Al-Doped SrMoO3 Perovskites as Promising Anode Materials in Solid Oxide Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Structural Characterization
2.3. Thermal Expansion Coefficients
2.4. Thermal Analysis
2.5. DC-Conductivity
2.6. Single-Cell Performance
2.7. Scanning Electron Microscopy
3. Result and Discussion
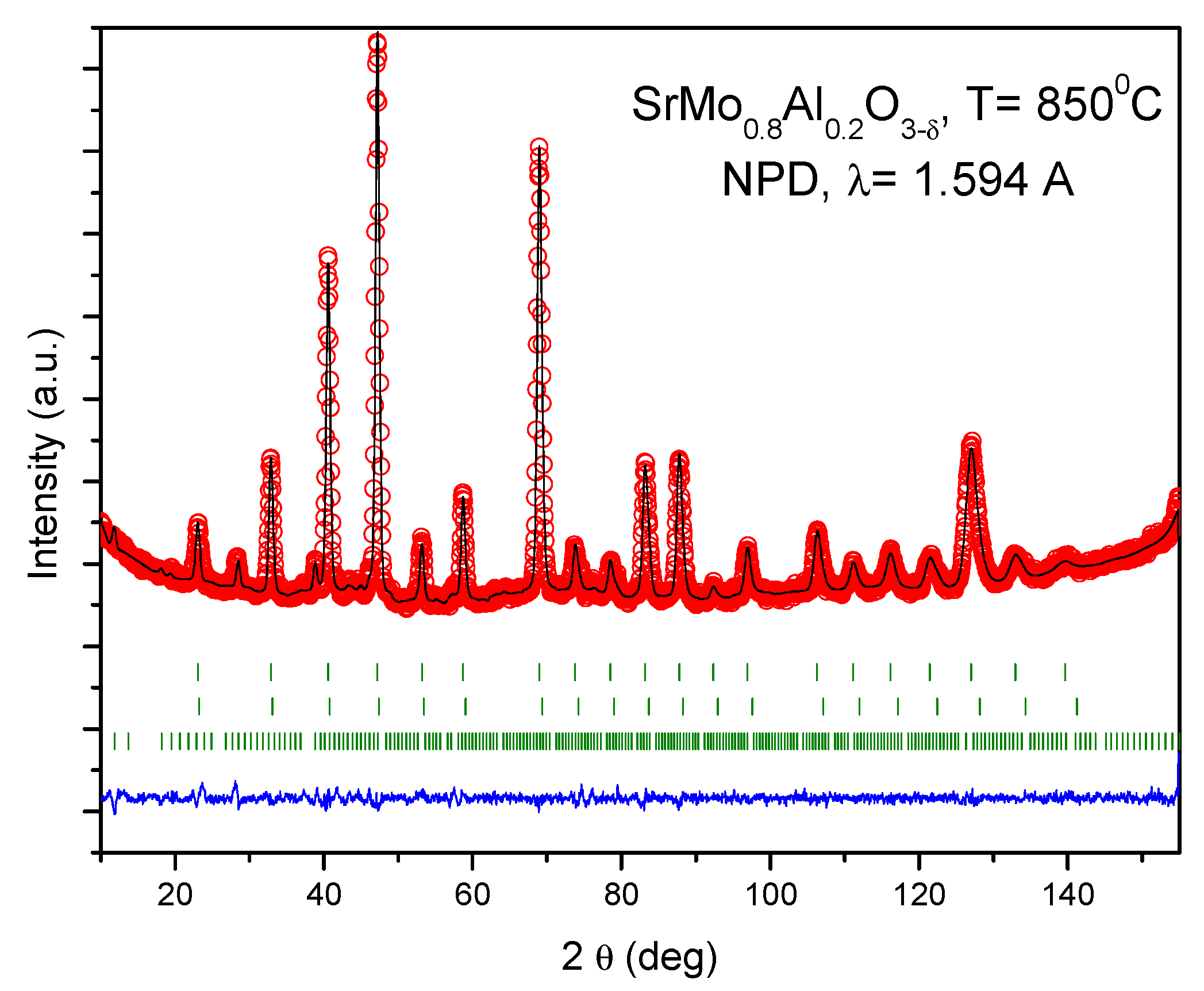
3.1. Crystallographic Characterization
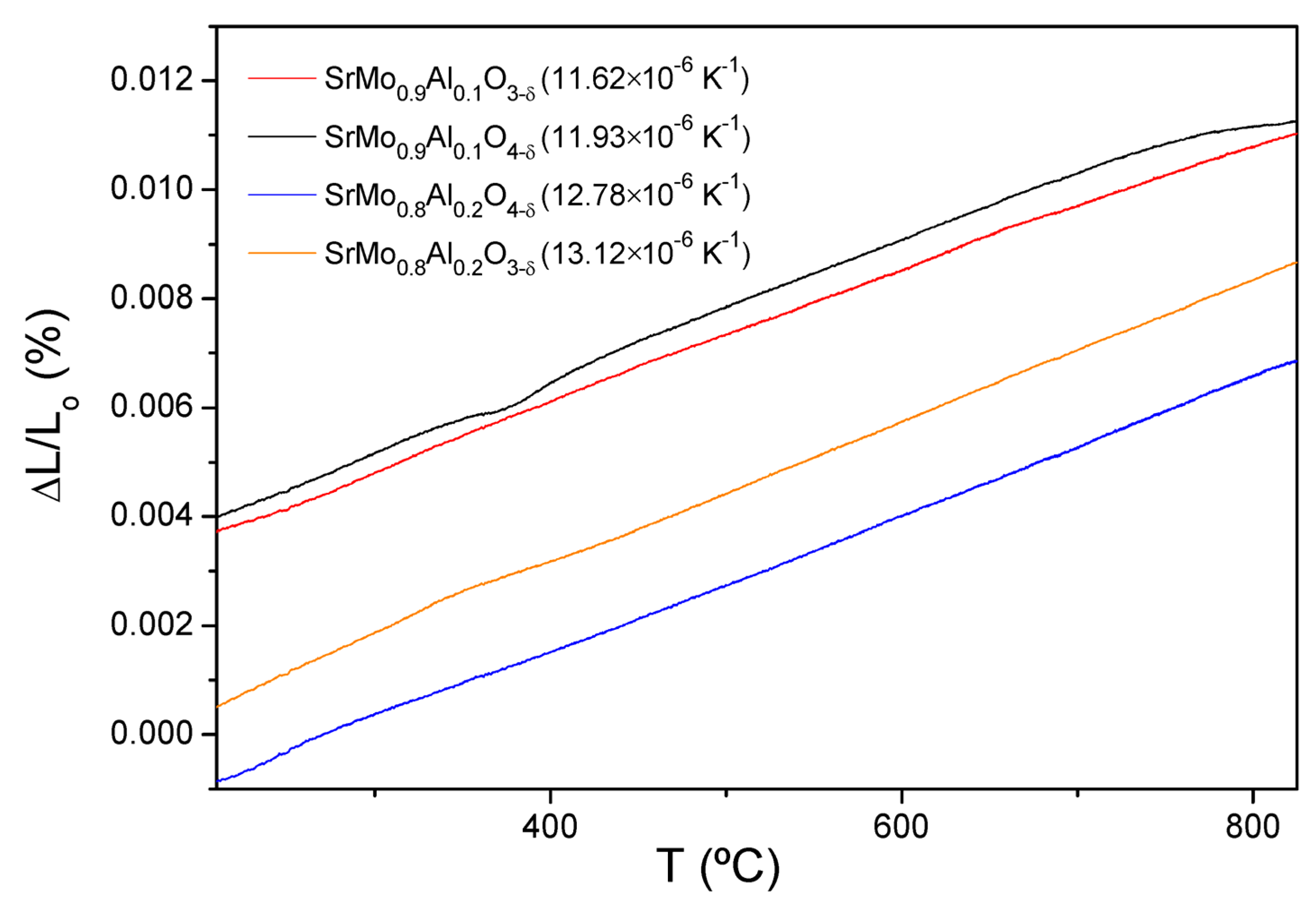
3.2. Thermal Expansion Measurements
3.3. Chemical Compatibility
3.4. Thermal Analysis
3.5. Electrical Conductivity Measurements
3.6. Fuel-Cell Evaluation
3.7. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghassemi, M.; Kamvar, M.; Steinberge-Wilckens, R. Fundamentals of Heat and Fluid Flow in High Temperature Fuel Cells; Academic Press: Cambridge, MA, USA, 2020; pp. 17–46. [Google Scholar]
- Cowin, P.I.; Petit, C.T.G.; Lan, R.; Irvine, J.T.S.; Tao, S. Recent Progress in the Development of Anode Materials for Solid Oxide Fuel Cells. Adv. Energy Mater. 2011, 1, 314–332. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chan, S.H. A review of anode materials development in solid oxide fuel cells. J. Mater. Sci. 2004, 39, 4405–4439. [Google Scholar] [CrossRef]
- Huijsmans, J.P.P.; Van Berkel, F.P.F.; Christie, G.M. Intermediate temperature SOFC—A promise for the 21st century. J. Power Sources 1998, 71, 107–110. [Google Scholar] [CrossRef]
- Brett, D.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Zhang, Z.; Baiyee, Z.M.; Ciucci, F.; Shao, Z. Compositional Engineering of Perovskite Oxides for Highly Efficient Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2015, 7, 8562–8571. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Alonso, J.A.; Bian, J. Recent Advances in Perovskite-Type Oxides for Energy Conversion and Storage Applications. Adv. Energy Mater. 2020, 10, 202000459. [Google Scholar] [CrossRef]
- Nagaia, I.; Shirakawa, N.; Ikeda, S.; Iwasaki, R.; Nishimura, H.; Kosaka, M. Highest conductivity oxide SrMoO3 grown by a floating-zone method under ultralow oxygen partial pressure. Appl. Phys. Lett. 2005, 87, 024105. [Google Scholar] [CrossRef]
- Hayashi, S.; Aoki, R.; Nakamura, T. Metallic conductivity in perovskite-type compounds AMoO3 (A = Ba, Sr, Ca) down to 2.5K. Mater. Res. Bull. 1979, 14, 409–413. [Google Scholar] [CrossRef]
- Chamberland, B.L.; Danielson, P.S. Alkaline-earth vanadium (IV) oxides having the AVO3 composition. J. Solid State Chem. 1971, 3, 243–247. [Google Scholar] [CrossRef]
- Feng, M.; Huang, J.; Peng, Y.; Huang, C.; Yue, X.; Huang, S. Tuning the electronic structures of cobalt-molybdenum bimetallic carbides to boost the hydrogen oxidation reaction in alkaline medium. Chem. Eng. J. 2022, 428, 131206. [Google Scholar] [CrossRef]
- Li, B.; Higgins, D.C.; Yang, D.; Lin, R.; Yu, Z.; Ma, J. New non-platinum Ir-V-Mo electro-catalyst, catalytic activity and CO tolerance in hydrogen oxidation reaction. Int. J. Hydrogen Energy 2012, 37, 18843–18850. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Son, D.; KilKim, T.S. Facile fabrication of amorphous NiMo catalysts for alkaline hydrogen oxidation reaction. J. Ind. Eng. Chem. 2021, 94, 309–316. [Google Scholar] [CrossRef]
- Martínez-Coronado, R.; Alonso, J.A.; Aguadero, A.; Fernández-Díaz, M.T. Optimized energy conversion efficiency in solid oxide fuel cells implementing SrMo1-xFexO3−δ perovskites as anodes. J. Power Sources 2012, 208, 153–158. [Google Scholar] [CrossRef]
- Martínez-Coronado, R.; Alonso, J.A.; Aguadero, A.; Fernández-Díaz, M.T. New SrMo1−xCrxO3−δ perovskites as anodes in solid-oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 4067–4073. [Google Scholar] [CrossRef]
- Cascos, V.; Alonso, J.A.; Fernández-Díaz, M.T. Novel Mg-Doped SrMoO3 Perovskites Designed as Anode Materials for Solid Oxide Fuel Cells. Materials 2016, 9, 588. [Google Scholar] [CrossRef] [Green Version]
- Cascos, V.; Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T. Design of new Ga-doped SrMoO3 Perovskites performing as anode materials in SOFC. Renew. Energy 2017, 111, 476–483. [Google Scholar] [CrossRef]
- Martínez-Coronado, R.; Alonso, J.A.; Fernández-Díaz, M.T. SrMo0.9Co0.1O3−δ: A potential anode for intermediate-temperature solid-oxide fuel cells (IT-SOFC). J. Power Sources 2014, 258, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Sears, V.F. Neutron scattering lengths and cross sections. Neutron News 1992, 3, 26–37. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rietveld, H.M.A. Profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radio and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Serrano-Sánchez, F.; Prado-Gonjal, J.; Nemes, N.M.; Biskup, N.; Varela, M.; Dura, O.J.; Martínez, J.L.; Fernández-Díaz, M.T.; Fauth, F.; Alonso, J.A. Low thermal conductivity in La-filled cobalt antimonide skutterudites with an inhomogeneous filling factor prepared under high-pressure conditions. J. Mater. Chem. A 2018, 6, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Gainza, J.; Serrano-Sánchez, F.; Prado-Gonjal, J.; Nemes, N.M.; Biskup, N.; Dura, O.J.; Martínez, J.L.; Fauth, F.; Alonso, J.A. Substantial thermal conductivity reduction in mischmetal skutterudites MmxCo4Sb12 prepared under high-pressure conditions, due to uneven distribution of the rare-earth elements. J. Mater. Chem. C 2019, 7, 4124–4131. [Google Scholar] [CrossRef] [Green Version]
- Macquart, R.B.; Kennedy, B.J.; Avdeev, M. Neutron diffraction study of phase transitions in perovskite-type strontium molybdate SrMoO3. J. Solid State Chem. 2010, 183, 250–255. [Google Scholar] [CrossRef]
- Tietz, F. Thermal expansion of SOFC materials. Ionics 1999, 5, 129–139. [Google Scholar] [CrossRef]
- Rafique, A.; Raza, R.; Arifin, N.A.; Ullah, M.K.; Ali, A.; Steinberger-Wilckens, R. Electrochemical and thermal characterization of doped ceria electrolyte with lanthanum and zirconium. Ceram. Int. 2018, 44, 6493–6499. [Google Scholar] [CrossRef]
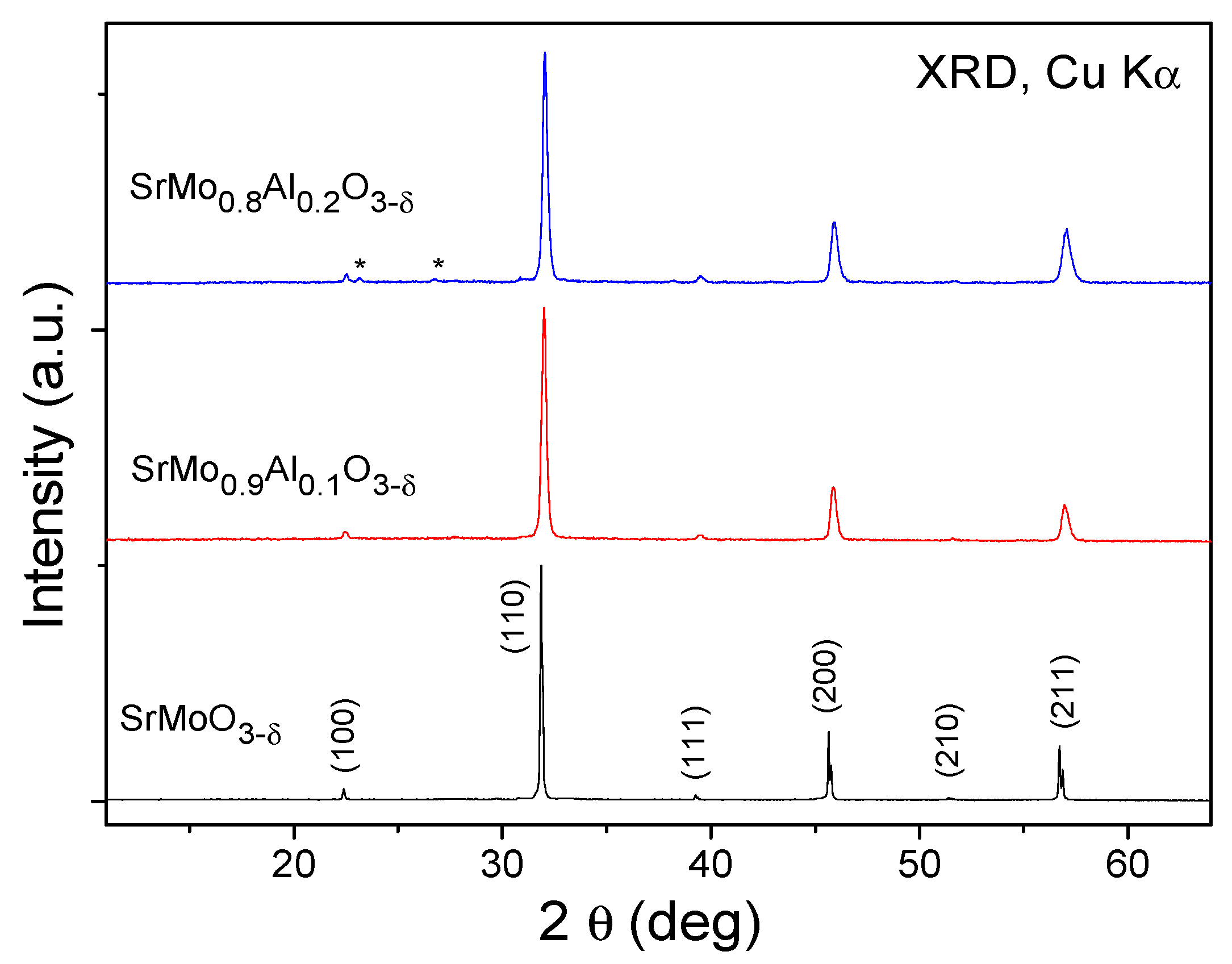

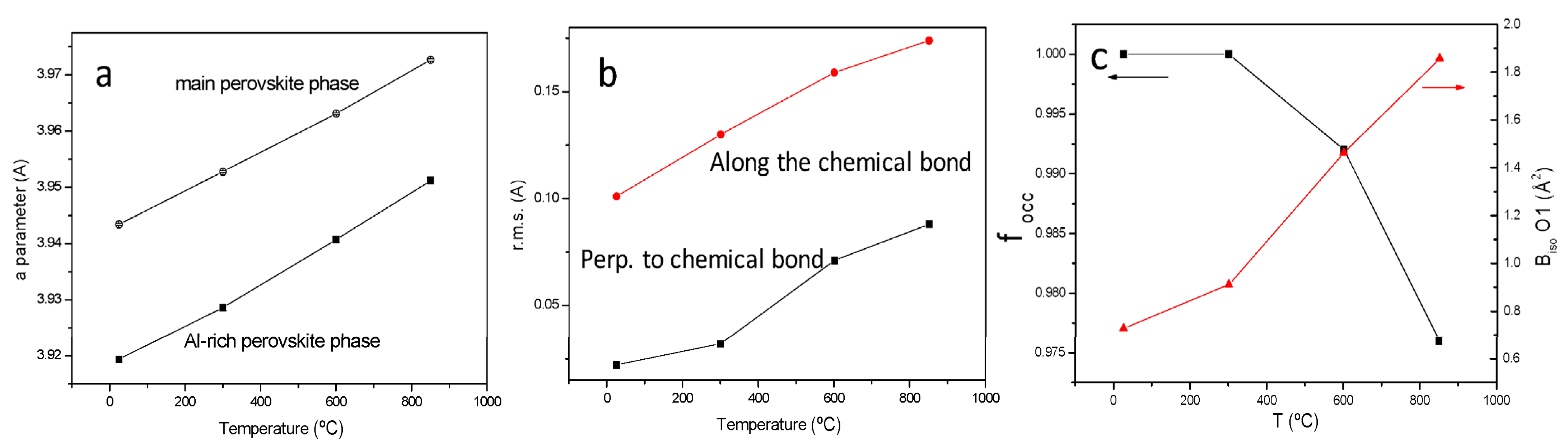

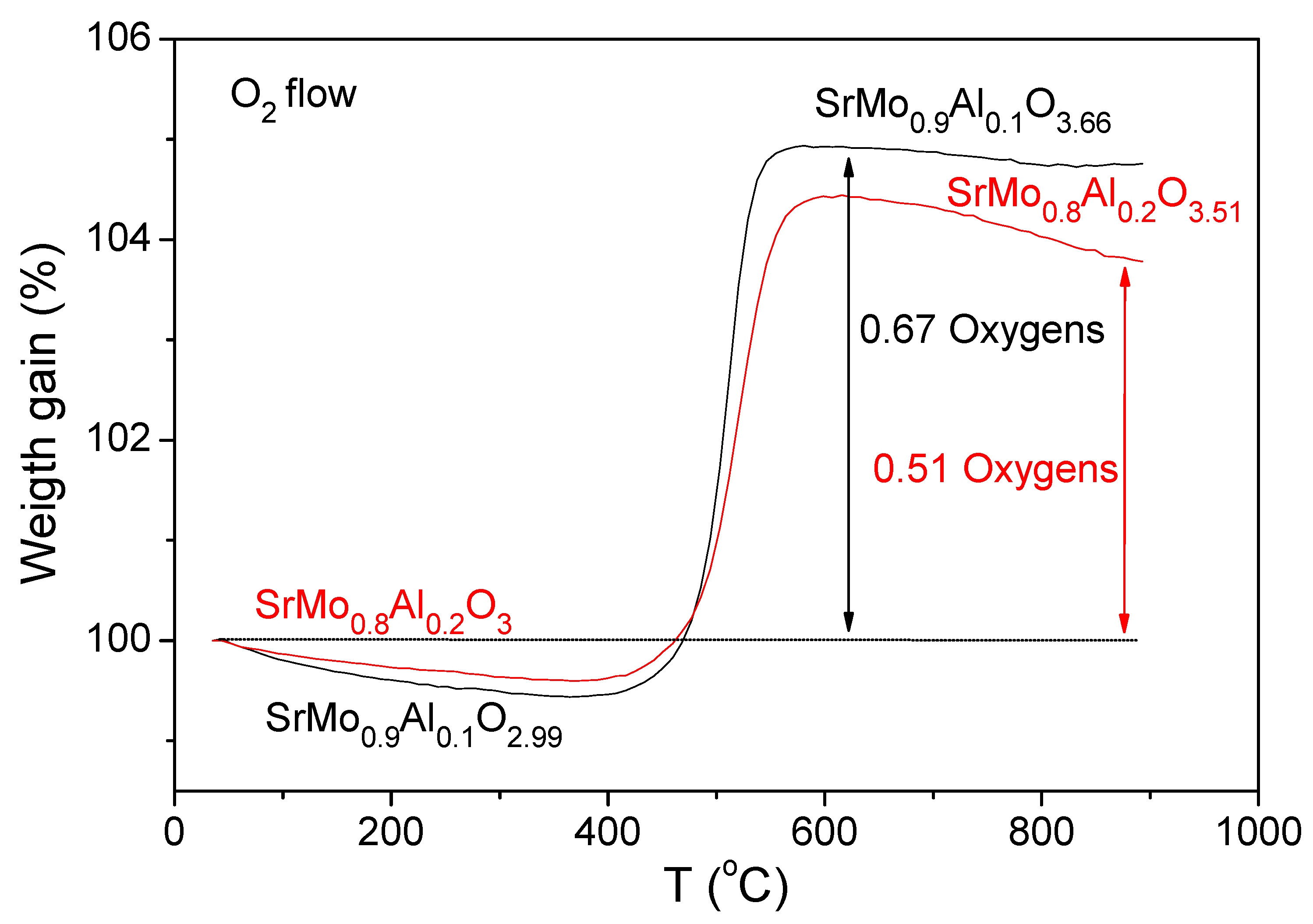
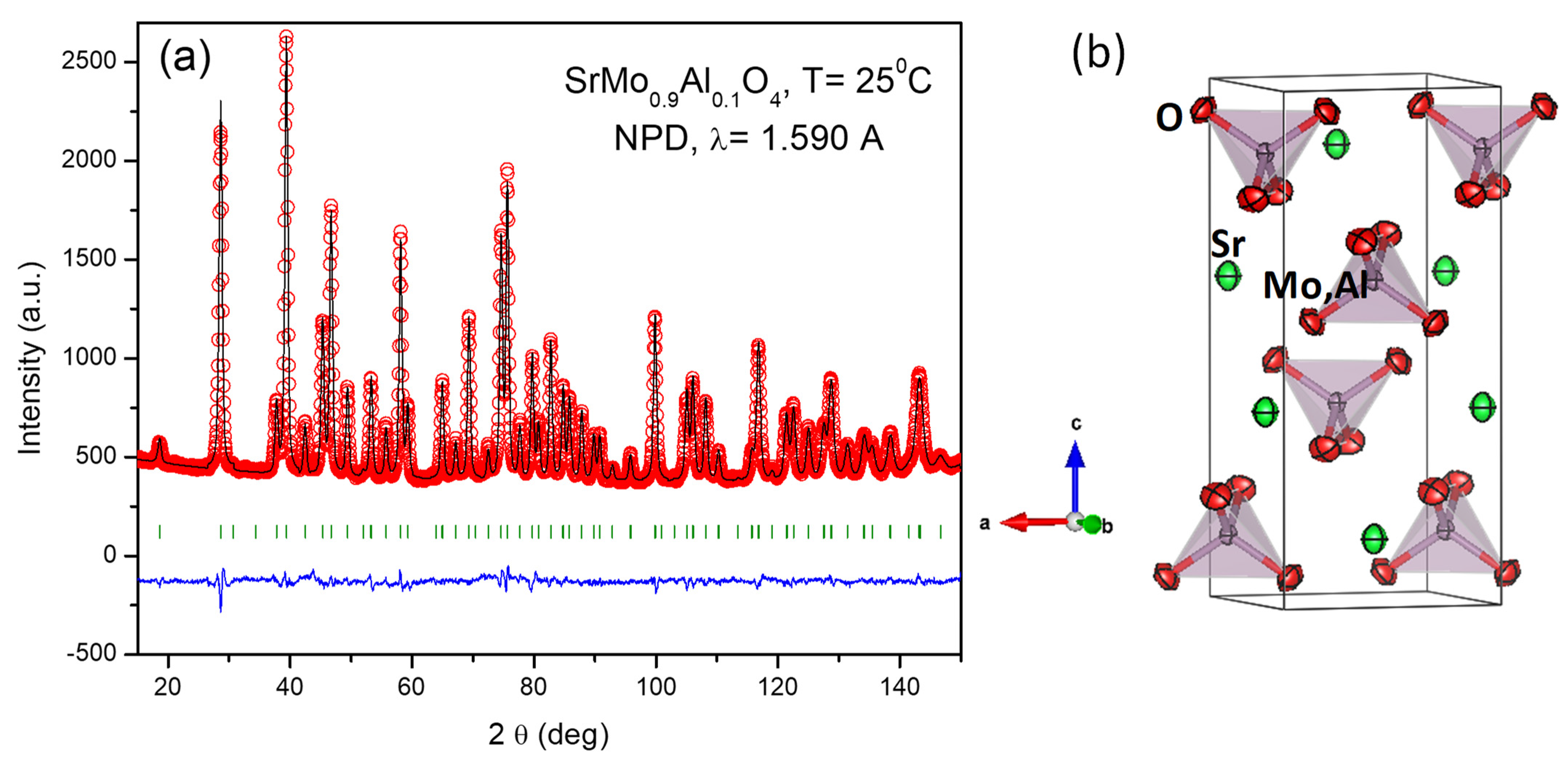
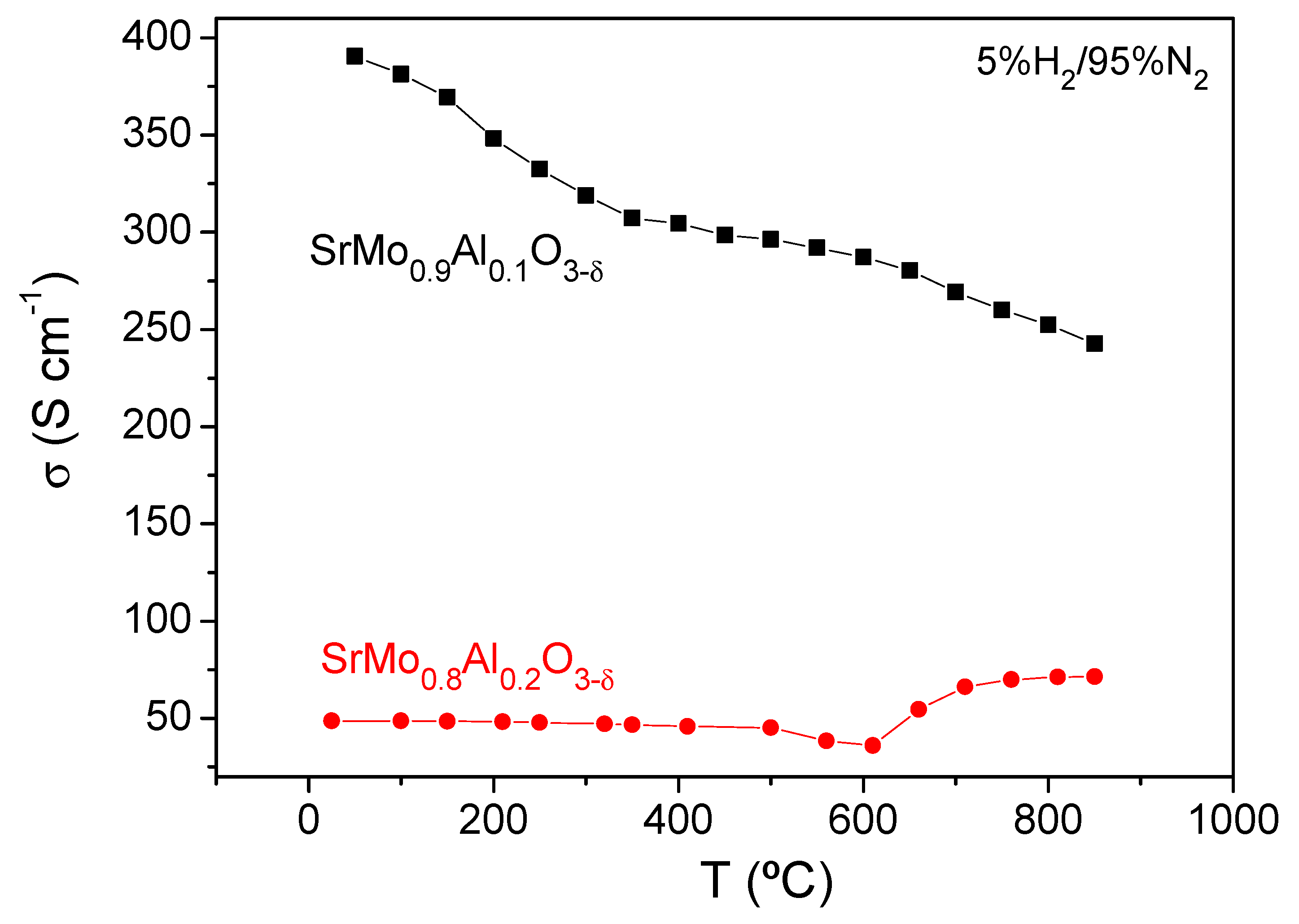
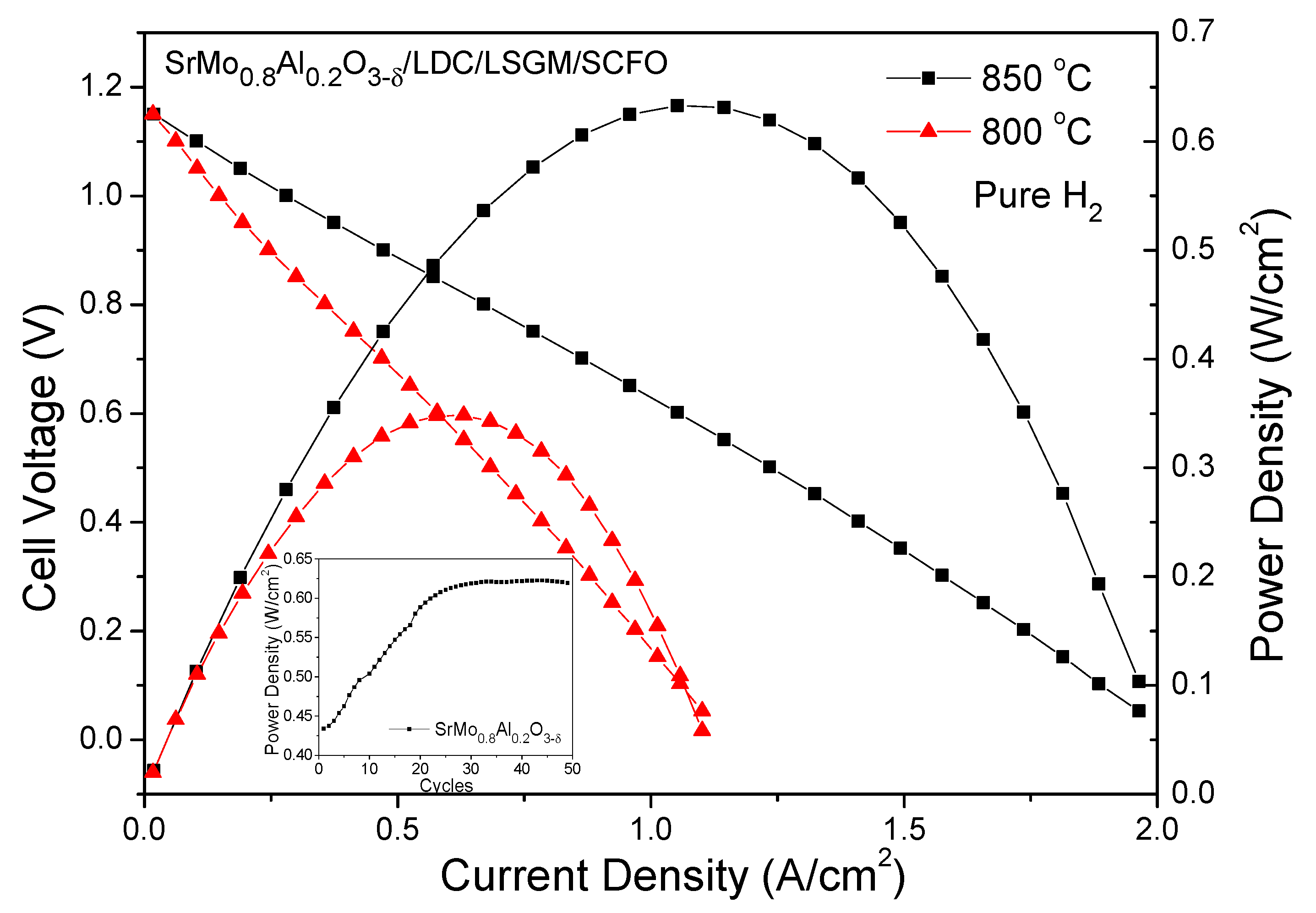


| SrMo1−xAlxO3−δ | x = 0 a | x = 0.1 | x = 0.2 |
|---|---|---|---|
| a (Å) | 3.97629(3) | 3.95893(8) | 3.9438(2) |
| V(Å)3 | 62.869(7) | 62.049(2) | 61.342(4) |
| Sr 1b (½, ½, ½) | |||
| Biso (Å2) | 0.77(3) | 0.743(3) | 0.95(1) |
| focc | 1.00 | 1.00 | 1.00 |
| Mo/Al 1a (0, 0, 0) | |||
| Biso (Å2) | 0.55(4) | 0.198(3) | 0.35(1) |
| Mo/Al focc | 1.00 | 0.97 5(1)/0.025(1) | 0.874/0.127 |
| O1 3d (½, 0, 0) | |||
| β11 * | - | 30(7) | 34(8) |
| β22 * | - | 159(5) | 159(6) |
| β33 * | - | 159(5) | 159(6) |
| Beq (Å2) | 0.75(10) | 0.7273 | 0.6560 |
| focc | 1.00 | 0.997(1) | 1.001(1) |
| Reliability factors | |||
| χ2 | - | 5.84 | 1.52 |
| Rp(%) | - | 3.71 | 1.71 |
| Rwp (%) | - | 5.02 | 2.18 |
| Rexp(%) | - | 2.07 | 1.77 |
| RBragg(%) | - | 2.85 | 1.80 |
| Distances (Å) | |||
| (Sr)-(O1) | - | 2.79939(4) | 2.7873(4) |
| (Mo/Al)-(O1) | 1.98814(1) | 1.97947(4) | 1.9719(4) |
| SrMo0.8Al0.2O3−δ | 300 °C | 600 °C | 850 °C |
|---|---|---|---|
| a (Ǻ) | 3.9527(2) | 3.9631(3) | 3.9722(4) |
| V(Ǻ)3 | 61.758(6) | 62.245(8) | 62.68(1) |
| Sr 1b (½, ½, ½) | |||
| Biso (Å2) | 1.156(7) | 1.739(8) | 2.25(1) |
| focc | 1.00 | 1.00 | 1.00 |
| Mo/Al 1a (0, 0, 0) | |||
| Biso (Å2) | 0.307(6) | 0.488(7) | 0.659(9) |
| Mo/Al focc | 0.874/0.127 | 0.874/0.127 | 0.874/0.127 |
| O1 3d (½, 0, 0) | |||
| β11 * | 13(13) | 64(15) | 153(20) |
| β22 * | 212(10) | 317(12) | 365(15) |
| β33 * | 212(10) | 317(12) | 365(15) |
| Beq (Å2) | 0.9111 | 1.4624 | 1.8578 |
| focc | 1.00 | 0.992(4) | 0.976 |
| Reliability factors | |||
| χ2 | 1.92 | 1.84 | 1.44 |
| Rp(%) | 1.91 | 1.87 | 1.78 |
| Rwp (%) | 2.53 | 2.55 | 2.27 |
| Rexp(%) | 1.83 | 1.88 | 1.89 |
| RBragg(%) | 3.13 | 2.20 | 1.62 |
| Distances (Å) | |||
| (Sr)-(O1) | 2.7950(1) | 2.8023(2) | 2.8088(2) |
| (Mo/Al)-(O1) | 1.9764(1) | 1.9816(2) | 1.9861(2) |
| Crystal Data | ||||||
|---|---|---|---|---|---|---|
| Tetragonal, I41/a | NPD, λ = 1.594 Å | |||||
| a = 5.37903 (9) Å | c = 12.0190(3) Å | |||||
| V = 347.76 (1) Å3 | Z = 4 | |||||
| Refinement | ||||||
| Rp = 1.98% | Rwp = 2.56% | |||||
| Rexp = 1.35% | RBragg = 3.85% | |||||
| χ2 = 3.76 | 3199 data points | |||||
| Fractional atomic coordinates and equivalent isotropic displacement parameters (Å2) | ||||||
| x | y | z | Ueq | Occ. (<1) | ||
| Sr | 0 | 0.25 | 0.625 | 0.0127(9) | ||
| Mo | 0 | 0.25 | 0.125 | 0.0054(9) | 0.9 | |
| Al | 0 | 0.25 | 0.125 | 0.0054(9) | 0.1 | |
| O | 0.2370(2) | 0.11321(16) | 0.04330(10) | 0.0135(5) | 0.9841(7) | |
| Atomic displacement parameters (Å2) | ||||||
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sr | 0.0118(6) | 0.0118(6) | 0.0143(14) | 0 | 0 | 0 |
| Mo | 0.0050(6) | 0.0050(6) | 0.0061(13) | 0 | 0 | 0 |
| Al | 0.0050(6) | 0.0050(6) | 0.0061(13) | 0 | 0 | 0 |
| O | 0.0144(5) | 0.0134(4) | 0.0127(4) | 0.0044(5) | 0.0038(3) | −0.0011(5) |
| Anode Material | Power Density |
|---|---|
| SrMo0.8Cr0.2O3−δ | 527 mW cm−2 [15] |
| SrMo0.8Co0.2O3−δ | 545 mW cm−2 [18] |
| SrMo0.8Fe0.2O3−δ | 551 mW cm−2 [14] |
| SrMo0.8Mg0.2O3−δ | 684 mW cm−2 [16] |
| SrMo0.8Ga0.2O3−δ | 560 mW cm−2 [17] |
| SrMo0.8Al0.2O3−δ | 633 mW cm−2 [material of this work] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascos, V.; Fernández-Díaz, M.T.; Alonso, J.A. Al-Doped SrMoO3 Perovskites as Promising Anode Materials in Solid Oxide Fuel Cells. Materials 2022, 15, 3819. https://doi.org/10.3390/ma15113819
Cascos V, Fernández-Díaz MT, Alonso JA. Al-Doped SrMoO3 Perovskites as Promising Anode Materials in Solid Oxide Fuel Cells. Materials. 2022; 15(11):3819. https://doi.org/10.3390/ma15113819
Chicago/Turabian StyleCascos, Vanessa, María Teresa Fernández-Díaz, and José Antonio Alonso. 2022. "Al-Doped SrMoO3 Perovskites as Promising Anode Materials in Solid Oxide Fuel Cells" Materials 15, no. 11: 3819. https://doi.org/10.3390/ma15113819
APA StyleCascos, V., Fernández-Díaz, M. T., & Alonso, J. A. (2022). Al-Doped SrMoO3 Perovskites as Promising Anode Materials in Solid Oxide Fuel Cells. Materials, 15(11), 3819. https://doi.org/10.3390/ma15113819







