Abstract
Over the past years, research attention has been focusing more on waste-derived, naturally derived, and renewable materials, in the view of a more sustainable economy. In this work, different topical formulations were obtained from the valorization of marine and agro-industrial by-products and the use of Carbopol 940 as gelling agent. In particular, the combination of extracts obtained from the marine snail, Rapanosa venosa, with Cladophora vagabunda and grape pomace extracts, was investigated for wound healing purposes. Rapana venosa has demonstrated wound healing properties and antioxidant activity. Similarly, grape pomace extracts have been shown to accelerate the healing process. However, their synergic use has not been explored yet. To this aim, four different formulations were produced. Three formulations differed for the presence of a different extract of Rapana venosa: marine collagen, marine gelatin, and collagen hydrolysate, while another formulation used mammalian gelatin as further control. Physico-chemical properties of the extracts as well as of the formulations were analyzed. Furthermore, thermal stability was evaluated by thermogravimetric analysis. Antioxidant capacity and biological behavior, in terms of cytocompatibility, wound healing, and antimicrobial potential, were assessed. The results highlighted for all the formulations (i) a good conservation and thermal stability in time, (ii) a neutralizing activity against free radicals, (iii) and high degree of cytocompatibility and tissue regeneration potential. In particular, collagen, gelatin, and collagen hydrolysate obtained from the Rapana venosa marine snail represent an important, valuable alternative to mammalian products.
1. Introduction
Skin tissue represents the largest human organ reaching ten percent of the total body mass. Its main role is acting as a protective barrier against the environment, but it is also responsible for sensory detection, fluid homeostasis, thermo- and immuno-regulation [1]. In physiological circumstances, after an injury, skin integrity should be easily restored by the human body with a minimal scar, through a complex and interactive process [2]. However, its self-regeneration ability can be seriously compromised under specific conditions, such as extensive skin loss, deep burns, chronic wounds, non-healing ulcers, and other diseases such as diabetes [2].
Considering that a series of factors may affect wound healing, a medical treatment is often necessary. In this scenario, over the past years, different wound care products have been developed to improve the quality of life. Since ancient times, owning to their therapeutic activities, availability and affordability with relative low cost, natural compounds have been commonly employed as emulsions and ointments in direct contact with skin [3]. Commonly, these treatments involved the uses of natural compounds obtained from different sources (i.e., herbal-derived and animal-derived compounds). Among the herbal-origin products, it is worth noting to cite herbs containing biologically active compounds (i.e., aloe vera, Calendula officinalis), while among the animal-origin compounds, honey and propolis certainly emerged with their known healing properties [4]. Nowadays, given the great attention on the environmental decline occurring worldwide, exploring the waste-derived, naturally derived, and renewable products, in view of a more sustainable and circular economy, has driven an outstanding research curiosity through the scientific community.
However, even though it is expected that natural compounds will assume a pivotal role in the healthcare, as they are a valuable origin of therapeutic substances for direct applications as topical wound healing agents, for the development of new classes of drugs with specific activities for each phase of the wound healing process they still highlight some limitations. If applied alone and not in combination with a natural or synthetic biomaterial wound dressing, they are not able to ensure an optimal treatment. Indeed, an ideal wound dressing should be able to provide a moist wound environment, offer protective role in secondary infections, remove wound exudate, and promote tissue regeneration [2]. Considering the above factors, modern wound dressings employ many biopolymers and bioactive compounds obtained from natural resources to develop different types of dressing biomaterials [5].
In particular, hydrophilic polymer networks, which confer an insoluble behavior to the polymeric system, are mainly employed in dry-to-moderately draining wounds to promote autolytic debridement in necrotic wounds and in granulating wounds [6]. Due to the composition and mechanical behavior, these biomaterials show properties which are similar to those of the natural extracellular matrix. In such a way, the smart dressing not only acts as the supporting material for cells in the tissue regeneration process but can also serve as the delivery platform of a bioactive compound [6]. Synthetic polymers (i.e., poly(acrylate acid) (PAA), poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), or carboxypolymethylene (Carbopol or Carbomer)) have been widely employed for wound healing application as they possess significant mechanical strength and durability [7,8,9]. However, natural polymers synthesized from plants, animals, and microbial sources (i.e., collagen, gelatin, chitosan, alginate, hyaluronic acid, or gellun-gum) and their modified derivatives are emerging as powerful platforms in wound healing treatment as they are non-toxic and biocompatible substrates equivalent to macromolecules recognized by the human body [10,11,12,13]. In this scenario, recent trends are moving towards the development of more specialized wound healing treatments involving the combined use of natural compounds, synthetic/natural biopolymers, and tissue engineering strategies [14].
Hence, the aim of the present study was to develop innovative topical formulations for efficient wound healing management. The main active components of the proposed formulations were derived from valorization of marine resources, Rapana venosa and Cladophora vagabunda, and the valorization of specific agro-industrial by-products, the grape pomace.
Rapana venosa is a predatory and invasive marine snail belonging to the Muricidae family which has a negative impact on different mussels and mollusks populations inhabiting the Black Sea [15]. Besides its nutritional value, when consumed as a seafood [16,17,18] Rapana venosa has been also reported to be a promising source of active biological compounds. Previous studies showed that amino acids, lipids, and proteins, extracted from the soft tissue of Rapana venosa, demonstrated wound healing properties and antioxidant activity [19,20,21]. Furthermore, a recent study from Gaspar-Pintiliescu et al. reported that the marine snail could represent an alternative source of collagen and its derivatives (e.g., gelatin and peptides) [22].
The interest in these naturally derived biopolymers in healthcare related sectors has had very fast growth in recent years. Collagen-based products obtained from marine organisms are considered compatible, pathogen free, and have no religious restrictions compared to mammalian collagen [23,24]. In addition, collagen extraction from by-products of fish processing industry, such as skin and scales, can significantly reduce environmental pollution. Besides different species of fish, other marine sources have also been reported to contain collagen and gelatin, such as sponges [25,26], jellyfishes [27,28], squids [29], and snails [22,30]. In particular, marine collagen and its derivatives have been reported to promote wound healing in vitro by improving cell proliferation and migration, up-regulating the expression of chemotactic factors (i.e., β-Fibroblast growth factors (FGFs) and Transforming growth factor (TGF)-β1), or important growth factors (i.e., Vascular endothelial growth factor (VEGF)) and stimulating the recruitment of inflammatory cells. Different formulations of collagen-based products, applied on wounded animal models, showed an increased re-epithelization rate, wound closure, and granular tissue deposition compared to control groups [24,31].
Cladophora vagabunda is a green alga found in abundance on the coastal waters of the Romanian Black Sea shore. Previous studies showed that Cladophora vagabunda is an important source of volatiles compounds, fatty acids, terpenoids, sterols, phenols, carbohydrates, vitamins, and minerals, with potential for developing new therapeutics or production of biodiesel [32,33,34].
Grape pomace, which is the solid waste generated from pressing and fermentation processes in the vinification of grape varieties, is an important source of fibers, polyphenolic compounds, soluble sugars, and minerals with strong antioxidant and antimicrobial effects [35]. Topical application of grape pomace powder from Cabernet Sauvignon variant on excision wounds created on rats has been reported to accelerate the healing process [36].
However, the combination of algal extracts with marine collagen or gelatin from Rapana venosa and grape pomace extract has not been investigated yet for wound healing purposes.
2. Materials and Methods
2.1. Materials and Chemicals
Rapana venosa specimens and Cladophora vagabunda algae were collected during the summer period from the Romanian seacoast of the Black Sea between the 2 Mai and Vama Veche areas. The snails were washed with cold distilled water (diH2O) and stored at −20 °C until use. Similarly, the marine algae were washed with cold diH2O and dried at room temperature (Troom).
Red grapes pomace (Vitis vinifera L.) Mamaia variety was provided by Murfatlar Research Station for Viticulture and Oenology, Constanta County, Romania.
Mammalian gelatin from pig skin (Type A) was purchased from Sigma-Aldrich (Saint Louis, MO, USA), as well as all the chemicals, unless otherwise specified.
2.2. Preparation and Characterization of Naturally Derived Extracts
Preparation of Rapana venosa extracts. The soft tissue of marine snails was removed from the hard shell, washed with diH2O, and cut into small pieces (2–5 mm). The cleaned tissue was subjected to alkaline pre-treatment with 0.5 M sodium hydroxide (NaOH) solution in a ratio of 1:10 (w/v) at Troom for 24 h in order to remove non-collagenous proteins.
Marine Gelatin extraction. Gelatin was extracted using acidic treatment, as previously described [22]. Briefly, the pre-treated tissue was extracted with 0.5 M acetic acid solution (1:10, w/v) at Troom for 24 h. After centrifugation at 8000× g for 40 min, the sample was heated at 60 °C. The resulting gelatin solution was dialyzed diH2O, and freeze-dried at −40 °C for 48 h.
Marine Collagen extraction. Collagen extraction was performed according to an adapted protocol from Gaspar-Pintiliescu et al. [37]. The pre-treated tissue was extracted with 0.5 M acetic acid solution (1:15, w/v) at 4 °C for 48 h. After centrifugation at 5000× g for 20 min, the supernatant was precipitated with 0.7 M sodium chloride (NaCl). The precipitate was collected after centrifugation at 5000× g, for 20 min, solubilized in 0.1 M acetic acid, and dialyzed against diH2O. All processes were carried out at 4 °C. The obtained collagen solution was freeze-dried at −40 °C for 48 h.
Collagen Hydrolysate. Collagen hydrolysate was obtained by enzymatic treatment. Briefly, samples of Rapana venosa collagen were dissolved in 0.2 M phosphate buffer, pH 6, containing papain with an enzyme substrate ratio of 25:1 (w/w), at 55 °C, for 6 h. The enzyme was inactivated by heating at 100 °C for 10 min. The resulting hydrolysate was then filtered, concentrated by centrifugal ultrafiltration at 7500× g for 25 min using filter units with cellulose membranes of 3000 Da MW, cut off, and dried at −40 °C.
Preparation of Cladophora vagabunda ethanolic extract. Grinded algae (10 g) were mixed with 100 mL ethanol/water solution (70/30, v/v) and placed in an ultrasonic bath for 30 min at 30 °C. Then, the extract was centrifuged at 3000× g for 15 min, and the resulting solution was filtered through Whatman filter paper to remove any remaining residues. The algal extract was kept at −4 °C until use.
Preparation of grape pomace ethanolic extract. Dried pomace (10 g) was placed into a volumetric flask (100 mL) and 70% aqueous ethanolic solution was added up to the mark. The extraction was carried out for 12 days, at Troom, in the dark, with periodic stirring. The pomace extract was then filtered and stored at −4 °C until use.
Characterization of marine-derived extracts. Ash and moisture content of marine-derived extracts were analyzed at 600 °C and 120 °C, respectively, according to AOAC standard methodology [38]. Dry substance (DS, %) content was calculated by subtracting the moisture percentages from the weight of the original sample. Total protein content was determined using the Lowry protein assay and bovine serum albumin (BSA) as standard in the range of concentration of 0–10 mg/mL.
2.3. Preparation of Topical Formulations
The sequential steps of preparation are indicated in Figure 1A–E. For the preparation of the different formulations, a specific amount of an acrylic acid polymer, Carbopol 940 (0.5 g), was dispersed in purified water (100 mL) at 40 °C and was mixed by a mixer at 1200 g for 30 min (Figure 1A). Carbopol 940 was selected as the thickener agent due to its easy and fast dispersion properties at Troom, its wide use in the pharmaceutical, cosmetic, and dermocosmetic areas for the development of topical formulations containing hydroalcoholic extracts [39,40], and because it provides gels with good rheological properties. Furthermore, it is biodegradable, bioadhesive, biocompatible, and it has non-irritant properties [40]. The neutral pH was obtained by adding a required amount of triethanolamine (0.75 g), an organic amine, to gain a transparent gel and to obtain an adequate semisolid formulation for skin administration. Furthermore, in order to maintain the stability of active ingredients, a preservative, citric acid (0.75 g), was added to all formulations. The formulations were prepared according to the quali-quantitative formula described in Table 1. After neutralization, the three extracts (marine collagen, marine gelatin, and collagen hydrolysate) from Rapana venosa were added, obtaining formulation II, III, and IV, respectively. Formulation I employed mammalian gelatin as further control (Figure 1B). After cooling down the temperature, mammalian gelatin and each protein from Rapana venosa were combined with Cladophora vagabunda and grape pomace extracts in a ratio of 1:2:2 (w/w/w) under stirring (Figure 1C). Final formulations were prepared in triplicate for further analyses (Figure 1D,E).
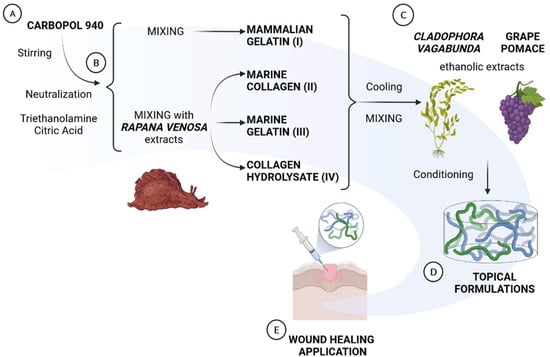
Figure 1.
Schematic representation of the sequential steps in the preparation of the four different formulations: (A) Carbopol 940 dispersion in water, neutralization and stabilization; (B) mixing with mammalian gelatin (Formulation 1) or with Rapana venosa extracts (Formulations II, III and IV); (C) combination of the three formulations with Cladophora vagabunda and grape pomace extracts; (D) cooling and realization of the final formulations; (E) characterization of the formulations.

Table 1.
Nomenclature and quali-quantitative chemical composition of the different formulations.
Table 1 summarizes the different formulations with related composition and nomenclature.
2.4. Physico-Chemical Characterization of the Topical Formulations
Organoleptic evaluation. The formulations were analyzed for smell, homogeneity, color, and consistency by visual inspection.
Thermal stability evaluation. The thermal stability of the different formulations was assessed by thermogravimetric analysis (TGA). TGA coupled with differential thermal analyses (DTA) was carried out using a Mettler Toledo TGA/SDTA851e thermogravimeter, under 80 mL min−1 synthetic air atmosphere, at a heating rate of 10 °C min−1.
Antioxidant capacity. In order to evaluate the antioxidant potential of the different formulations, cyclic voltammetry (CV) and Differential Pulse Voltammetry (DPV) were used. The voltammetric measurements were performed in a three electrochemical cell using a potentiostat-galvanostat Autolab 302N controlled by GPES software. In particular, the electrochemical cell consisted of a glassy carbon electrode (GCE, 3 mm diameter, an auxiliary electrode consisting of a glassy carbon rod and Ag/AgCl (Metrohm) as a reference electrode. Before measurements, the surface of GCE was polished with 1–0.05 µm alumina powder, then washed with doubly diH20 and stabilized by sweeping the potential between 0.1 V and 0.95 V for 5 consecutive scans with 50 mV/s in 0.1 M phosphate buffer pH 7. The stock solutions were obtained by dissolving of 0.1 g of each sample in 5 mL ethanol in an ultrasonic bath 20 min at Troom. In order to assess the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capability, 2 mL of sample stock solutions were added to 8 mL of a solution containing 0.02 mg/mL DPPH radical in ethanol–phosphate buffer solution pH 7 (1:1 v/v). The sample was subjected to electrochemical measurements after dark incubation for 60 min. Similar procedure was performed in order to determine the redox properties by electrochemical methods, when 2 mL of stock solutions were diluted with 8 mL of 0.1 M phosphate buffer pH 7.0. The antioxidant activity (AA%) was calculated relative to DPPH from the anodic peak currents, according to Equation (1):
AA (%) = (IDPPH • IDPPH-SAMPLE)/IDPPH• × 100
where: IDPPH• and IDPPH-SAMPLE are the anodic peak currents of DPPH• and of DPPH•, respectively, in the presence of sample.
2.5. Biological Characterization of the Formulations
In vitro cell culture. Biological assays were performed using a stabilized cell line of rat fibroblasts NCTC clone L929 (ATCC) for cytocompatibility test, and human keratinocytes (HaCaT, ECACC via Addex Bio) for wound healing test. NCTC clone L929 derived from mouse connective tissue (normal subcutaneous areolar and adipose tissue of 100 day old male C3H/An, passage number: 565) with a morphology of fibroblast, while HaCaT derived from human keratinocytes (in vitro spontaneously transformed keratinocytes from histologically normal skin of male Caucasian of 62 years old). NCTC clone L929 cells were cultivated in Minimum Essential Medium Eagle (MEM) growth medium, supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic solution (streptomycin 100 mg/mL and penicillin 100 U/mL), and maintained at 37 °C in a humid atmosphere with 5% CO2 and 95% air humidity. HaCaT cells were cultivated in RPMI growth medium (Biochrom, Berlin, Germany), supplemented with 10% FBS and 1% antibiotic solution (streptomycin 100 mg/mL and penicillin 100 U/mL), and maintained at 37 °C in standard conditions.
Indirect cytotoxicity assay. The method of the indirect contact was used according to ISO 10993:5 (Biological evaluation of medical devices). For each sample, a stock solution of 10 mg/mL was prepared in MEM supplemented with 10% FBS at 37 °C for 24 h. Prior to the in vitro evaluation, the extracts were diluted in order to obtain different concentrations ranging from 10–500 µg/mL. The solutions were sterile filtered with 0.22 µm Millipore membranes.
Fibroblast cells were seeded in 96 well plates at a cellular density of 4 × 104 cells/mL and after 24 h, time necessary for cell adhesion, the growth medium was removed and different concentrations of conditioned media from the four formulations (10, 50, 100, and 500 µg/mL) were added. Cells were maintained in standard conditions for 24 and 48 h, and afterwards the MTT (Sigma, Steinheim, Germany) test was performed according to the manufacturer’s protocol. Briefly, cells were incubated with a 0.25 mg/mL MTT solution for 3 h at 37 °C in a humid atmosphere. The formazan crystals formed in the active metabolic cells were dissolved in acetic acid and the absorbance read at 570 nm with a Mithras LB 940 microplate reader (Berthold Technologies, Bad Wildbad, Germany). The results were reported as viability percentages relative to the control (untreated cells) considered to have a 100% viability.
Wound healing evaluation, Migration potential assay. In order to evaluate the influence of the different formulations on the migration and repair ability of an injured cellular monolayer, the Scratch assay was used. Briefly, HaCaT were seeded in 24 well plates at a cellular density of 1.5 × 105 cells/well and cultivated in RPMI growth medium at 37 °C. After 24 h, a perpendicular linear wound (scratch) was created by means of a 200 µL sterile pipette tip on the cellular monolayer. A blank line perpendicular to the scratch, painted under the bottom of the plate, ensured the recording of the same wound area per well. After a gentle PBS wash for cellular debris removal, the formulation at the concentration of 100 µg/mL, which was previously sterile filtered, was added, and cells were maintained in standard conditions at 37 °C for 24 h.
After the scratch, wound closure was monitored collecting digitalized images at beginning of the experiment (time 0—immediately after the scratch) and after 24 h in order to evaluate the migration status of the cells and the coverage of the wounded area. To estimate cell migration, images of two sites per well through an inverted microscope (Axio Star Plus) and digital camera (Carl Zeiss, Jena, Germany) (10× objective) were captured. The wound areas were analyzed using ImageJ 1.51 software for quantitative analysis of the wound closure percentage, according to Equation (2). Four replicates per condition were used and three experiments were done.
Repair rate of scratch (%) = (ATo − ATh)/AT0 × 100
where AT0 corresponds to the whole wound taken immediately after the scratch, and ATh corresponds to the whole wound taken after 24 h.
Antimicrobial potential assessment. The antimicrobial activity was assessed via a modified version of the diffusimetric antibiogram method Kirby–Bauer on pathogenic strains, specific for skin and mucous membranes, Gram-positive bacteria (Staphylococcus aureus, ATCC 25923), Gram-negative bacteria (Escherichia coli, ATCC 25922), and fungal (Candida albicans, ATCC 10231), respectively. Briefly, standardized microbial suspensions corresponding to 0.5 McFarland density were prepared from fresh (24 h) solid cultures of Staphylococcus aureus grown on trypticase soy agar (TSA) nutrient medium by aerobic incubation at 37 °C. The bacterial inoculum was added to give a final concentration of 1 × 108 colony forming units per mL (CFU/mL) and 10 μL of each formulation at the concentration of 100 µg/mL was incubated for 24 h on inoculated agar plates. The diameter of the growth inhibition zones was monitored for three independent measurements.
2.6. Statistics and Data Analysis
For each experiment, results are presented as mean ± standard deviation (SD). For cytocompatibility and wound healing analyses, statistical analysis of variance of the means compared to untreated samples was assessed by two-way and one-way ANOVA, respectively, followed by Tukey’s post-hoc test, by using GraphPad Prism software (version 7.0). Different levels of significance were considered 95–99.9999% (* p < 0.05, # p < 0.001; ° p < 0.0001) among the different results.
3. Results and Discussions
3.1. Characterization of Naturally Derived Extracts
In the present study, collagen from the soft tissue of Rapana venosa was obtained according to the following steps: removal of non-collagenous proteins, extraction by chemical method with acetic acid and salt precipitation with NaCl, while gelatin was obtained by heat extraction with acetic acid. Both extracts were previously characterized, presenting a good cytocompatibility, promoting cell-induced capacity and lacking irritant potential when tested in vitro and compared with mammalian collagen and gelatin [22]. Collagen hydrolysate was obtained by papain hydrolysis of collagen samples. Papain is a plant-derived protease that was also successfully used in preparation of collagen hydrolysates derived from yellowfin tuna (Thunnus albacares) skin and Malaysian jellyfish, Rhopilema hispidum [41,42]. Cladophora vagabunda and pomace extracts were obtained using 70% ethanolic solution. This extraction method is preferred due to non-toxic nature of the solvents and is frequently used for recovering higher concentrations of analytes, especially polyphenols [43]. Previous studies reported that Cladophora vagabunda extracts containing polyphenols presented antioxidant and antimicrobial properties against two bacterial strains Staphylococcus aureus and Escherichia coli [44], while in the case of grape pomace from Mamaia variety it was shown that antioxidant activity of the ethanolic extract positively correlated with its total phenolic content [45].
The analysis of biomasses was carried out to determine dry substance (DS), ash-mineral substance, and total protein content in both marine sources: Rapana venosa and Clodophora vagabunda. The main biochemical properties are reported in Table 2. For Rapana venosa, the ash content and DS were low as the Asian marine gasteropods were collected during summer. Indeed, it was clearly reported that dry weight and ash content drop their lowest level during the spawning period, which falls between May and August. This result was confirmed by other previous studies [46,47]. The analysis of the biochemical composition indicated a high concentration of protein in Rapana venosa. Indeed, the high nutritional value of the marine organism is given by the high concentration of protein in the marine snail, as confirmed by the specialized literature [47]. In the present study, the total protein content of Rapana venosa was 56.87% comparable to the values found in the literature [22].

Table 2.
Biochemical parameters of marine sources (correlated to the dry substance, DS %).
Total protein content of Clodophora vagabuda was 38.79%, higher than that found for other Clodophora microalgae, which showed a value ranging from 26.8% and 27.8% [48] or Clodophora glomerata, which was 14.45% [49]. Indeed, Rapana venosa and Cladophora macroalgae contain a high number of proteins and feature high moisture (around 60–90%) [50].
3.2. Physico-Chemical Characterization of the Formulations
Considering the biocompatibility of Rapana venosa extracts and their ability to promote cell adhesion [22] alongside with the antioxidant and antimicrobial potential of Cladophora vagabunda [33] and pomace extracts [35,44], these molecules were embedded in a formulation intended for wound healing application. Different formulations were prepared using Carbopol 940 as gelling polymer.
The macroscopic evaluation of the formulations showed satisfactory organoleptic characteristics in terms of odor, color, and appearance, indicating a smooth, homogenous, and slightly turbid, colored texture, associated with the algal and pomace extracts. These properties are very important to obtain good patient acceptance.
From the thermogravimetric analyses, the formulations showed a loss of mass in the temperature range between 25 and 150 °C caused by water and volatile components’ (i.e., solvents) evaporation, associated with an endothermic effect. The combustion of organic components took place in the temperature range between 150 and 550 °C in at least three distinct steps, associated with exothermic effects (Figure 2). The thermal behavior of the four formulations was similar to that of Carbopol 940, with the exception of the solvent evaporation step (Supplementary Information, Figure S1). Representative samples of formulations I and III were dried at 40 °C for 14 days and remeasured by TGA (Supplementary Information, Figure S1). The water content decreased to 10–15% w, while the same thermal behavior was observed above 100% w. The thermal degradation of the dried formulations starts at ~140 °C, which is lower than the 180 °C temperature in the case of pure Carbopol 940 (Supplementary Information, Figure S1C). However, since the formulations are envisioned for body temperature applications, this decrease in thermal stability is not expected to significantly affect their potential applications.
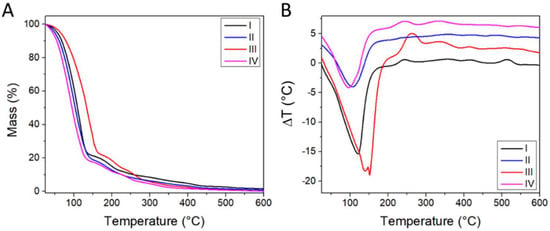
Figure 2.
(A) Thermogravimetric Analysis (TGA) and (B) Differential Thermal Analyses (DTA) results for the different formulations. Each curve in (B), with the exception of formulation I, is shifted by +2 °C with respect to the previous one, for clarity.
The organic fraction and inorganic residue at 600 °C were computed based on the TGA data (Table 3).

Table 3.
Results highlighting organic fraction and inorganic residue at 600 °C from Thermogravimetric Analysis (TGA) and antioxidant activity electrochemical (AA%). Standard uncertainty of TGA measurements was 0.5% w, while the SD for the electrochemical measurements was 4.5% (n = 3).
The antioxidant properties of different formulations have been evaluated by electrochemical analyses (CV and DPV) and by determination of DPPH radical scavenging capacity. The electrochemical methods are attractive tools for the determination of antioxidant activity owing to their simplicity, sensitivity, selectivity, low probe consumption, and rapidity of analysis. Moreover, the electrochemical signal is not affected by the sample complexity and by the turbidity. The representative cyclic voltammograms exhibited two anodic peaks situated at 0.37 V, 0.52 V, and 0.54 V, for the formulations I and II, whilst for the formulation III they were slightly displaced toward more electropositive potentials, i.e., 0.39 V and 0.58 V, respectively (Figure 3). In the case of formulation IV, only a broad peak at about 0.55 V was observed. The shifting of peak potentials could be probably due to the nature of polymer matrix used for the immobilization of bioactive compounds. The cyclic voltammogram of Carbopol 940 in ethanolic phosphate buffer of pH 7 was also performed (Supplementary information, Figure S2). The anodic current starts to increase after a potential of 0.3 V but a well-defined anodic peak cannot be observed for Carbopol 940. The anodic peaks of formulations I-IV indicated the presence of antioxidants belonging from different classes such as polyphenols and carotenoids. The low oxidation potentials revealed the capability of the bioactive compounds of formulations (I–IV) to donate electrons and to exhibit antioxidant activity. On the other hand, the absence of any peak on reverse scan indicated that the oxidized compounds were not involved in subsequent electrochemical reduction reaction [51]. Despite information regarding the reversibility of redox processes, CV has limitations with respect to peak resolution. Accordingly, DP voltammograms of the formulations (I–IV) were recorded within the range between 0.1 V and 0.7 V with a step potential of 9 mV and a modulation of amplitude of 0.03 V. The three anodic peaks located at approximately 0.28 V, 0.38 V, and 0.62 V underlined the antioxidant properties in aqueous buffered solutions and demonstrated that DPV is more sensitive than CV. The presence of an only weak anodic peak on the DP voltammogram of formulation IV suggested a lower concentration of antioxidant species, probably due to the consumption by O2 dissolved in aqueous buffered solutions.
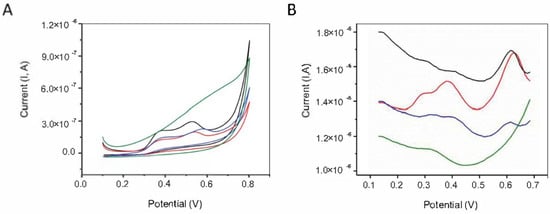
Figure 3.
(A) Cyclic Voltammetry (CV) and (B) Differential Pulse Voltammetry (DP) of formulations: I (black line), II (red line), III (blue line), and IV (green line) in ethanol (0.1 M phosphate buffer of pH 7 (1/1 v/v)) at glassy carbon electrode (GCE). Scan rate 50 mV/s.
However, all the formulations exhibited antioxidant activity due to their content of antioxidants, which can scavenge the DPPH radicals. In particular, the antioxidant potential is mediated by the ability of the bioactive compounds contained in the vegetal extracts (algae and pomace) to donate hydrogen atoms to DPPH. The voltammetric measurements indicated that the DPPH scavenging capacity varied from 20% to 52.49%, with the highest value obtained for formulation III (Table 3). The unexpected superior DPPH scavenging capacity observed for the formulation III, characterized by the highest redox potentials, could be assigned either to the synergistic effects of polymer matrix or to the different radicals quenching rates.
3.3. Biological Characterization of the Formulations
Cytocompatibility analysis. Non-cytotoxicity, biocompatibility, mild processing conditions, and physico-chemical properties similar to the natural tissues are among the major features which make collagen and gelatin suitable for skin tissue regeneration. In view of this, the basic cytocompatibility of the different formulations was assessed by evaluating the metabolic activity of rat fibroblasts NCTC clone L929. Results revealed that all the four tested formulations were biocompatible at both time points (Figure 4). Cell viability was >90% for the concentrations ranging between 10–100 µg/mL. Furthermore, the formulations II and IV containing gelatin and collagen hydrolysate from Rapana venosa, respectively, supported cell proliferation; higher cell viability percentages were registered for the first three tested concentrations (109.41–102.54%), compared to untreated control (100%), after 48 h.
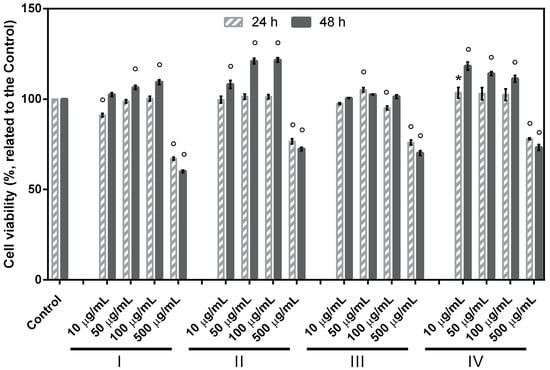
Figure 4.
Viability of fibroblasts (NCTC clone L929, ATCC) cultivated in the presence of different concentrations of formulations I, II, III, IV, evaluated by MTT test at 24 and 48 h. Data represent the mean ± SD (n = 3). (* p < 0.05; ° p < 0.0001 compared to untreated control).
Wound healing potential. Based on the cytocompatibility data, the 100 µg/mL concentration was selected for a wound healing test. Results indicated that the repair rate of scratch (%) was higher for the treated cells when compared to the control, suggesting a beneficial effect of the formulations on the healing process. Hence, the highest closure area was observed for cells treated with formulation IV (89.35%), containing collagen hydrolysate, which stimulated a high rate of cell migration, followed by formulation II (83.75%) containing Rapana venosa gelatin. Mammalian gelatin and marine collagen-based formulations I and III presented a lower effect on cell migration (Figure 5 and Figure 6).
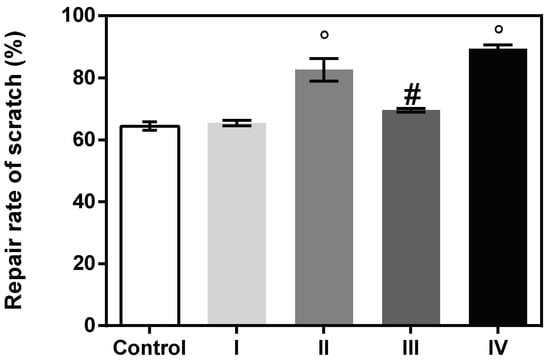
Figure 5.
Repair rate of scratch (%) by cellular migration of human keratinocytes (HaCaT, ECACC) assessed by means of ImageJ 1.51 software. Data represent the mean ± SD. (# p < 0.001; ° p < 0.0001) compared to control.
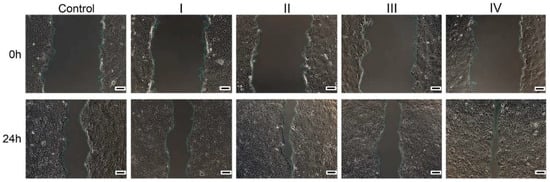
Figure 6.
Optical microscopy images of wounded human keratinocyte monolayers (HaCaT, ECACC) (Control) and treated with the four formulations at time 0, immediately after the scratch, and after 24 h of cultivation. Scale Bar = 100 µm.
Keratinocyte’s migration was observed for all the conditions tested, and formulations II, III, and IV proved to be efficient in stimulating the closure rate of the wounded cell monolayer. The most powerful formulation seems to be formulation IV with a considerable percentage of migrated cells. The positive effect on tissue regeneration can be explained by a synergic activity of the different components embedded in the formulation. Moreover, the collagen hydrolysate from Rapana venosa presented the highest regenerative potential, in accordance with previous studies indicating that collagen hydrolysate from Oreochromis nilocitus was a suitable microenvironment to diminish the inflammatory phase duration, promoting wound healing [52,53]. Furthermore, in vivo tests on protein extracts from Rapana venosa showed that the samples reduced inflammatory infiltration, stimulated dermal and epidermal regeneration, and promoted angiogenesis, overall accelerating the time of healing from 22–24 days to 12–18 days [20]. The results suggested that marine gelatin and hydrolysate containing bioactive compounds of lower molecular mass could be more effective signaling molecules than large polymers such as marine collagen or mammalian gelatin. In addition, previous studies have shown that Cladophora vagabunda extracts containing bioactive compounds, such as tocopherol, carotenoids, and polyphenols, exhibited antioxidant and anti-inflammatory activity, which could accelerate the wound healing process [54]. Regarding formulations II and III, a good in vitro cytocompatibility with HaCaT cells and an increased proliferation and cell adhesion capacity for gelatin obtained Rapana venosa have been also confirmed [22].
Antimicrobial activity. The interference with the microbial growth was monitored and the results are summarized in Table 4. The experimental data showed that all four formulations exhibited a clear antibacterial activity against both Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus. However, the antifungal properties were more limited and moderate for all tested formulations against the growth and multiplication of Candida albicans. Escherichia coli, the Gram-negative bacterial strain, proved to be more sensitive to the bioactive principles incorporated in the formulations, with larger growth inhibition zones when compared to the ones developed on Gram-positive Staphylococcus aureus. This effect can be correlated with the structural features of the bacterial species used in the study, respectively, the simpler cell wall architecture specific to Gram-negative strains that could be associated with an increased permissiveness to bioactive natural compounds.

Table 4.
Diameter of microbial growth inhibition zone (mm) by using the four different formulations. Experimental data are reported as mean ± SD (n = 3).
The clear antibacterial activity of all the formulations could be due to the effect of bioactive compounds present in grape pomace and algal extracts, but also to their incorporation in Carbopol 940, which facilitated their gradual diffusion in the growth medium. Previous studies reported that Cladophora vagabunda extracts containing polyphenols presented antimicrobial properties against both bacterial strains of Escherichia coli and Staphylococcus aureus [44]. Grape pomace extract presented significant influence on the bacterial growth of Staphylococcus aureus and Escherichia coli [55].
4. Conclusions
In this study, different formulations were obtained from sustainable bioresources and industrial wastes with low environmental impact by marine sources valorization, collagen, gelatin, and collagen hydrolysate from Rapana venosa and ethanolic extracts from Cladophora vagabunda, respectively. Mamaia variety grape pomace, specific to the Black Sea region, was successfully added to the compositions. Despite the limitations of the current research, the following conclusions were reached:
- all the tested formulations exhibited a good conservation and thermal stability over time,
- they showed a neutralizing activity against free radicals,
- they were characterized by a high degree of cytocompatibility and tissue regeneration potential.
The present results suggest that collagen, gelatin, and collagen hydrolysate obtained from the Rapana venosa marine snail represent an important, valuable alternative to mammalian products. Furthermore, the different formulations could be employed as valuable candidates for smart wound dressings.
For future perspectives, the spreadability, as well as the mechanical and rheological behavior of the materials, will be optimized in view of their use as suitable bioinks for the design of 3D printed patches/wound dressings with tailored physico-chemical and mechanical properties for skin regeneration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma15103507/s1, Figure S1. Results from (A) Thermogravimetric Analysis (TGA), (B) Differential Thermal Analyses (DTA) and (C) the first derivative of the TGA signal (dTG) of the Carbopol 940, formulation I and III, dried at 40 °C for 14 days. Figure S2. Cyclic Voltammetry (CV) of Carbopol 940.
Author Contributions
Conceptualization, A.-M.P. and U.D.; methodology, A.-M.P., A.I., A.G.-P., T.N.-P., B.N.-P., R.-A.M., M.M.; validation, A.-M.P., A.I., A.G.-P., T.N.-P., B.N.-P., R.-A.M., M.M.; investigation, A.-M.P., A.I., A.G.-P., T.N.-P., B.N.-P., R.-A.M., M.M.; data curation, A.-M.P., A.I., A.G.-P., T.N.-P., B.N.-P., R.-A.M., M.M., U.D.; writing—original draft preparation, A.-M.P., U.D.; writing—review and editing, L.M., O.C., U.D.; supervision, L.M., O.C., U.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Romanian Ministry of Research and Innovation, UEFISCDI, project No. PN-III-P1-1.2-PCCDI-2017-0701, grant No. 85PCCDI/2018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bartolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [Green Version]
- Razia, S.; Park, H.; Shin, E.; Shim, K.S.; Cho, E.; Kang, M.C.; Kim, S.Y. Synergistic effect of Aloe vera flower and Aloe gel on cutaneous wound healing targeting MFAP4 and its associated signalling pathway: In-vitro study. J. Ethnopharmacol. 2022, 290, 115096. [Google Scholar] [CrossRef]
- Kim, H. Wound dressing materials: The essentials. J. Wound Manag. Res. 2018, 14, 141–142. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhu, C.; Fan, D.; Fu, R.; Ma, P.; Duan, Z.; Li, X.; Lei, H.; Chi, L. A Bi-Layer PVA/CMC/PEG Hydrogel with Gradually Changing Pore Sizes for Wound Dressing. Macromol. Biosci. 2019, 19, 1800424. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.R.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Mandal, U.K.; Taher, M.; Susanti, D.; Jaffri, J.M. PVA-PEG physically cross-linked hydrogel film as a wound dressing: Experimental design and optimization. Pharm. Dev. Technol. 2018, 23, 751–760. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Chen, C.Y.; Shi, B.H.; Zheng, B.; Wang, S.J.; Tan, W.Q.; Wu, L.H. Effects of chitosan-collagen dressing on wound healing in vitro and in vivo assays. J. Appl. Biomater. Funct. Mater. 2021, 19, 2280800021989698. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; D’Amora, U.; Ronca, A.; Li, Y.; Mo, X.; Zhou, F.; Yuan, M.; Ambrosio, L.; Wu, J.; Raucci, M.G. In vitro and in vivo biocompatibility and inflammation response of methacrylated and maleated hyaluronic acid for wound healing. RSC Adv. 2020, 10, 32183–32192. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.Y.; Zhu, X.; Mukherjee, D.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L. Pristine gellan gum–collagen interpenetrating network hydrogels as mechanically enhanced anti-inflammatory biologic wound dressings for burn wound therapy. ACS Appl. Bio Mater. 2021, 4, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and synthetic polymer-based nanocomposites in wound dressing applications: A review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef]
- Zolotarev, V. The Black Sea ecosystem changes related to the introduction of new mollusc species. Mar. Ecol. 1996, 17, 227–236. [Google Scholar] [CrossRef]
- Luo, F.; Xing, R.; Wang, X.; Peng, Q.; Li, P. Proximate composition, amino acid and fatty acid profiles of marine snail Rapana venosa meat, visceral mass and operculum. J. Sci. Food Agric. 2017, 97, 5361–5368. [Google Scholar] [CrossRef]
- Merdzhanova, A.; Panayotova, V.; Dobreva, D.A.; Stancheva, R.; Peycheva, K. Lipid composition of raw and cooked Rapana venosa from the Black Sea. Ovidius Univ. Ann. Chem. 2018, 29, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Leontowicz, M.; Leontowicz, H.; Namiesnik, J.; Apak, R.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Rapana venosa consumption improves the lipid profiles and antioxidant capacities in serum of rats fed an atherogenic diet. Nutr. Res. 2015, 35, 592–602. [Google Scholar] [CrossRef]
- Badiu, D.L.; Balu, A.M.; Barbes, L.; Luque, R.; Nita, R.; Radu, M.; Rosoiu, N. Physico-chemical characterisationof lipids from Mytilus galloprovincialis (L.) and Rapana venosa and their healing properties on skin burns. Lipids 2008, 43, 829–849. [Google Scholar] [CrossRef]
- Badiu, D.L.; Luque, R.; Dumitrescu, E.; Craciun, A.; Dinca, D. Amino acids from Mytilus galloprovincialis (L.) and Rapana venosa molluscs accelerate skin wounds healing via enhancement of dermal and epidermal neoformation. Protein J. 2010, 29, 81–92. [Google Scholar] [CrossRef]
- Luo, F.; Xing, R.; Wang, X.; Yang, H.; Li, P. Antioxidant activities of Rapana venosa meat and visceral mass during simulated gastrointestinal digestion and their membrane ultrafiltration fractions. Int. J. Food Sci. Technol. 2018, 53, 395–403. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stefan, L.M.; Anton, E.D.; Berger, D.; Matei, C.; Negreanu-Pirjol, T.; Moldovan, L. Physicochemical and biological properties of gelatin extracted from marine snail Rapana venosa. Mar. Drugs 2019, 17, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef] [PubMed]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the marine sponges Axinella cannabina and Suberites carnosus: Isolation and morphological, biochemical, and biophysical characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.C.; Barros, A.A.; Aroso, I.M.; Fassini, D.; Silva, T.H.; Reis, R.L.; Duarte, A.R.C. Extraction of collagen/gelatin from the marine demosponge Chondrosia reniformis (Nardo, 1847) using water acidified with carbon dioxide–process optimization. Ind. Eng. Chem. Res. 2016, 55, 6922–6930. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum Kishinouye for use in hemostatic applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef] [Green Version]
- Chancharern, P.; Laohakunjit, N.; Kerdchoechuen, O.; Thumthanaruk, B. Extraction of type A and type B gelatin from jellyfish (Lobonema smithii). Int. Food Res. J. 2016, 23, 419. [Google Scholar]
- Sun, B.; Li, C.; Mao, Y.; Qiao, Z.; Jia, R.; Huang, T.; Yang, W. Distinctive characteristics of collagen and gelatin extracted from Dosidicus gigas skin. Int. J. Food Sci. Technol. 2021, 56, 3443–3454. [Google Scholar] [CrossRef]
- Nazeer, R.A.; Suganya, U.S. Porous scaffolds of gelatin from the marine gastropod Ficus variegate with commercial cross linkers for biomedical applications. Food Sci. Biotechnol. 2014, 23, 327–335. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horincar, V.B.; Parfene, G.; Tyagi, A.K.; Gottardi, D.; Dinică, R.; Guerzoni, M.E.; Bahrim, G. Extraction and characterization of volatile compounds and fatty acids from red and green macroalgae from the Romanian Black Sea in order to obtain valuable bioadditives and biopreservatives. J. Appl. Phycol. 2014, 26, 551–559. [Google Scholar] [CrossRef]
- Elenkov, I.; Georgieva, T.; Hadjieva, P.; Dimitrova-Konaklieva, S.; Popov, S. Terpenoids and sterols in Cladophora vagabunda. Phytochemistry 1995, 38, 457–459. [Google Scholar] [CrossRef]
- Sharmila, S.; Jeyanthi, R.L. GC-MS analysis of esters of fatty acid present in biodiesel produced from Cladophora vagabunda. J. Chem. Pharm. Res. 2012, 4, 4883–4887. [Google Scholar]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape pomace valorization: A systematic review and meta-analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Ramdath, D.D.; Marshall, J.R.; Isitor, G.N.; Eversley, M.; Xue, S.; Shi, J. Wound-healing activity of the skin of the common grape (Vitis Vinifera) variant, cabernet sauvignon. Phytother. Res. 2010, 24, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Pintiliescu, A.; Anton, E.D.; Iosageanu, A.; Berger, D.; Matei, C.; Mitran, R.A.; Negreanu-Pirjol, T.; Craciunescu, O.; Moldovan, L. Enhanced wound healing activity of undenatured type I collagen isolated from discarded skin of Black Sea gilthead bream (Sparus aurata) conditioned as 3D porous dressing. Chem. Biodivers. 2021, 18, e2100293. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemistry, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Poulose, N.; Sajayan, A.; Ravindran, A.; Sreechithra, T.V.; Vardhan, V.; Selvin, J.; Kiran, G.S. Photoprotective effect of nanomelanin-seaweed concentrate in formulated cosmetic cream: With improved antioxidant and wound healing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111816. [Google Scholar] [CrossRef]
- Gavan, A.; Colobatiu, L.; Hanganu, D.; Bogdan, C.; Olah, N.K.; Achim, M.; Mirel, S. Development and Evaluation of Hydrogel Wound Dressings Loaded with Herbal Extracts. Processes 2022, 10, 242. [Google Scholar] [CrossRef]
- Nurilmala, M.; Pertiwi, R.M.; Nurhayati, T.; Fauzi, S.; Batubara, I.; Ochiai, Y. Characterization of collagen and its hydrolysate from yellowfin tuna Thunnus albacares skin and their potencies as antioxidant and antiglycation agents. Fish. Sci. 2019, 85, 591–599. [Google Scholar] [CrossRef]
- Ab Aziz, N.A.; Salim, N.; Zarei, M.; Saari, N.; Yusoff, F.M. Extraction, anti-tyrosinase, and antioxidant activities of the collagen hydrolysate derived from Rhopilema hispidum. Prep. Biochem. Biotechnol. 2021, 51, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.N.; Cadavez, V.; Ferreira-Santos, P.; Alves, M.J.; Ferreira, I.C.; Barros, L.; Gonzales-Barron, U. Chemical profile and bioactivities of extracts from edible plants readily available in Portugal. Foods 2021, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, R.; Stanciu, G.; Tomescu, A.; Ionescu, A.M.; Cadar, E. Evaluation of antioxidant and antimicrobial activity in relation to total phenolic content of green algae from Black Sea. Rev. Chim. 2019, 70, 1197–1203. [Google Scholar] [CrossRef]
- Artem, V.; Negreanu–Pirjol, T.; Ranca, A.; Ciobanu, C.; Abduraman, A.; Bratu, M.M.; Popoviciu, D.R.; Moldovan, L.; Negreanu-Pirjol, B.S. Total phenolic content correlated with antioxidant activity of some grape pomace biomass hydroalcoholic extracts, white and red varieties. UPB. Sci. Bull. Ser. B 2021, 83, 61–72. [Google Scholar]
- Roşioru, D.M. The biochemical characterization of the marine environment and the main commercial mollusks from Romanian Black Sea coast area. Cercet. Mar.-Mar. Rech. 2012, 42, 103–114. [Google Scholar]
- Roşioru, D.M.; Oros, A.; Coatu, V.; Stoica, E.; Negreanu-Pîrjol, T. Estimation of Rapana Venosa (Valenciennes, 1846) quality, a marine living resource from the Romanian Black Sea with bioeconomic importance. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2020, 82, 39–46. [Google Scholar]
- Machado, L.; Kinley, R.D.; Magnusson, M.; de Nys, R.; Tomkins, N.W. The potential of macroalgae for beef production systems in Northern Australia. J. Appl. Phycol. 2015, 27, 2001–2005. [Google Scholar] [CrossRef]
- Messyasz, B.; Leska, B.; Fabrowska, J.; Pikosz, M.; Roj, E.; Cieslak, A.; Schroeder, G. Biomass of freshwater Cladophora as a raw material for agriculture and the cosmetic industry. Open Chem. 2015, 13, 1108–1118. [Google Scholar] [CrossRef]
- Srimaroeng, C.; Ontawong, A.; Saowakon, N.; Vivithanaporn, P.; Pongchaidecha, A.; Amornlerdpison, D.; Soodvilai, S.; Chatsudthipong, V. Antidiabetic and Renoprotective Effects of Cladophora glomerata Kützing Extract in Experimental Type 2 Diabetic Rats: A Potential Nutraceutical Product for Diabetic Nephropathy. J. Diabetes Res. 2015, 2015, 320167. [Google Scholar] [CrossRef] [Green Version]
- Vasyliev, G.S.; Vorobyova, V.I.; Linyucheva, O.V. Evaluation of Reducing Ability and Antioxidant Activity of Fruit Pomace Extracts by Spectrophotometric and Electrochemical Methods. J. Anal. Methods Chem. 2020, 2020, 88694436. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine collagen peptides from the skin of Nile Tilapia (Oreochromis niloticus): Characterization and wound healing evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-based biomaterial for skin wound healing (gelatin vs. collagen): Expert review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, R.; Negreanu-Pirjol, T.; Mirea, M.; Negreanu-Pirjol, B.S. Bioactive Compounds from Three Green Algae Species Along Romanian Black Sea Coast with Therapeutically Properties. Eur. J. Nat. Sci. Med. 2020, 3, 87–106. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric Optimization of Biologically Active Compounds Extraction from Grape Marc: Composition and Antimicrobial Activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).