A Systematical Evaluation of the Crystallographic Orientation Relationship between MC Precipitates and Ferrite Matrix in HSLA Steels
Abstract
:1. Introduction
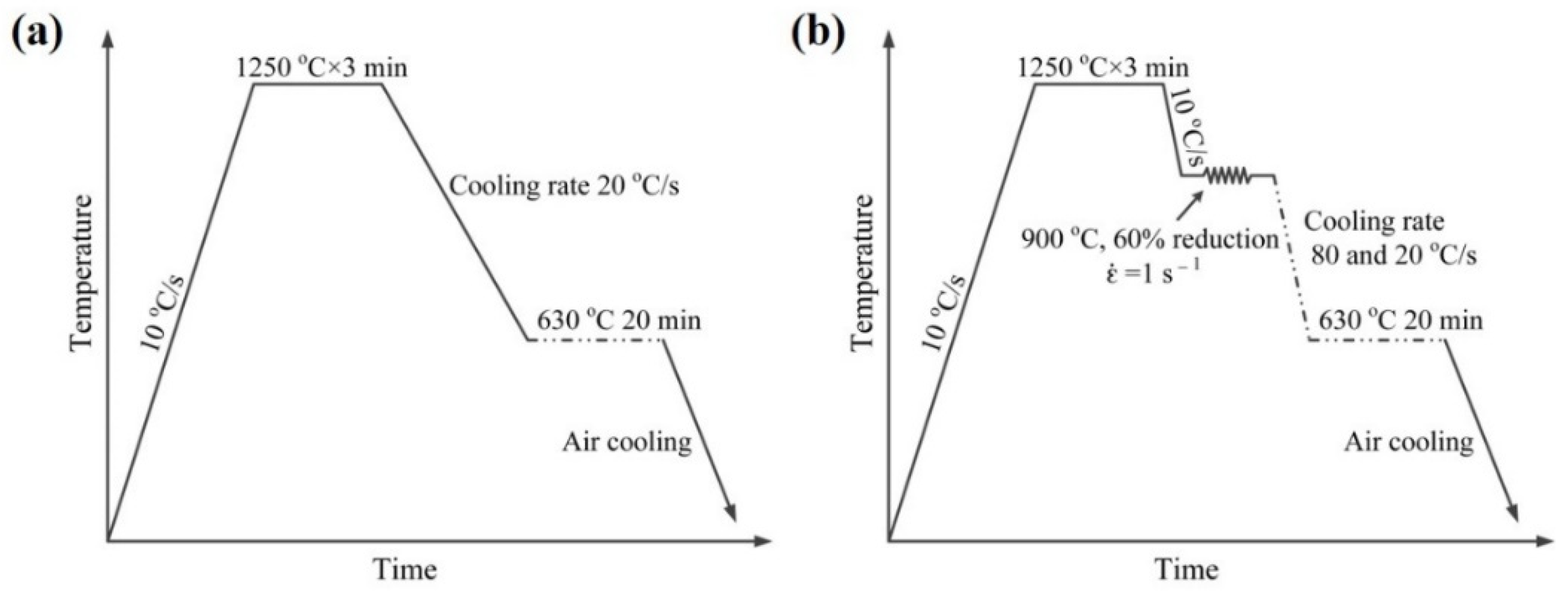
2. Materials and Experimental Procedure
3. Results and Discussions
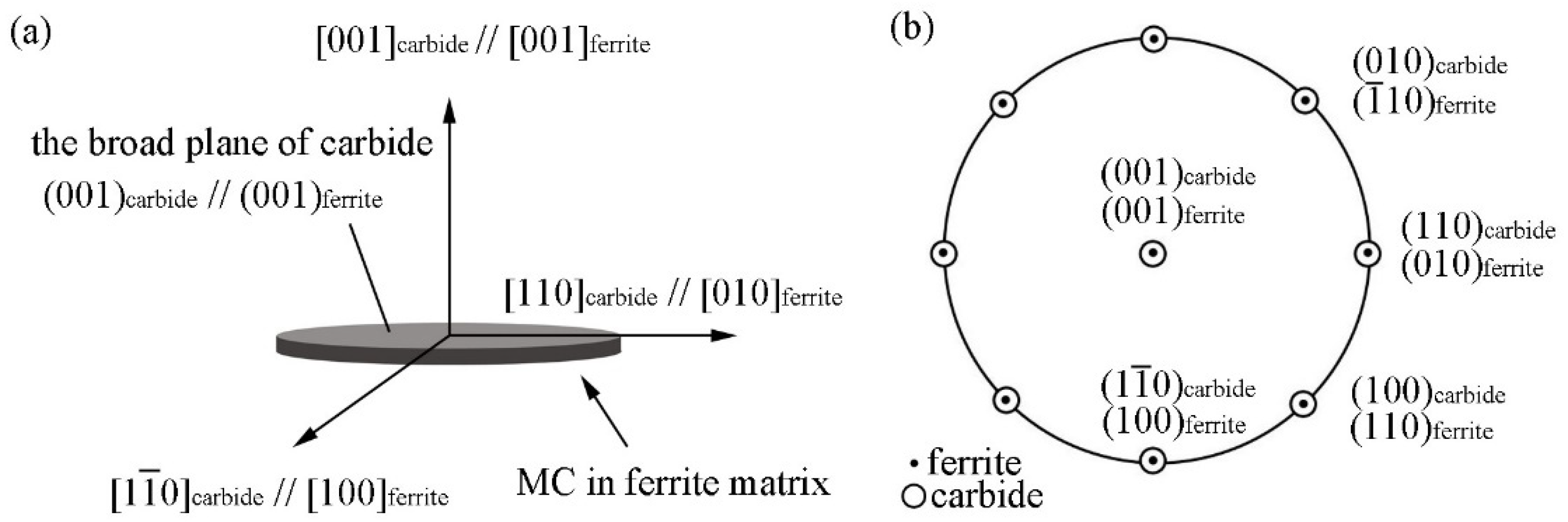
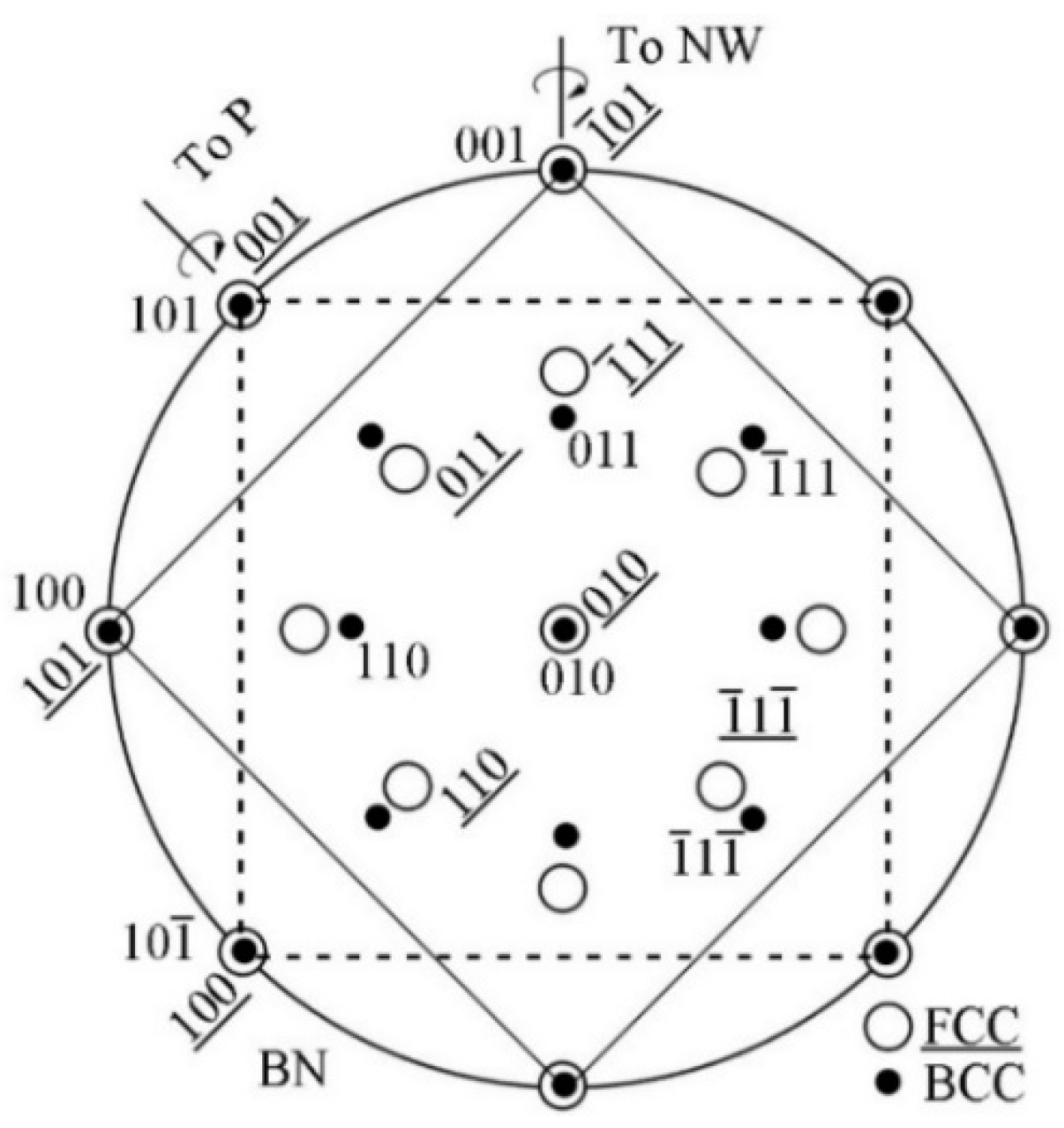
3.1. Summary of Common ORs in MC/Ferrite System
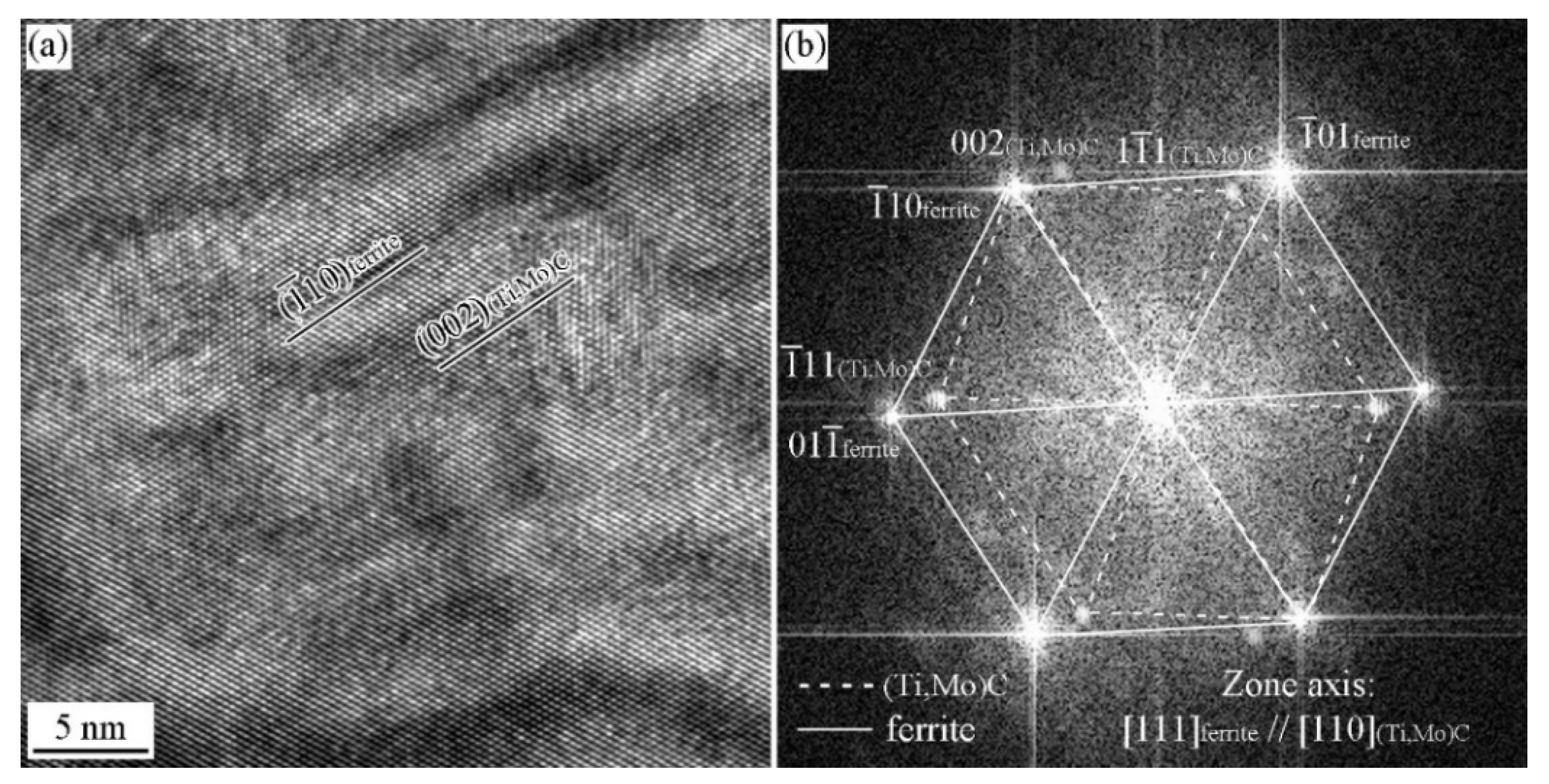
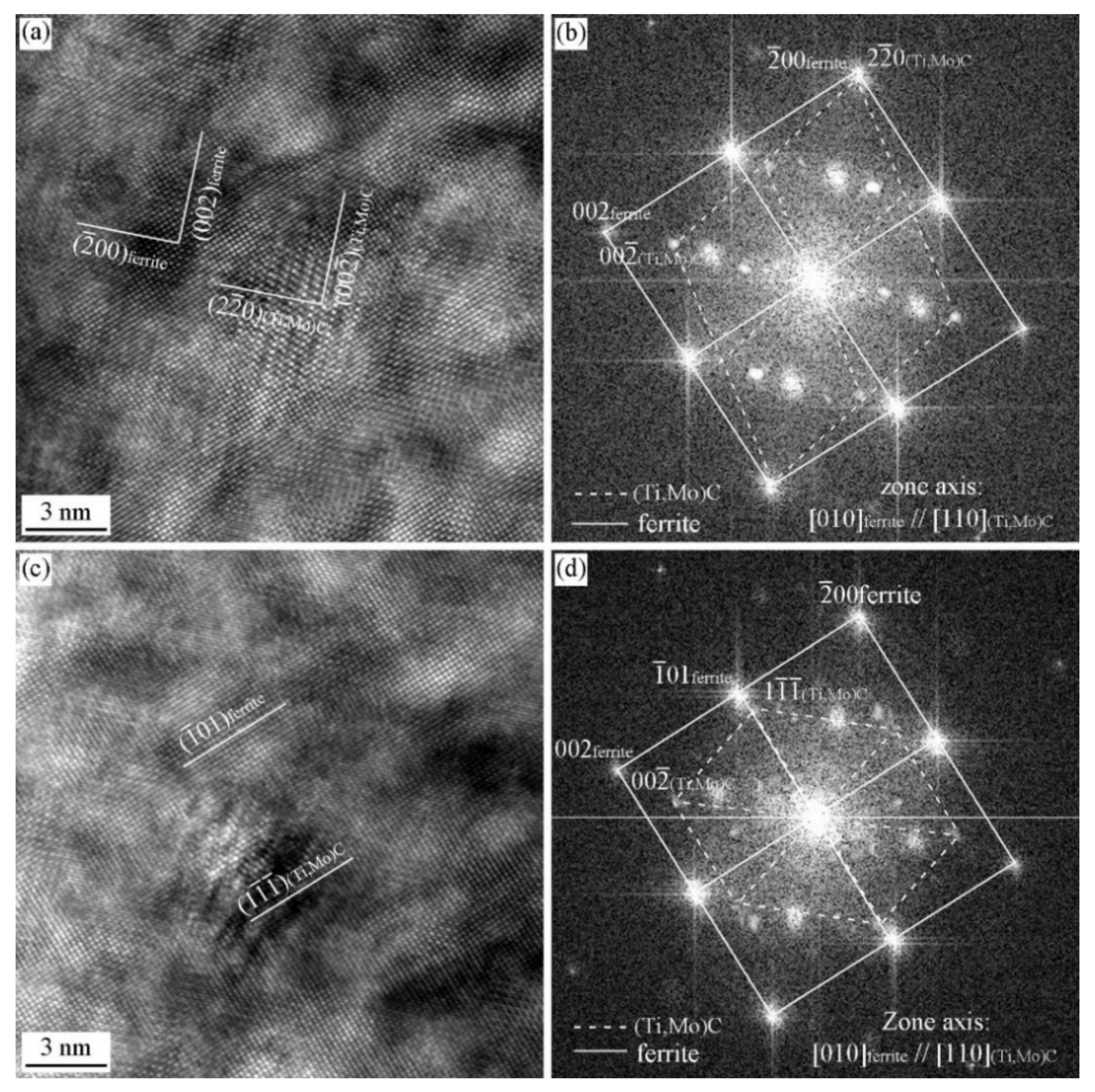
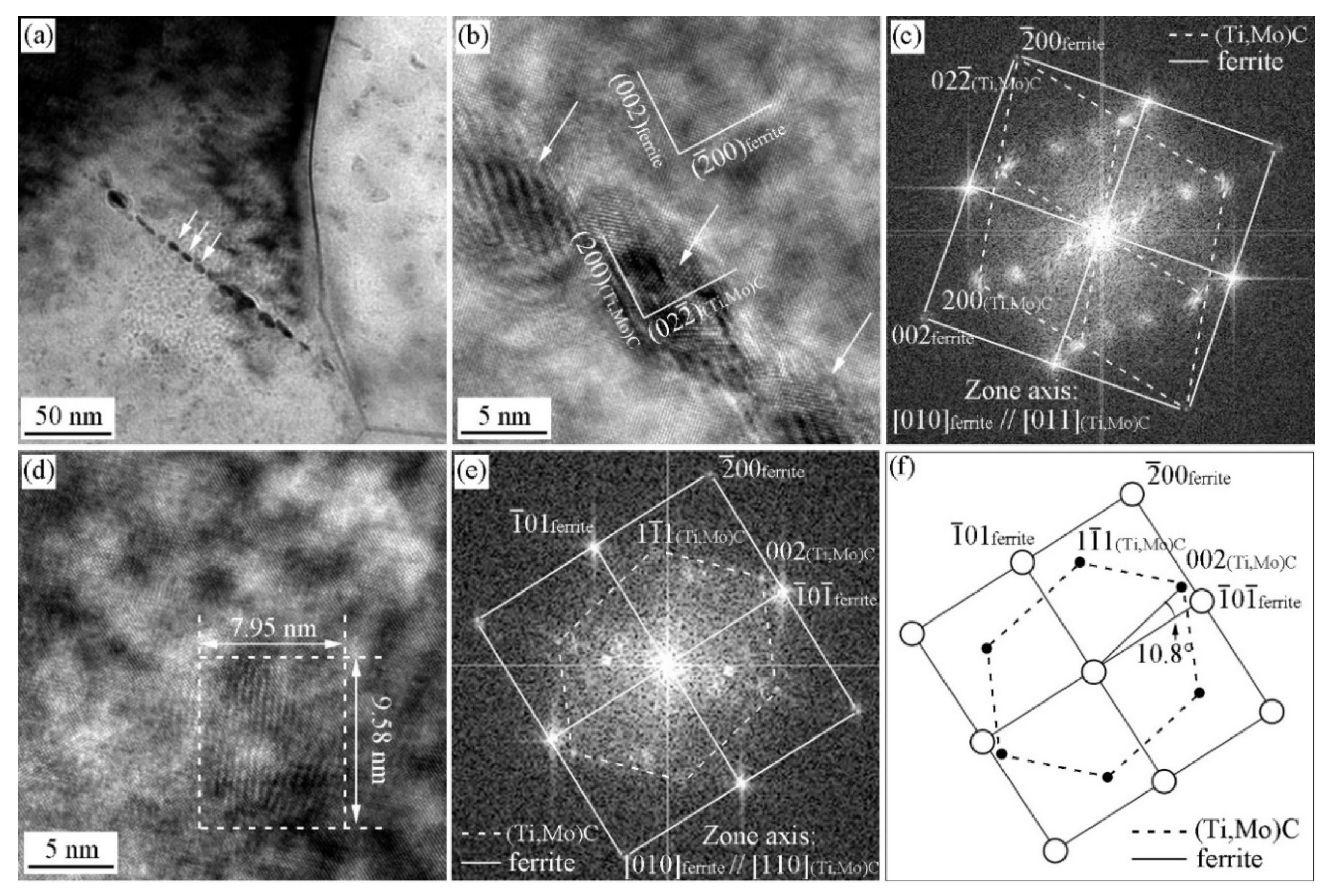
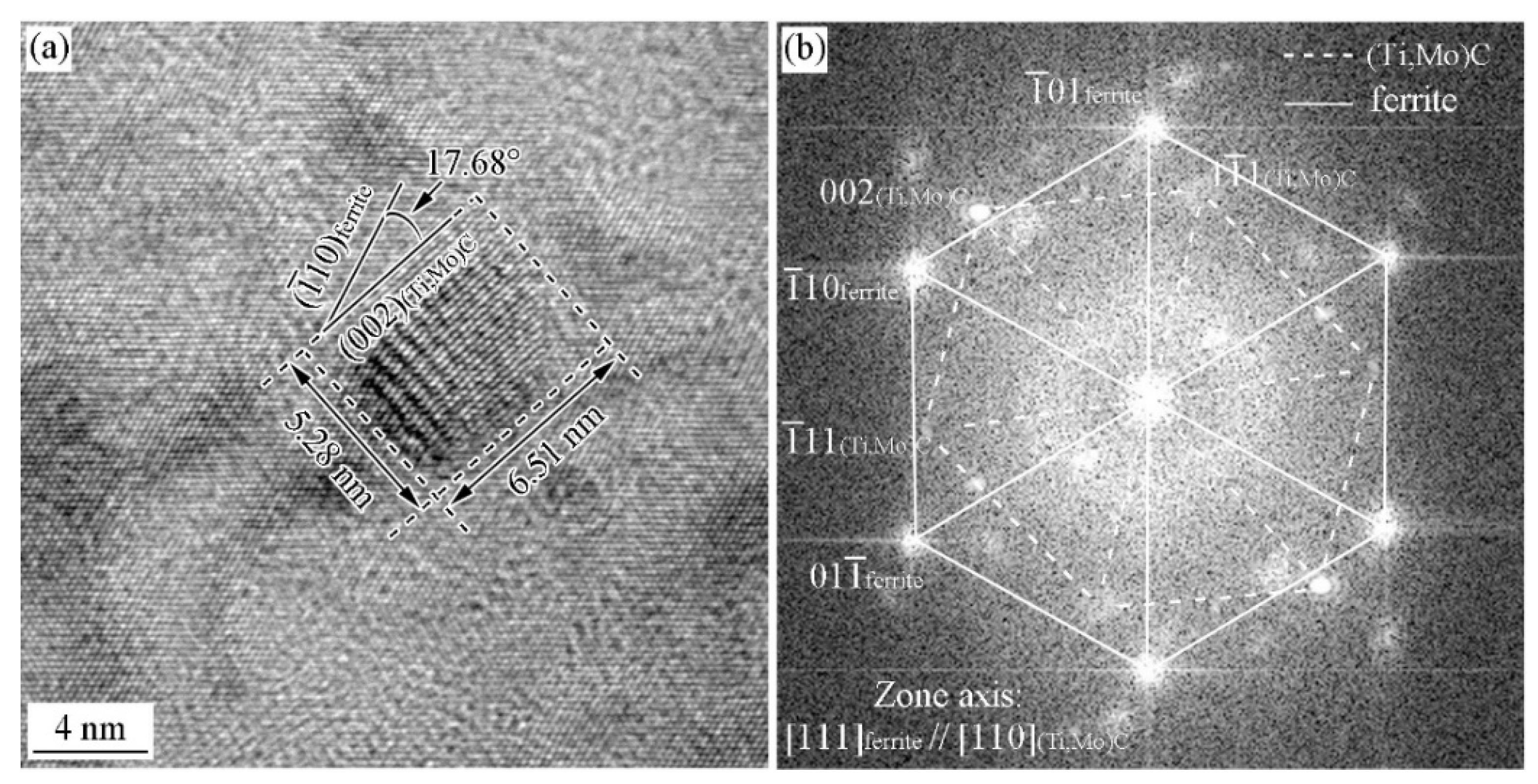
3.2. Precipitation Behavior in the Specimen with and without Deformation
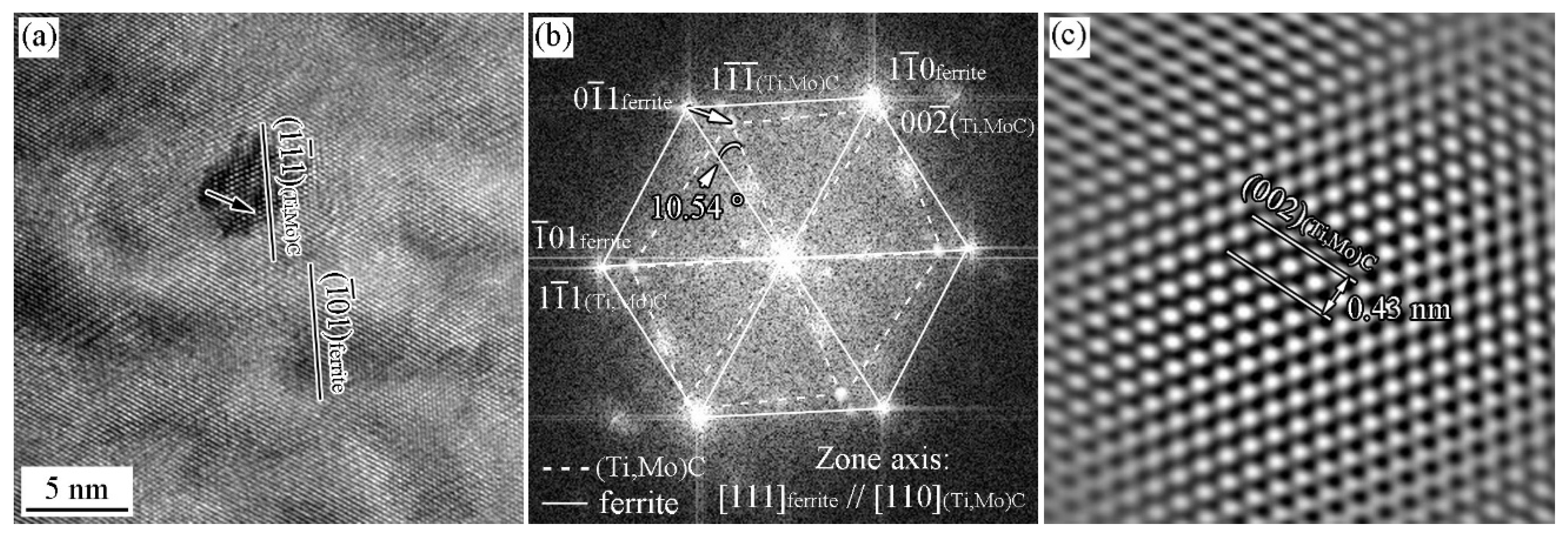
3.3. ORs between MC and Ferrite in Specimen with Process I
3.4. ORs between MC and Ferrite in Specimen with Process II
4. Conclusions
- In the isothermal holding process without austenite deformation, interphase precipitation can be obtained, and these precipitates obey BN ORs with ferrite matrix.
- In the isothermal holding process with austenite deformation, supersaturate precipitates have been obtained, and these precipitates can obey the BN, NW, KS, and P ORs in the same specimens.
- With the cooling rate of 20 °C/s after austenite deformation, the precipitates and ferrite can strictly satisfy the specific OR, while at the cooling rate of 80 °C/s, precipitates and ferrite can roughly satisfy these OR with the deviation ~10°.
Author Contributions
Funding
Conflicts of Interest
References
- Hajisafari, M.; Nategh, S.; Yoozbashizadeh, H.; Ekrami, A. Improvement in Mechanical Properties of Microalloyed Steel 30MSV6 by a Precipitation Hardening Process. J. Iron Steel Res. Int. 2013, 20, 66–73. [Google Scholar] [CrossRef]
- Mirzadeh, H.; Cabrera, J.M.; Prado, J.; Najafizadeh, A. Hot deformation behavior of a medium carbon microalloyed steel. Mater. Sci. Eng. A 2011, 528, 3876–3882. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.-X.; Xie, H.; Gao, X.-H.; Misra, R. Microstructure and mechanical properties of TMCP heavy plate microalloyed steel. Mater. Sci. Eng. A 2014, 607, 122–131. [Google Scholar] [CrossRef]
- Ormston, D.; Schwinn, V.; Hulka, K. Development of low carbon microalloyed bainitic steels for structural plate applications. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2005; pp. 573–580. [Google Scholar] [CrossRef]
- Shen, X.; Tang, S.; Chen, J.; Liu, Z.; Misra, R.; Wang, G. Grain refinement in surface layers through deformation-induced ferrite transformation in microalloyed steel plate. Mater. Des. 2017, 113, 137–141. [Google Scholar] [CrossRef]
- Wu, K.; Li, Z.; Guo, A.M.; He, X.; Zhang, L.; Fang, A.; Cheng, L. Microstructure Evolution in a Low Carbon Nb–Ti Microalloyed Steel. ISIJ Int. 2006, 46, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Li, L.; Gao, J.; Huo, X. Precipitation strengthening of titanium microalloyed high-strength steel plates with isothermal treatment. Mater. Sci. Eng. A 2016, 657, 413–421. [Google Scholar] [CrossRef]
- Perez-Prado, M.T.; Kassner, M.E. Chapter 9—Creep of intermetallics. In Fundamentals of Creep in Metals and Alloys, 3rd ed.; Kassner, M.E., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2015; pp. 189–232. [Google Scholar]
- Bailey, D.J.; Stevenson, R. High strength low carbon sheet steel by thermomechanical treatment: I. Strengthening mechanisms. Metall. Mater. Trans. A 1979, 10, 47–55. [Google Scholar] [CrossRef]
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of High Strength Hot-rolled Sheet Steel Consisting of Ferrite and Nanometer-sized Carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Seol, J.-B.; Na, S.-H.; Gault, B.; Kim, J.-E.; Han, J.-C.; Park, C.-G.; Raabe, D. Core-shell nanoparticle arrays double the strength of steel. Sci. Rep. 2017, 7, srep42547. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Li, G.; Mao, X.; Fang, K. Nanoscale Cementite Precipitates and Comprehensive Strengthening Mechanism of Steel. Met. Mater. Trans. A 2011, 42, 3797–3812. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Liu, L.; Deng, X.; Wang, Z. The formation mechanism of complex carbides in Nb-V microalloyed steel. Mater. Lett. 2021, 311, 131544. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, H.; Wu, Y.; Liu, X.; Chen, H.; Yao, M.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A.; et al. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Deng, X.-T.; Lei, C.-S.; Wang, Z.-D. New orientation relationship with low interfacial energy in MC/ferrite system observed in Nb-Ti bearing steel during isothermal quenching process. Scr. Mater. 2019, 163, 101–106. [Google Scholar] [CrossRef]
- Enomoto, M. Nucleation of phase transformations at intragranular inclusions in steel. Met. Mater. 1998, 4, 115–123. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, C.Y.; Wang, T.Y.; Huang, C.Y.; Yang, J.R. Orientation relationship transition of nanometre sized interphase precipitated TiC carbides in Ti bearing steel. Mater. Sci. Technol. 2010, 26, 421–430. [Google Scholar] [CrossRef]
- Dahmen, U. Orientation relationships in precipitation systems. Acta Metall. 1982, 30, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.-G.; Hara, T.; Tsuzaki, K. High-resolution transmission electron microscopy study of crystallography and morphology of TiC precipitates in tempered steel. Philos. Mag. 2004, 84, 1735–1751. [Google Scholar] [CrossRef]
- Yen, H.-W.; Chen, P.-Y.; Huang, C.-Y.; Yang, J.-R. Interphase precipitation of nanometer-sized carbides in a titanium–molybdenum-bearing low-carbon steel. Acta Mater. 2011, 59, 6264–6274. [Google Scholar] [CrossRef]
- Grajcar, A.; Skrzypczyk, P.; Kuziak, R.; Gołombek, K. Effect of finishing hot-working temperature on microstructure of thermomechanically processed Mn–Al multiphase Steels. Steel Res. Int. 2014, 85, 1058–1069. [Google Scholar] [CrossRef]
- Opiela, M.; Grajcar, A. Elaboration of forging conditions on the basis of the precipitation analysis of MX-type phases in microalloyed steels. Arch. Civ. Mech. Eng. 2012, 12, 427–435. [Google Scholar] [CrossRef]
- Headley, T.J.; Brooks, J.A. A new Bcc-Fcc orientation relationship observed between ferrite and austenite in solidification structures of steels. Metall. Mater. Trans. A 2002, 33, 5–15. [Google Scholar] [CrossRef]
- Yen, H.-W.; Huang, C.-Y.; Yang, J.-R. Characterization of interphase-precipitated nanometer-sized carbides in a Ti–Mo-bearing steel. Scr. Mater. 2009, 61, 616–619. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phase in Steels; Metallurgical Industry Press: Beijing, China, 2006. [Google Scholar]
- Yen, H.W.; Huang, C.Y.; Yang, J.R. The Nano Carbide Control: Design of Super Ferrite in Steels. Adv. Mater. Res. 2010, 89–91, 663–668. [Google Scholar] [CrossRef]
- Xiao, F.; Liao, B.; Qiao, G.; Guan, S. Effect of hot deformation on phase transformation kinetics of 86CrMoV7 steel. Mater. Charact. 2006, 57, 306–313. [Google Scholar] [CrossRef]






| Name | Orientation Relationship | Variant | Close-Packed Components | Smallest Misfit |
|---|---|---|---|---|
| BN | {001}MC//{001}α <110> MC//<100>α | 3 | None | {011}MC//{001}α |
| P | {001}MC//{101}α <10> MC//<11>α | 12 | CP directions | {002}MC//{011}α |
| NW | {111}MC//{110}α <01> MC//<001>α | 12 | CP planes | {111}MC//{110}α |
| KS | {111}MC//{110}α <10> MC//<11>α | 24 | CP planes/directions | {111}MC//{110}α |
| BN Variant | Parallel Plane | Parallel Direction | Parallel Direction |
|---|---|---|---|
| BN 1a | (001)MC//(001)α | [110]MC//[010]α | [100]MC//[110]α |
| BN 1b | (001)MC//(001)α | [10]MC//[010]α | [00]MC//[110]α |
| BN 2a | (100)MC//(001)α | [011]MC//[010]α | [010]MC//[110]α |
| BN 2b | (100)MC//(001)α | [01]MC//[010]α | [00]MC//[110]α |
| BN 3a | (010)MC//(001)α | [101]MC//[010]α | [001]MC//[110]α |
| BN 3b | (010)MC//(001)α | [10]MC//[010]α | [100]MC//[110]α |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, J.; Li, Y.; Liu, L.; Jin, C.; Gao, X.; Deng, X.; Wang, Z. A Systematical Evaluation of the Crystallographic Orientation Relationship between MC Precipitates and Ferrite Matrix in HSLA Steels. Materials 2022, 15, 3967. https://doi.org/10.3390/ma15113967
Li X, Yang J, Li Y, Liu L, Jin C, Gao X, Deng X, Wang Z. A Systematical Evaluation of the Crystallographic Orientation Relationship between MC Precipitates and Ferrite Matrix in HSLA Steels. Materials. 2022; 15(11):3967. https://doi.org/10.3390/ma15113967
Chicago/Turabian StyleLi, Xiaolin, Jiawei Yang, Yating Li, Linxi Liu, Chi Jin, Xiangyu Gao, Xiangtao Deng, and Zhaodong Wang. 2022. "A Systematical Evaluation of the Crystallographic Orientation Relationship between MC Precipitates and Ferrite Matrix in HSLA Steels" Materials 15, no. 11: 3967. https://doi.org/10.3390/ma15113967
APA StyleLi, X., Yang, J., Li, Y., Liu, L., Jin, C., Gao, X., Deng, X., & Wang, Z. (2022). A Systematical Evaluation of the Crystallographic Orientation Relationship between MC Precipitates and Ferrite Matrix in HSLA Steels. Materials, 15(11), 3967. https://doi.org/10.3390/ma15113967







