Thermal Stability of Fluorescent Chitosan Modified with Heterocyclic Aromatic Dyes
Abstract
1. Introduction
2. Materials and Methods
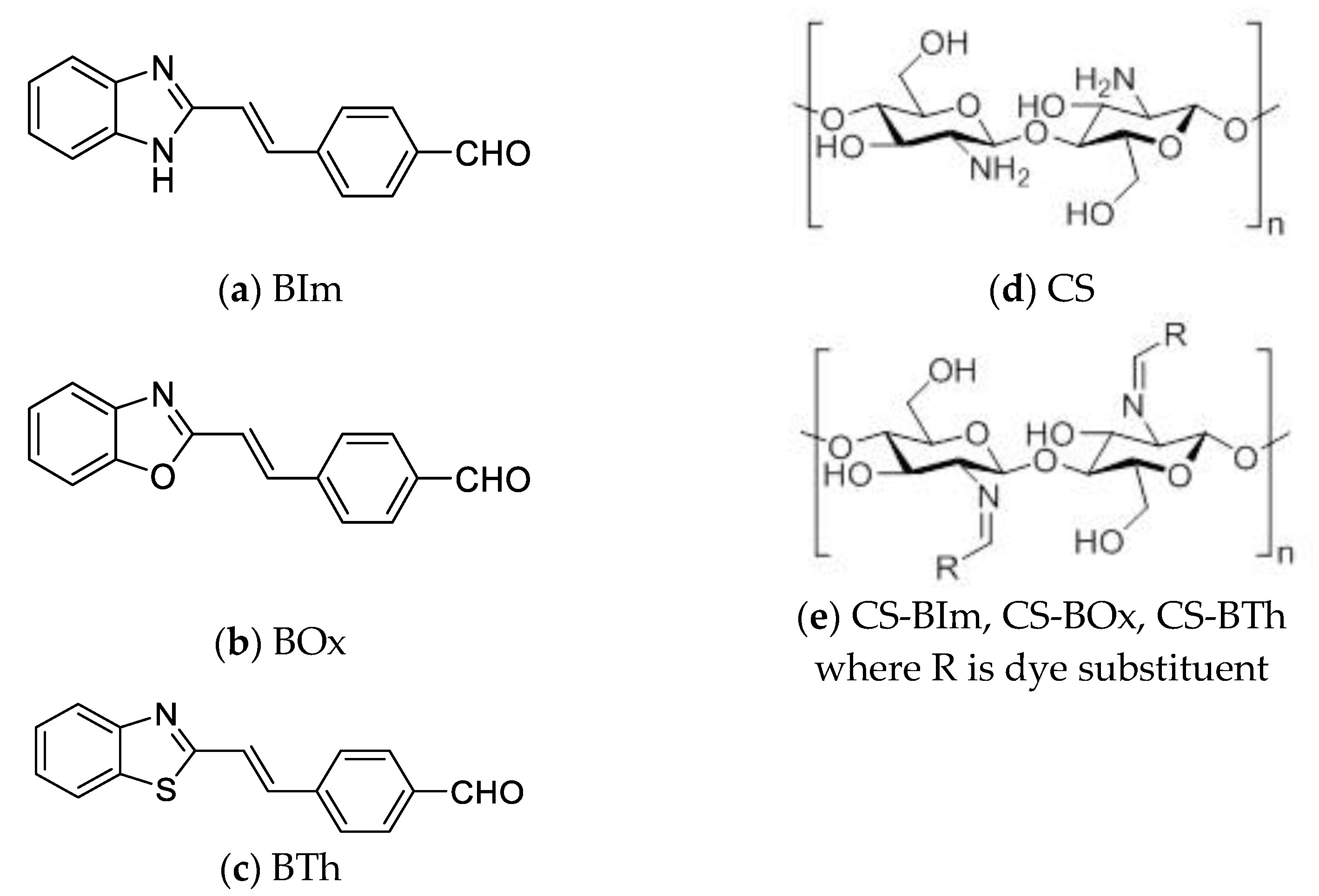
2.1. Materials
2.2. Thermal Analysis
3. Results and Discussion
3.1. Thermal Stability of Chitosan Modifiers: Low-Molecular-Weight Heterocyclic Compounds—BIm, Box, and BTh
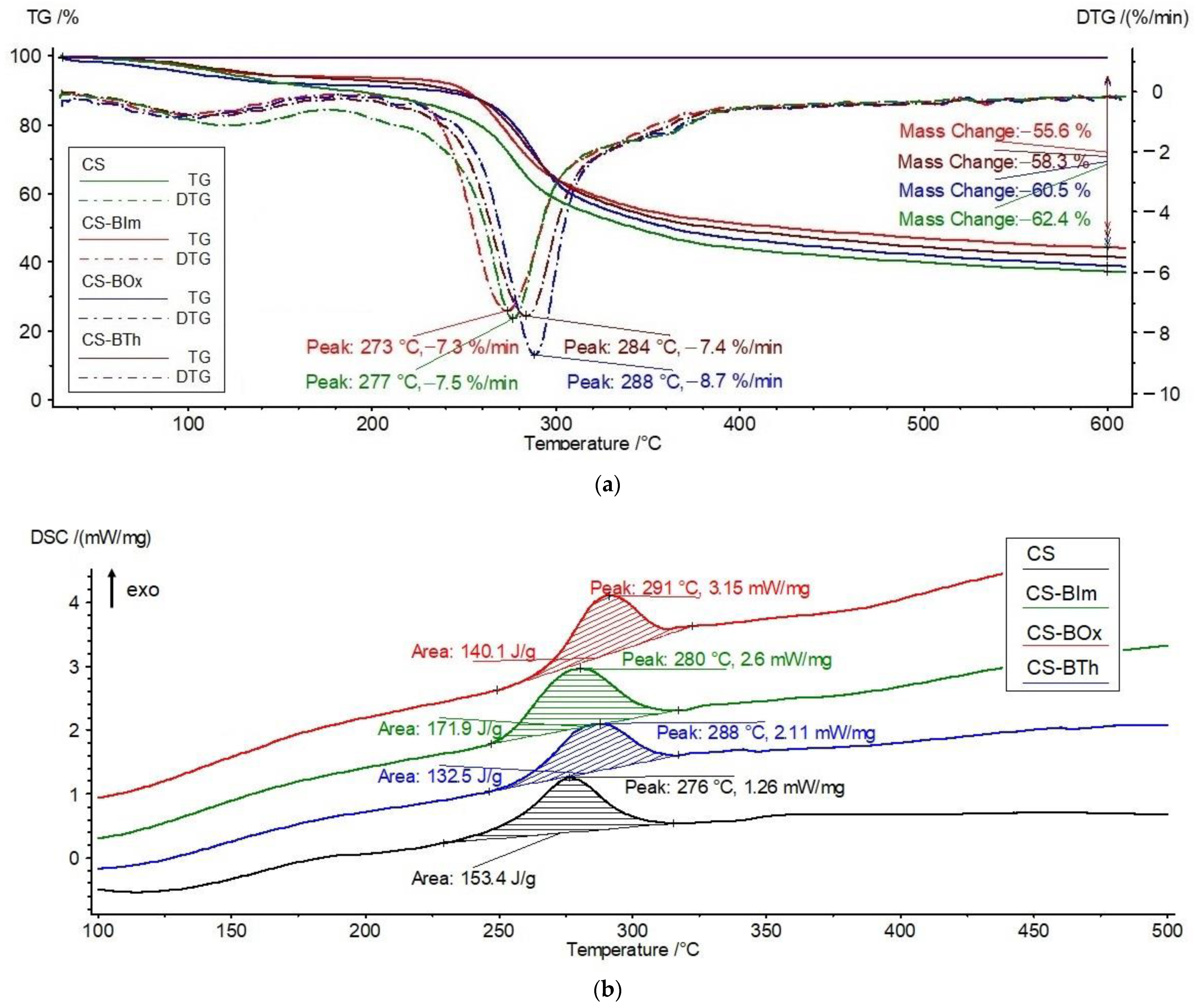
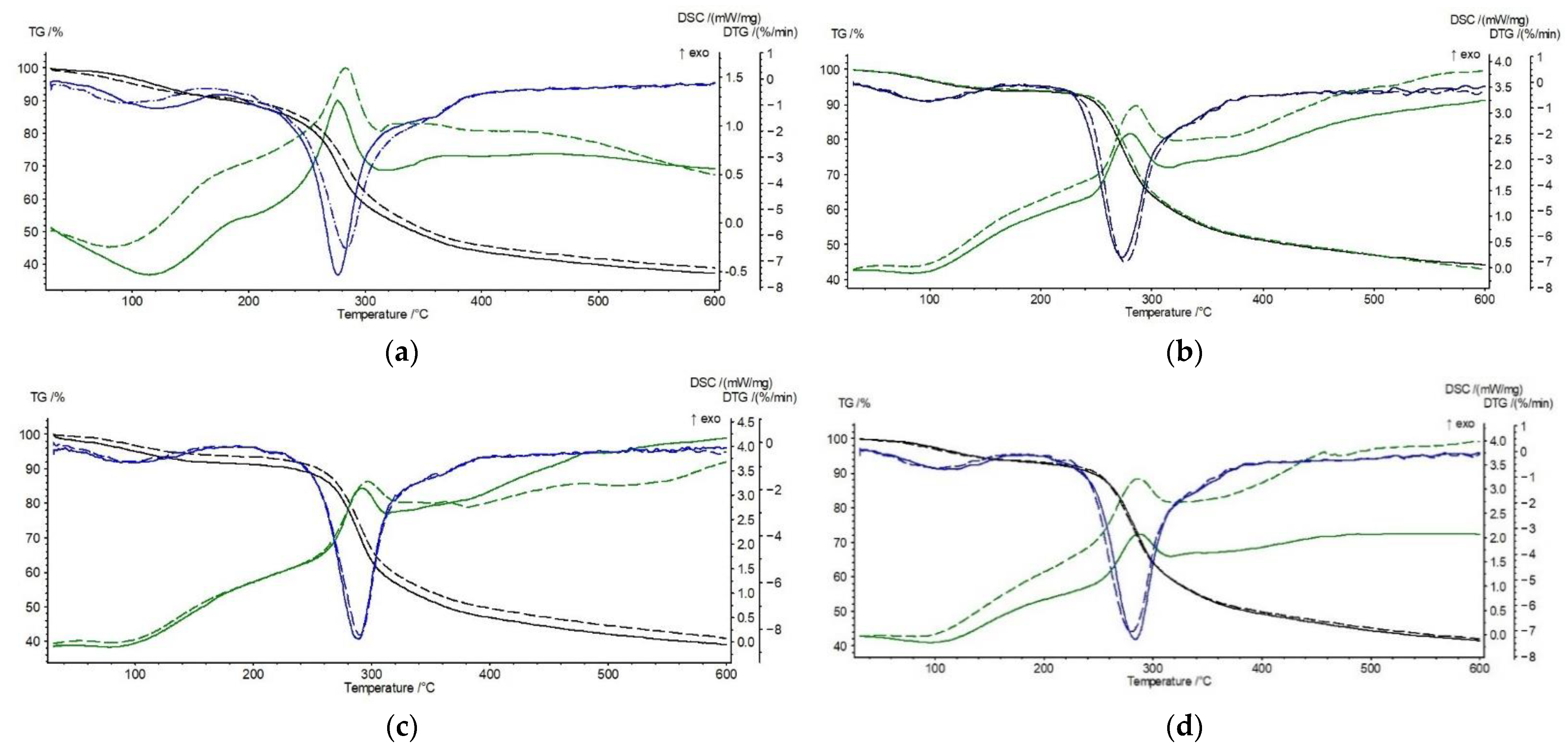
3.2. Thermal Stability of Chitosan Derivatives—CS-BIm, CS-BOx, CS-BTh
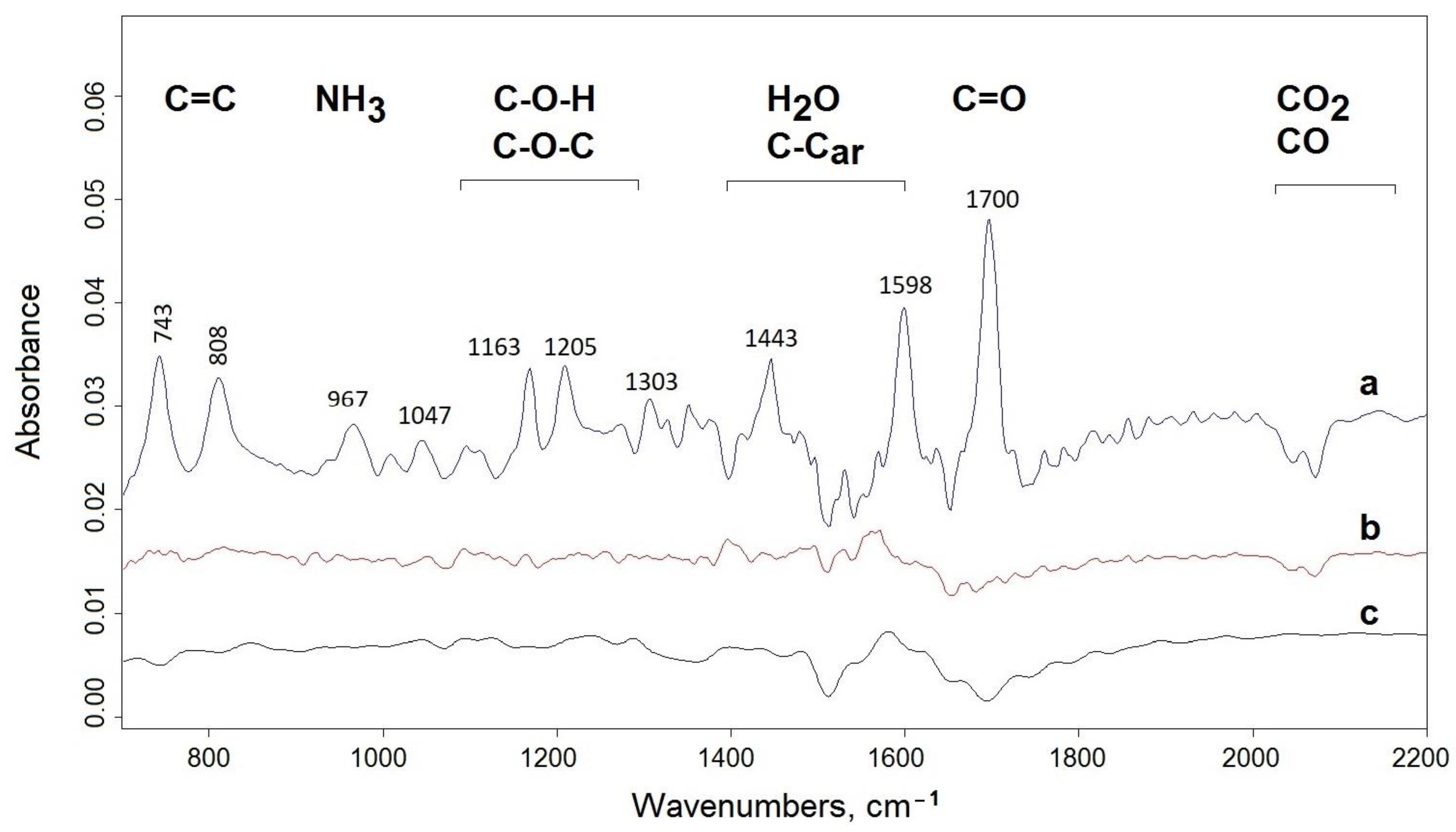
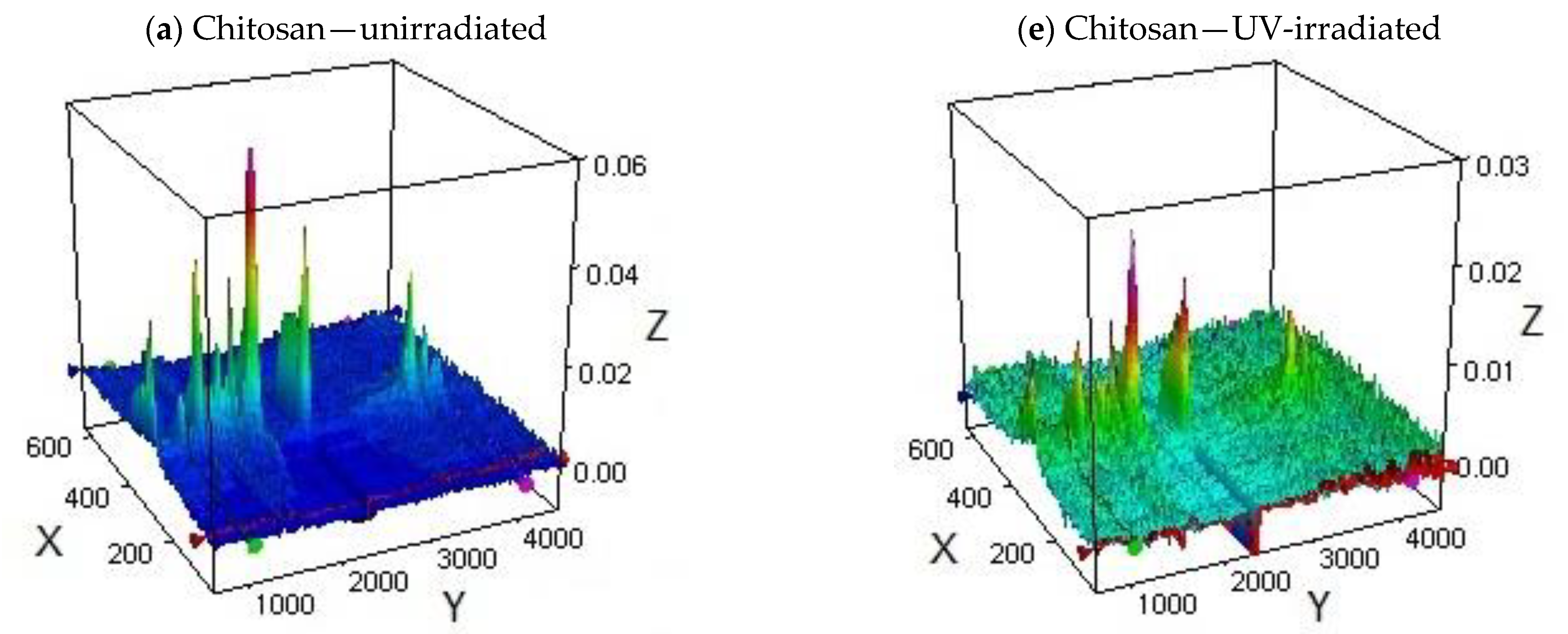
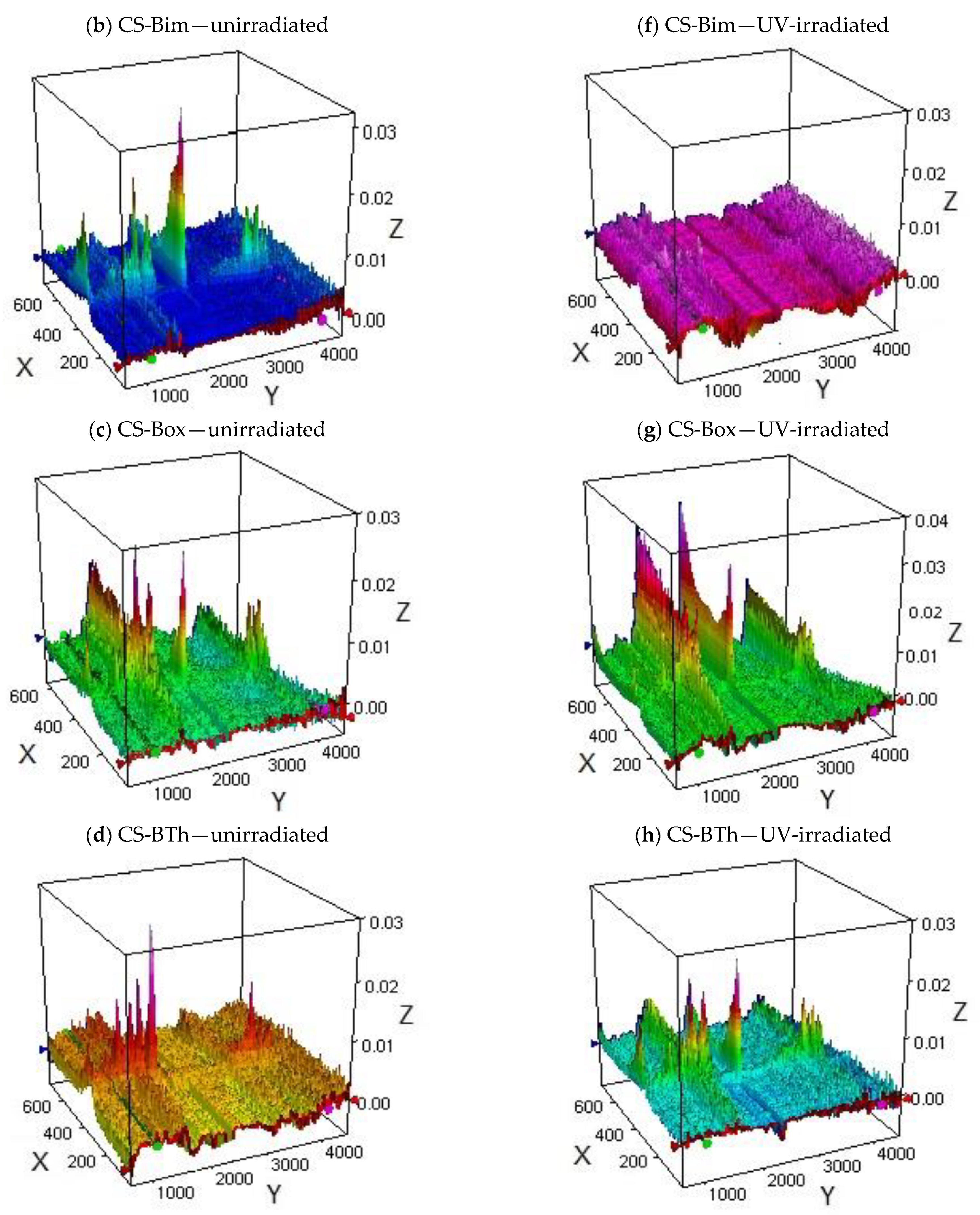
3.3. Effect of UV-Irradiation on the Thermal Stability of Chitosan Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fei, X.; Gu, Y. Progress in modifications and applications of fluorescent dye probe. Prog. Nat. Sci. 2009, 19, 1–7. [Google Scholar] [CrossRef]
- Taraska, J.W.; Zagotta, W.N. Fluorescence applications in molecular neurobiology. Neuron 2010, 66, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Zhao, B.; Xia, Y.; Li, D. Organic Fluorescent Compounds that Display Efficient Aggregation-Induced Emission Enhancement and Intramolecular Charge Transfer. Molecules 2018, 23, 1446. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.S.T. Fluorescent Labeling of Biomolecules with Organic Probes. Chem. Rev. 2008, 109, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Q.; Li, M.; Liu, M.D.; Zhuang, W.M.; Li, G.K. A highly sensitive fluorescent acidic pH probe based on rhodamine B diethyl-2-aminobutenedioate conjugate and its application in living cells. Dyes Pigm. 2013, 96, 71–75. [Google Scholar] [CrossRef]
- Zhu, S.L.; Zhang, J.T.; Janjanam, J.; Bi, J.H.; Vegesna, G.; Tiwari, A.; Luo, F.T.; Wei, J.J.; Liu, H.Y. Highly water-soluble, near-infrared emissive BODIPY polymeric dye bearing RGD peptide residues for cancer imaging. Anal. Chim. Acta 2013, 758, 138–144. [Google Scholar] [CrossRef]
- Woo, Y.; Chaurasiya, S.; O’Leary, M.; Han, E.; Fong, Y. Fluorescent imaging for cancer therapy and cancer gene therapy. Mol. Ther. Oncolytics 2021, 23, 231–238. [Google Scholar] [CrossRef]
- Lifson, M.A.; Ozen, M.O.; Inci, F.; Wang, S.Q.; Inan, H.; Baday, M.; Henrich, T.J.; Demirci, U. Advances in biosensing strategies for HIV detection, diagnosis, and therapeutic monitoring. Adv. Drug Deliv. Rev. 2016, 103, 90–104. [Google Scholar] [CrossRef]
- Putlyaeva, L.V.; Lukyanov, K.A. Studying SARS-CoV-2 with Fluorescence Microscopy. Int. J. Mol. Sci. 2021, 22, 6558. [Google Scholar] [CrossRef]
- Samacoits, A.; Nimsamer, P.; Mayuramart, O.; Chantaravisoot, N.; Sitthi-amorn, P.; Nakhakes, C.; Luangkamchorn, L.; Tongcham, P.; Zahm, U.; Suphanpayak, S.; et al. Machine Learning-Driven and Smartphone-Based Fluorescence Detection for CRISPR Diagnostic of SARS-CoV-2. ACS Omega 2021, 6, 2727–2733. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; He, Y.; Zhang, G.; Wen, H.; Wang, Y.; Wu, Q.-P.; Cui, H. Determining Essential Requirements for Fluorophore Selection in Various Fluorescence Applications Taking Advantage of Diverse Structure–Fluorescence Information of Chromone Derivatives. J. Med. Chem. 2021, 64, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Rininsland, F.H.; Wittenburg, S.K.; Achyuthan, K.E.; Duncan, W.; McBranch, D.W.; Whitten, D.G. Biocidal Activity of a Light-Absorbing Fluorescent Conjugated Polyelectrolyte. Langmuir 2005, 21, 10154–10159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, L.; Yang, Q.; Fengting Lv, F.; Wang, S. Water-Soluble Conjugated Polymers for Imaging, Diagnosis, and Therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-S. Fluorescent Compounds Having the Spaced and Integrated Type Receptors. Korean Soc. Photoscience 2016, 5, 1–7. [Google Scholar] [CrossRef][Green Version]
- Zheng, H.; Zhan, X.-Q.; Bian, Q.-N.; Zhang, X.-J. Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors. Chem. Commun. 2013, 49, 429–447. [Google Scholar] [CrossRef]
- Wagh, S.B.; Maslivetc, V.A.; La Clair, J.J.; Kornienko, A. Lessons in Organic Fluorescent Probe Discovery. ChemBioChem 2021, 22, 3109–3139. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Pakhira, M.; Nandi, A.K. Fluorescence in “Nonfluorescent” Polymers. ACS Omega 2020, 5, 30747–30766. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Baic, W.; Bao, Y. Fluorescent chemosensors based on conjugated polymers with N-heterocyclic moieties: Two decades of progress. Polym. Chem. 2020, 11, 3095–3114. [Google Scholar] [CrossRef]
- Ahumada, G.; Borkowska, M. Fluorescent Polymers Conspectus. Polymers 2022, 14, 1118. [Google Scholar] [CrossRef]
- Hai, T.A.P.; Sugimoto, R. Fluorescence control of chitin and chitosan fabricated via surface functionalization using direct oxidative polymerization. RSC Adv. 2018, 8, 7005–7013. [Google Scholar] [CrossRef]
- Marpu, S.B.; Benton, E.N. Shining Light on Chitosan: A Review on the Usage of Chitosan for Photonics and Nanomaterials Research. Int. J. Mol. Sci. 2018, 19, 1795. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, S.; Chan, H.S.O.; Ng, S.-C. Novel Fluorescent Carbazolyl−Pyridinyl Alternating Copoloymers: Synthesis, Characterization, and Properties. Macromolecules 2005, 38, 7629–7635. [Google Scholar] [CrossRef]
- Seifi, H.; Gholami, T.; Seifi, S.; Ghoreishi, S.M.; Salavati-Niasari, M. A review on current trends in thermal analysis and hyphenated techniques in the investigation of physical, mechanical and chemical properties of nanomaterials. J. Anal. Appl. Pyrolysis 2020, 149, 104840. [Google Scholar] [CrossRef]
- Saikia, P. Chapter 5-Physical and thermal analysis of polymer. In Polymer Science and Innovative Applications. Materials, Techniques, and Future Developments, 1st ed.; AlMaadeed, M.A.A., Ponnamma, D., Carignano, M.A., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2020; pp. 153–205. [Google Scholar]
- Pielichowski, K.; Pielichowska, K. Thermal Analysis of Polymeric Materials-Methods and Developments; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2022. [Google Scholar]
- Liu, Z. Review and prospect of thermal analysis technology applied to study thermal properties of energetic materials. FirePhysChem 2021, 1, 129–138. [Google Scholar] [CrossRef]
- Ozawa, T. Thermal analysis-Review and prospect. Thermochim. Acta 2000, 355, 35–42. [Google Scholar] [CrossRef]
- Gabbott, P. Principles and Applications of Thermal Analysis; Blackwell Publishing Ltd.: Singapore, 2008. [Google Scholar]
- Gianotti, V.; Antonioli, D.; Sparnacci, K.; Laus, M.; Cassino, C.; Marsano, F.; Seguini, G.; Perego, M. TGA-GC–MS quantitative analysis of phosphorus-end capped functional polymers in bulk and ultrathin films. J. Anal. Appl. Pyrolysis 2017, 128, 238–245. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Moo Espinosa, J.I.; Cauich-Rodríguez, J.V.; Ávila-Ortega, A.; Vázquez-Torres, H.; Marcos-Fernández, A.; San Román, J. TGA/FTIR studies of segmented aliphatic polyurethanes and their nanocomposites prepared with commercial montmorillonites. Polym. Degrad. Stab. 2009, 94, 1666–1677. [Google Scholar] [CrossRef]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Using TGA/FTIR TGA/MS and cone calorimetry to understand thermal degradation and flame retardancy mechanism of polycarbonate filled with solid bisphenol A bis(diphenyl phosphate) and montmorillonite. Polym. Degrad. Stab. 2012, 97, 605–614. [Google Scholar] [CrossRef]
- Singh, S.; Wu, C.; Williams, P.T. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J. Anal. Appl. Pyrolysis 2012, 94, 99–107. [Google Scholar] [CrossRef]
- Mansa, R.; Zou, S. Thermogravimetric analysis of microplastics: A mini review. Environ. Adv. 2021, 5, 100117. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Yu, J.; Ni, F.; Sheng, Y.; Scircle, A.; Cizdziel, J.V.; Zhou, Y. Separation and identification of microplastics in marine organisms by TGA-FTIR-GC/MS: A case study of mussels from coastal China. Environ. Pollut. 2021, 272, 115946. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, H.; Tafelska-Kaczmarek, A.; Roszek, K.; Czarnecka, J.; Jędrzejewska, B.; Zblewska, K. Fluorescent Chitosan Modified with Heterocyclic Aromatic Dyes. Materials 2021, 14, 6429. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lei, H.; Li, N.; Qiu, Z.; Wang, H.; Guo, J.; Luo, Y.; Zhong, Z.; Liuc, X.; Zhi-Hong, Z. Novel heterocycle-based organic molecules with two-photon induced blue fluorescent emission. J. Mater. Chem. 2003, 13, 708–711. [Google Scholar] [CrossRef]
- Li, W.; Xiao, L.; Qin, C. The Characterization and Thermal Investigation of Chitosan-Fe3O4 Nanoparticles Synthesized Via A Novel One-step Modifying Process. J. Macromol. Sci. A 2010, 48, 57–64. [Google Scholar] [CrossRef]
- Neto, C.G.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M.R. Thermal Analysis of Chitosan Based Networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kędziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposite with magnetite. J. Therm. Anal. Calor. 2016, 124, 1267–1280. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Bourakhouadar, M. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym. Degrad. Stab. 2016, 130, 1–9. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Zawadzki, J. Chitosan pyrolysis and adsorption properties of chitosan and its carbonizate. Carbohydr. Res. 2010, 345, 941–947. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 395–401. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A new look towards the thermal decomposition of chitins and chitosans with different degrees of deacetylation by coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Qin, C.; Wang, L.; Li, W. Volatile compounds formed from the pyrolysis of chitosan. Carbohydr. Polym. 2011, 83, 1553–1557. [Google Scholar] [CrossRef]
- Bussiere, P.-O.; Gardette, J.-L.; Rapp, G.; Masson, C.; Therias, S. New insights into the mechanism of photodegradation of chitosan. Carbohydr. Polym. 2021, 259, 117715. [Google Scholar] [CrossRef] [PubMed]
- Bussière, P.O.; Rivaton, A.; Sandrine Thérias, S.; Gardette, J.-L. Multiscale Investigation of the Poly(N-vinylcarbazole) Photoageing Mechanism. J. Phys. Chem. B 2012, 116, 802–812. [Google Scholar] [CrossRef] [PubMed]
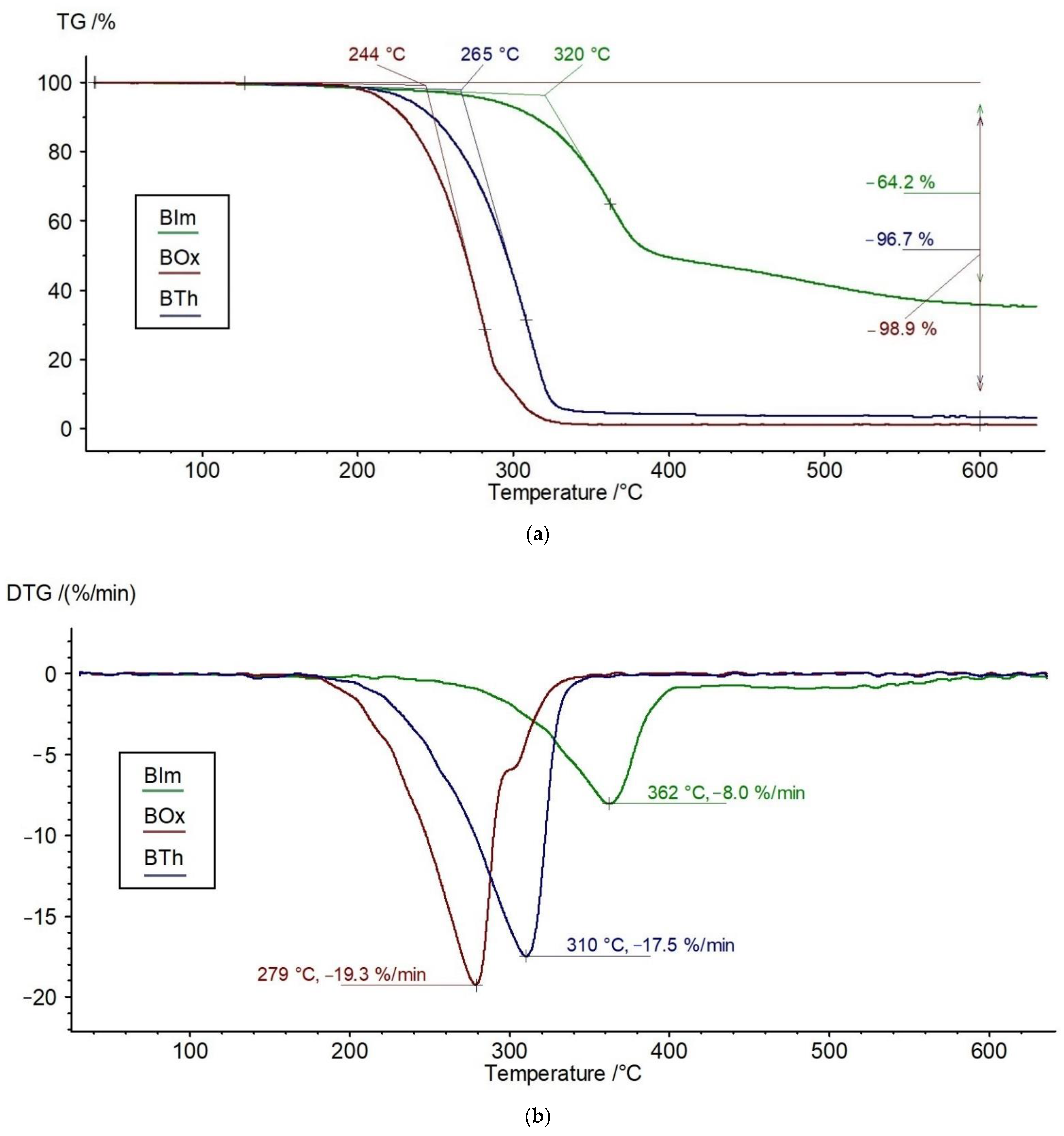

| Sample | To, °C | Tmax, °C | ∆m, % | Vmax, %/min | Melting Point, °C | Gram–Schmidt Curve |
|---|---|---|---|---|---|---|
| BIm | 320 | 362 | 64.2 | 8.0 | 196 | two peaks of similar intensity at 393 and 488 °C; |
| BOx | 244 | 279, 300 | 98.9 | 19.3 | 187 | two peaks at 342 °C (main) and 549 °C (weak); |
| BTh | 265 | 310 | 96.7 | 17.5 | 156, 174 | lack of peaks, monotonic growth without extremum |
| Sample 0 h | I Step | II Step | Total ∆m, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| To, °C | Tmax, °C | ∆m, % | To, °C | Tmax, °C | ∆m, % | Texo, °C; ΔH, J/g | Vmax, %/min | ||
| CS | 80 | 121 | 7.7 | 254 | 277 | 54.8 | 276; 153 | 7.5 | 62.4 |
| CS-BIm | 75 | 94 | 5.3 | 254 | 273 | 51.7 | 280; 172 | 7.3 | 55.6 |
| CS-BOx | 73 | 103 | 7.9 | 265 | 288 | 53.0 | 291; 140 | 8.7 | 60.5 |
| CS-BTh | 75 | 110 | 5.7 | 259 | 284 | 52.6 | 288; 132 | 7.4 | 58.3 |
| Sample 8 h UV | I Step | II Step | Total ∆m, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| To, °C | Tmax, °C | ∆m, % | To, °C | Tmax, °C | ∆m, % | Texo,°C; ΔH, J/g | Vmax, %/min | ||
| CS | 83 | 92 | 8.1 | 254 | 283 | 52.9 | 283; 140 | 6.5 | 60.7 |
| CS-BIm | 73 | 94 | 5.3 | 254 | 276 | 51.7 | 286; 285 | 7.5 | 57.1 |
| CS-BOx | 73 | 101 | 5.6 | 266 | 290 | 53.5 | 296; 402 | 8.2 | 59.2 |
| CS-BTh | 71 | 103 | 5.6 | 255 | 280 | 52.2 | 287; 290 | 7.1 | 58.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajer, D.; Kaczmarek, H. Thermal Stability of Fluorescent Chitosan Modified with Heterocyclic Aromatic Dyes. Materials 2022, 15, 3667. https://doi.org/10.3390/ma15103667
Bajer D, Kaczmarek H. Thermal Stability of Fluorescent Chitosan Modified with Heterocyclic Aromatic Dyes. Materials. 2022; 15(10):3667. https://doi.org/10.3390/ma15103667
Chicago/Turabian StyleBajer, Dagmara, and Halina Kaczmarek. 2022. "Thermal Stability of Fluorescent Chitosan Modified with Heterocyclic Aromatic Dyes" Materials 15, no. 10: 3667. https://doi.org/10.3390/ma15103667
APA StyleBajer, D., & Kaczmarek, H. (2022). Thermal Stability of Fluorescent Chitosan Modified with Heterocyclic Aromatic Dyes. Materials, 15(10), 3667. https://doi.org/10.3390/ma15103667







