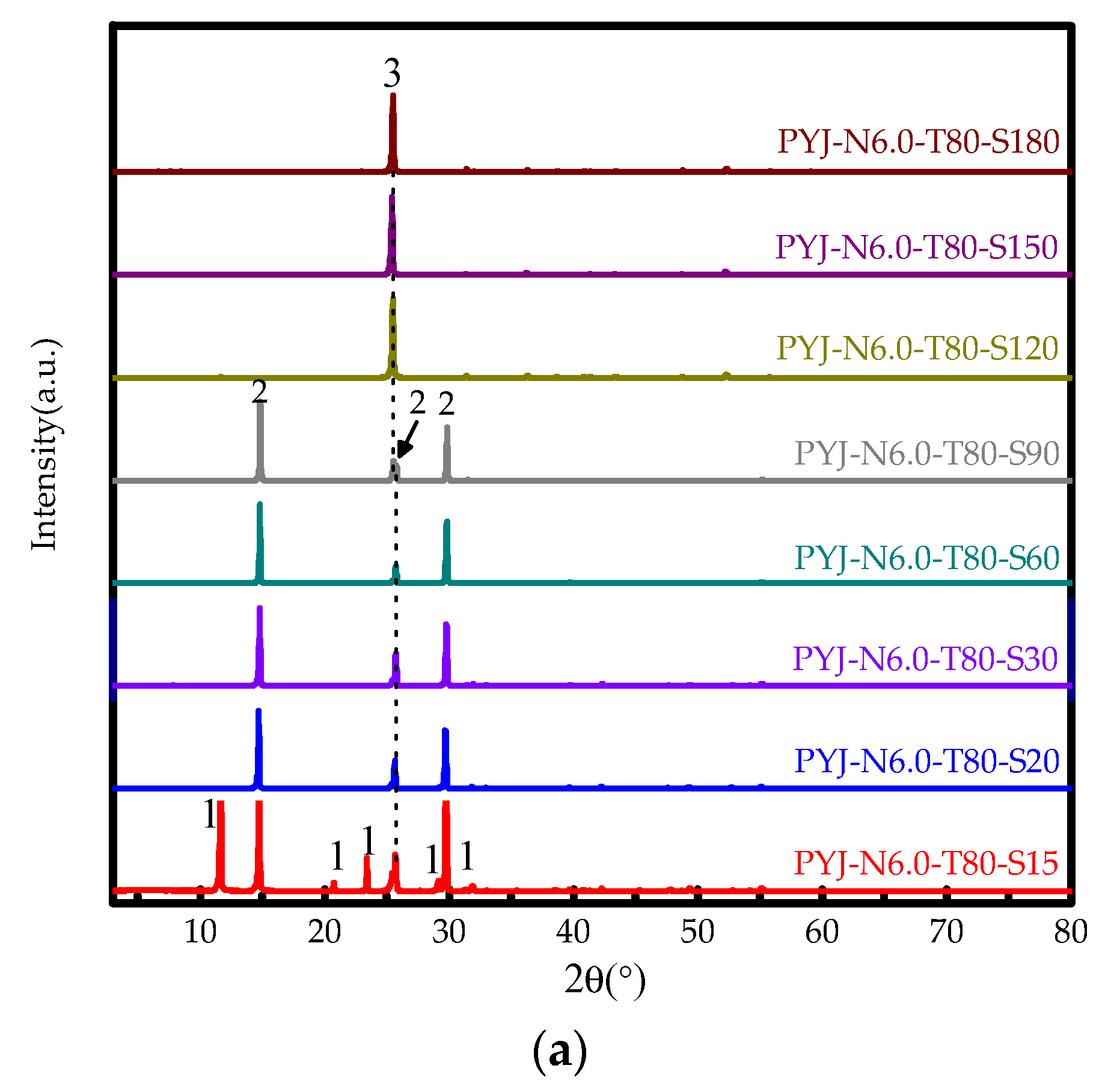
3.2.2. Effect of Reaction Time
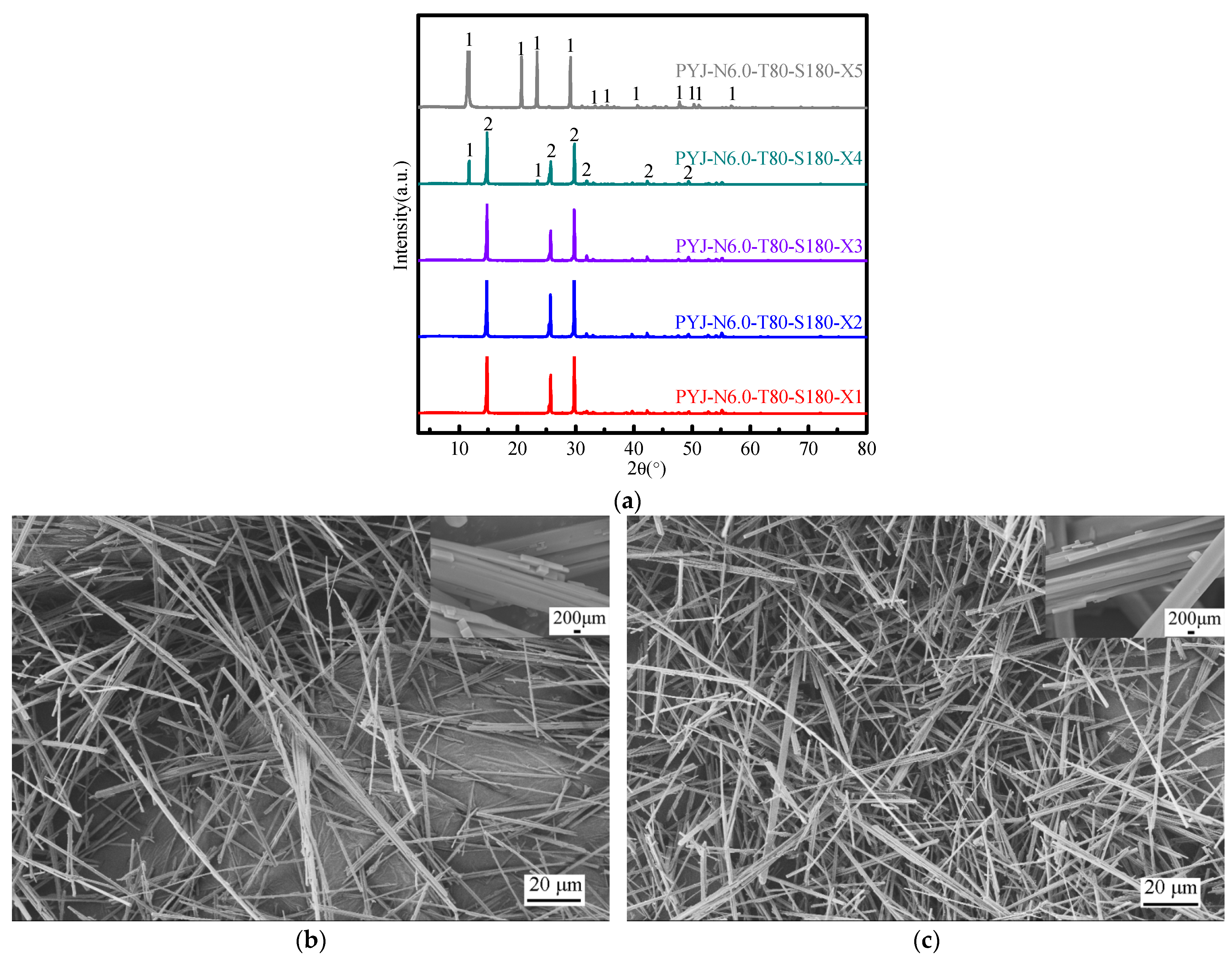
3.2.3. Effect of Reaction Cycle Number
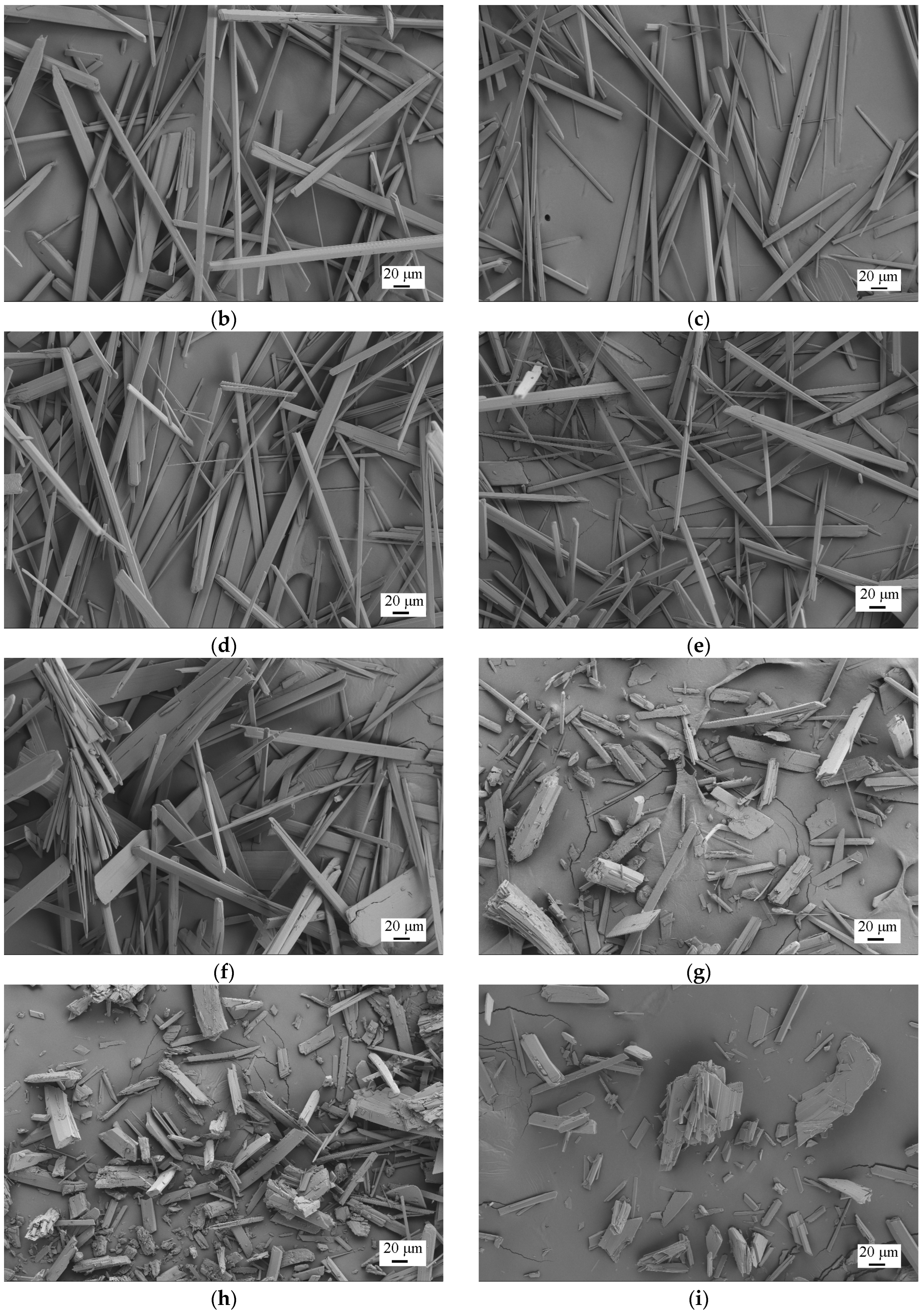
3.2.4. Effect of Reaction Time on the Crystal Habit of HH
3.3. Analysis of PYJB Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [Green Version]
- Arocena, J.; Rutherford, P.; Dudas, M. Heterogeneous distribution of trace elements and fluorine in phosphogypsum by-product. Sci. Total Environ. 1995, 162, 149–160. [Google Scholar] [CrossRef]
- Tsioka, M.; Voudrias, E.A. Comparison of alternative management methods for phosphogypsum waste using life cycle analysis. J. Clean. Prod. 2020, 266, 121386. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Jalali, J. Isolation and screening of indigenous bacteria from phosphogypsum-contaminated soils for their potential in promoting plant growth and trace elements mobilization. J. Environ. Manag. 2020, 260, 110063. [Google Scholar] [CrossRef] [PubMed]
- Trifi, H.; BEN Salem, I.; Benzina, N.K.; Fourati, A.; Costa, M.C.; Achouak, W.; Sghaier, H.; Saidi, M. Effectiveness of plant growth-promoting rhizobacterium Pantoea sp. BRM17 in enhancing canola growth on phosphogypsum-amended soil. Pedosphere 2017, 30, 570–576. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; López-Coto, I.; Aguado, J.L.; Bolívar, J.P.; Santisteban, M. Dynamics of contaminants in phosphogypsum of the fertilizer industry of Huelva (SW Spain): From phosphate rock ore to the environment. Appl. Geochem. 2010, 25, 705–715. [Google Scholar] [CrossRef]
- Saadaoui, E.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N. Phosphogypsum: Potential uses and problems—A review. Int. J. Environ. Stud. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Zhao, Y.; Shu, Z.; Wang, Y.; Zhang, Y.; Shen, X. Preparation of paper-free and fiber-free plasterboard with high strength using phosphogypsum. Constr. Build. Mater. 2020, 243, 118091. [Google Scholar] [CrossRef]
- Haneklaus, N.; Barbossa, S.; Basallote, M.D.; Bertau, M.; Bilal, E.; Chajduk, E.; Chernysh, Y.; Chubur, V.; Cruz, J.; Dziarczykowski, K.; et al. Closing the upcoming EU gypsum gap with phosphogypsum. Resour. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Romero-Hermida, M.I. Environmental Impact of Phospho-gypsum-Derived Building Materials. Int. J. Environ. Res. Public Health 2020, 17, 4248. [Google Scholar] [CrossRef]
- Mymrin, V.; Aibuldinov, E.K.; Avanci, M.A.; Alekseev, K.; Argenda, M.A.; Carvalho, K.Q.; Erbs, A.; Catai, R.E. Material cycle realization by hazardous phosphogypsum waste, ferrous slag, and lime production waste application to produce sustainable construction materials. J. Mater. Cycles Waste Manag. 2021, 23, 591–603. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Zhang, L.; Xiao, B. Preparation of load-bearing building materials from autoclaved phosphogypsum. Constr. Build. Mater. 2009, 23, 687–693. [Google Scholar] [CrossRef]
- Degirmenci, N.; Okucu, A.; Turabi, A. Application of phosphogypsum in soil stabilization. Build. Environ. 2006, 42, 3393–3398. [Google Scholar] [CrossRef]
- Amrani, M.; Taha, Y.; Kchikach, A.; Benzaazoua, M.; Hakkou, R. Phosphogypsum recycling: New horizons for a more sustainable road material application. J. Build. Eng. 2020, 30, 101267. [Google Scholar] [CrossRef]
- Silva, M.V.; de Rezende, L.R.; Mascarenha, M.M.D.A.; de Oliveira, R.B. Phosphogypsum, tropical soil and cement mixtures for asphalt pavements under wet and dry environmental conditions. Resour. Conserv. Recycl. 2019, 144, 123–136. [Google Scholar] [CrossRef]
- Mesić, M. The Application of Phosphogypsum in Agriculture. Agric. Conspec. Sci. 2016, 81, 7–13. Available online: https://hrcak.srce.hr/file/248582 (accessed on 5 January 2022).
- Papastefanou, C.; Stoulos, S.; Ioannidou, A.; Manolopoulou, M. The application of phosphogypsum in agriculture and the radiological impact. J. Environ. Radioact. 2006, 89, 188–198. [Google Scholar] [CrossRef]
- Wang, J. Retracted: Utilization effects and environmental risks of phosphogypsum in agriculture: A review. J. Clean. Prod. 2020, 276, 123337. [Google Scholar] [CrossRef]
- Maierdan, Y.; Haque, M.A.; Chen, B.; Maimaitiyiming, M.; Ahmad, M.R. Recycling of waste river sludge into unfired green bricks stabilized by a combination of phosphogypsum, slag, and cement. Constr. Build. Mater. 2020, 260, 120666. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Fourie, A.; Xin, C. Utilization of phosphogypsum and phosphate tailings for cemented paste backfill. J. Environ. Manag. 2017, 201, 19–27. [Google Scholar] [CrossRef]
- Chen, Q. Utilization of Phosphogypsum to Prepare High-Purity CaCO3 in the NH4Cl–NH4OH–CO2 System. ACS Sustain. Chem. Eng. 2020, 8, 11649–11657. [Google Scholar] [CrossRef]
- Lu, W.; Ma, B.; Su, Y.; He, X.; Jin, Z.; Qi, H. Preparation of α-hemihydrate gypsum from phosphogypsum in recycling CaCl2 solution. Constr. Build. Mater. 2019, 214, 399–412. [Google Scholar] [CrossRef]
- Ma, B.; Lu, W.; Su, Y.; Li, Y.; Gao, C.; He, X. Synthesis of α-hemihydrate gypsum from cleaner phosphogypsum. J. Clean. Prod. 2018, 195, 396–405. [Google Scholar] [CrossRef]
- Dondi, M. Soluble salts and efflorescence in structural clay products: A scheme to predict the risk of efflorescence. Bol. De La Soc. Esp. De Ceram. Y Vidr. 1997, 36, 619–629. [Google Scholar]
- Li, Z.; Demopoulos, G.P. Model-Based Construction of Calcium Sulfate Phase-Transition Diagrams in the HCl−CaCl2−H2O System between 0 and 100 °C. Ind. Eng. Chem. Res. 2006, 45, 4517–4524. [Google Scholar] [CrossRef]
- Hammas, I.; Horchani-Naifer, K.; Férid, M. Solubility study and valorization of phosphogypsum salt solution. Int. J. Miner. Process. 2013, 123, 87–93. [Google Scholar] [CrossRef]
- Gayevskii, V.; Kochmarskii, V.; Gayevska, S. Nucleation and crystal growth of calcium sulfate dihydrate from aqueous solutions: Speciation of solution components, kinetics of growth, and interfacial tension. J. Cryst. Growth 2020, 548, 125844. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, L.; Wang, L.; Huang, X.; Zhang, Y.; Feng, Z.; Cui, D. Precipitation-dissolution behaviors of rare earth ions in H3PO4Ca(H2PO4)2 solutions. J. Rare Earths 2019, 37, 520–527. [Google Scholar] [CrossRef]
- Al-Othman, A. Gypsum crystallization and hydrochloric acid regeneration by reaction of calcium chloride solution with sulfuric acid. Hydrometallurgy 2009, 96, 95–102. [Google Scholar] [CrossRef]
- Eun, H.-M. Enzymes and Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 1996; pp. 47–48. [Google Scholar] [CrossRef]
- Song, X. Preparation of calcium sulfate whiskers using waste calcium chloride by reactive crystallization. Cryst. Res. Technol. 2011, 46, 166–172. [Google Scholar] [CrossRef]
- Adekola, F.A.; Olosho, A.I.; Baba, A.A.; Adebayo, S.A. Dissolution kinetics studies of Nigerian gypsum ore in hydrochloric acid. J. Chem. Technol. Metall. 2018, 53, 845–855. Available online: https://dl.uctm.edu/journal/node/j2018-5/8_17-191_p845-855.pdf (accessed on 1 January 2022).
- Walawalkar, M.; Nichol, C.K.; Azimi, G. Process investigation of the acid leaching of rare earth elements from phosphogypsum using HCl, HNO3, and H2SO4. Hydrometallurgy 2016, 166, 195–204. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and resource utilization of flue gas desulfurized gypsum in China—A review. Environ. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef]
- Jia, C.; Wu, L.; Chen, Q.; Lin, J.; Yang, L.; Song, Z.; Guan, B. Distribution behavior of arsenate into α-calcium sulfate hemihydrate transformed from gypsum in solution. Chemosphere 2020, 255, 126936. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Li, J.; Li, T.; Zheng, S.; Han, W.; Geng, Q.; Guo, H. Influence of crystal modifier on the preparation of α-hemihydrate gypsum from phosphogypsum. Constr. Build. Mater. 2017, 133, 323–329. [Google Scholar] [CrossRef]
- Weiss, H.; Bräu, M.F. How Much Water Does Calcined Gypsum Contain? Angew. Chem. Int. Ed. 2009, 48, 3520–3524. [Google Scholar] [CrossRef]
- Chen, Y. Gypsum Building Materials, 2nd ed.; Chemical Industry Press: Beijing, China, 2010; pp. 77–80. Available online: https://www.wl.cn/7661701 (accessed on 1 January 2021).
- Lv, Y.; Li, N.; Tang, M.; Liu, Z.-H. Thermodynamic properties of microporous crystals for two hydrated aluminoborates, K2[Al(B5O10)]·4H2O and (NH4)2 [Al(B5O10)]·4H2O. J. Chem. Thermodyn. 2013, 58, 129–133. [Google Scholar] [CrossRef]



| Chemical Composition | SO3 | CaO | SiO2 | Al2O3 | P2O5 | Fe2O3 | SrO | F | K2O | TiO2 | MgO | BaO | Other | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt % | 39.36 | 29.60 | 5.03 | 1.85 | 1.60 | 0.86 | 0.24 | 0.20 | 0.17 | 0.13 | 0.11 | 0.05 | 0.06 | 20.74 |
| Test Items | GB6656-2010 | Test Results | |||||
|---|---|---|---|---|---|---|---|
| Radioactivity | A | B | C | ||||
| Internal Exposure Index (IRa) | ≤1 | ≤1.3 | Does not satisfy class A | - | Does not satisfy class A or B | 0.3 | |
| External Exposure Index(Iγ) | ≤1.3 | ≤1.9 | ≤2.8 | 0.4 | |||
| No. | HCl Concentration (mol/L) | Temperature (°C) | Time (min) | Cycles |
|---|---|---|---|---|
| 1 | 1.0 | 70 | 60 | 1 |
| 2 | 1.5 | 70 | 60 | 1 |
| 3 | 2.0 | 70 | 60 | 1 |
| 4 | 2.5 | 70 | 60 | 1 |
| 5 | 3.0 | 70 | 60 | 1 |
| 6 | 3.5 | 70 | 60 | 1 |
| 7 | 4.0 | 70 | 60 | 1 |
| 8 | 4.5 | 70 | 60 | 1 |
| 9 | 5.0 | 70 | 60 | 1 |
| 10 | 3.5 | 60 | 60 | 1 |
| 11 | 3.5 | 70 | 60 | 1 |
| 12 | 3.5 | 80 | 60 | 1 |
| 13 | 3.5 | 90 | 60 | 1 |
| 14 | 3.5 | 100 | 60 | 1 |
| 15 | 3.5 | 90 | 5 | 1 |
| 16 | 3.5 | 90 | 10 | 1 |
| 17 | 3.5 | 90 | 20 | 1 |
| 18 | 3.5 | 90 | 30 | 1 |
| 19 | 3.5 | 90 | 60 | 1 |
| 20 | 3.5 | 90 | 20 | 1 |
| 21 | 3.5 | 90 | 20 | 2 |
| 22 | 3.5 | 90 | 20 | 3 |
| 23 | 3.5 | 90 | 20 | 4 |
| 24 | 3.5 | 90 | 20 | 5 |
| 25 | 3.5 | 90 | 20 | 6 |
| 26 | 3.5 | 90 | 20 | 7 |
| 27 | 3.5 | 90 | 20 | 8 |
| No. | HCl Concentration (mol/L) | Temperature (°C) | Time (min) | Cycles |
|---|---|---|---|---|
| 1 | 5.0 | 80 | 180 | 1 |
| 2 | 5.5 | 80 | 180 | 1 |
| 3 | 6.0 | 80 | 180 | 1 |
| 4 | 6.5 | 80 | 180 | 1 |
| 5 | 7.0 | 80 | 180 | 1 |
| 6 | 6.0 | 60 | 180 | 1 |
| 7 | 6.0 | 70 | 180 | 1 |
| 8 | 6.0 | 80 | 180 | 1 |
| 9 | 6.0 | 90 | 180 | 1 |
| 10 | 6.0 | 100 | 180 | 1 |
| 11 | 6.0 | 80 | 10 | 1 |
| 12 | 6.0 | 80 | 20 | 1 |
| 13 | 6.0 | 80 | 30 | 1 |
| 14 | 6.0 | 80 | 60 | 1 |
| 15 | 6.0 | 80 | 90 | 1 |
| 16 | 6.0 | 80 | 120 | 1 |
| 17 | 6.0 | 80 | 150 | 1 |
| 18 | 6.0 | 80 | 180 | 1 |
| 19 | 6.0 | 80 | 30 | 1 |
| 20 | 6.0 | 80 | 30 | 2 |
| 21 | 6.0 | 80 | 30 | 3 |
| 22 | 6.0 | 80 | 30 | 4 |
| 23 | 6.0 | 80 | 30 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Sun, H.; Peng, T.; Ding, W.; Li, X.; Xiao, S. A Simple and Efficient Method for Preparing High-Purity α-CaSO4·0.5H2O Whiskers with Phosphogypsum. Materials 2022, 15, 4028. https://doi.org/10.3390/ma15114028
Lin Y, Sun H, Peng T, Ding W, Li X, Xiao S. A Simple and Efficient Method for Preparing High-Purity α-CaSO4·0.5H2O Whiskers with Phosphogypsum. Materials. 2022; 15(11):4028. https://doi.org/10.3390/ma15114028
Chicago/Turabian StyleLin, Yan, Hongjuan Sun, Tongjiang Peng, Wenjin Ding, Xiang Li, and Sha Xiao. 2022. "A Simple and Efficient Method for Preparing High-Purity α-CaSO4·0.5H2O Whiskers with Phosphogypsum" Materials 15, no. 11: 4028. https://doi.org/10.3390/ma15114028
APA StyleLin, Y., Sun, H., Peng, T., Ding, W., Li, X., & Xiao, S. (2022). A Simple and Efficient Method for Preparing High-Purity α-CaSO4·0.5H2O Whiskers with Phosphogypsum. Materials, 15(11), 4028. https://doi.org/10.3390/ma15114028






