Femtosecond Laser-Ablated Copper Surface as a Substrate for a MoS2-Based Hydrogen Evolution Reaction Electrocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Femtosecond Blackening
2.2. MoS2 Electrodeposition
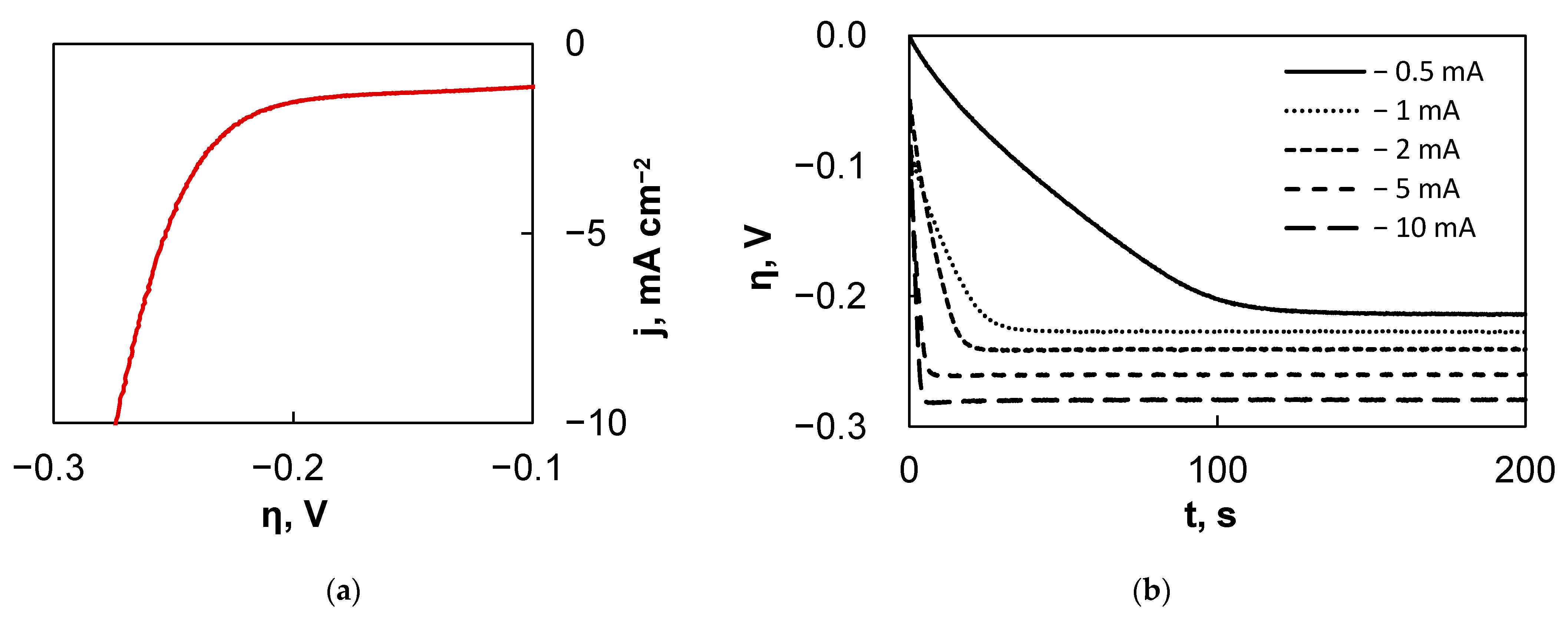
2.3. Characterization of HER Electrocatalytic Activity
2.4. Profilometrical Analysis
2.5. Scanning Electrochemical Microscopy (SECM)
3. Results and Discussion
3.1. Profilometrical Analysis, Surface Morphology and Structure
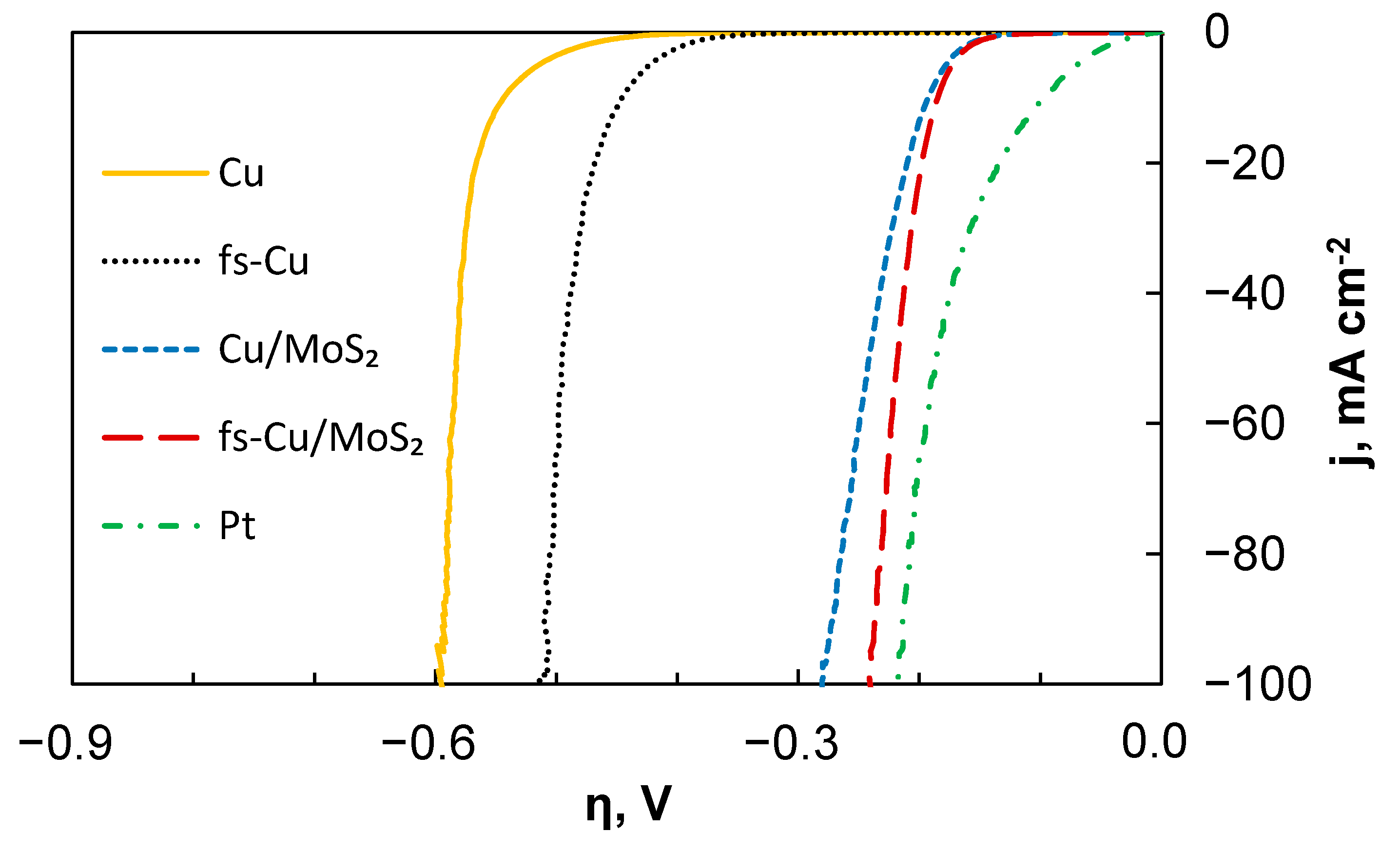
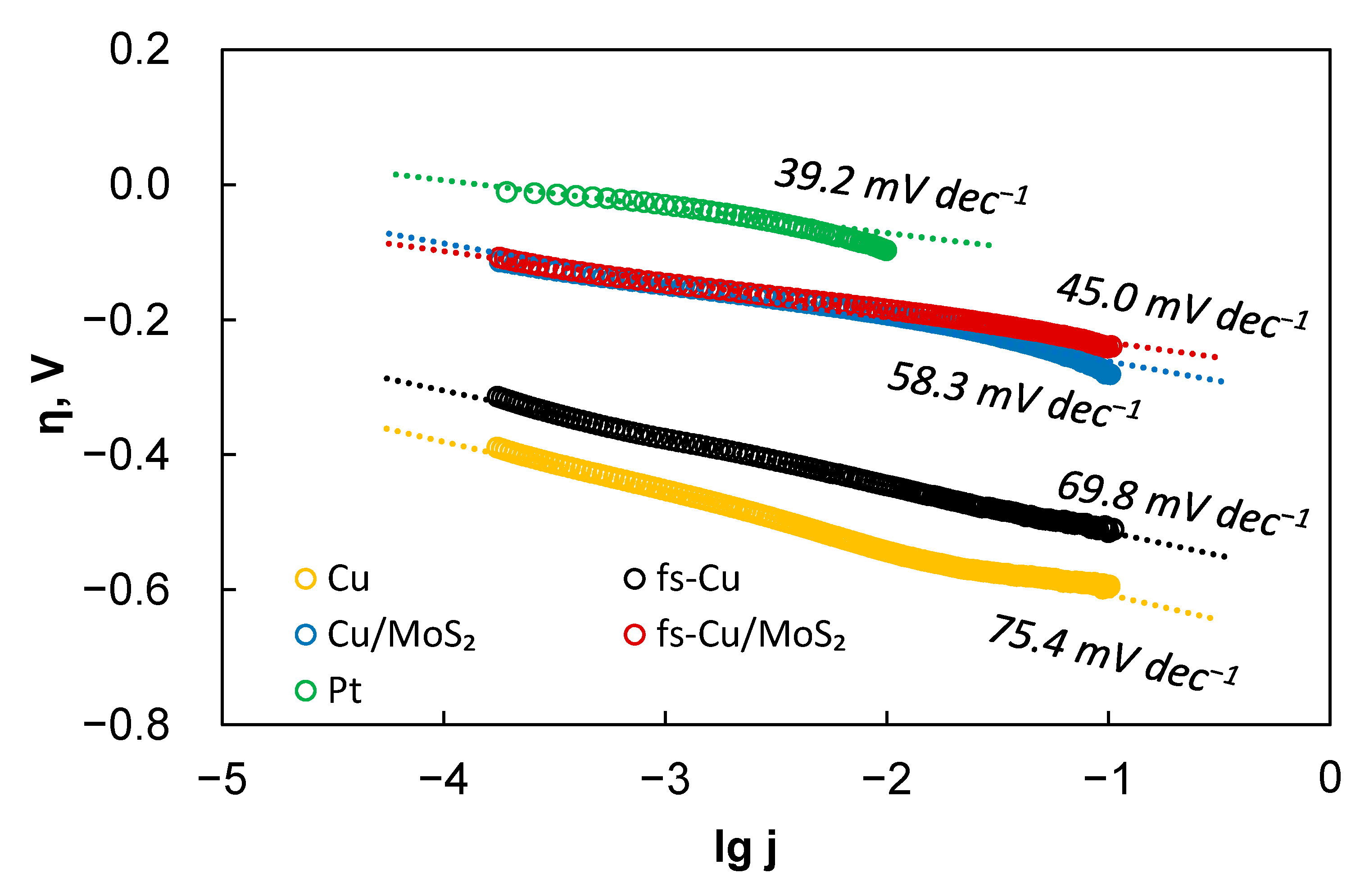
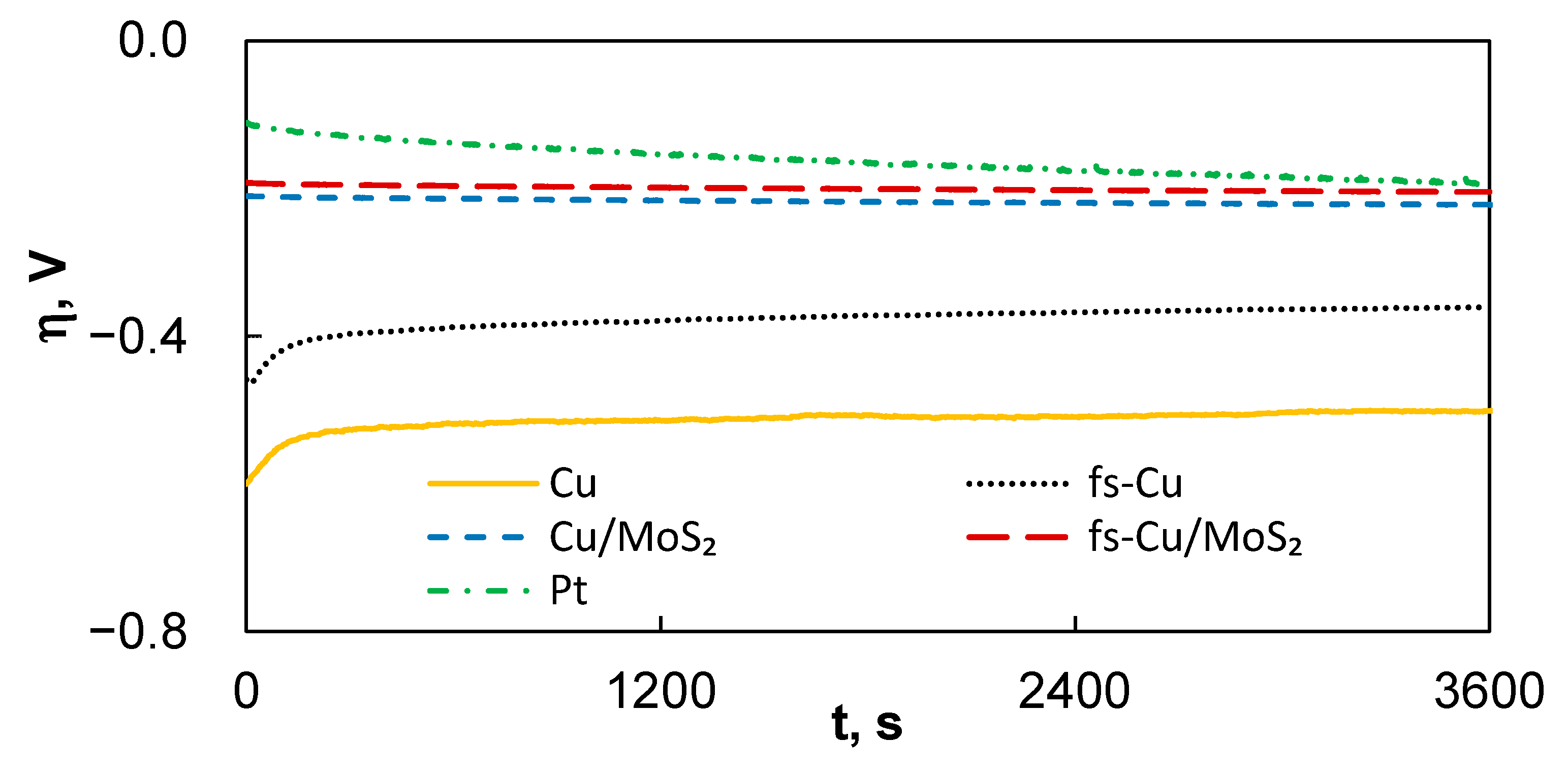
3.2. Electrocatalytic Activity
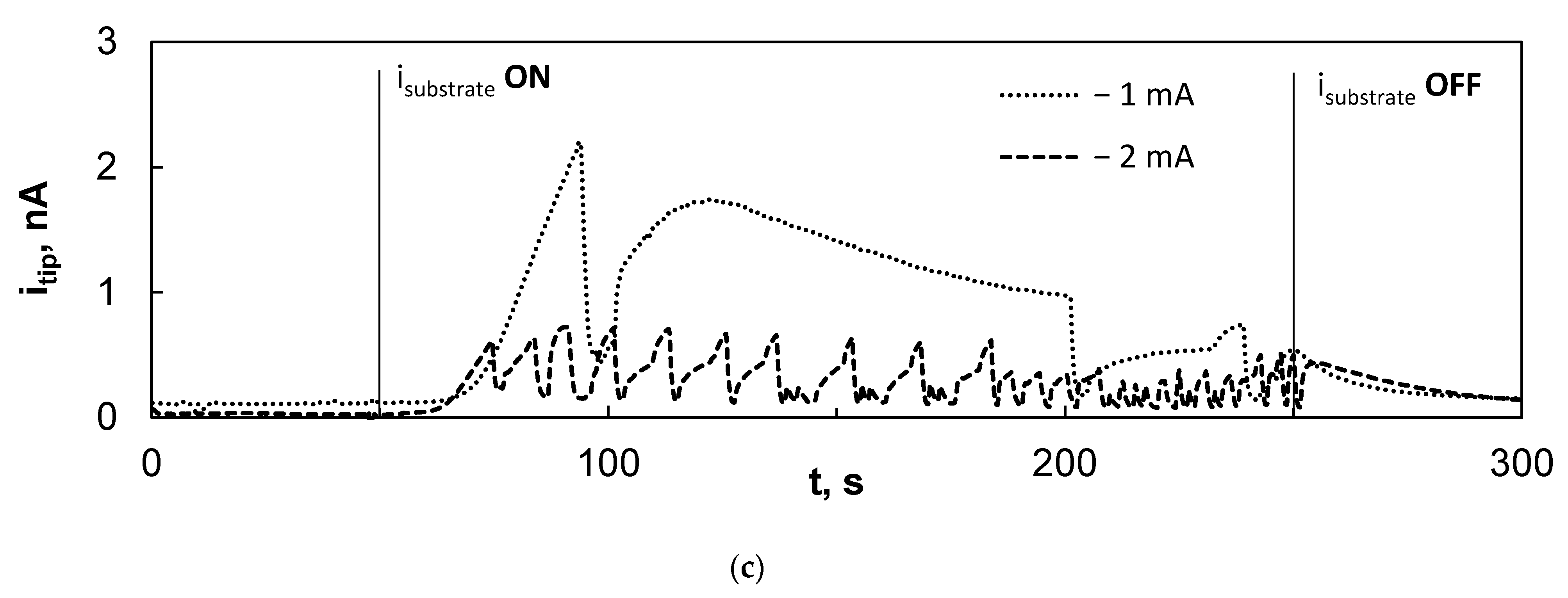
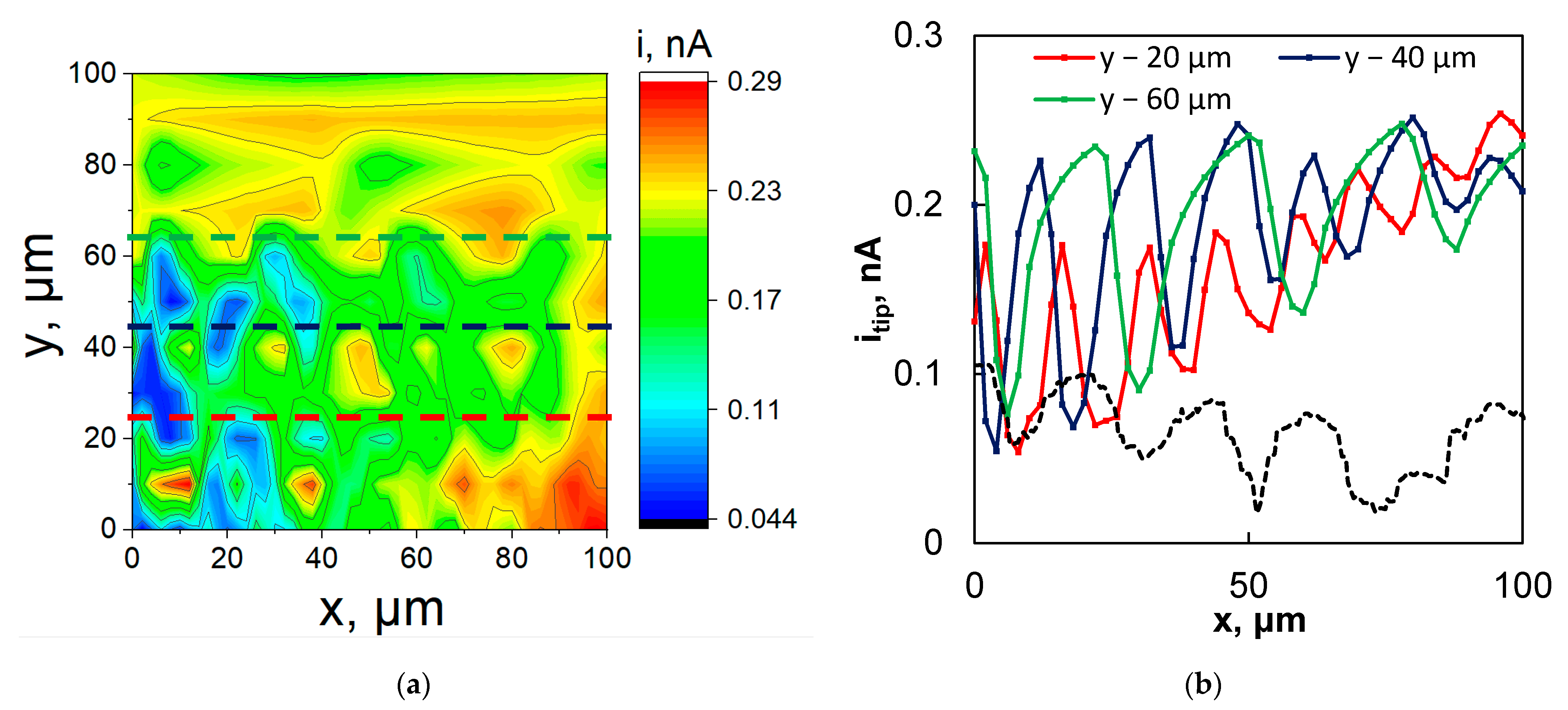
3.3. Scanning Electrochemical Microscopy Study
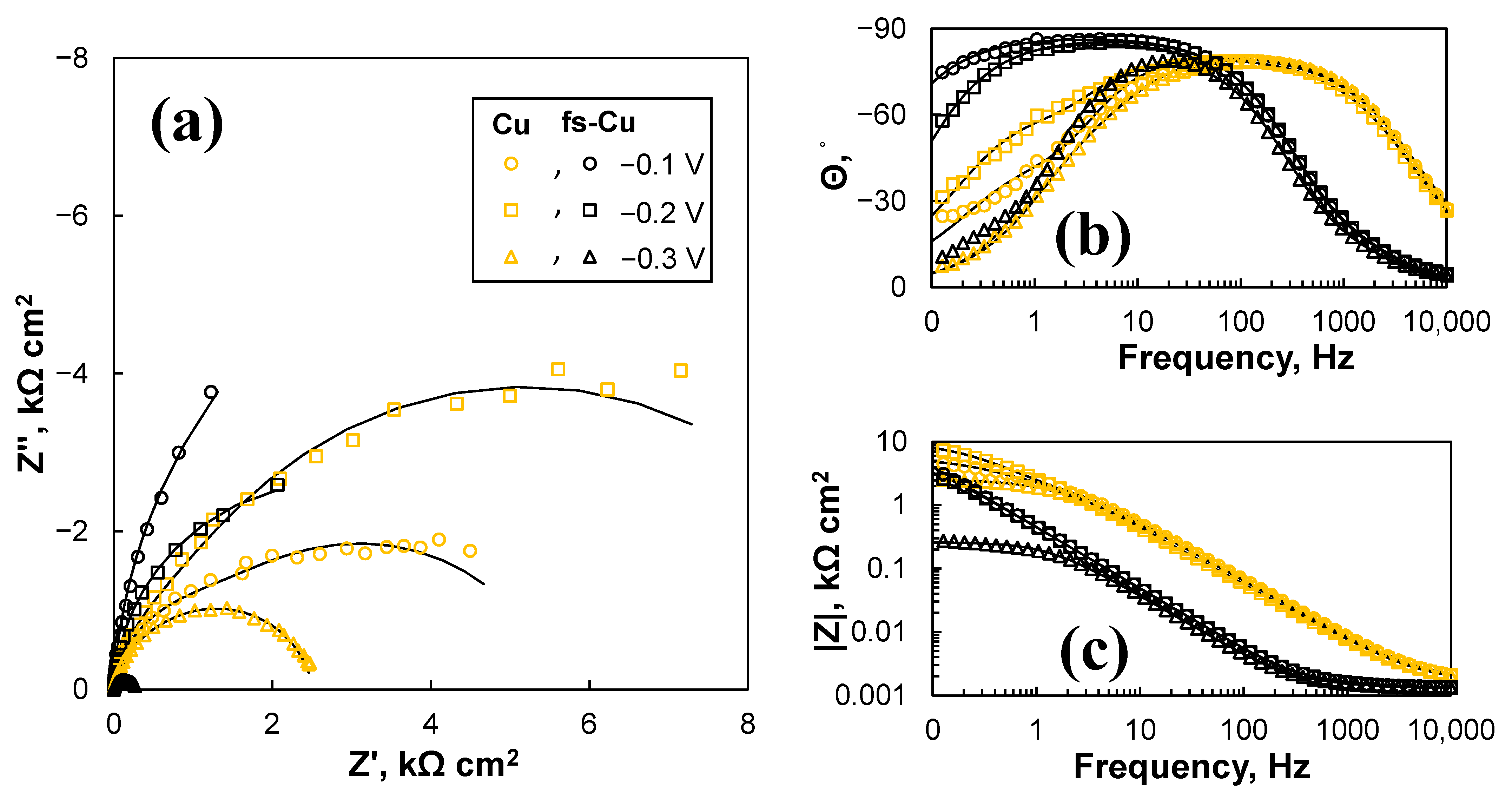
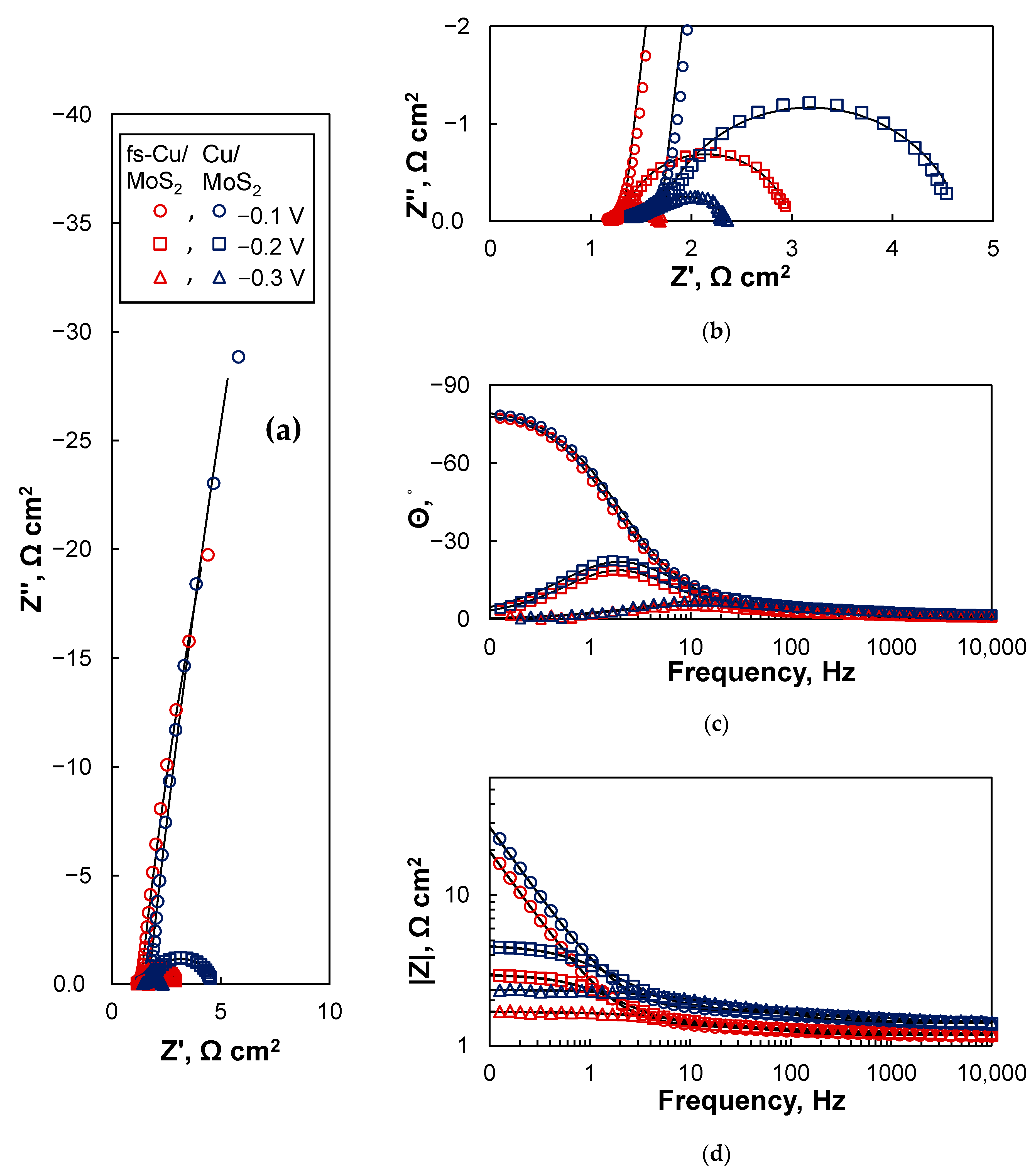
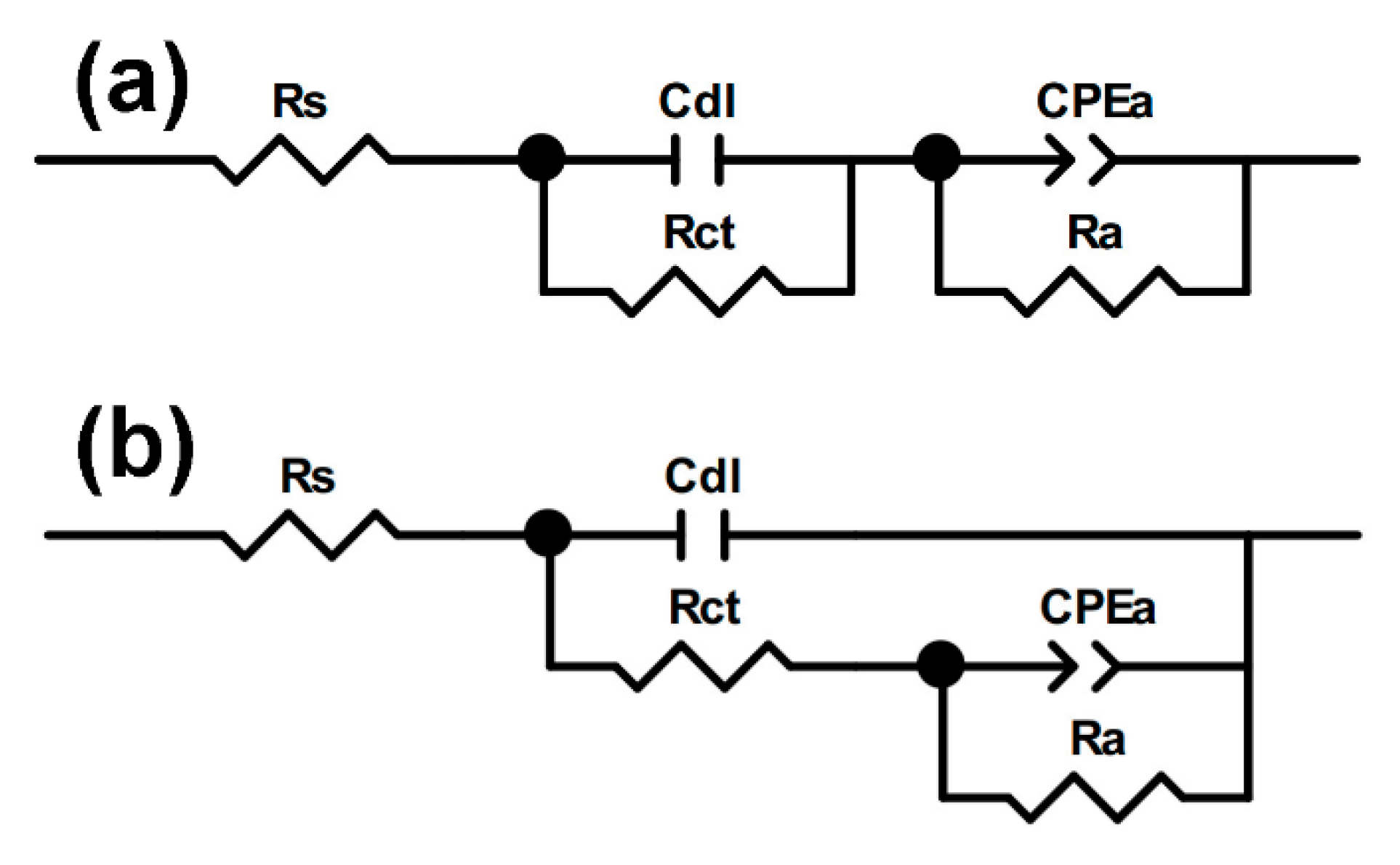
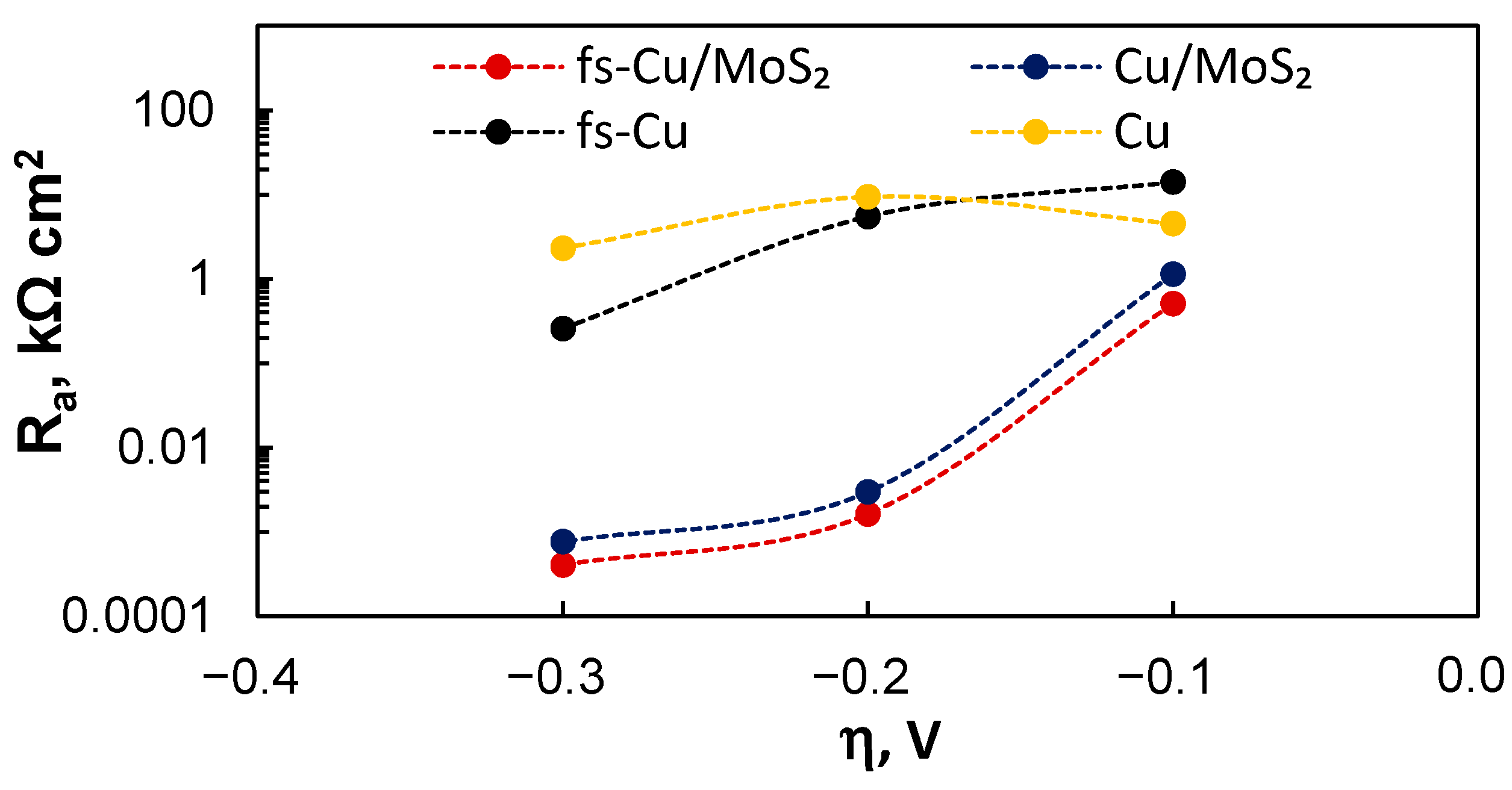
3.4. Electrochemical Impedance Spectroscopy Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirk, M.D.; Molian, P.A. A Review of Ultrashort Pulsed Laser Ablation of Materials. J. Laser Appl. 1998, 10, 18–28. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Ultrafast Lasers—Reliable Tools for Advanced Materials Processing. Light Sci. Appl. 2014, 3, e149. [Google Scholar] [CrossRef]
- Phillips, K.C.; Gandhi, H.H.; Mazur, E.; Sundaram, S.K. Ultrafast Laser Processing of Materials: A Review. Adv. Opt. Photonics 2015, 7, 684. [Google Scholar] [CrossRef]
- Amoako, G. Femtosecond Laser Structuring of Materials: A Review. APR 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Jonušauskas, L.; Mackevičiūtė, D.; Kontenis, G.; Purlys, V. Femtosecond Lasers: The Ultimate Tool for High-Precision 3D Manufacturing. Adv. Opt. Technol. 2019, 8, 241–251. [Google Scholar] [CrossRef]
- Shukla, P.; Waugh, D.G.; Lawrence, J.; Vilar, R. Laser Surface Structuring of Ceramics, Metals and Polymers for Biomedical Applications. In Laser Surface Modification of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 281–299. ISBN 978-0-08-100883-6. [Google Scholar]
- Yavtushenko, I.O.; Yavtushenko, M.S.; Zolotovskii, I.O.; Novikov, S.G.; Berintsev, A.V.; Kadochkin, A.S.; Stoliarov, D.A.; Kostishko, B.B.; Bunakov, N.A. Features of Metal Surface Structuring by High-Power Femtosecond Laser Pulses. Tech. Phys. Lett. 2015, 41, 743–746. [Google Scholar] [CrossRef]
- Vanagas, E.; Kudryashov, I.; Tuzhilin, D.; Juodkazis, S.; Matsuo, S.; Misawa, H. Surface Nanostructuring of Borosilicate Glass by Femtosecond NJ Energy Pulses. Appl. Phys. Lett. 2003, 82, 2901–2903. [Google Scholar] [CrossRef]
- Hrabovsky, J.; Liberatore, C.; Mirza, I.; Sladek, J.; Beranek, J.; Bulgakov, A.V.; Bulgakova, N.M. Surface Structuring of Kapton Polyimide with Femtosecond and Picosecond IR Laser Pulses. Interfacial Phenom. Heat Transf. 2019, 7, 113–121. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.; Bian, H.; Ou, Y.; Si, J.; Du, G.; Hou, X. Stable Superhydrophobic Surface with Hierarchical Mesh-Porous Structure Fabricated by a Femtosecond Laser. Appl. Phys. A 2013, 111, 243–249. [Google Scholar] [CrossRef]
- Volpe, A.; Gaudiuso, C.; Ancona, A. Laser Fabrication of Anti-Icing Surfaces: A Review. Materials 2020, 13, 5692. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Z. Bioinspired Surfaces with Wettability for Antifouling Application. Nanoscale 2019, 11, 22636–22663. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Colorizing Metals with Femtosecond Laser Pulses. Appl. Phys. Lett. 2008, 92, 041914. [Google Scholar] [CrossRef]
- Lee, B.E.J.; Exir, H.; Weck, A.; Grandfield, K. Characterization and Evaluation of Femtosecond Laser-Induced Sub-Micron Periodic Structures Generated on Titanium to Improve Osseointegration of Implants. Appl. Surf. Sci. 2018, 441, 1034–1042. [Google Scholar] [CrossRef]
- Duriagina, Z.; Kulyk, V.; Kovbasiuk, T.; Vasyliv, B.; Kostryzhev, A. Synthesis of Functional Surface Layers on Stainless Steels by Laser Alloying. Metals 2021, 11, 434. [Google Scholar] [CrossRef]
- Battiato, S.; Bruno, L.; Terrasi, A.; Mirabella, S. Superior Performances of Electroless-Deposited Ni–P Films Decorated with an Ultralow Content of Pt for Water-Splitting Reactions. ACS Appl. Energy Mater. 2022, 5, 2391–2399. [Google Scholar] [CrossRef]
- Li, Z.; Jalil, S.A.; Singh, S.C.; Li, W.; Wei, X.; Guo, C. Significantly Enhanced Electrocatalytic Activity of Copper for Hydrogen Evolution Reaction through Femtosecond Laser Blackening. Int. J. Hydrog. Energy 2021, 46, 10783–10788. [Google Scholar] [CrossRef] [PubMed]
- Rieck genannt Best, F.; Koch, J.; Lilienkamp, G.; Körkemeyer, F.; Maier, H.J.; Caro, J.; Lange, K. Spiky Nickel Electrodes for Electrochemical Oxygen Evolution Catalysis by Femtosecond Laser Structuring. Int. J. Electrochem. 2018, 2018, 9875438. [Google Scholar] [CrossRef]
- Koj, M.; Gimpel, T.; Schade, W.; Turek, T. Laser Structured Nickel-Iron Electrodes for Oxygen Evolution in Alkaline Water Electrolysis. Int. J. Hydrog. Energy 2019, 44, 12671–12684. [Google Scholar] [CrossRef]
- Gabler, A.; Müller, C.I.; Rauscher, T.; Köhring, M.; Kieback, B.; Röntzsch, L.; Schade, W. Ultrashort Pulse Laser-Structured Nickel Surfaces as Hydrogen Evolution Electrodes for Alkaline Water Electrolysis. Int. J. Hydrog. Energy 2017, 42, 10826–10833. [Google Scholar] [CrossRef]
- Ou, G.; Fan, P.; Zhang, H.; Huang, K.; Yang, C.; Yu, W.; Wei, H.; Zhong, M.; Wu, H.; Li, Y. Large-Scale Hierarchical Oxide Nanostructures for High-Performance Electrocatalytic Water Splitting. Nano Energy 2017, 35, 207–214. [Google Scholar] [CrossRef]
- Hoffmann, V.; Hoffmann, L.; Schade, W.; Turek, T.; Gimpel, T. Simple Femtosecond Laser-Based Production of Enlarged Nickel Surfaces Alloyed with Molybdenum, Iron and Cobalt Using Aqueous Solutions and Metal Foils. Appl. Surf. Sci. 2021, 541, 148481. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Hu, J.; Qiu, Z.; Liu, W.; Lu, J.; Yin, J. Self-Supported Cobalt Oxide Electrocatalysts with Hierarchical Chestnut Burr-like Nanostructure for Efficient Overall Water Splitting. Chem. Eng. J. 2022, 435, 134995. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Wang, D.; Xu, H.; Wang, B.; Meng, F.; Zhang, J.; An, M. Electrodeposition: Synthesis of Advanced Transition Metal-Based Catalyst for Hydrogen Production via Electrolysis of Water. J. Energy Chem. 2021, 57, 547–566. [Google Scholar] [CrossRef]
- Maniam, K.K.; Chetty, R.; Thimmappa, R.; Paul, S. Progress in the Development of Electrodeposited Catalysts for Direct Liquid Fuel Cell Applications. Appl. Sci. 2022, 12, 501. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, C.; Liu, C.; Zuo, M.; Qin, L.; Yan, X.; Xing, Y.; Li, H.; Si, R.; Zhou, S.; et al. Electrochemical Deposition as a Universal Route for Fabricating Single-Atom Catalysts. Nat. Commun. 2020, 11, 1215. [Google Scholar] [CrossRef] [Green Version]
- Do, H.H.; Ha, T.D.C.; Jo, H.; Ok, K.M.; Cho, J.H.; Ahn, S.H.; Kim, M.-G.; Kim, S.Y. Low-Temperature Synthesis of Molybdenum Sulfides, Tungsten Sulfides, and Composites Thereof as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Appl. Surf. Sci. 2022, 576, 151828. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Li, J.; Lei, J. Electrodeposition of Amorphous Molybdenum Sulfide Thin Film for Electrochemical Hydrogen Evolution Reaction. BMC Chem. 2019, 13, 88. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, S.; Kang, G.-S.; Lee, Y.-K.; Park, G.G.; Lee, D.C.; Joh, H.-I. Insight into the Superior Activity of Bridging Sulfur-Rich Amorphous Molybdenum Sulfide for Electrochemical Hydrogen Evolution Reaction. Appl. Catal. B Environ. 2019, 258, 117995. [Google Scholar] [CrossRef]
- Bose, R.; Balasingam, S.K.; Shin, S.; Jin, Z.; Kwon, D.H.; Jun, Y.; Min, Y.-S. Importance of Hydrophilic Pretreatment in the Hydrothermal Growth of Amorphous Molybdenum Sulfide for Hydrogen Evolution Catalysis. Langmuir 2015, 31, 5220–5227. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, J.; Li, B.; Shi, L.; Ji, H.; Song, L.; Xu, H. Amorphous Molybdenum Sulfide/Carbon Nanotubes Hybrid Nanospheres Prepared by Ultrasonic Spray Pyrolysis for Electrocatalytic Hydrogen Evolution. Small 2017, 13, 1700111. [Google Scholar] [CrossRef]
- Wang, T.; Zhuo, J.; Du, K.; Chen, B.; Zhu, Z.; Shao, Y.; Li, M. Electrochemically Fabricated Polypyrrole and MoSx Copolymer Films as a Highly Active Hydrogen Evolution Electrocatalyst. Adv. Mater. 2014, 26, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Mabayoje, O.; Liu, Y.; Wang, M.; Shoola, A.; Ebrahim, A.M.; Frenkel, A.I.; Mullins, C.B. Electrodeposition of MoSx Hydrogen Evolution Catalysts from Sulfur-Rich Precursors. ACS Appl. Mater. Interfaces 2019, 11, 32879–32886. [Google Scholar] [CrossRef] [PubMed]
- Levinas, R.; Tsyntsaru, N.; Cesiulis, H. The Characterisation of Electrodeposited MoS2 Thin Films on a Foam-Based Electrode for Hydrogen Evolution. Catalysts 2020, 10, 1182. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.-M.; Bai, S.; Liu, J. Blackening of Metals Using Femtosecond Fiber Laser. Appl. Opt. 2015, 54, 324. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, E.A.; Neumann-Spallart, M.; Hodes, G.; Lévy-Clément, C. Electrochemical Deposition of MoS2 Thin Films by Reduction of Tetrathiomolybdate. Thin Solid Film. 1996, 280, 86–89. [Google Scholar] [CrossRef]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous Molybdenum Sulfide Films as Catalysts for Electrochemical Hydrogen Production in Water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Lamouchi, A.; Assaker, I.B.; Chtourou, R. Effect of Annealing Temperature on the Structural, Optical, and Electrical Properties of MoS2 Electrodeposited onto Stainless Steel Mesh. J. Mater. Sci. 2017, 52, 4635–4646. [Google Scholar] [CrossRef]
- Fletcher, S. Tafel Slopes from First Principles. J. Solid State Electrochem. 2009, 13, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [Green Version]
- Prats, H.; Chan, K. The Determination of the HOR/HER Reaction Mechanism from Experimental Kinetic Data. Phys. Chem. Chem. Phys. 2021, 23, 27150–27158. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S.; Driess, M.; Menezes, P.W. The Pitfalls of Using Potentiodynamic Polarization Curves for Tafel Analysis in Electrocatalytic Water Splitting. ACS Energy Lett. 2021, 6, 1607–1611. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Medina, M.; Corradini, P.G.; de Brito, J.F.; Sousa Santos, H.L.; Mascaro, L.H. The Substrate Morphology Effect for Sulfur-Rich Amorphous Molybdenum Sulfide for Electrochemical Hydrogen Evolution Reaction. J. Electrochem. Soc. 2022, 169, 026519. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Chen, J.; Xing, X.; Wang, Y.; Zhang, Y.; Yang, M.; Zhang, G. Confined Synthesis of MoS2 with Rich Co-Doped Edges for Enhanced Hydrogen Evolution Performance. J. Energy Chem. 2022, 70, 18–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Hwang, J.; Tang, M.T.; Chun, H.; Wang, X.; Zhao, H.; Chan, K.; Han, B.; Gao, P.; Li, H. Ultrastable Molybdenum Disulfide-Based Electrocatalyst for Hydrogen Evolution in Acidic Media. J. Power Sources 2020, 456, 227998. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Huang, Y.; Nielsen, R.A.; Goddard, W.A.; Li, Y.; Cao, L. Engineering the Composition and Crystallinity of Molybdenum Sulfide for High-Performance Electrocatalytic Hydrogen Evolution. ACS Catal. 2015, 5, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Abidi, N.; Bonduelle-Skrzypczak, A.; Steinmann, S.N. How Stable Are 2H-MoS2 Edges under Hydrogen Evolution Reaction Conditions? J. Phys. Chem. C 2021, 125, 17058–17067. [Google Scholar] [CrossRef]
- Filotás, D.; Fernández-Pérez, B.M.; Nagy, L.; Nagy, G.; Souto, R.M. A Novel Scanning Electrochemical Microscopy Strategy for the Investigation of Anomalous Hydrogen Evolution from AZ63 Magnesium Alloy. Sens. Actuators B Chem. 2020, 308, 127691. [Google Scholar] [CrossRef]
- Liang, Z.; Ahn, H.S.; Bard, A.J. A Study of the Mechanism of the Hydrogen Evolution Reaction on Nickel by Surface Interrogation Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 4854–4858. [Google Scholar] [CrossRef]
- Fernández, J.L.; Zoski, C.G. Voltammetric and Scanning Electrochemical Microscopy Investigations of the Hydrogen Evolution Reaction in Acid at Nanostructured Ensembles of Ultramicroelectrode Dimensions: Theory and Experiment. J. Phys. Chem. C 2018, 122, 71–82. [Google Scholar] [CrossRef]
- Levinas, R.; Tsyntsaru, N.; Cesiulis, H. Insights into Electrodeposition and Catalytic Activity of MoS2 for Hydrogen Evolution Reaction Electrocatalysis. Electrochim. Acta 2019, 317, 427–436. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All The Catalytic Active Sites of MoS 2 for Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef] [PubMed]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications. In Modern Aspects of Electrochemistry; Conway, B.E., Bockris, J.O., White, R.E., Eds.; Modern Aspects of Electrochemistry; Kluwer Academic Publishers: Boston, MA, USA, 2002; Volume 32, pp. 143–248. ISBN 978-0-306-45964-1. [Google Scholar]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The Analysis of Electrode Impedances Complicated by the Presence of a Constant Phase Element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Kakaei, M.N.; Neshati, J.; Rezaierod, A.R. On the Extraction of the Effective Capacitance from Constant Phase Element Parameters. Prot. Met. Phys. Chem. Surf. 2018, 54, 548–556. [Google Scholar] [CrossRef]
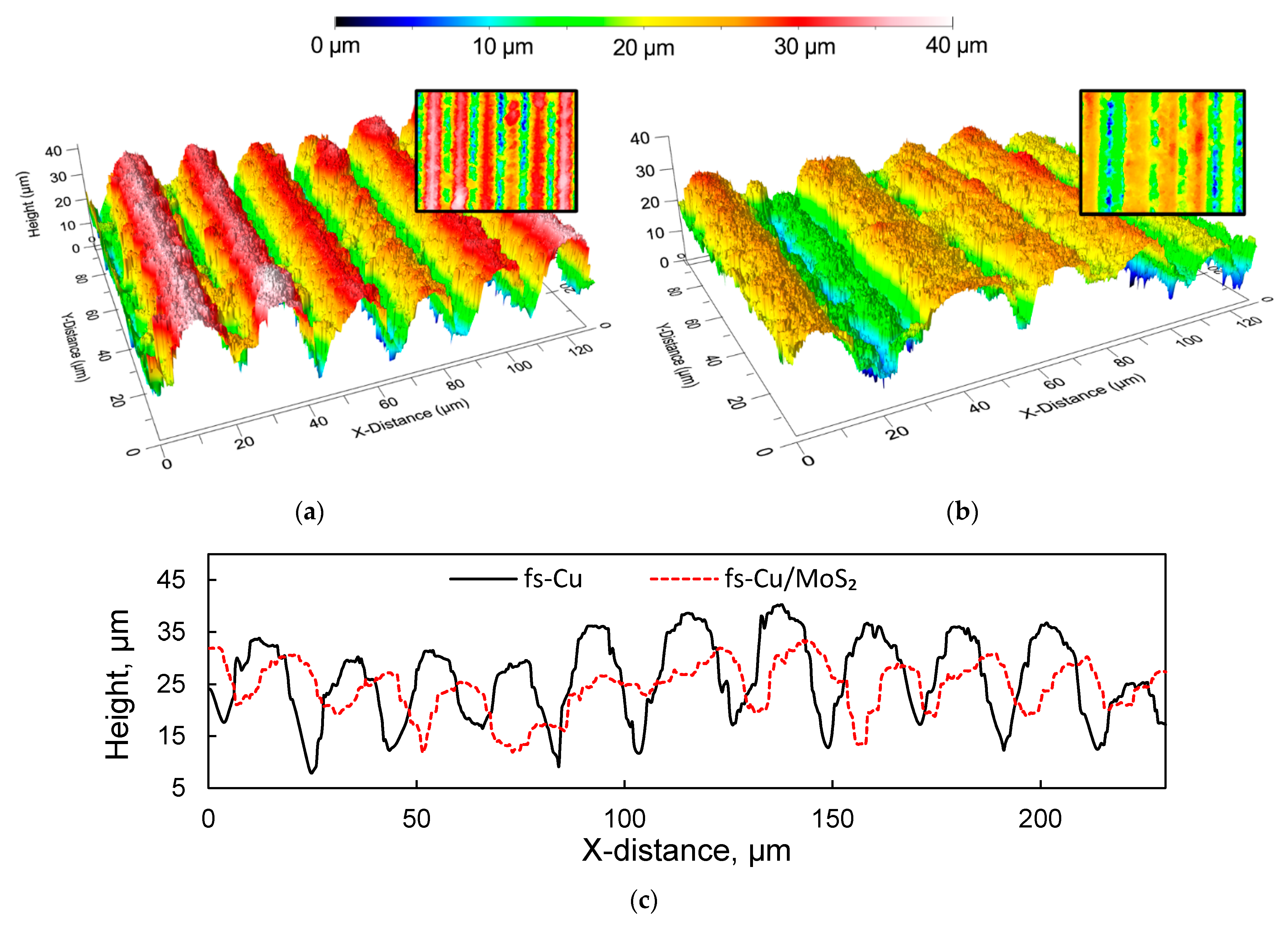
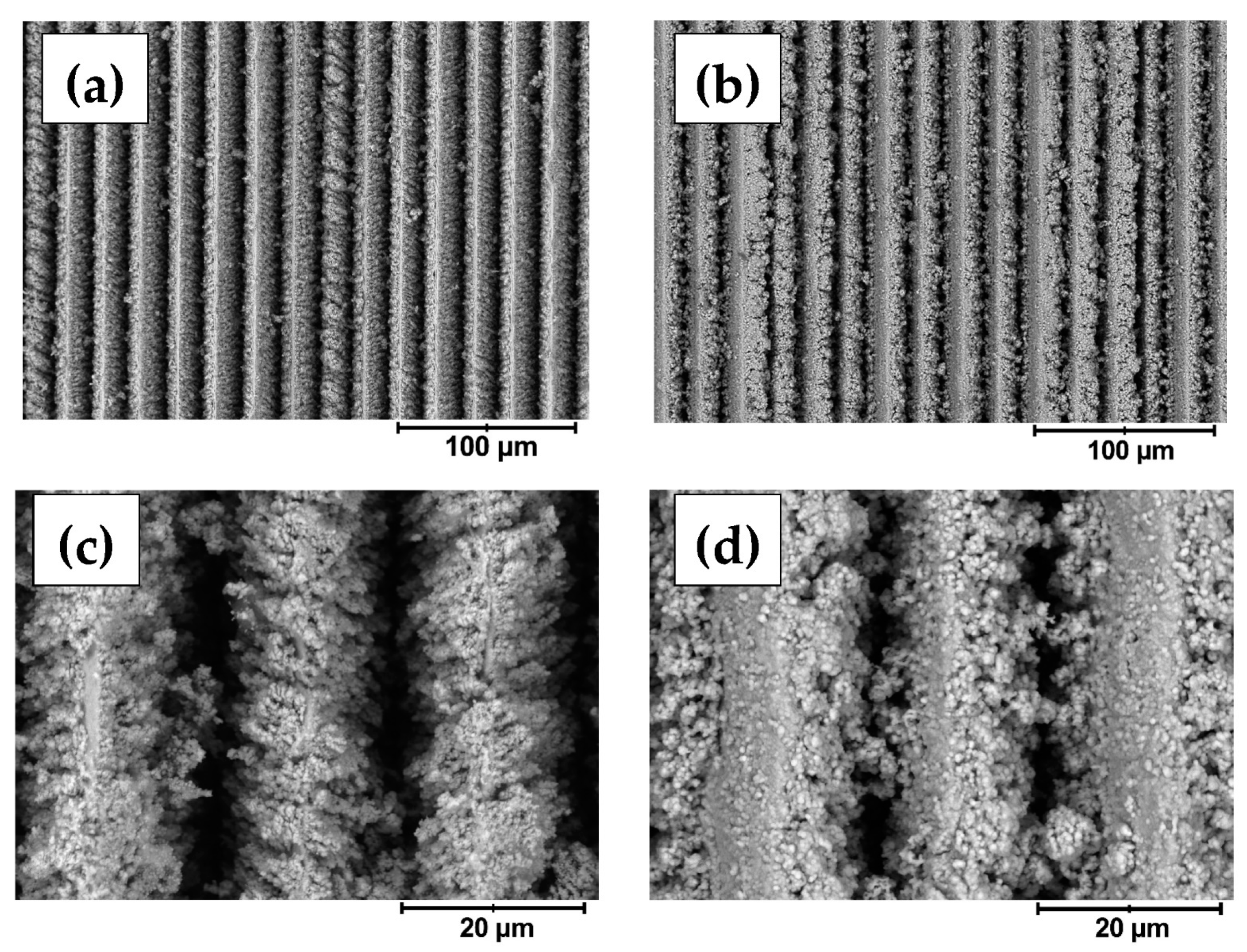
| Sa, µm | Sv, µm | Sp, µm | Spar, mm2 | Sdar, mm2 | |
|---|---|---|---|---|---|
| fs-Cu | 6.86 | 53.11 | 24.26 | 0.25 | 10.46 |
| fs-Cu/MoS2 | 4.94 | 48.36 | 16.21 | 0.25 | 4.68 |
| Sample | Tafel Slope, mV dec−1 | j0, µA cm−2 | η10mA, mV |
|---|---|---|---|
| Cu | 75.4 | 0.00089 | −552 |
| fs-Cu | 69.8 | 0.0043 | −445 |
| Cu/MoS2 | 58.3 | 3.23 | −193 |
| fs-Cu/MoS2 | 45.0 | 0.65 | −185 |
| Pt | 39.2 | 150 | −97 |
| Sample | Synthesis Method | Tafel Slope, mV dec−1 | η10mA, mV | Reference |
|---|---|---|---|---|
| rGO/MoSx−100 °C | Hydrothermal | 48.8 | −125 | [27] |
| MoSx | Electrodeposition | 55 | −211 | [28] |
| 67% Sbr-MoSx | Chemical synthesis | 46 | −96 | [29] |
| MoS6 | Anodic electrodeposition | - | −161 | [33] |
| Cu-foam/MoS2 | Electrodeposition | 49 | −142 | [34] |
| MoSx-TiO2NT | Electrodeposition | 43 | −73 | [44] |
| Co-doped MoS2 | In situ sulfuration | - | −155 | [45] |
| Pd0.2-MoS2 | Solvothermal | 60 | −106 | [46] |
| Cu/MoS2 | Electrodeposition | 58.3 | −193 | This work |
| fs-Cu/MoS2 | Electrodeposition | 45.0 | −185 | This work |
| Cu | ||||
| η, V | Cdl, mF cm−2 | Rct, Ω cm2 | Ca, mF cm−2 | Ra, kΩ cm2 |
| −0.1 | 0.0529 ± 0.0035 | 1033 ± 32 | 0.176 ± 0.0091 | 4.53 ± 0.049 |
| −0.2 | 0.114 ± 0.0089 | 546 ± 21 | 0.113 ± 0.0022 | 9.41 ± 0.069 |
| −0.3 | 0.126 ± 0.018 | 191 ± 13 | 0.072 ± 0.0021 | 2.32 ± 0.011 |
| fs-Cu | ||||
| −0.1 | 0.607 ± 0.063 | 0.164 ± 0.0096 | 0.387 ± 0.0013 | 14.1 ± 0.33 |
| −0.2 | 0.582 ± 0.063 | 0.169 ± 0.010 | 0.390 ± 0.0015 | 5.49 ± 0.063 |
| −0.3 | 0.644 ± 0.23 | 0.138 ± 0.027 | 0.544 ± 0.010 | 0.258 ± 0.0028 |
| Cu/MoS2 | ||||
| −0.1 | 3.76 ± 0.33 | 0.320 ± 0.013 | 49.6 ± 0.53 | 1.14 ± 0.068 |
| −0.2 | 2.03 ± 0.19 | 0.264 ± 0.012 | 58.9 ± 1.7 | 0.00299 ± 4.3 × 10−5 |
| −0.3 | 0.805 ± 0.073 | 0.149 ± 0.0083 | 33.6 ± 1.8 | 0.000768 ± 1.3 × 10−5 |
| fs-Cu/MoS2 | ||||
| −0.1 | 10.2 ± 0.90 | 0.206 ± 0.011 | 64.9 ± 0.97 | 0.510 ± 0.16 |
| −0.2 | 6.69 ± 0.51 | 0.155 ± 0.0069 | 82.7 ± 1.8 | 0.00163 ± 1.7 × 10−5 |
| −0.3 | 2.64 ± 0.49 | 0.073 ± 0.0082 | 63.3 ± 5.6 | 0.000405 ± 1.3 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levinas, R.; Grigucevičienė, A.; Kubilius, T.; Matijošius, A.; Tamašauskaitė-Tamašiūnaitė, L.; Cesiulis, H.; Norkus, E. Femtosecond Laser-Ablated Copper Surface as a Substrate for a MoS2-Based Hydrogen Evolution Reaction Electrocatalyst. Materials 2022, 15, 3926. https://doi.org/10.3390/ma15113926
Levinas R, Grigucevičienė A, Kubilius T, Matijošius A, Tamašauskaitė-Tamašiūnaitė L, Cesiulis H, Norkus E. Femtosecond Laser-Ablated Copper Surface as a Substrate for a MoS2-Based Hydrogen Evolution Reaction Electrocatalyst. Materials. 2022; 15(11):3926. https://doi.org/10.3390/ma15113926
Chicago/Turabian StyleLevinas, Ramūnas, Asta Grigucevičienė, Tadas Kubilius, Aidas Matijošius, Loreta Tamašauskaitė-Tamašiūnaitė, Henrikas Cesiulis, and Eugenijus Norkus. 2022. "Femtosecond Laser-Ablated Copper Surface as a Substrate for a MoS2-Based Hydrogen Evolution Reaction Electrocatalyst" Materials 15, no. 11: 3926. https://doi.org/10.3390/ma15113926
APA StyleLevinas, R., Grigucevičienė, A., Kubilius, T., Matijošius, A., Tamašauskaitė-Tamašiūnaitė, L., Cesiulis, H., & Norkus, E. (2022). Femtosecond Laser-Ablated Copper Surface as a Substrate for a MoS2-Based Hydrogen Evolution Reaction Electrocatalyst. Materials, 15(11), 3926. https://doi.org/10.3390/ma15113926








