Synthesis and Electrochemical Characterization of LiNi0.5Co0.2Mn0.3O2 Cathode Material by Solid-Phase Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cathode
2.2. Ex Site Characterizations
2.3. Electrochemical Measurements
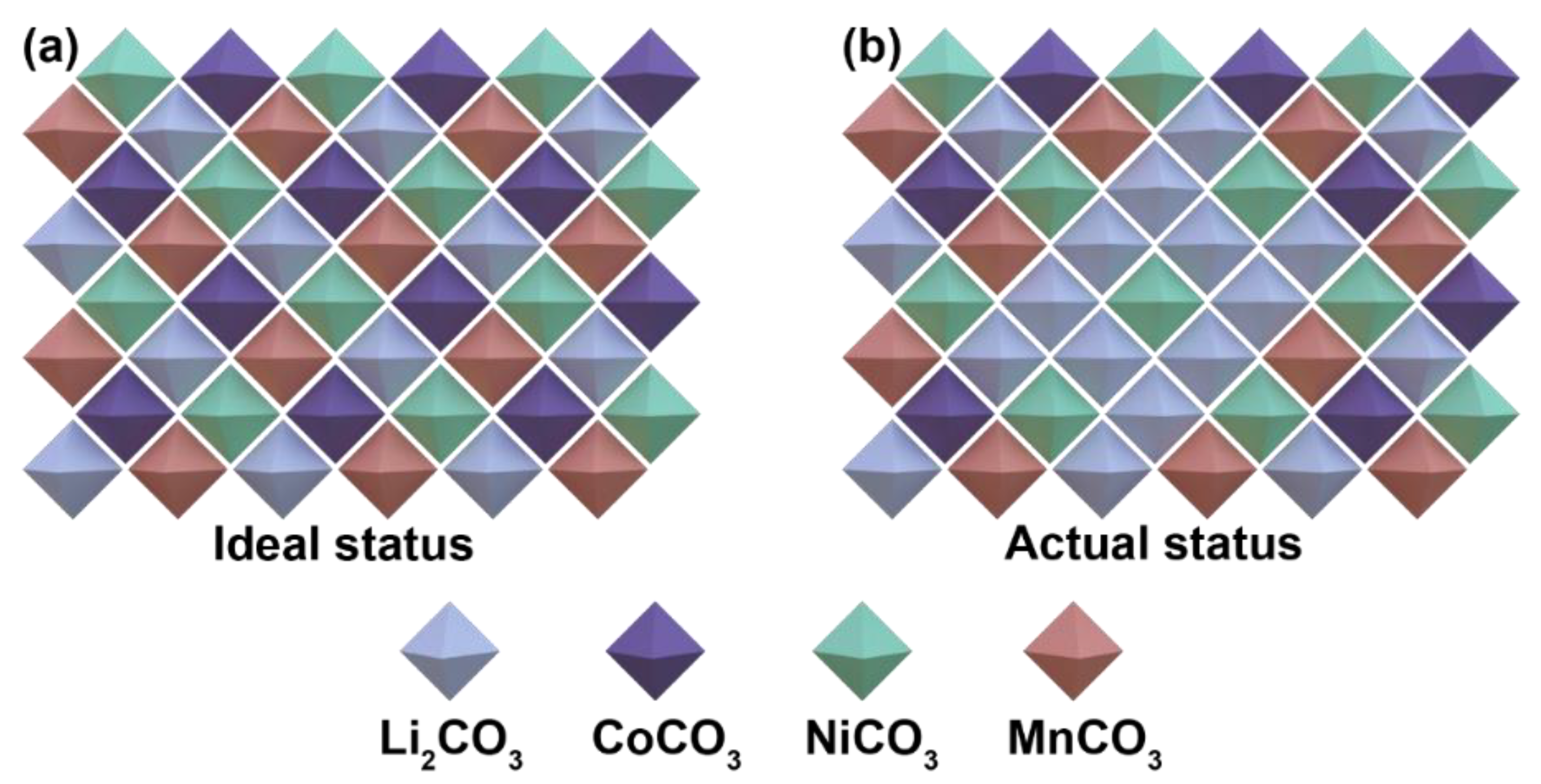
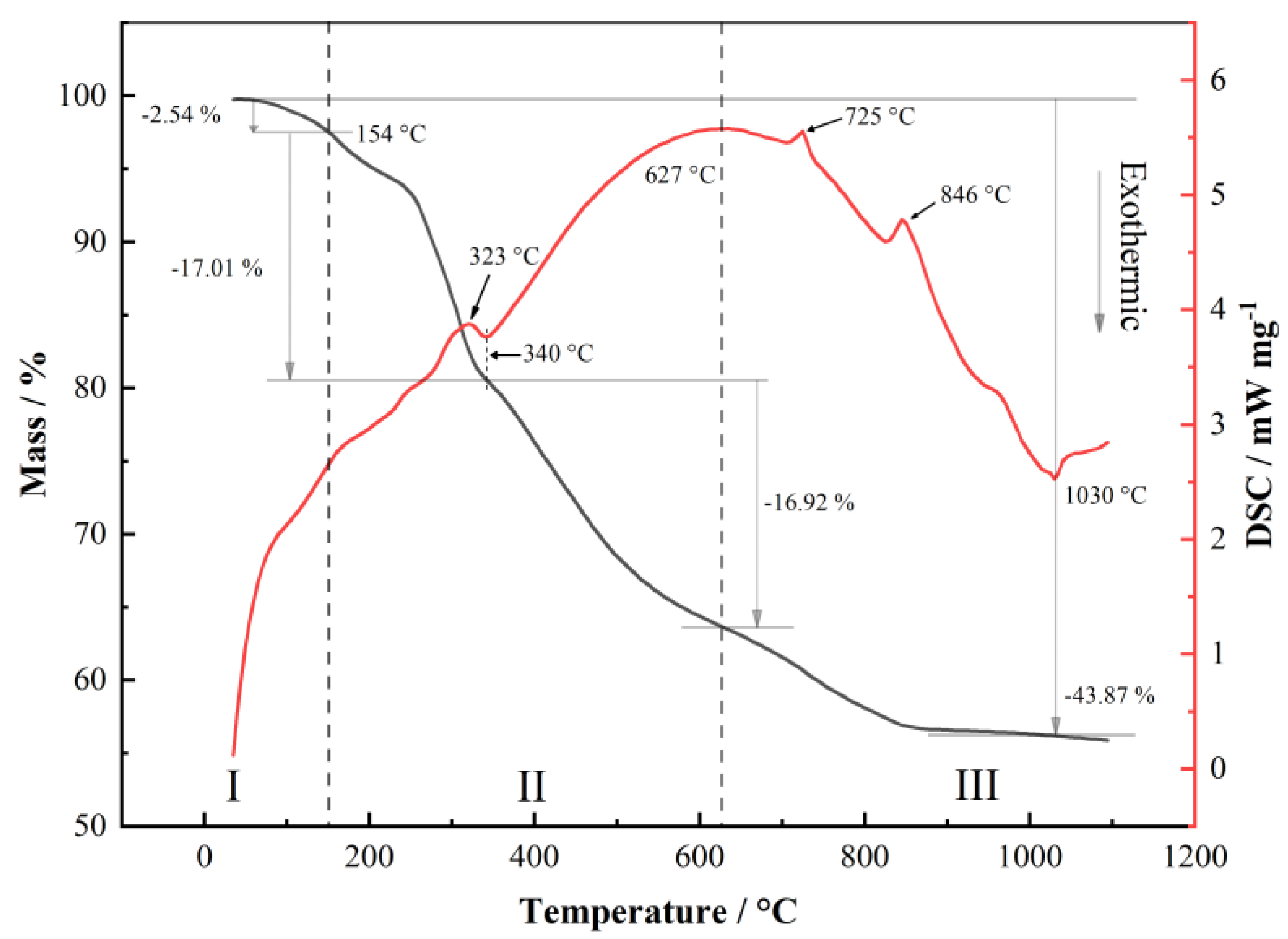
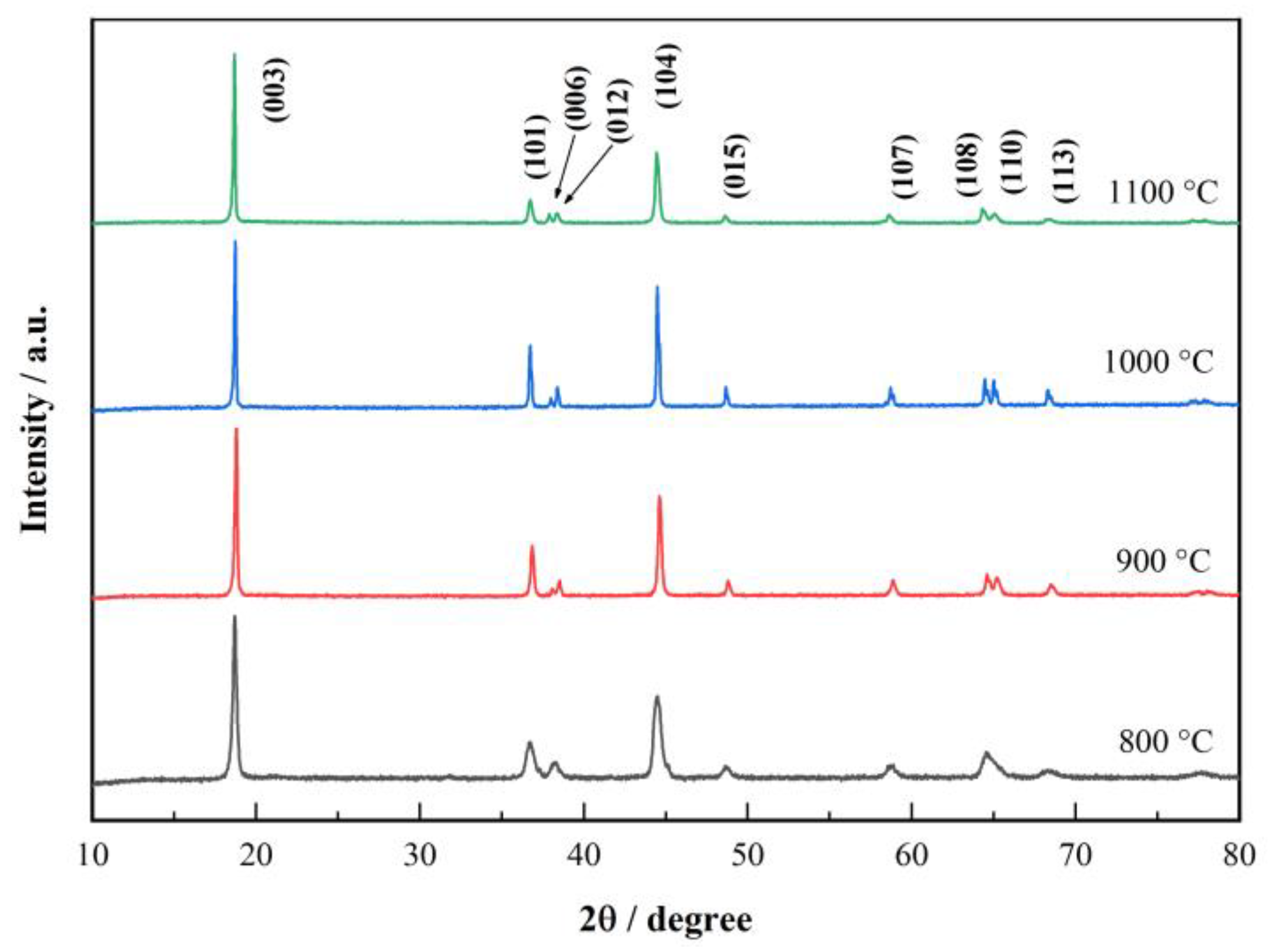
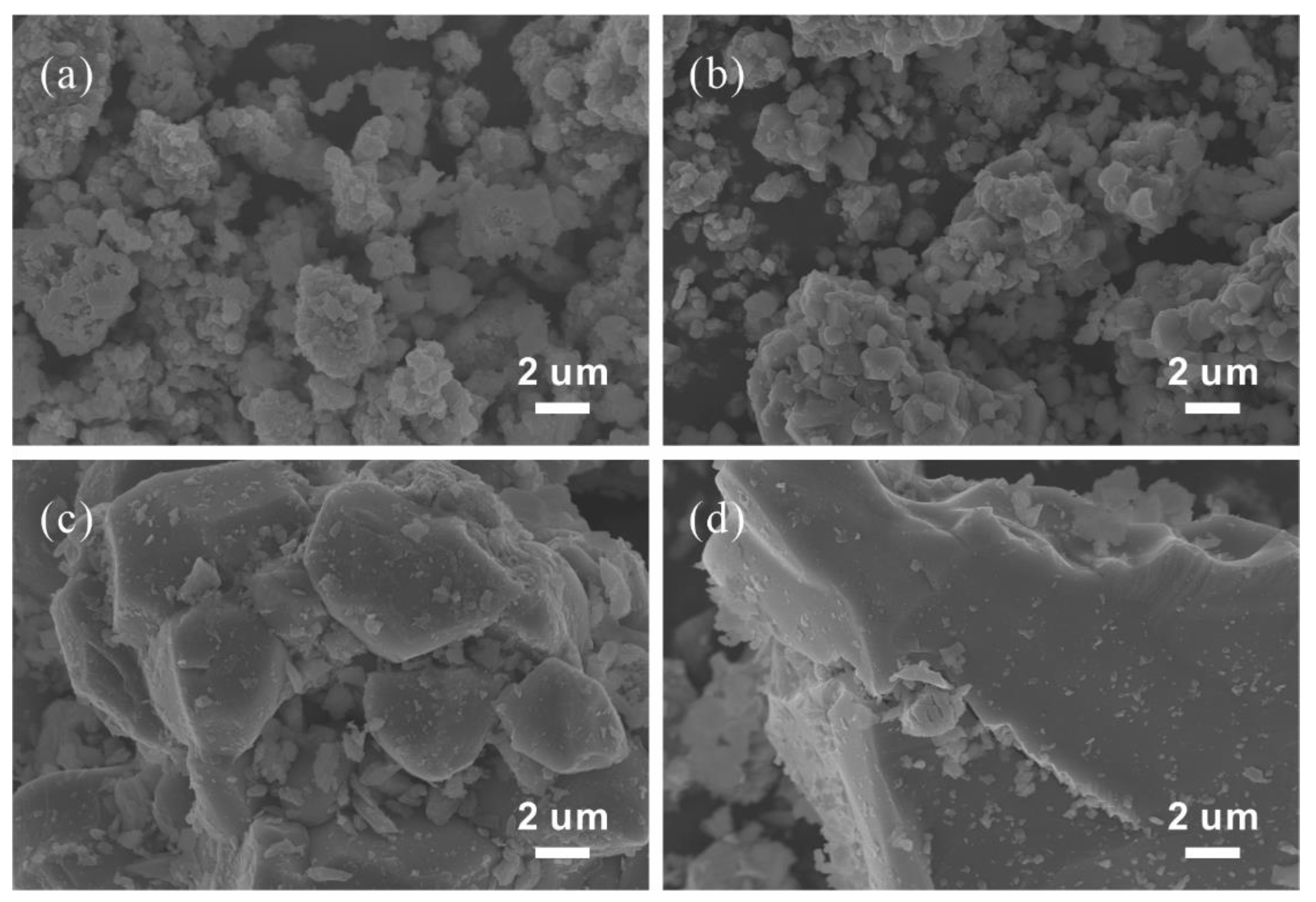
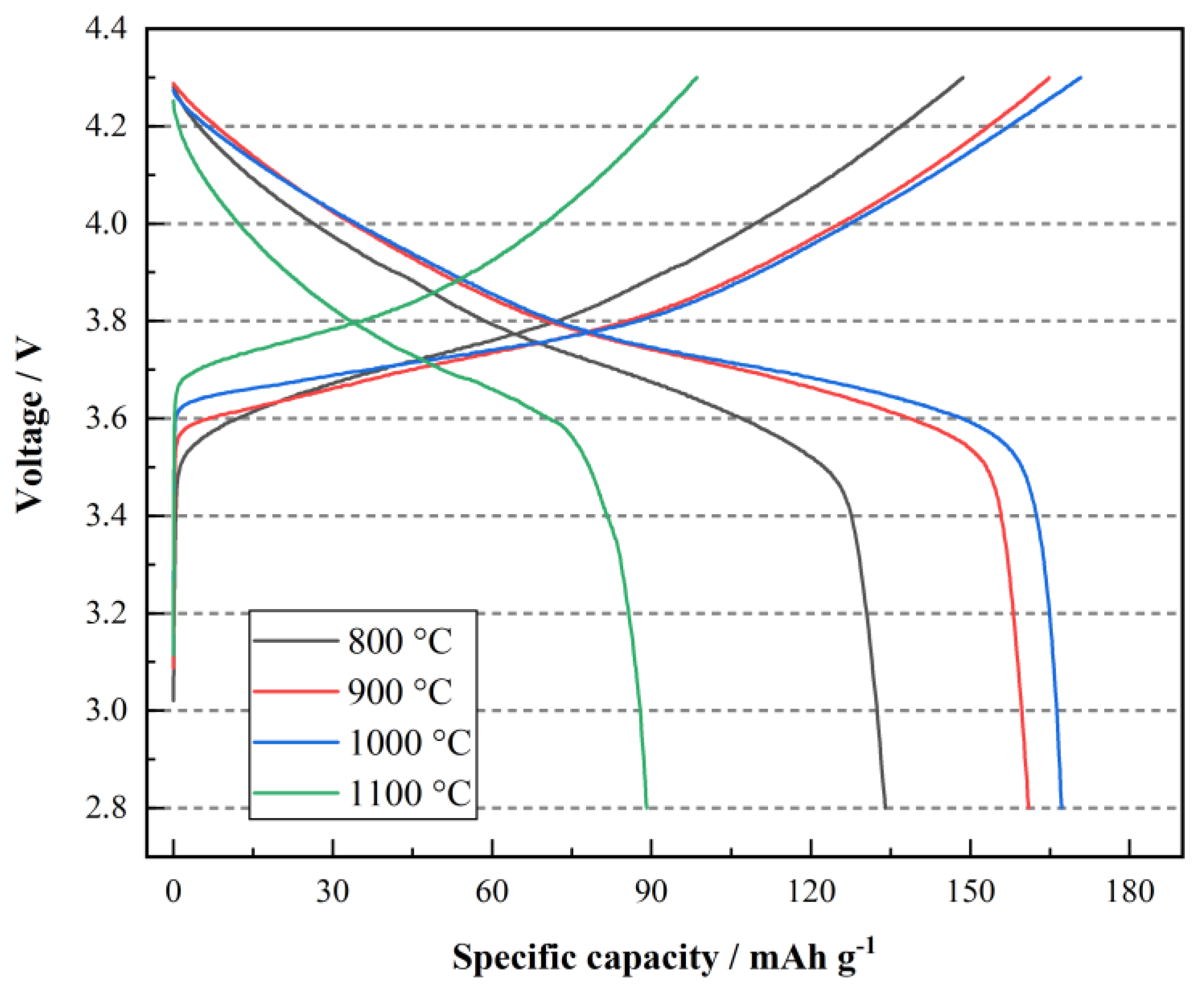
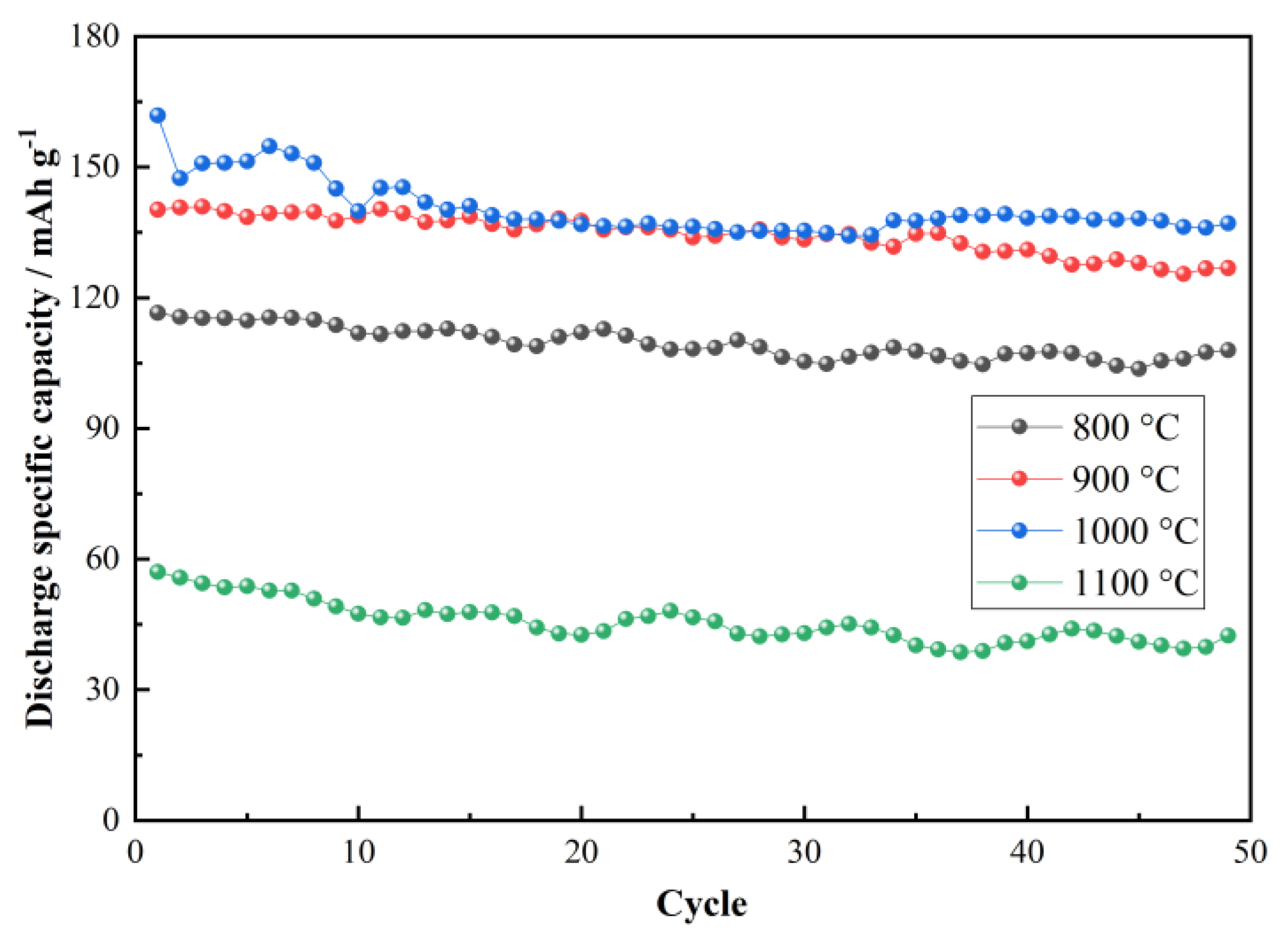
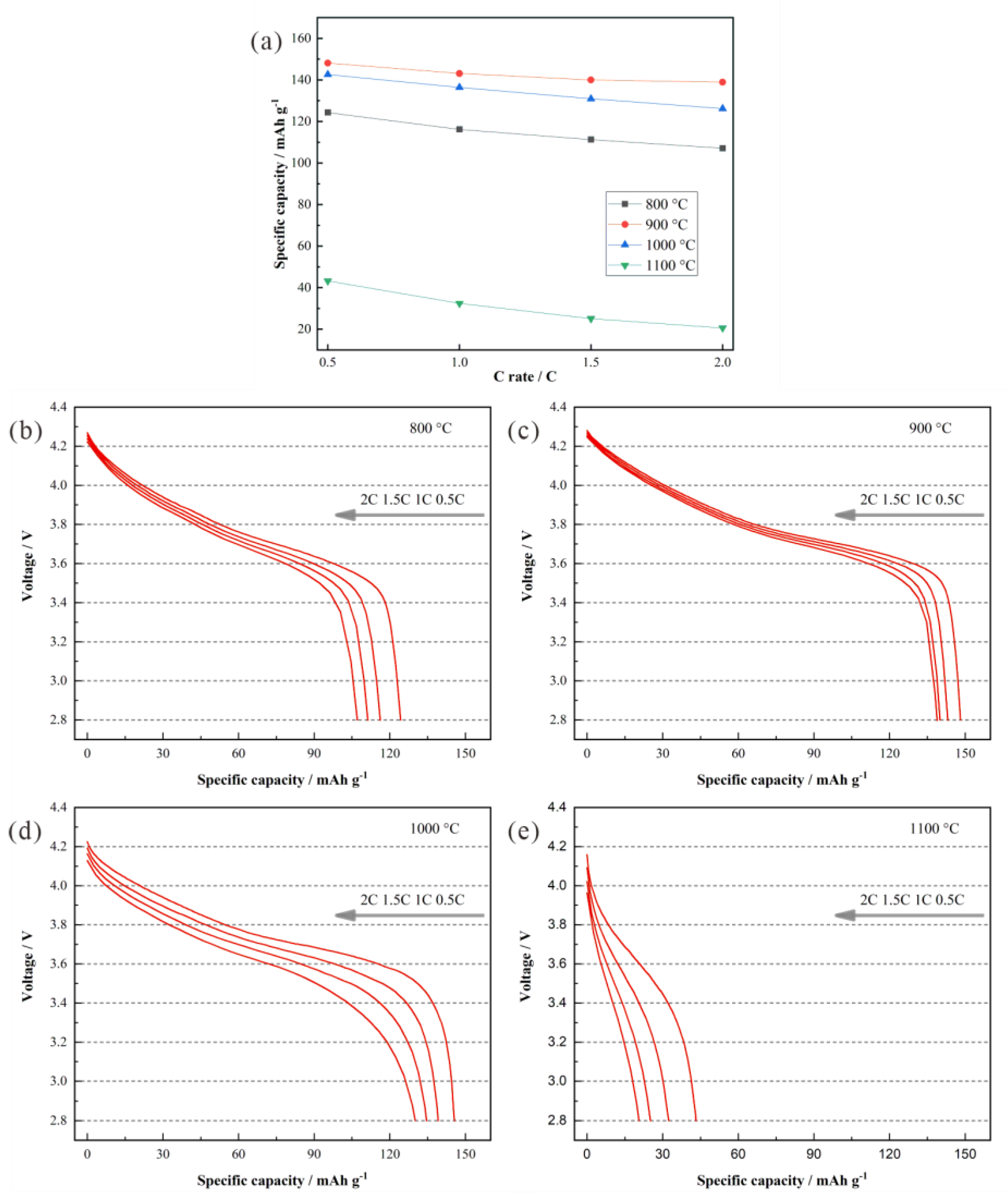
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Choi, S.; Wang, G. Advanced lithium-Ion batteries for practical applications: Technology, development, and future perspectives. Adv. Mater. Technol. 2018, 3, 1700376. [Google Scholar] [CrossRef]
- Dang, R.; Qu, Y.; Ma, Z.; Yu, L.; Duan, L.; Lü, W. The effect of elemental doping on nickel-rich NCM cathode materials of lithium ion batteries. J. Phys. Chem. C 2021, 126, 151–159. [Google Scholar] [CrossRef]
- Chen, C.; Tao, T.; Qi, W.; Zeng, H.; Wu, Y.; Liang, B.; Yao, Y.; Lu, S.; Chen, Y. High-performance lithium ion batteries using SiO2-coated LiNi0.5Co0.2Mn0.3O2 microspheres as cathodes. J. Alloys Compd. 2017, 709, 708–716. [Google Scholar] [CrossRef]
- Zhao, R.; Miao, J.; Lan, W.; Wu, Z.; Hung, I.M.; Lv, D.; Zeng, R.; Shi, G.; Chen, H. Synthesis of layered materials by ultrasonic/microwave-assisted coprecipitation method: A case study of LiNi0.5Co0.2Mn0.3O2. Sustain. Mater. Technol. 2018, 18, e00083. [Google Scholar] [CrossRef]
- Jung, S.K.; Gwon, H.; Hong, J.; Park, K.Y.; Seo, D.H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Du, K.; Hua, C.; Tan, C.; Peng, Z.; Cao, Y.; Hu, G. A high-powered concentration-gradient Li(Ni0.85Co0.12Mn0.03)O2 cathode material for lithium ion batteries. J. Power Sources 2014, 263, 203–208. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, X.; He, Z.; Weng, D.; Zhu, W. Nanoparticled Li(Ni1/3Co1/3Mn1/3)O2 as cathode material for high-rate lithium-ion batteries. J. Power Sources 2006, 153, 350–353. [Google Scholar] [CrossRef]
- Hu, S.K.; Cheng, G.H.; Cheng, M.Y.; Hwang, B.J.; Santhanam, R. Cycle life improvement of ZrO2-coated spherical LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries. J. Power Sources 2009, 188, 564–569. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.; Kim, S.I.; Martin, S.W. Enhanced electrochemical properties of LiNi(1/3)Co(1/3)Mn(1/3)O2 cathode material by coating with LiAlO2 nanoparticles. J. Power Sources 2006, 161, 623–627. [Google Scholar] [CrossRef]
- Yao, C.; Mo, Y.; Jia, X.; Chen, X.; Xia, J.; Chen, Y. LiMnPO4 surface coating on LiNi0.5Co0.2Mn0.3O2 by a simple sol-gel method and improving electrochemical properties. Solid State Ion. 2018, 317, 156–163. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Fang, C.; Meng, Y.S. Urea-based hydrothermal synthesis of LiNi0.5Co0.2Mn0.3O2 cathode material for Li-ion battery. J. Power Sources 2018, 394, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qiu, W.; Yu, L.; Zhao, H.; Li, T. Synthesis and electrochemical characterization of layered Li(Ni1/3Co1/3Mn1/3)O2 cathode materials by low-temperature solid-state reaction. J. Alloys Compd. 2008, 449, 326–330. [Google Scholar] [CrossRef]
- Tan, L.; Liu, H. High rate charge–discharge properties of LiNi1/3Co1/3Mn1/3O2 synthesized via a low temperature solid-state method. Solid State Ion. 2010, 181, 1530–1533. [Google Scholar] [CrossRef]
- Noh, H.J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Li, L.; Xia, L.; Yang, H.; Zhan, X.; Chen, J.; Chen, Z.; Duan, J. Solid-state synthesis of lanthanum-based oxides Co-coated LiNi0.5Co0.2Mn0.3O2 for advanced lithium ion batteries. J. Alloys Compd. 2020, 832, 154959. [Google Scholar] [CrossRef]
- He, Y.S.; Ma, Z.F.; Liao, X.Z. Synthesis and characterization of submicron-sized by a simple self-propagating solid-state metathesis method. J. Power Sources 2007, 163, 1053–1058. [Google Scholar] [CrossRef]
- Javanmardi, M.; Emadi, R.; Ashrafi, H. Synthesis of nickel aluminate nanoceramic compound from aluminum and nickel carbonate by mechanical alloying with subsequent annealing. Trans. Nonferrous Met. Soc. China 2016, 26, 2910–2915. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, H.G. Thermal and carbothermic decomposition of Na2CO3 and Li2CO3. Metall. Mater. Trans. B 2001, 32, 17–24. [Google Scholar] [CrossRef]
- Ouyang, Z.; Wen, P.; Chen, Y.; Ye, L. Study on thermodynamic equilibrium and character inheritance of cobalt carbonate decomposition. Vacuum 2020, 179, 109559. [Google Scholar] [CrossRef]
- Zaki, M.I.; Nohman, A.K.H.; Kappenstein, C.; Wahdan, T.M. Temperature-programmed characterization studies of thermochemical events occurring in the course of decomposition of Mn oxysalts. J. Mater. Chem. 1995, 5, 1081–1088. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.H. Surface modification of LiNi0.5Co0.2Mn0.3O2 cathode materials with Li2O-B2O3-LiBr for lithium-ion batteries. Int. J. Energy Res. 2019, 43, 4644–4651. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Li, W.; Cao, G.; Tang, S.; Su, Q.; Deng, S.; Guo, J. High-voltage electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material via the synergetic modification of the Zr/Ti elements. Electrochim. Acta 2018, 281, 48–59. [Google Scholar] [CrossRef]
- Hao, J.; Yu, Z.; Liu, H.; Song, W.; Liu, J.; Kong, L.; Li, C. Enhancing electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials derived from NiF2 artificial interface at elevated voltage. J. Alloys Compd. 2019, 806, 814–822. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhong, Y.; Wu, H.; Li, S.; Cheng, Q.; Guo, P. Coating for improving electrochemical performance of NCM523 cathode for lithium-ion batteries. Ionics 2020, 27, 13–20. [Google Scholar] [CrossRef]
- Jia, X.; Yan, M.; Zhou, Z.; Chen, X.; Yao, C.; Li, D.; Chen, D.; Chen, Y. Nd-doped LiNi0.5Co0.2Mn0.3O2 as a cathode material for better rate capability in high voltage cycling of Li-ion batteries. Electrochim. Acta 2017, 254, 50–58. [Google Scholar] [CrossRef]
- Breuer, O.; Chakraborty, A.; Liu, J.; Kravchuk, T.; Burstein, L.; Grinblat, J.; Kauffman, Y.; Gladkih, A.; Nayak, P.; Tsubery, M.; et al. Understanding the role of minor molybdenum doping in LiNi0.5Co0.2Mn0.3O2 electrodes: From structural and surface analyses and theoretical modeling to practical electrochemical cells. ACS Appl. Mater. Interfaces 2018, 10, 29608–29621. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Chen, L.; Huang, X. Electrochemical characterization of positive electrode material LiNi[1/3]Co[1/3]Mn[1/3]O[2] and compatibility with electrolyte for lithium-ion batteries. J. Electrochem. Soc. 2004, 151, A914. [Google Scholar] [CrossRef]
- Trevisanello, E.; Ruess, R.; Conforto, G.; Richter, F.H.; Janek, J. Polycrystalline and single crystalline NCM cathode materials—Quantifying particle cracking, active surface area, and lithium diffusion. Adv. Energy Mater. 2021, 11, 2003400. [Google Scholar] [CrossRef]
- He, L.P.; Li, K.; Zhang, Y.; Liu, J. Substantial doping engineering in layered LiNi0.5+xCo0.2-xMn0.3O2 materials for lithium-ion batteries. J. Electrochem. Soc. 2021, 168, 060534. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Su, B.; Xue, W.; Zhang, J. Synthesis and Electrochemical Characterization of LiNi0.5Co0.2Mn0.3O2 Cathode Material by Solid-Phase Reaction. Materials 2022, 15, 3931. https://doi.org/10.3390/ma15113931
Li X, Su B, Xue W, Zhang J. Synthesis and Electrochemical Characterization of LiNi0.5Co0.2Mn0.3O2 Cathode Material by Solid-Phase Reaction. Materials. 2022; 15(11):3931. https://doi.org/10.3390/ma15113931
Chicago/Turabian StyleLi, Xinli, Ben Su, Wendong Xue, and Junnan Zhang. 2022. "Synthesis and Electrochemical Characterization of LiNi0.5Co0.2Mn0.3O2 Cathode Material by Solid-Phase Reaction" Materials 15, no. 11: 3931. https://doi.org/10.3390/ma15113931
APA StyleLi, X., Su, B., Xue, W., & Zhang, J. (2022). Synthesis and Electrochemical Characterization of LiNi0.5Co0.2Mn0.3O2 Cathode Material by Solid-Phase Reaction. Materials, 15(11), 3931. https://doi.org/10.3390/ma15113931




