Nanoparticles of Bioactive Metals/Metal Oxides and Their Nanocomposites with Antibacterial Drugs for Biomedical Applications
Abstract
:1. Introduction
2. INPs as Promising Antibacterial Agents
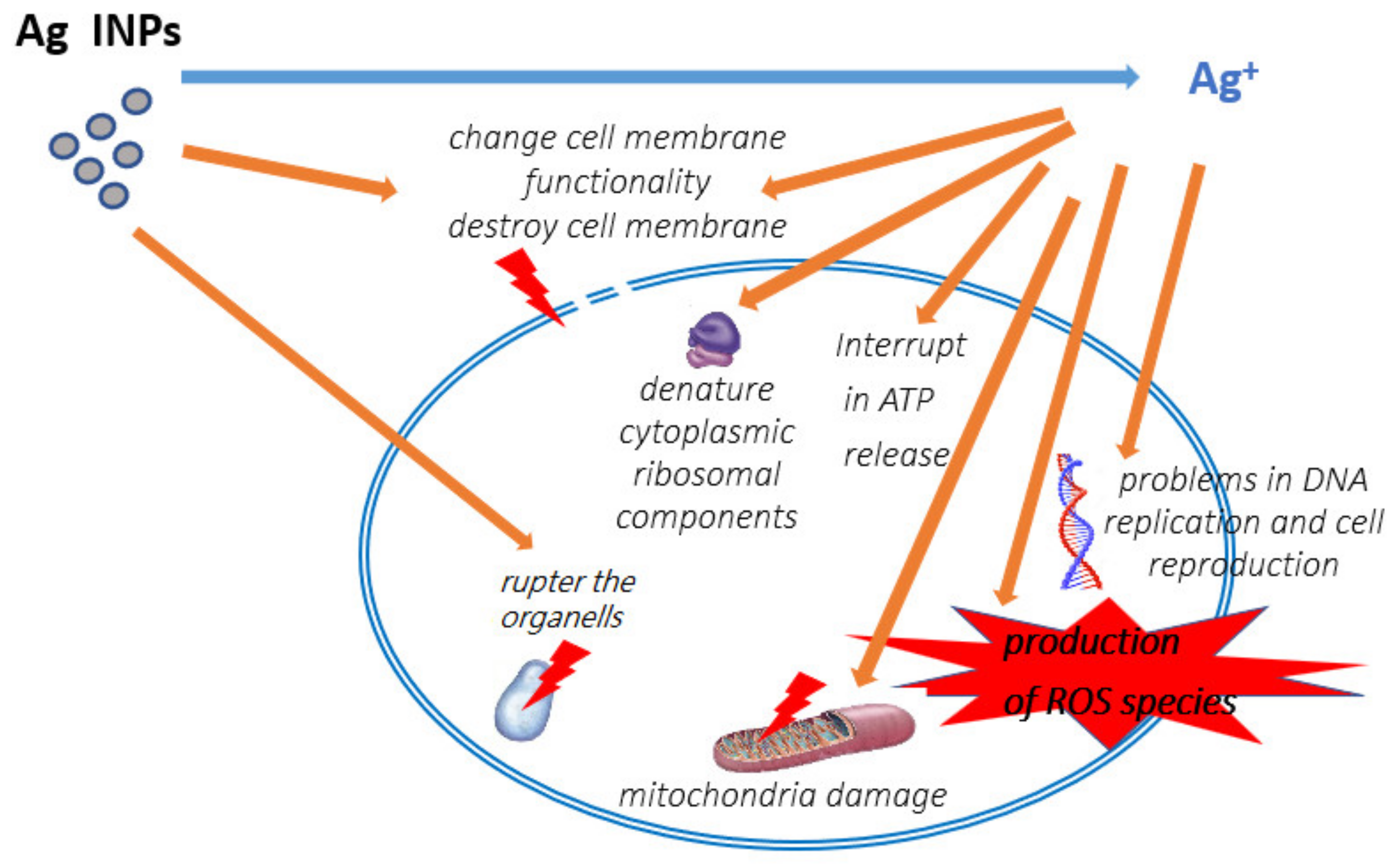
2.1. Ag INPs
2.2. Cu, Cu2O, and CuO INPs
2.3. Au INPs
2.4. ZnO INPs
2.5. TiO2 INPs
2.6. Other Metal INPs
2.7. Metal Oxide INPs
2.8. Magnetic INPs
3. Complex INPs
4. Nanocomposites of INPs with Antibacterial Drug Substances
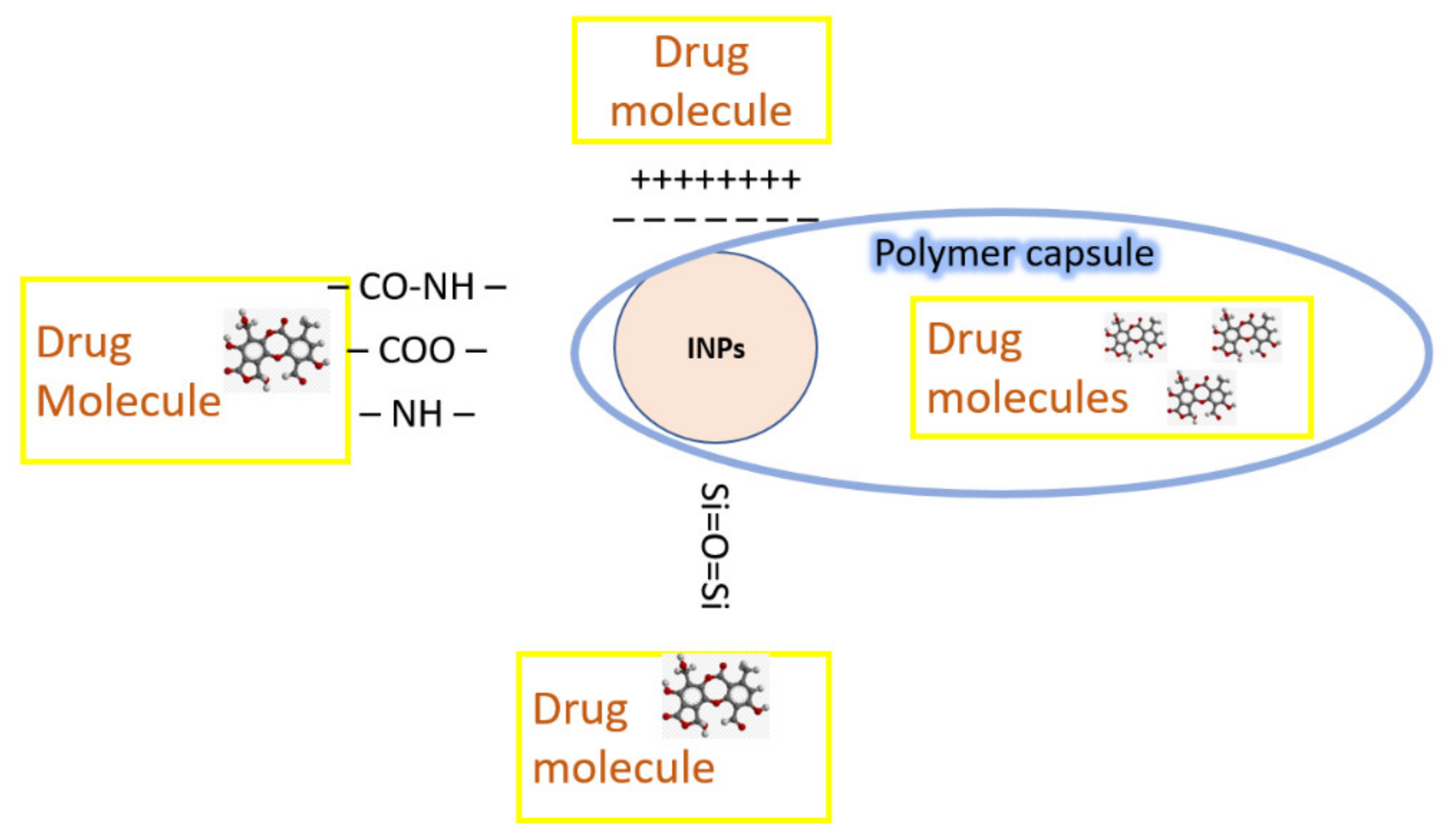
4.1. Drug Carriers
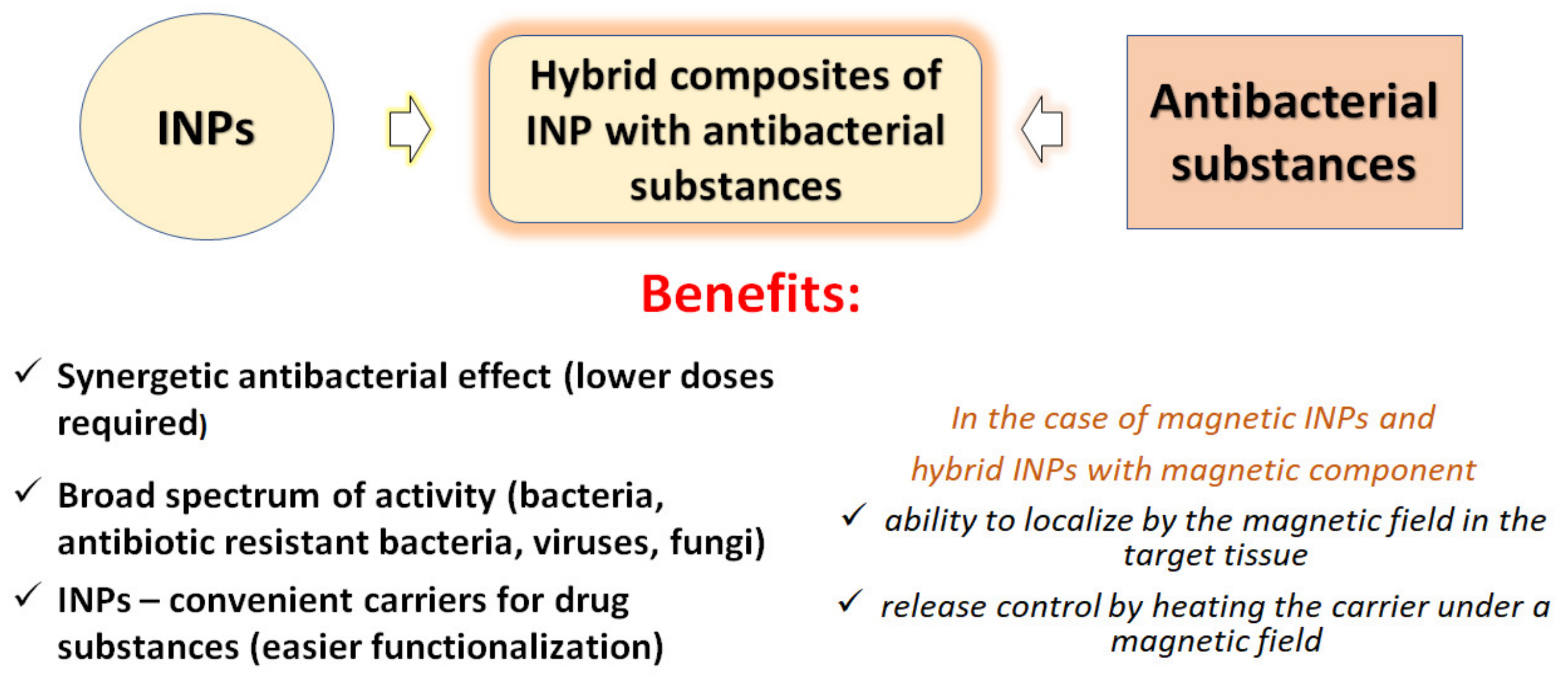
4.2. INPs as Components of Hybrid Nanocomposites (HNCs)
5. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Hyeon, T.; Rotello, V.M. Nanomedicine. Chem. Soc. Rev. 2012, 41, 2537–2538. [Google Scholar] [CrossRef] [PubMed]
- Jalili, H.; Aslibeiki, B.; Eskandarzadeh, N. Advanced applications of spinel ferrite nanoparticles in medicine: Magnetic hyperthermia, magnetic resonance imaging and targeted drug delivery. Iran. J. Phys. Res. 2021, 21, 219–242. [Google Scholar]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2022, 10, 3824. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic nanocarriers: Evolution of spinel ferrites for medical applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar]
- Shaw, S.; Shit, G.C.; Tripathi, D. Impact of drug carrier shape, size, porosity and blood rheology on magnetic nanoparticle-based drug delivery in a microvessel. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128370. [Google Scholar]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, 1906539. [Google Scholar] [CrossRef]
- Díez-Villares, S.; Ramos-Docampo, M.A.; da Silva-Candal, A.; Hervella, P.; Vázquez-Ríos, A.J.; Dávila-Ibáñez, A.B.; López-López, R.; Iglesias-Rey, R.; Salgueiriño, V.; Fuente, M. Manganese Ferrite Nanoparticles Encapsulated into Vitamin E/Sphingomyelin Nanoemulsions as Contrast Agents for High-Sensitive Magnetic Resonance Imaging. Adv. Healthc. Mater. 2021, 10, 2101019. [Google Scholar] [CrossRef]
- Farzaneh, S.; Hosseinzadeh, S.; Samanipour, R.; Hatamie, S.; Ranjbari, J.; Khojasteh, A. Fabrication and characterization of cobalt ferrite magnetic hydrogel combined with static magnetic field as a potential bio-composite for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2021, 64, 102525. [Google Scholar]
- Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials 2021, 11, 2337. [Google Scholar]
- Umut, E.; Coşkun, M.; Pineider, F.; Berti, D.; Güngüneş, H. Nickel ferrite nanoparticles for simultaneous use in magnetic resonance imaging and magnetic fluid hyperthermia. J. Colloid Interface Sci. 2019, 550, 199–209. [Google Scholar] [CrossRef]
- Khizar, S.; Ahmad, N.M.; Ahmed, N.; Manzoor, S.; Hamayun, M.A.; Naseer, N.; Tenório, M.K.L.; Lebaz, N.; Elaissari, A. Aminodextran coated CoFe2O4 nanoparticles for combined magnetic resonance imaging and hyperthermia. Nanomaterials 2020, 10, 2182. [Google Scholar] [CrossRef]
- Li, Z.; Yang, C.; Lu, W.; Chu, Z.; Zhang, J.; Li, M.; Wang, Q. Ultrasensitive immuno-PCR for detecting aflatoxin B1 based on magnetic separation and barcode DNA. Food Control 2022, 138, 109028–109030. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahreinizad, H.; Amiri, Z.; Aliabadi, M.; Salimi-Bani, M.; Nakisa, A.; Davoodi, F.; Tahmasebi, B.; Ahmadpour, F.; Radinekiyan, F.; et al. Functionalized magnetic nanoparticles for the separation and purification of proteins and peptides. Trends Anal. Chem. 2021, 141, 116291. [Google Scholar]
- Wang, L.; Lin, J. Recent advances on magnetic nanobead based biosensors: From separation to detection. Trends Anal. Chem. 2020, 128, 115915. [Google Scholar]
- Prescott, J.F. Outpacing the resistance tsunami: Antimicrobial stewardship in equine medicine, an overview. Equine Vet. Educ. 2021, 33, 539–545. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Wahab, S.; Khan, T.; Adil, M.; Khan, A. Mechanistic aspects of plant-based silver nanoparticles against multi-drug resistant bacteria. Heliyon 2021, 7, e07448. [Google Scholar]
- Jha, D.; Thiruveedula, P.K.; Pathak, R.; Kumar, B.; Gautam, H.K.; Agnihotri, S.; Sharma, A.K.; Kumar, P. Multifunctional biosynthesized silver nanoparticles exhibiting excellent antimicrobial potential against multi-drug resistant microbes along with remarkable anticancerous properties. Mater. Sci. Eng. C 2017, 80, 659–669. [Google Scholar]
- Spirescu, V.; Chircov, C.; Grumezescu, A.; Vasile, B.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar]
- Maliszewska, I.; Sadowski, Z. Synthesis and antibacterial activity of of silver nanoparticles. J. Phys. Conf. Ser. 2009, 146, 012024. [Google Scholar] [CrossRef]
- Almasoud, N.; Alhaik, H.; Almutairi, M.; Houjak, A.; Hazazi, K.; Alhayek, F.; Aljanoubi, S.; Alkhaibari, A.; Alghamdi, A.; Soliman, D.A.; et al. Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity. Green Process. Synth. 2021, 10, 510–528. [Google Scholar]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar]
- Kaur, N. Nanoantimicrobials: An Emerging Technological Approach in Food Preservation. Technol. Dev. Food Preserv. Process. Storage 2020, 20, 146–165. [Google Scholar]
- Yin, I.; Zhang, J.; Zhao, S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Syed, A.; Sabya, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [PubMed]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Ghetas, A.; Abdel-Razek, N.; Shakweer, M.S.; Abotaleb, M.; Ahamad Paray, B.; Ali, S.; Eldessouki, E.A.; Dawood, M.A.O.; Khalil, R.H. Antimicrobial activity of chemically and biologically synthesized silver nanoparticles against some fish pathogens. Saudi J. Biol. Sci. 2022, 29, 1298–1305. [Google Scholar] [PubMed]
- Hossain, M.; Polash, S.; Takikawa, M.; Shubhra, R.D.; Saha, T.; Islam, Z.; Hossain, S.; Hasan, A.; Takeoka, S.; Sarker, S.R. Investigation of the Antibacterial Activity and in vivo Cytotoxicity of Biogenic Silver Nanoparticles as Potent Therapeutics. Front. Bioeng. Biotechnol. 2019, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.A.R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro antimicrobial activity of green synthesized silver nanoparticles against selected Gram-negative foodborne pathogens. Front. Microbiol. 2018, 7, 1555. [Google Scholar] [CrossRef]
- Chudasama, B.; Vala, A.K.; Andhariya, N.; Mehta, R.V.; Upadhyay, R.V. Highly bacterial resistant silver nanoparticles: Synthesis and antibacterial activities. J. Nanoparticle Res. 2010, 12, 1677–1685. [Google Scholar]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar]
- Nasiri, A.; Afsar, G.R.; Nojoumi, S.A.; Akbarizadeh, M.; Harirchi, S.; Arefnezhad, M.; Sahraei, S.; Hesaraki, M.; Afshari, M.; Javadian, F.; et al. Evaluation of the Antimicrobial Activity of Silver Nanoparticles on Antibiotic-Resistant Pseudomonas aeruginosa. Int. J. Basic Sci. Med. 2016, 1, 25–28. [Google Scholar] [CrossRef]
- El-Gohary, F.A.; Abdel-Hafez, L.J.M.; Zakaria, A.I.; Shata, R.R.; Tahoun, A.; El-Mleeh, A.; Abo Elfadl, E.A.; Elmahallawy, E.K. Enhanced antibacterial activity of silver nanoparticles combined with hydrogen peroxide against multidrug-resistant pathogens isolated from dairy farms and beef slaughterhouses in Egypt. Infect. Drug Resist. 2020, 13, 3485–3499. [Google Scholar]
- Lakkim, V.; Reddy, M.C.; Pallavali, R.R.; Reddy, K.R.; Reddy, C.V.; Inamuddin; Bilgrami, A.L.; Lomada, D.A. Green synthesis of silver nanoparticles and evaluation of their antibacterial activity against multidrug-resistant bacteria and wound healing efficacy using a murine model. Antibiotics 2020, 9, 902. [Google Scholar] [CrossRef]
- Singh, K.; Panghal, K.; Chaudhary, Y.; Jaya, P.A. Antibacterial activity of synthesized silver nanoparticles from Tinospora cordifolia against multi drug resistant strains of Pseudomonas aeruginosa isolated from burn patients. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Qamar, H.; Rehman, S.; Chauhan, D.K.; Tiwari, A.K.; Upmanyu, V. Green synthesis, characterization and antimicrobial activity of copper oxide nanomaterial derived from Momordica charantia. Int. J. Nanomed. 2020, 15, 2541–2553. [Google Scholar] [CrossRef] [Green Version]
- Agarwala, M.; Choudhury, B.; Yadav, R.N.S. Comparative Study of Antibiofilm Activity of Copper Oxide and Iron Oxide Nanoparticles Against Multidrug Resistant Biofilm Forming Uropathogens. Indian J. Microbiol. 2014, 54, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Abdulazeem, L.; Abdalkareem, J.; Saade, R.; Wurood, J. Anti-bacterial activity of gold nanoparticles against two type of antibiotic resistance pathogenic bacteria in Al-Hilla city. Mater. Today Proc. 2021, 5, 1–4. [Google Scholar] [CrossRef]
- Nazari, P.; Dowlatabadi-Bazaz, R.; Mofid, M.R.; Pourmand, M.R.; Daryani, N.E.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shahverdi, A.R. The antimicrobial effects and metabolomic footprinting of carboxyl-capped bismuth nanoparticles against Helicobacter pylori. Appl. Biochem. Biotechnol. 2014, 172, 570–579. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar]
- Ferreyra Maillard, A.P.V.; Gonçalves, S.; Santos, N.C.; López de Mishima, B.A.; Dalmasso, P.R.; Hollmann, A. Studies on interaction of green silver nanoparticles with whole bacteria by surface characterization techniques. Biochim. Et Biophys. Acta 2019, 1861, 1086–1092. [Google Scholar]
- Raj Dwivedi, G.; Sanchita; Singh, P.D.; Sharma, A.; Darokar, M.; Srivastava, K.S. Nano Particles: Emerging Warheads Against Bacterial Superbugs: Ingenta Connect. Bentham Sci. Publ. 2016, 13, 1963–1975. [Google Scholar]
- Khalandi, B.; Asadi, N.; Milani, M.; Davaran, S.; Abadi, A.J.N.; Abasi, E.; Akbarzadeh, A. A Review on Potential Role of Silver Nanoparticles and Possible Mechanisms of their Actions on Bacteria. Drug Res. 2017, 67, 70–76. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Si, Y.; Shu, K. Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: Growth inhibition, cell injury, oxidative stress and internalization. PLoS ONE 2018, 13, e0209020. [Google Scholar]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar]
- Galdiero, Stefania; Falanga, Annarita; Vitiello, Mariateresa; Cantisani, Marco; Marra, Veronica; Galdiero, Massimiliano Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918.
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.M.; Lau, G.K.; Che, C.-M. Silver Nanoparticles Inhibit Hepatitis B virus Replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [CrossRef]
- Mehrbod, P.; Motamed, N.; Tabatabaeian, M.; Soleymanier, R.; Amini, E.; Shahidi, M.; Kheyri, M.T. In vitro antiviral effect of “Nanosilver” on influenza virus. DARU J. Pharm. Sci. 2015, 17, 88–93. [Google Scholar]
- Lara, H.H.; Ixtepan-Turrent, L.; Garza-Treviño, E.N.; Rodriguez-Padilla, C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J. Nanobiotechnol. 2010, 8, 15. [Google Scholar]
- Aboubakr, H.A.; Williams, P.; Gangal, U.; Youssef, M.M.; El-Sohaimy, S.A.; Bruggeman, P.J.; Goyal, S.M. Virucidal effect of cold atmospheric gaseous plasma on feline calicivirus, a surrogate for human norovirus. Appl. Environ. Microbiol. 2015, 81, 3612–3622. [Google Scholar] [PubMed] [Green Version]
- Bhella, D.; Gatherer, D.; Chaudhry, Y.; Pink, R.; Goodfellow, I.G. Structural insights into calicivirus attachment and uncoating. J. Virol. 2008, 82, 8051–8058. [Google Scholar] [CrossRef] [Green Version]
- Ratan, A.; Mashrur, R.; Chhoan, P.; Shahriar, S.; Haidere, F.; Runa, N.J.; Kim, S.; Kweon, D.; Hosseinzadeh, H.; Cho, J. Silver Nanoparticles as Potential Antiviral Agents. Pharmaceutics 2021, 13, 2034. [Google Scholar]
- Eduardo, J.J.; Afrânio, F.; Bruno, N.M.F.; Auberson, M.; Albuquerque, E.; Almeida, A. Antifungal activity of silver nanoparticles obtained by green synthesis. Rev. Inst. Med. Trop. São Paulo 2015, 2, 165–167. [Google Scholar]
- Skóra, B.; Krajewska, U.; Nowak, A.; Dziedzic, A.; Barylyak, A.; Kus-Liśkiewicz, M. Noncytotoxic silver nanoparticles as a new antimicrobial strategy. Sci. Rep. 2021, 11, 1. [Google Scholar]
- Akpinar, I.; Unal, M.; Sar, T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021, 3, 4. [Google Scholar]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal ac tivity and mode of action of silver nano-particles on Candida albicans. BioMetals 2009, 22, 235–242. [Google Scholar] [CrossRef]
- Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar]
- Dorau, B.; Arango, R.; Green, F. An investigation into the potential of ionic silver as a wood preservative. In Proceedings of the 2nd Wood-Frame Housing Durability and Disaster Issues Conference. For. Prod. Soc. 2004, 2004, 133–145. [Google Scholar]
- Saikova, S.V.; Vorob’Ev, S.A.; Nikolaeva, R.B.; Mikhlin, Y.L. Conditions for the formation of copper nanoparticles by reduction of copper(II) ions with hydrazine hydrate solutions. Russ. J. Gen. Chem. 2010, 80, 1122–1127. [Google Scholar] [CrossRef]
- Zia, R.; Riaz, M.; Farooq, N.; Qamar, A.; Anjum, S. Antibacterial activity of Ag and Cu nanoparticles synthesized by chemical reduction method: A comparative analysis. Mater. Res. Express 2020, 5, 7. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.; Hameed, A.; Yawar, W.; Masood ul Hasan, M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Sci. Rep. 2020, 10, 16680–16698. [Google Scholar]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper nanoparticles with high antimicrobial activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Rosenberg, M.; Vija, H.; Kahru, A.; Keevil, C.; Ivask, A. Rapid in situ assessment of Cu-ion mediated effects and antibacterial efficacy of copper surfaces. Sci. Rep. 2018, 8, 8172. [Google Scholar] [CrossRef]
- Tortella, G.R.; Pieretti, J.C.; Rubilar, O.; Fernández-Baldo, M.; Benavides-Mendoza, A.; Diez, M.C.; Seabra, A.B. Silver, copper and copper oxide nanoparticles in the fight against human viruses: Progress and perspectives. Crit. Rev. Biotechnol. 2021, 42, 431–449. [Google Scholar]
- Viet, P.V.; Nguyen, H.; Cao, T.M.; Hieu, L.V. Antifungal Activities of Copper Nanoparticles Synthesized by a Chemical Reduction Method. J. Nanomater. 2016, 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Pham, N.D.; Duong, M.; Le, M.V.; Hoang, A.; Pham, L. Preparation and characterization of antifungal colloidal copper nanoparticles and their antifungal activity against Fusarium oxysporum and Phytophthora capsic. Comptes Rendus Chim. 2019, 22, 786–793. [Google Scholar] [CrossRef]
- Muñoz-Escobar, A.; Reyes-López, S.Y. Antifungal susceptibility of Candida species to copper oxide nanoparticles on polycaprolactone fibers (PCL-CuONPs). PLoS ONE 2020, 15, e0228864. [Google Scholar]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2020, 19, 167–187. [Google Scholar]
- Kuo, Y.-L.; Wang, S.-G.; Wu, C.-Y.; Lee, K.-C.; Jao, C.-J.; Chou, S.-H.; Chen, Y.-C. Functional gold nanoparticle-based antibacterial agents for nosocomial and antibiotic-resistant bacteria. Nanomedicine 2016, 11, 2497–2510. [Google Scholar] [CrossRef]
- Singh, R.; Behera, S.; Singh, K.; Mishra, S.; Panigrahi, B.; Sahoo, T.; Parhi, P.; Mandal, D. Biosynthesized gold nanoparticles as photocatalysts for selective degradation of cationic dye and their antimicrobial activity. J. Photochem. Photobiol. A Chem. 2020, 400, 112704. [Google Scholar]
- Lavaee, F.; Ranjbar, Z.; Modarezi, F.; Keshavarz, F. The Effect of Gold Nano Particles with Different Sizes on Streptococcus Species. J. Dent. 2021, 4, 235–242. [Google Scholar]
- Hameed, S.; Wang, Y.; Zhao, L.; Xie, L.; Ying, Y. Shape-dependent significant physical mutilation and antibacterial mechanisms of gold nanoparticles against foodborne bacterial pathogens (Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus) at lower concentrations. Mater. Sci. Eng. C 2020, 108, 110338. [Google Scholar] [CrossRef]
- Meléndez-Villanueva, A.; Morán-Santibañez, K.; Martínez-Sanmiguel, J.; Rangel-López, J.; Garza-Navarro, R.; Rodríguez-Padilla, M.A.; Zarate-Triviño, C.; Trejo-Ávila, M.; Laura, M. Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses 2019, 11, 12–24. [Google Scholar]
- Kim, J.; Yeom, M.; Lee, T.; Kim, H.-O.; Na, W.; Kang, L.J.W.; Park, G.; Park, C.; Song, D.; Haam, S. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020, 18, 54. [Google Scholar]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral potential of nanoparticles—can nanoparticles fight against coronaviruses. Nanomaterials 2020, 10, 1645. [Google Scholar]
- Wani, I.A.; Ahmad, T. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B Biointerfaces 2013, 101, 162–170. [Google Scholar] [PubMed]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanotechnol. 2011, 7, 184–192. [Google Scholar]
- Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2010, 94, 22–31. [Google Scholar]
- Mendes, C.; Dilarri, G.; Forsan, C.; Sapata, V.; Lopes, P.; de Matos, M.; Peterson, B.; Montagnolli, R.; Ferreira, H.; Bidoia, E. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Jpn. 1996, 29, 627–633. [Google Scholar]
- Wahab, R.; Kim, Y.-S.; Mishra, A.; Yun, S.-I.; Shin, H.-S. Formation of ZnO micro-flowers prepared via solution process and their antibacterial activity. Nanoscale Res. Lett. 2010, 5, 1675–1681. [Google Scholar]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 49–56. [Google Scholar]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar]
- Melk, M.; El-Hawary, S.; Melek, F.R.; Saleh, D.O.; Ali, O.M.; El Raey, M. Antiviral Activity of Zinc Oxide Nanoparticles Mediated by Plumbago indica L. Extract Against Herpes Simplex Virus Type 1 (HSV-1). Int. J. Nanomed. 2021, 16, 8221–8233. [Google Scholar]
- Selim, N.M.; Saleh, D.; Ali, O.M.; Raey, M.A. Nano Zinc Oxide Green-Synthesized from Plumbago auriculata Lam. Alcoholic Extract. Plants 2021, 10, 2447. [Google Scholar]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerrero-Vargas, J.A.; Rodríguez-Páez, J.E. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar]
- Hosseini, S.S.; Joshaghani, H.; Shokohi, T.; Ahmadi, A.; Mehrbakhsh, Z. Antifungal activity of ZnO nanoparticles and nystatin and downregulation of SAP1–3 genes expression in fluconazole-resistant candida albicans isolates from vulvovaginal candidiasis. Infect. Drug Resist. 2020, 13, 385–394. [Google Scholar]
- Attia Gouda, H.; Moemen, Y.S.; Youns, M.; Ibrahim, A.M.; Abdou, R.; El Raey, M.A. Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surf. B Biointerfaces 2021, 203, 111724. [Google Scholar]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2016, 166, 207–215. [Google Scholar]
- Heidary, M.; Zaker, B.; Amini, S.M.; Jafari, A.; Ghalami, N.M.; Ghodousi, A. The anti-mycobacterial activity of Ag, ZnO, and Ag- ZnO nanoparticles against MDR- and XDR-Mycobacterium tuberculosis. Infect. Drug Resist. 2019, 12, 3435–3500. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barajas, N.; Anaya-Esparza, L.; Villagrán-de la Mora, Z.; Sánchez-Burgos, J.; Pérez-Larios, A. Review of therapies using TiO2 nanomaterials for increased anticancer capability. Anti-Cancer Agents Med. Chem. 2021, 22, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Chai, M.; Yao, X.; Chen, W.; Chu, P.K. Synergistic antibacterial activity of physical-chemical multi-mechanism by TiO2 nanorod arrays for safe biofilm eradication on implant. Bioact. Mater. 2021, 6, 12–25. [Google Scholar] [PubMed]
- Yin, M.; Li, Z.; Ju, E.; Wang, Z.; Dong, K.; Ren, J.; Qu, X. Multifunctional upconverting nanoparticles for near-infrared triggered and synergistic antibacterial resistance therapy. Chem. Commun. 2014, 50, 10448–10490. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria andleishmaniaparasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef]
- Sodagar, A.; Akhoundi, M.; Bahador, A.; Jalali, Y.; Behzadi, Z.; Elhaminejad, F.; Mirhashemi, A. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in orthodontics. Dent. Press J. Orthod. 2017, 22, 67–74. [Google Scholar]
- Yasuyuki, M.; Kunihiro, K.; Kurissery, S.; Kanavillil, N.; Sato, Y.; Kikuchi, Y. Antibacterial properties of nine pure metals: A laboratory study using Staphylococcus aureus and Escherichia coli. Biofouling 2010, 26, 851–858. [Google Scholar] [CrossRef]
- Sadiq, I.M.; Chowdhury, B.; Chandrasekaran, N.; Mukherjee, A. Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomedicine: Nanotechnology. Biol. Med. 2009, 5, 282–286. [Google Scholar]
- Roy, A.; Gauri, S.S.; Bhattacharya, M.; Bhattacharya, J. Antimicrobial activity of CaO nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 1570–1578. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S.A. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar]
- Ismail, R.A.; Sulaiman, G.M.; Abdulrahman, S.A.; Marzoog, T.R.A. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater. Sci. Eng. 2015, 53, 286–297. [Google Scholar]
- Golabiazar, R.; Omar, Z.A.; Ahmad, R.N.; Hasan, S.A.; Sajadi, S. Mohammad Synthesis and characterization of antibacterial magnetite-activated carbon nanoparticles. J. Chem. Res. 2020, 44, 80–87. [Google Scholar]
- Ramteke, C.; Sarangi, B.K.; Chakrabarti, T.; Mudliar, S.; Satpute, D.; Pandey, R.A. Synthesis and Broad Spectrum Antibacterial Activity of Magnetite Ferrofluid. Curr. Nanosci. 2010, 6, 587–591. [Google Scholar] [CrossRef]
- Gole, D.A.; Kapatkar, S.B.; Mathad, S.N.; Chavan, R.R. In vitro antimicrobial activity of cobalt ferrite nanoparticles synthesized by co-precipitation method. Acta Chem. IASI 2020, 28, 225–236. [Google Scholar] [CrossRef]
- Gheidari, D.; Mehrdad, M.; Maleki, S.; Hosseini, S. Synthesis and potent antimicrobial activity of CoFe2O4 nanoparticles under visible light. Heliyon 2020, 6, e05058. [Google Scholar]
- Manju, B.G.; Raji, P. Biological synthesis, characterization, and antibacterial activity of nickel-doped copper ferrite nanoparticles. Appl. Phys. A Mater. Sci. Process. 2019, 125, 313. [Google Scholar]
- Soleimani, L.; Mohammad, K.; Ghorbani, M.; Naghibi, H.; Khalaj, P. Synthesis and characterization of polyrhodanine/nickel ferrite nanocomposite with an effective and broad spectrum antibacterial activity. Polym.-Plast. Technol. Mater. 2019, 58, 1461–1470. [Google Scholar]
- Koli, P.B.; Kapadnis, K.H. Synthesis, Characterization& Antimicrobial Activity of Mixed Metal Oxides of Iron Cobalt Nickel and Zinc. Int. J. Chem. Phys. Sci. 2016, 4, 357–360. [Google Scholar]
- Haghniaz, R.; Rabbani, A.; Vajhadin, F.; Khan, T.; Kousar, R.; Khan, A.R.; Montazerian, H.; Iqbal, J.; Libanori, A.; Kim, H.J.; et al. Anti-bacterial and wound healing-promoting effects of zinc ferrite nanoparticles. J. Nanobiotechnol. 2021, 19, 38. [Google Scholar]
- Maksoud, M.I.A.; El-Sayyad, S.; El-Bastawisy, S.; Fathy, M. Antibacterial and antibiofilm activities of silver-decorated zinc ferrite nanoparticles synthesized by a gamma irradiation-coupled sol-gel method against some pathogenic bacteria from medical operating room surfaces. RSC Adv. 2021, 11, 28361–28374. [Google Scholar] [PubMed]
- Morais, D.O.; de Pancotti, A.S.; Guilherme, S.S.; Marielena, V.; Alexandre, L.S.; de Mauro, V.B.; da Costa, V.G.; Wang, J. Synthesis, characterization, and evaluation of antibacterial activity of transition metal oxyde nanoparticles. J. Mater. Sci. Mater. Med. 2021, 32, 101. [Google Scholar] [PubMed]
- Majhi, R.K.; Mohanty, S.; Khan, I.; Mishra, A.; Brauner, A. Ag@ZnO Nanoparticles Induce Antimicrobial Peptides and Promote Migration and Antibacterial Activity of Keratinocytes. ACS Infect. Dis. 2021, 7, 2068–2072. [Google Scholar] [CrossRef] [PubMed]
- Gingasu, D.; Mindru, I.; Patron, L.; Marinescu, G.; Preda, S.; Calderon-Moreno, J.M.; Osiceanu, P.; Somacescu, S.; Stanica, N.; Popa, M.; et al. Soft chemistry routes for the preparation of Ag-CoFe2O4 nanocomposites. Ceram. Int. 2017, 43, 3284–3291. [Google Scholar]
- Gingasu, D.; Mindru, I.; Patron, L.; Calderon-Moreno, J.M.; Mocioiu, O.C.; Preda, S.; Stanica, N.; Nita, S.; Dobre, N.; Popa, M.; et al. Green Synthesis Methods of CoFe2O4 and Ag-CoFe2O4 Nanoparticles Using Hibiscus Extracts and Their Antimicrobial Potential. J. Nanomater. 2016, 2016, 12–25. [Google Scholar]
- Bednář, J.; Svoboda, L.; Rybková, Z.; Dvorský, R.; Malachová, K.; Stachurová, T.; Matýsek, D.; Foldyna, V. Antimicrobial synergistic effect between Ag and Zn in Ag-ZnO.mSiO2 silicate composite with high specific surface area. Nanomaterials 2019, 9, 1265. [Google Scholar]
- Bankier, C.; Matharu, R.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef] [Green Version]
- Rekha, K.; Nirmala, M.; Nair, M.G.; Anukaliani, A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B Condens. Matter 2010, 15, 3180–3185. [Google Scholar] [CrossRef]
- Dutta, R.K.; Sharma, P.K.; Bhargava, R.; Kumar, N.; Pandey, A.C. Differential Susceptibility of Escherichia coli Cells toward Transition Metal-Doped and Matrix-Embedded ZnO Nanoparticles. J. Phys. Chem. B 2010, 114, 5594–5599. [Google Scholar] [CrossRef]
- Pant, H.; Pant, B.; Sharma, R.; Amarjargal, A.; Kim, H.; Park, C. Antibacterial and photocatalytic properties of Ag/TiO2/ZnO nano-flowers prepared by facile one-pot hydrothermal process. Ceram. Int. 2013, 39, 1503–1510. [Google Scholar] [CrossRef]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar]
- Kotrange, H.; Najda, A.; Bains, A.; Gruszecki, R.; Chawla, P.; Tosif, M.M. Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics. Int. J. Mol. Sci. 2021, 22, 9596. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar]
- Kalimuthu, K.; Lubin, B.-C.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M.A. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J. Nanobiotechnol. 2018, 16, 34. [Google Scholar]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic nanoparticles for biomedical purposes: Modern trends and prospects. Magnetochemistry 2020, 6, 30. [Google Scholar]
- Rivero, M.; Marín-Barba, M.; Gutiérrez, L.; Lozano-Velasco, E.; Wheeler, G.N.; Sánchez-Marcos, J.; Muñoz-Bonilla, A.; Morris, C.; Ruiz, A. Toxicity and biodegradation of zinc ferrite nanoparticles in Xenopus laevis. J. Nanoparticle Res. 2019, 21, 181. [Google Scholar]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef]
- Panácek, A.; Smékalová, M.; Kilianová, M.; Prucek, R.; Bogdanová, K.; Věcěrová, R.; Kolár, M.; Havrdová, M.; Płaza, G.A.; Chojniak, J.; et al. Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules 2016, 21, 26. [Google Scholar]
- Zou, L.; Wang, J.; Gao, Y.; Ren, X.; Rottenberg, E.; Lu, J.; Holmgren, A. Synergistic antibacterial activity of silver with antibiotics correlating with the upregulation of the ROS production. Sci. Rep. 2018, 8, 11131. [Google Scholar]
- Brasil, S.L.; Filgueiras, A.L.; Campos, M.B.; Neves, S.L.; Eugênio, M.; Sena, L.A.; Sant’Anna, B.; Da Silva, V.L.; Diniz, C.G.; Sant’Ana, A.C. Synergism in the antibacterial action of ternary mixtures involving silver nanoparticles, chitosan and antibiotics. J. Braz. Chem. Soc. 2018, 29, 2026–2033. [Google Scholar] [CrossRef]
- Kiranmai, M.; Kadimcharla, K.; Keesara, R.; Fatima, S.N.; Bommena, P.; Batchu, U.R. Green Synthesis of Stable Copper Nanoparticles and Synergistic Activity with Antibiotics. Indian J. Pharm. Sci. 2017, 79, 695–700. [Google Scholar]
- Khurana, C.; Sharma, P.; Pandey, O.P.; Chudasama, B. Synergistic Effect of Metal Nanoparticles on the Antimicrobial Activities of Antibiotics against Biorecycling Microbes. J. Mater. Sci. Technol. 2016, 32, 524–532. [Google Scholar] [CrossRef]
- Vernaya, O.I.; Shabatin, V.P.; Semenov, A.M.; Shabatina, T.I. Cryochemical synthesis and antibacterial activity of a hybrid composition based on Ag nanoparticles and dioxidine. Mosc. Univ. Chem. Bull. 2017, 72, 6–9. [Google Scholar]
- Fadwa, A.O.; Albarag, A.M.; Alkoblan, D.K.; Mateen, A. Determination of synergistic effects of antibiotics and Zno NPs against isolated E. Coli and A. Baumannii bacterial strains from clinical samples. Saudi J. Biol. Sci. 2021, 28, 5332–5337. [Google Scholar]
- Thamer, R.; Alsammak, E. Synergistic effect of Zinc Oxide nanoparticles and Vancomycin on Methicillin resistant Staphylococcus aureus. J. Life Bio Sci. Res. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Abdeen, E.E.; Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.; Obare, S.O.; Scott, M.E. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar]
- Vernaya, O.I.; Khvatov, D.I.; Nuzhdina, A.V.; Fedorov, V.V.; Shabatin, V.P.; Semenov, A.M.; Shabatina, T.I. Cu/dioxidine hybrid nanocomposites: Cryochemical synthesis. Mosc. Univ. Chem. Bull. 2017, 72, 224–226. [Google Scholar] [CrossRef]
- Vernaya, O.I.; Shabatin, V.P.; Nuzhdina, A.V. Cryochemical synthesis and antibacterial activity of hybrid nanocomposites based on dioxidine containing Ag and Cu nanoparticles incorporated in biopolymer cryostructurates. Russ. Chem. Bull. 2017, 66, 2152–2156. [Google Scholar] [CrossRef]
- Vernaya, O.I.; Shabatin, V.P.; Semenov, A.M. Low-temperature synthesis and antibacterial activity of hybrid systems of gentamicin sulfate with copper and iron nanoparticles. Mosc. Univ. Chem. Bull. 2020, 75, 258–260. [Google Scholar]
- Shabatina, T.I.; Vernaya, O.I.; Karlova, D.L.; Nuzhdina, A.V.; Shabatin, V.P.; Semenov, A.M.; Lozinskii, V.I.; Mel’Nikov, M.Y. Hybrid Systems of Delivery of Long-Acting Drugs Based on Gentamicin Sulfate, Silver, and Copper Nanoparticles, and Gelatin Biopolymer Matrices. Nanotechnol. Russ. 2018, 13, 546–550. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Karlova, D.L.; Nuzhdina, A.V.; Shabatin, V.P.; Semenov, A.M.; Lozinskii, V.I.; Mel’nikov, M.Y. A Hybrid Nanosystems Based on an Antibacterial Preparation of Dioxydine and Metal Nanoparticles (Ag and Cu) Included in Biopolymer Cryostructures. Nanotechnol. Russ. 2018, 13, 182–188. [Google Scholar] [CrossRef]
- Rastogi, L.; Jyothi, K.; Sashidhar, R.B. Antibacterial effects of gum kondagogu reduced/stabilized silver nanoparticles in combination with various antibiotics: A mechanistic approach. Appl. Nanosci. 2015, 5, 535–543. [Google Scholar]
- Alizadeh, A.; Salouti, M.; Alizadeh, H.; Kazemizadeh, A.R.; Safari, A.A.; Mahmazi, S. Enhanced antibacterial effect of azlocillin in conjugation with silver nanoparticles against Pseudomonas aeruginosa. IET Nanobiotechnol. 2017, 11, 942–947. [Google Scholar] [CrossRef]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar]
- Ashmore, D.; Chaudhari, A.; Barlow, B.; Barlow, B.; Harper, T.; Vig, K.; Miller, M.; Singh, S.; Nelson, E.; Pillai, S. Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles. Rev. Inst. Med. Trop. São Paulo 2018, 60, e18. [Google Scholar]
- Abo-Shama, U.H.; El-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F. Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Luan, Z.; Sun, G.; Huang, Y.; Yang, Y.; Ruifu, Y.; Li, C.; Tingting, W.; Tan, D.; Qi, S.; Jun, C.; et al. Metagenomics Study Reveals Changes in Gut Microbiota in Centenarians: A Cohort Study of Hainan Centenarians. Front. Microbiol. 2020, 11, 1474. [Google Scholar]
- Kaur, A.; Kumar, R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv. 2019, 9, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Pauer, A.C.; Gonzales, A.A.; Fenniri, H. Enhanced antibiotic activity of ampicillin conjugated to gold nanoparticles on PEGylated rosette nanotubes. Int. J. Nanomed. 2019, 14, 7281–7289. [Google Scholar]
- Payne, J.N.; Waghwani, H.K.; Connor, M.G.; Hamilton, W.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R.A. Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front. Microbiol. 2016, 7, 607. [Google Scholar]
| NPs, Size | Bacteria Tested (Drug to Which It Is Resistant) | Method and Concentrations | Reference |
|---|---|---|---|
| Ag 5–20 nm | Staphylococcus aureus (methicillin) | MIC: 32 μg/mL | [33] |
| Ag - | P. aeruginosa (ampicillin, nitrofurantoin, nalidixic acid, ciprofloxacin) | MIC: 12.5 μg/mL | [34] |
| Ag 45 nm | E. coli (erythromycin, amoxicillin, tetracycline, and streptomycin) L. monocytogenes (rifampicin, cefotaxime, tetracycline, gentamycin, and chloramphenicol) S. typhimurium (norfloxacin, amoxicillin, ciprofloxacin, chloramphenicol, and trimethoprim/sulphamethoxazole) P. aeruginosa (ciprofloxacin, norfloxacin, streptomycin, and levofloxacin.) K. pneumoniae (neomycin, kanamycin, tetracycline, nalidixic acid, amoxicillin, and gentamycin) | MIC: E. coli 6.25 μg/mL L. monocytogenes 12.5 μg/mL S. typhimurium 3.125 μg/mL P. aeruginosa 6.25 μg/mL K. pneumoniae 25 μg/mL | [35] |
| Ag 20 nm | E. coli, K. pneumoniae, S. aureus, P. aeruginosa (tetracycline, ampicillin, and erythromycin) | Disk diffusion method, Ag NPs showed bacteriolytic activity at all tested concentrations: 10, 30, 60, 90, and 120 µg/µL | [36] |
| Ag 12 nm | Pseudomonas aeruginosa (amikacin, aztreonam, ceftizoxime, cefepime, gentamicin, imipenem, netilmicin, ofloxacin, piperacillin and tazobactam; the strains which were resistant to 6 or 7 antibiotics from the list above were used) | MIC: 6.25 μg/mL | [37] |
| CuO 62 | Staphylococcus aureus, Streptococcus mutans, Streptococcus pyogenes, Streptococcus viridans, Staphylococcus epidermidis, Corynebacterium xerosis, and Bacillus cereus, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Proteus vulgaris (multidrug-resistant clinical bacterial strains) | Disk diffusion method, concentration of CuO NRs 1.25 mg/50 µL DMSO | [38] |
| CuO 25–30 nm Fe2O3 25–30 nm | Staphylococcus aureus (methicillin), Staphylococcus epidermidis (methicillin), Enterococcus faecalis (vancomycin) | Disk diffusion method, MIC 30–40 μg/mL | [39] |
| Au 25 nm | Pseudomonas aeruginosa and Staphylococcus aureus (azithromycin, chloramphenicol, tetracycline, nitrofurantoin, cefotaxime, amoxicillin, sulphamethoxazole, novobiocin, cephalothin, methicillin, bacitracin, ampicillin and aztreonam) | Disk diffusion method | [40] |
| Bi 1–5 nm | Helicobacter pylori (multiple-antibiotic) | MIC: 100 μg/mL | [41] |
| NPs | NPs Size | Bacteria Tested | Method and Concentrations | Reference |
|---|---|---|---|---|
| Fe3O4/γ-Fe2O3 | 10–20 nm | Escherichia coli, Bacillus subtilis (Relatively high ROS production was indicated in upon Fe2O3 treatment of the bacteria) | The BacLight fluorescence assay, bacterial growth kinetic and colony-forming unit studies, 2.5, 5, 10, 25, and 50 μM | [111] |
| Fe2O3 | 50–110 nm | Staphylococcus aureus, Escherichia coli | Disk diffusion method, 4 mg/mL | [112] |
| Fe3O4 | 14 nm | Staphylococcus aureus, Proteus mirabilis, Pseudomonas aureginosa | Disk diffusion method, 11.50–36.30 mg/mL | [113] |
| Fe3O4 | 10–100 nm | E. coli, Staphylococcus aureus, Bacillus subtilis and Pseudomonas aeruginosa | Disk diffusion method, 0.029–0.047 mg/mL | [114] |
| CoFe2O4 | 35 nm | Staphylococcus aureus, Candida albicans and Rhizopus oryzae fungal strain | MIC: 25 μg/mL | [115] |
| CoFe2O4 | 20–30 nm | Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Bacillus cereus | MIC (μg/mL): Pseudomonas aeruginosa 0.24, Escherichia coli 0.12, Staphylococcus aureus 0.24, Bacillus cereus 0.06 | [116] |
| Cu1−xNixFe2O4 | 20–60 nm | Escherichia coli, Klebsiella pneumonia, Staphylococcus aureus, Bacillus subtilis | Disk diffusion method | [117] |
| NiFe2O4 | 15–50 nm | Escherichia coli, Staphylococcus aureus | MIC: 5 mg/mL | [118] |
| Ni, Co, Fe, Zn ferrites | Escherichia coli, Staphylococcus aureus | Disk diffusion method | [119] | |
| ZnFe2O4 | Staphylococcus aureus, Streptococcus Pneumoniae, and carbapenem-resistant Enterobacteriaceae | Disk diffusion method, 12.5–100 mg/mL | [120] | |
| Ag-decorated ZnFe2O4 | 25 nm | Staphylococcus vitulinus, S. aureus, Enterococcus columbae, Staphylococcus lentus | MIC: 0.4–1.5 μg/mL | [121] |
| ZnFe2O4, CoFe2O4, Zn0.5Co0.5Fe2O4 | Escherichia coli, Staphylococcus au-reus | IC50 (µg/mL): ZnFe2O4–460, CoFe2O4–980, Zn0.5Co0.5Fe2O4–465 | [122] | |
| Ag@ZnO | Staphylococcus aureus | Noncytotoxic doses of Ag@ZnO stimulate proliferation and migration of human keratinocytes. Ag@ZnO increases the expression of antimicrobial peptides hBD2 and RNase7 and lysosomal degradation of intracellular bacteria. | [123] | |
| Ag-CoFe2O4 | 23–29 nm | E. coli, Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis, Candida albicans | MIC: 2 μg/mL | [124] |
| NPs | Antibiotic | Bacteria or Fungi Tested | Reference |
|---|---|---|---|
| Ag, Cu | Tetracycline, kanamycin | Bacillus subtilis, Pseudomonas fluorescens | [143] |
| Ag, Cu | Gentamicin, dioxidine | S. aureus, E. coli | [151,152] |
| Ag | Ciprofloxacin, streptomycin and gentamicin | Staphylococcus aureus, Pseudomonas. Aeruginosa, Escherichia coli | [153] |
| Ag | Azlocillin | P. aeruginosa | [154] |
| Ag | Erythromycin, ampicillin, chloramphenicol, cephalothin, clindamycin, tetracycline, gentamycin, amoxicillin, ciprofloxacin, cefpodoxime, cefuroxime | Multiresistant S. aureus, S. mutans, S. oralis, S. gordonii, Enterococcus faecalis, E. coli, A. actinomycetemcomitans, P. aeruginosa | [155,156,157] |
| Ag | Vancomycin, ampicillin, penicillin | S. aureus, E. coli, K. pneumoniae | [158] |
| Ag | Vancomycin, amikacin | E. coli, S. aureus | [159] |
| Au | Ampicillin | S. aureus | [160] |
| Au | Kanamycin | Staphylococcus epidermidis, Streptococcus bovis, Enterobacter aerogenes, Pseudomonas aeruginosa | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabatina, T.; Vernaya, O.; Shumilkin, A.; Semenov, A.; Melnikov, M. Nanoparticles of Bioactive Metals/Metal Oxides and Their Nanocomposites with Antibacterial Drugs for Biomedical Applications. Materials 2022, 15, 3602. https://doi.org/10.3390/ma15103602
Shabatina T, Vernaya O, Shumilkin A, Semenov A, Melnikov M. Nanoparticles of Bioactive Metals/Metal Oxides and Their Nanocomposites with Antibacterial Drugs for Biomedical Applications. Materials. 2022; 15(10):3602. https://doi.org/10.3390/ma15103602
Chicago/Turabian StyleShabatina, Tatyana, Olga Vernaya, Aleksei Shumilkin, Alexander Semenov, and Mikhail Melnikov. 2022. "Nanoparticles of Bioactive Metals/Metal Oxides and Their Nanocomposites with Antibacterial Drugs for Biomedical Applications" Materials 15, no. 10: 3602. https://doi.org/10.3390/ma15103602
APA StyleShabatina, T., Vernaya, O., Shumilkin, A., Semenov, A., & Melnikov, M. (2022). Nanoparticles of Bioactive Metals/Metal Oxides and Their Nanocomposites with Antibacterial Drugs for Biomedical Applications. Materials, 15(10), 3602. https://doi.org/10.3390/ma15103602






