1. Introduction
The use of biomass in the energy sector can potentially reduce the emission of harmful substances into the air. It is assumed that the absorbed amount of CO
2 during plant growth balances the amount generated in the process of biomass combustion [
1]. Biomass is therefore considered a zero-emission fuel. This assumption is incorrect because burning biomass also produces other pollutants that the plant cannot absorb during the growing season. The pollutants emitted during the combustion process can be divided into primary and secondary groups. Primary pollutants are emitted into the atmosphere directly at the locations where they are produced. Primary pollutants remain in the atmosphere unchanged from the moment they are generated. The sources of primary pollution are power plants and heating devices for single-family houses. Secondary pollutants, however, are the products of physical changes and chemical reactions between the components of the atmosphere and the pollutants that flow into it. Continuous technological progress, new branches of industry, and the development of transport contribute to the increase in the number of point, line, and area emitters. The constant increase in the number of sources of harmful emissions has an adverse impact on air quality. In highly developed countries, legal directives and laws specify the emission limits for individual compounds related to the type of fuel burned or the nominal power of the emitter. Therefore, the growing number of emitters is not reflected in the amount of emissions [
2].
Research commissioned by the European Environment Agency (EEA) showed that air pollution is one of the leading environmental factors affecting human life and health [
2]. In highly developed countries, including EU countries, appropriate legal regulations were introduced to limit the problem of harmful emissions [
2,
3,
4]. During the combustion process, harmful substances emitted in exhaust gases include suspended dust (PM), carbon monoxide (CO), nitrogen oxides (NO
x), and sulfur oxides (SO
x). The hazardous compounds emitted in exhaust gases are polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs). The emissions of these compounds are incredibly high in the case of biomass combustion [
4,
5,
6,
7,
8]. Too high of a concentration in the air of the compounds mentioned above can potentially lead to deficiencies in the human circulatory and respiratory systems. Even a slight excess in the permissible concentrations of pollutants in the air may cause disturbances in concentration and perception [
8,
9,
10,
11,
12].
The implemented EU Emissions Trading System (EU ETS) is one of the main tools in the fight against air pollution. This system covers approximately 40% of all greenhouse gas (GHG) emissions in the European Economic Area. The emissions trading system, in addition to new legal regulations, enables the continuous reduction of permissible limit values in reducing the total emission of pollutants [
13]. According to the European Commission, all of these measures aim to bring about an economic transformation toward climate neutrality, which must be attained by 2050 [
14,
15].
Catalytic substances added to combusted energy carriers are a group of compounds that improve the efficiency of the combustion process. One of the tasks of catalytic additives is to increase the oxidation process of fuel particles together with products of incomplete combustion. Depending on the used catalyst, it is possible to reduce or oxidize selected harmful compounds. The use of co-combustion of active substances with fuels is able to reduce greenhouse gas emissions and eliminate carcinogenic, mutagenic, and toxic compounds [
16]. Catalytic additives based on copper (Cu) and manganese (Mn) oxide, with the use of aluminum oxide (Al
2O
2) as a carrier, demonstrated the effect of reducing carbon monoxide (CO) emission and particulate matter during the combustion of solid biomass. The great advantage of this type of catalyst is its low price [
16,
17,
18,
19]. During our research in 2021 at the University of Life Sciences in Wrocław, we prepared five different catalytic compounds based on TiO
2, MnO
2, Cu (NO
3)
2‧3H
2O, 8% K
2PtCl
2 solution, and 32% aqueous urea solution. Sodium aluminum silicate was used as the carrier for the active substance. The research indicated that the use of these catalytic additives in the combustion of sunflower husk pellets led to a reduction in the emission of harmful substances and increased the combustion temperature. The observed increase in combustion temperature caused a decrease in the carbon monoxide (CO) concentration. A reduction in the proportion of incomplete combustion in the biomass boiler indirectly contributed to a decrease in the amount of fuel burned [
20].
One method for managing and eliminating harmful emissions is promoting solutions based on renewable energy in heat and electricity generation systems. Mainly in large and low-power installations, this transformation consists of replacing fossil fuels, such as coal, with high-quality biomass fuel [
21].
During the combustion of biomass, the concentration of pollutants in exhaust gases is many times lower than the composition of exhaust gases from coal combustion [
22,
23]. Another advantage of the use of biomass energy is its local application, thereby reducing emissions produced through transportation. The use of biomass fuel produced from local raw materials also permits the diversification of energy sources, which is now a desirable operation [
24,
25].
Considering the current state of development in alternative energy sources, we may safely conclude that in the era of phasing out fossil fuels, fuels from biomass will soon take over the role of basic energy carrier, for example, in heat generation systems. Compared with conventional energy sources, biomass fuels are a low-emission alternative in the production of electricity and heat. Biomass with high energy potential is found in almost every corner of the world. Depending on its physicochemical properties, biomass can be transformed in thermal processes, for example, gasification, pyrolysis, and direct combustion, to exploit its energy [
26].
In the area where we conducted our research, sugar production is a major industry. In the production of sugar from sugar beet, waste is generated in the form of beet pulp. The companies that produce sugar have problems managing this type of biomass waste. Previously, sugar pulp was used as feed for farm animals, but due to decreasing numbers of pig and cattle breeders in the nearest region and the transition of farms to automated feeding with ready-mixed feed, the problem of beet pulp management has become significant.
The second local biomass waste that requires management is wheat bran, which is formed during the production of wheat flour. Local companies that produce flour sell a small amount of this waste as an additive to animal feed. However, the production of waste in the form of wheat bran is greater than the market demand and generates a large quantity of biomass waste that also requires management.
This paper consists of five sections.
Section 1 includes information about air pollution and its effect on health, the types of catalysts, and the methods of waste biomass energy management.
Section 2 describes the methods and equipment used for measuring exhaust gas quality and the temperatures in the combustion chamber and flue gas duct.
Section 3 discusses the results of our research, which investigated the effect of additives (potassium permanganate and calcium oxide) on emissions produced by biomass waste combustion.
Section 4 summarizes the research outcomes.
Section 5 presents conclusions drawn from the research and proposes follow-up studies in this field.
3. Results
We conducted a series of tests to determine and compare the effects of selected catalytic additives on the combustion process of wheat bran and beet pulp pellets. The measured values of individual pollutant emissions were converted to a reference oxygen content (10 vol.% of content in the exhaust gas).
3.1. Biofuel Physicochemical Analysis
Table 6 and
Table 7 present the results of our analysis of the agricultural origin raw materials.
Table 8 and
Table 9 present the results of our elemental analysis of the wheat bran and beet pulp.
3.2. Analysis of the Exhaust Gas Composition
We compared our measurements with the figures for burning A1 class wood pellets (used as a reference, as in [
29]), which are a popular type of energy carrier. Using the analyzer manufacturer’s software, the results were automatically converted into an oxygen content of 10 vol.% in the exhaust gases.
Table 10 provides a key to the terms used in the charts that indicate these results.
Figure 4 shows the relationship between the CO concentration in the exhaust gases and the type of biofuel and catalytic additive used in the experiment.
During the biomass waste combustion process, the CO emissions reached levels of 451 mg‧m−3 for wheat bran pellets and 746 mg‧m−3 for beet pulp pellets. Carbon monoxide emissions during the combustion of commercial fuel (wood pellets) reached a level of 252 mg‧m−3. The use of additives in combination with fuel during combustion reduced the CO concentration in the exhaust gases in each case. During the combustion of wheat bran pellets, carbon monoxide emissions decreased, on average, by 15–41% using a CaO-based additive and 20–45% using a KMnO4-based additive. When burning the beet pulp pellets, the CO emissions were reduced by 12–21% using a CaO-based additive, depending on the additive concentration, and 19–30% using a KMnO4-based additive, also depending on the concentration of the active substance. While feeding the boiler with wood pellets, the CO emissions into the atmosphere were reduced by 8–18% using CaO, depending on the concentration of the substance, and 3.5–30% using KMnO4. These data were the average values of two measurement sets.
Figure 5 indicates the relationship between the exhaust gas NO
x concentration, the type of biofuel, and the catalyst.
We recorded the concentrations of nitrogen oxides emitted during the combustion of the biomass pellets. NOx emissions varied around 559 mg‧m−3 during the combustion of wheat bran pellets, 341 mg‧m−3 in the case of beet pulp pellets, and 185 mg‧m−3 for wood pellets. The addition of the CaO-based additive reduced the NOx emissions by 7–16% (wheat bran pellets), 8–27% (beet pulp pellets), and 8–18% (wood pellets), depending on the concentration of the substance. Burning the biomass with the KMnO4-based additives also succeeded in reducing the concentrations of nitrogen oxides in the flue gas. Using this additive, NOx emissions were reduced by 24–36% (wheat bran), 12–35% (beet pulp), and 3.5–30% (wood pellets), depending on the concentration of the active substance. These data were the average values of two measurement sets.
Figure 6 indicates the effect of the biofuel and catalytic additives on the concentrations of sulfur dioxide (SO
2) in the exhaust gases.
We also recorded reduced concentrations of SO2 during the process of burning wheat bran and beet pulp with additives. No reduction in the composition of exhaust gases was observed while burning wood pellets in the boiler. The SO2 emissions were 13.4 mg‧m−3 during the combustion of wheat bran pellets and 9.8 mg‧m−3 in the case of beet pulp pellets. Each of the additives in combination with the biomass fuel produced a reduction in SO2 concentration in the exhaust gases. While burning wheat bran, the SO2 emissions decreased by 74–83% using the CaO-based additive and 15–42% using the KMnO4-based additive, depending on the concentration of the active substance. In the case of burning beet pulp pellets, the SO2 emissions were reduced by 71–87% using the CaO-based additive and 41–65% using KMnO4, depending on the concentration of the additive. These data were the average values of two measurement sets.
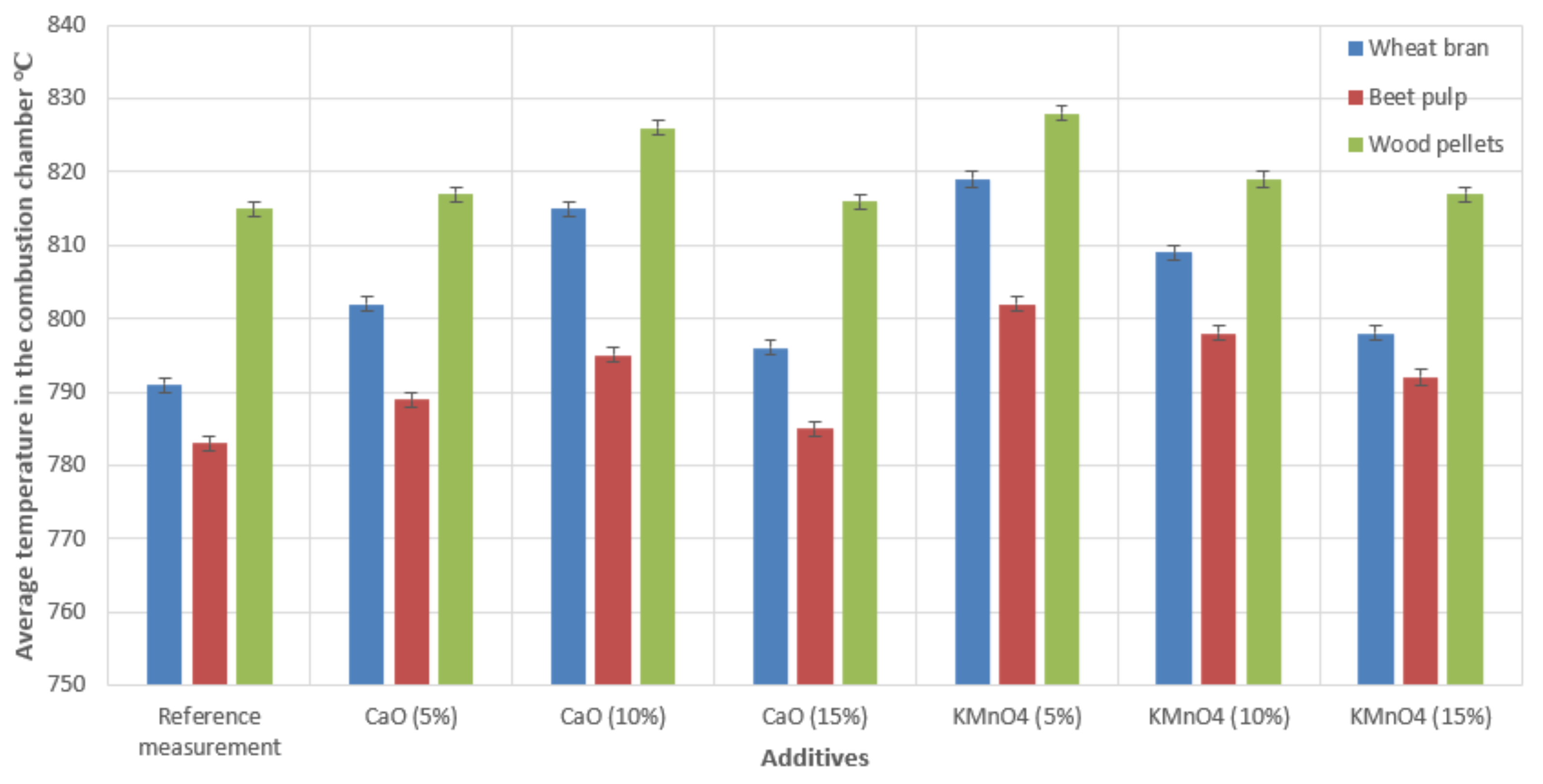
3.3. Measurement of the Combustion Process Temperature
Figure 7 presents the average temperature registered in the combustion chamber of the boiler after the stabilization of the combustion process.
We recorded increases in the average temperatures in the combustion chamber of the low-power boiler during the combustion of biomass fuels in combination with CaO and KMnO4 additives (in all concentrations). The average increase in the recorded temperatures in the boiler’s combustion chamber in the case of wheat bran pellets was 5 °C–24 °C depending on the CaO concentration and 7 °C–28 °C depending on KMnO4 concentration. In the case of beet pulp pellets, the temperature increased in the combustion chamber on average by 2 °C–12 °C with the addition of CaO, depending on its concentration, and 9 °C–19 °C with the addition of KMnO4, also depending on concentration. While feeding the boiler with the most popular biomass fuel, i.e., wood pellets, the temperature increased by 1 °C–11 °C using the CaO additive, varying with concentration, and 2 °C–13 °C while using the KMnO4 additive. These data were the average values of two measurement sets.
4. Discussion
This article’s subject matter is challenging to compare with the achievements published in other journals. The use of catalytic additives incinerated in combination with waste biomass in low-power boilers is a new approach to the problem of limiting emissions from combustion processes. The only similar studies were works that studied flue gas cleaning in large industrial power units. Due to the scale and different conditions in the combustion process in low-power boilers, these results cannot be used for making comparisons.
The catalysts (CaO and KMnO
4) used in our experiment improved the combustion process and reduced the concentrations of pollutants found in the exhaust gases. Managed through the energy process, these substances compensated for the adverse properties of biomass waste. Due to the high quantities of nitrogen and sulfur in the main mixture, the amount of emissions of nitrogen compounds during combustion was significant. According to the fuel mechanism of the formation of nitrogen oxides [
30], high nitrogen content in biomass results in increased NO
x emissions in exhaust gases. The use of catalytic additives in combination with fuel during the combustion process produced large reductions in emissions of these gases; in the best variants, CaO achieved a 41% reduction, and KMnO
4 achieved a 45% reduction. Sulfur contained in the two biomass wastes (wheat bran and beet pulp) produced SO
2 during the combustion process. The use of additives in combination with these materials during combustion reduced the sulfur oxide emissions into the atmosphere. In the best variants, the addition of CaO reduced SO
2 emissions by 83%. The addition of KMnO
4 produced the greatest reduction of SO
2 pollution, achieving slightly over 87%.
The use of catalytic additives increased the average temperature in the combustion chamber by 1 °C–13 °C, depending on the concentration and type of additive. By using catalytic additives, the combustion process was more efficient as a result of improvement in the reaction toward complete combustion. Therefore, the temperatures increased and the process reduced the concentrations of CO emissions. In the best variant, the addition of CaO reduced the CO emissions by 41%, and similarly, the addition of KMnO4 to the biomass pellets reduced the CO emissions by 45%. Improvement in the quality of combustion and consequent burning of flammable components in the exhaust gases increased the overall efficiency of the combustion process and reduced the chimney loss.
A comparison of the results in
Figure 3,
Figure 4,
Figure 5 and
Figure 6 indicated that the reduction in emissions was not always directly dependent on the quantity of the additive used. The addition of 10% CaO led to the largest reductions in CO and SO
2 emissions; however, a 15% addition to the combusted fuels produced the best reduction in NO
x emissions. In the case of KMnO
4, a 5% addition in all cases achieved the largest emissions reduction. A comparison of the results in
Figure 6 indicated that temperature correlated with the quantity of decreased emissions. We also observed that the emissions reduction and the efficiency of the entire combustion process were more significant at higher temperatures, (see
Figure 3, referring to CO emissions). The results also revealed that some additive quantities were too large, and consequently, the combustion process was not as efficient. However, it should be noted that all additives had a positive effect on reducing emissions (compared with emissions produced by burning raw materials only).
During the combustion of biomass waste in combination with the KMnO
4 additive, slight sintering of burned material occurred and may have slightly affected the combustion process. When CaO was used as an additive, ash agglomeration did not occur. This positive effect from CaO was also verified in the work [
31]; the ability of CaO to prevent sintering is a great advantage in obtaining a proper combustion process.
The use of catalytic additives positively affects the combustion process and reduces the emission of harmful substances into the atmosphere. The catalytic additives applied in the current study compensated for the adverse properties encountered in the waste materials from the food production and processing industry. Catalytic additives enable the use of waste as energy carriers in the incineration process. The widespread use of CaO and KMnO4 additives in other biomass waste treatments beyond only storage or neutralization would potentially benefit a large number of food-processing companies in managing their waste energy. In this way, manufacturers may be able to cover their heat requirements in whole or partially at no significant cost. The use of catalytic additives has a positive effect on the environment and the economy, and to some extent, enables the replacement of fossil fuels with ecological organic fuels.
5. Conclusions
The findings in other articles indicate that catalytic additives are a suitable technology for treating exhaust gases, including gases produced during the combustion of biomass waste. The main scientific challenge is determining the correct balance of catalytic compounds to combine high reduction efficiency with the low cost of the selected substance. The aim of the current study was to identify a suitable catalyst for the combustion of wheat bran and beet pulp biomass waste. The approach we proposed is innovative. To the best of our knowledge, no other research team has yet investigated the effect of CaO and KMnO4 catalytic additives on the quality of exhaust gases during the process of burning wheat bran and beet pulp.
From our research, we concluded that the use of catalytic additives in combination with biomass waste during the combustion process eliminated some of the adverse physicochemical properties of these materials. The use of this type of waste in energy production processes, including heat produced via direct combustion, is therefore possible and economically reasonable. The use of catalytic additives reduces the emission of harmful substances, increases the boiler’s efficiency, and reduces the consumption of biomass fuel.
Similar analyses were done on other catalytic additives used during the combustion of wood pellets [
32] and sunflower husks [
33]. Another study investigated the use of urea to reduce NO
x emissions [
34] from low-power biomass boilers. These catalytic additives reduced emissions effectively, and the profitability of their use increased as a consequence of reduced fuel consumption and increased combustion efficiency. Urea is also effective [
22], but the degree of emissions reduction relates to the optimal temperature in the combustion chamber.
The research describes a prospectively useful solution for entrepreneurs who produce this type of biomass waste in the technological processes of their food processing endeavors. However, the use of catalytic additives has some limitations. The active substance of the catalytic system is characterized by the most effective action in a specific temperature window. Too low or too high of a temperature may diminish its efficiency. Future research in this area could investigate the effect of catalytic additives on boiler efficiency, determine the effect of burning substances in combination on the lifespan of the heating surfaces in the boiler, the effect of additives on the composition of the ash resulting from the combustion process, or identify new types of catalytic additives (motivated by the need to increase the efficiency of flue gas cleaning). We plan to study, compare, and verify the results of using smaller additive quantities, for example, 0.5–5.0%, and investigate the effect of catalytic systems and other organic biomass waste materials on flue gas quality.