Plutonic Rocks as Protection Layers to Concrete Exposed to Ultra-High Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Studied
2.2. X-ray Diffraction
2.3. Thermal Gravimetric Analysis
2.4. Optical Microscopy
2.5. Microtomography
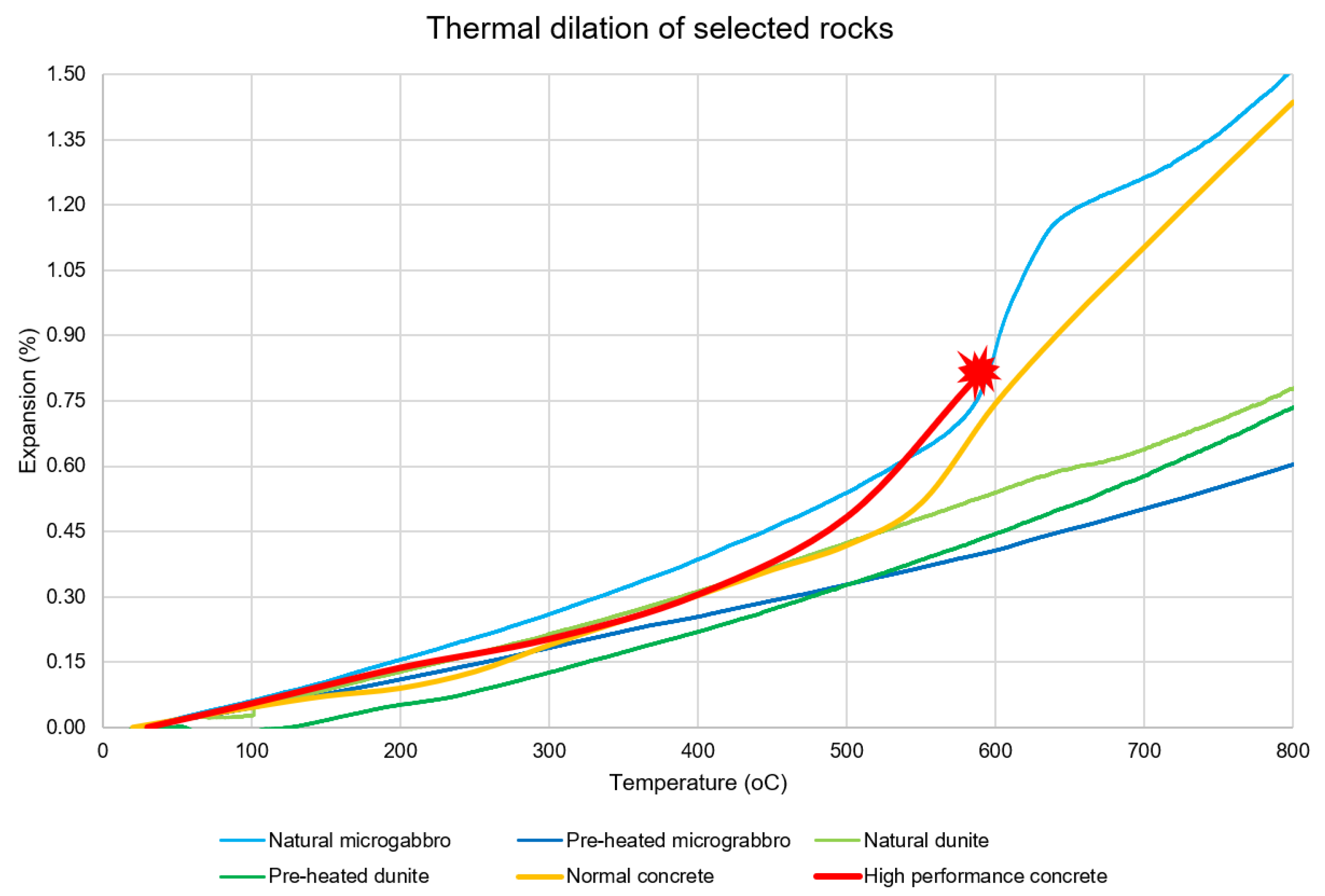
2.6. Thermal Dilation
2.7. Compressive Strength
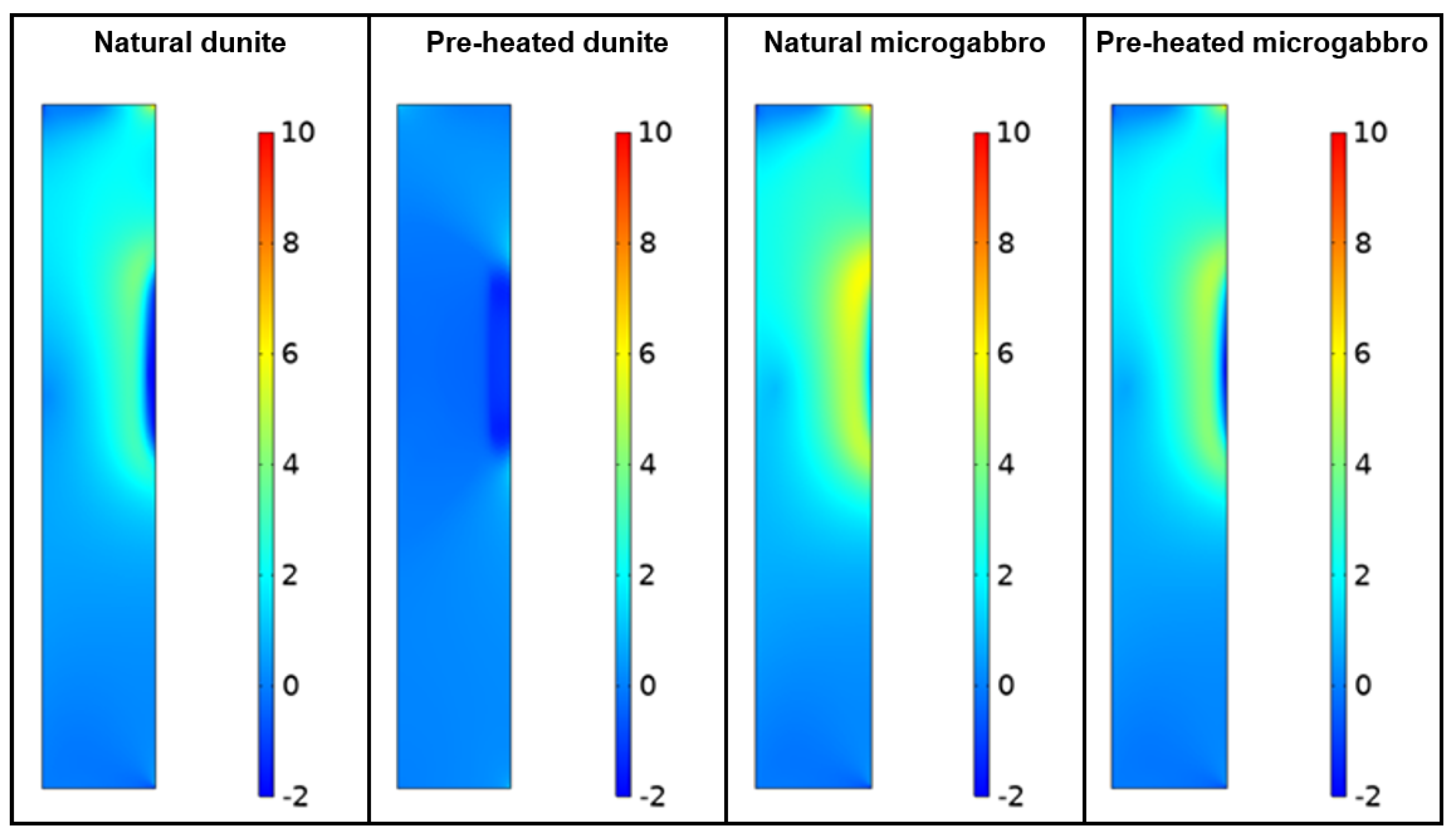
2.8. Stress Comparison
3. Results
3.1. X-ray Diffraction
3.2. Thermogravimetric Analysis
3.3. Optical Microscopy
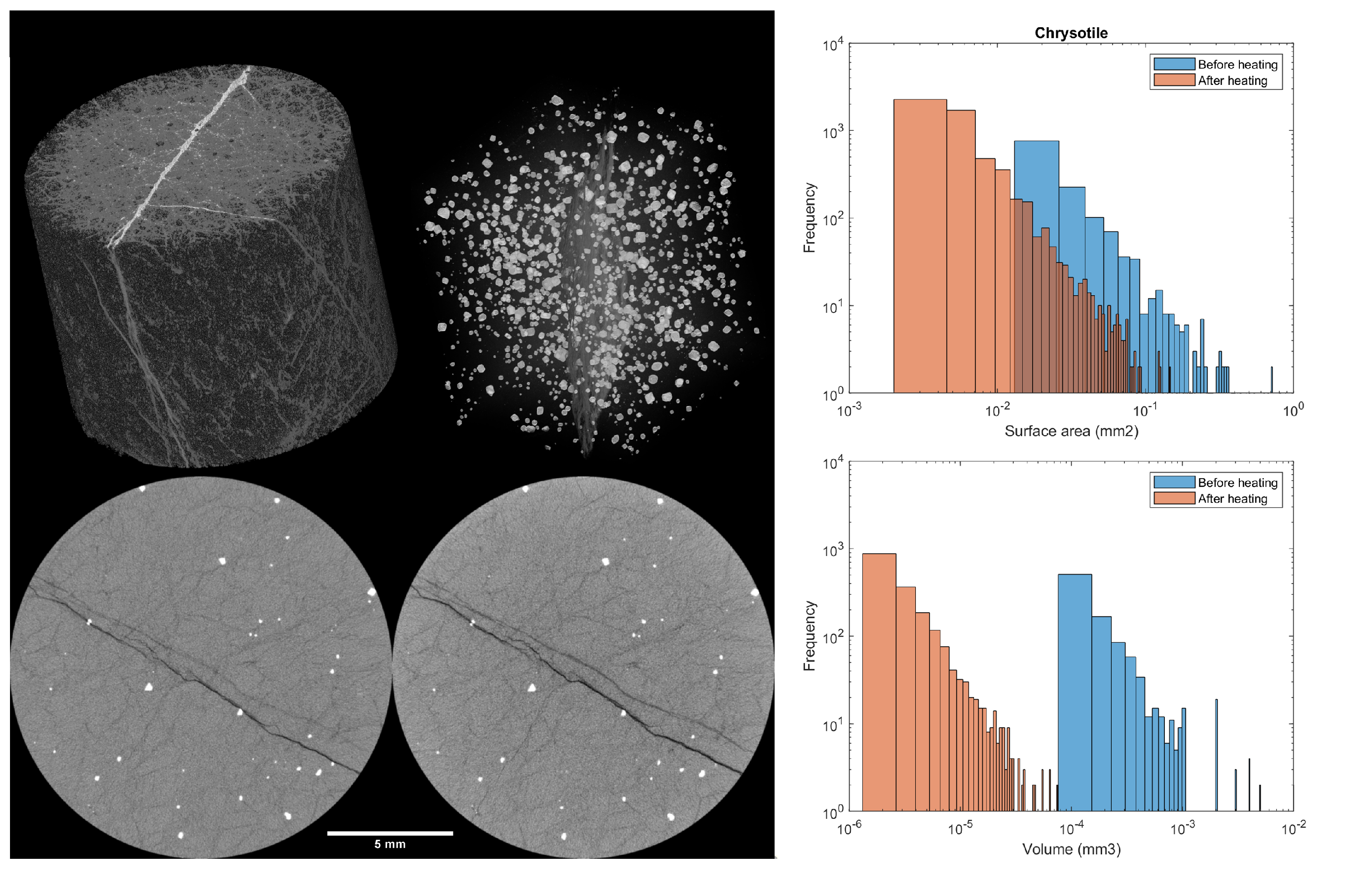
3.4. X-ray Microtomography
3.4.1. Dunite
3.4.2. Microgabbro
3.5. Compressive Strength, Thermal Dilation and Stresses Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XRD | X-ray diffraction spectroscopy |
| TGA | Thermal gravimetric analysis |
| DTG | Differential thermogravimetric analysis |
| PPL | Plain polarised light |
| XPL | Cross polarised light |
| CT | Computer tomography |
| WEKA | Waikato Environment for Knowledge Analysis |
| CTE | Coefficient of thermal expansion |
| FEM | Finite element model |
References
- Georgali, B.; Tsakiridis, P.E. Microstructure of fire-damaged concrete. A case study. Cem. Concr. Compos. 2005, 27, 255–259. [Google Scholar] [CrossRef]
- Ma, Q.; Guo, R.; Zhao, Z.; Lin, Z.; He, K. Mechanical properties of concrete at high temperature—A review. Constr. Build. Mater. 2015, 93, 371–383. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ingason, H. Overview of research on fire safety in underground road and railway tunnels. Tunn. Undergr. Space Technol. 2018, 81, 568–589. [Google Scholar] [CrossRef]
- Hertz, K.D. Limits of spalling of fire-exposed concrete. Fire Saf. J. 2003, 38, 103–116. [Google Scholar] [CrossRef]
- Fu, Y.; Li, L. Study on mechanism of thermal spalling in concrete exposed to elevated temperatures. Mater. Struct. 2011, 44, 361–376. [Google Scholar] [CrossRef]
- Bei, S.; Zhixiang, L. Investigation on spalling resistance of ultra-high-strength concrete under rapid heating and rapid cooling. Case Stud. Constr. Mater. 2016, 4, 146–153. [Google Scholar] [CrossRef]
- Luz, A.P.D.; Braulio, M.A.; Pandolfelli, V.C. Refractory Castable Engineering, 1st ed.; Göller Verlag GmbH: Baden-Baden, Germany, 2015; Volume 1. [Google Scholar]
- Lu, T.J.; Fleck, N.A. The Thermal Shock Resistance of Solids. Acta Mater. 1998, 46, 4755–4768. [Google Scholar] [CrossRef]
- Morsy, M.S.; Shebl, S.S. Effect of silica fume and metakaoline pozzolana on the performance of blended cement pastes against fire. Ceram. Silik. 2007, 51, 40–44. [Google Scholar]
- Nazari, A.; Bagheri, A.; Sanjayan, J.G.; Dao, M.; Mallawa, C.; Zannis, P.; Zumbo, S. Thermal shock reactions of Ordinary Portland cement and geopolymer concrete: Microstructural and mechanical investigation. Constr. Build. Mater. 2019, 196, 492–498. [Google Scholar] [CrossRef]
- Pimenta, P.; Mindeguia, J.C.; Behloul, M. Behaviour of UHPFRC at High Temperatures. In Designing and Building with UHPFRC; Toutlemonde, F., Resplendino, J., Eds.; Wiley: London, UK, 2011; Volume Chapter 40, pp. 579–599. [Google Scholar]
- Peng, G.F.; Niu, X.J.; Shang, Y.J.; Zhang, D.P.; Chen, X.W.; Ding, H. Combined curing as a novel approach to improve resistance of ultra-high performance concrete to explosive spalling under high temperature and its mechanical properties. Cem. Concr. Res. 2018, 109, 147–158. [Google Scholar] [CrossRef]
- Kalifa, P.; Menneteau, F.D.; Quenard, D. Spalling and pore pressure in HPC at high temperatures. Cem. Concr. Res. 2000, 30, 1915–1927. [Google Scholar] [CrossRef]
- Zeiml, M.; Lackner, R.; Mang, H.A. Experimental insight into spalling behavior of concrete tunnel linings under fire loading. Acta Geotech. 2008, 3, 295–308. [Google Scholar] [CrossRef]
- Hedayati, M.; Sofi, M.; Mendis, P.A.; Ngo, T. A comprehensive review of spalling and fire performance of concrete members. Electron. J. Struct. Eng. 2015, 15, 8–34. [Google Scholar]
- Li, Y.; Yang, E.H.; Zhou, A.; Liu, T. Pore pressure build-up and explosive spalling in concrete at elevated temperature: A review. Constr. Build. Mater. 2021, 284, 122818. [Google Scholar] [CrossRef]
- Abhilash, P.P.; Nayak, D.K.; Sangoju, B.; Kumar, R.; Kumar, V. Effect of nano-silica in concrete; a review. Constr. Build. Mater. 2021, 278, 122347. [Google Scholar] [CrossRef]
- Li, G.; Zhang, A.; Song, Z.; Shi, C.; Wang, Y.; Zhang, J. Study on the resistance to seawater corrosion of the cementitious systems containing ordinary Portland cement or/and calcium aluminate cement. Constr. Build. Mater. 2017, 157, 852–859. [Google Scholar] [CrossRef]
- Al-Swaidani, A.M. Use of micro and nano volcanic scoria in the concrete binder: Study of compressive strength, porosity and sulfate resistance. Case Stud. Constr. Mater. 2019, 11, e00294. [Google Scholar] [CrossRef]
- Yu, Z.; Ni, C.; Tang, M.; Shen, X. Relationship between water permeability and pore structure of Portland cement paste blended with fly ash. Constr. Build. Mater. 2018, 175, 458–466. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, J.J.; Peng, G.F.; Breugel, K.V. Numerical analysis of heating rate effect on spalling of high-performance concrete under high temperature conditions. Constr. Build. Mater. 2017, 152, 456–466. [Google Scholar] [CrossRef]
- Choe, G.; Kim, G.; Yoon, M.; Hwang, E.; Nam, J.; Guncunski, N. Effect of moisture migration and water vapor pressure build-up with the heating rate on concrete spalling type. Cem. Concr. Res. 2019, 116, 1–10. [Google Scholar] [CrossRef]
- Sarıfakıoğlu, E.; Özen, H.; Winchester, J. Whole Rock and Mineral Chemistry of Ultramafic-mafic Cumulates from the Orhaneli (Bursa) Ophiolite, NW Anatolia. Turk. J. Earth Sci. 2009, 18, 55–83. [Google Scholar]
- Robertson, A.H.; Parlak, O. Late Cretaceous-Palaeocene subduction-collision-exhumation of a microcontinent along the northern, active margin of the Southern Neotethys: Evidence from the Alanya Massif and the adjacent Antalya Complex (S Turkey). J. Asian Earth Sci. 2020, 201, 104467. [Google Scholar] [CrossRef]
- Mendonça Filho, F.; Romero Rodriguez, C.; Schlangen, E.; Çopuroğlu, O. Surface effects of molten slag spills on calcium aluminate cement paste. Mater. Des. 2022, 217, 110623. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; Volume 1. [Google Scholar]
- Poole, A.; Sims, I. Concrete Petrography: A Handbook of Investigative Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Romero Rodríguez, C.; França de Mendonça Filho, F.; Chaves Figueiredo, S.; Schlangen, E.; Šavija, B. Fundamental investigation on the frost resistance of mortar with microencapsulated phase change materials. Cem. Concr. Compos. 2020, 113, 103705. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Darbon, J.; Cunha, A.; Chan, T.; Osher, S.; Jensen, G. Fast nonlocal filtering applied to electron cryomicroscopy. In Proceedings of the 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, USA, 28 June–1 July 2008; pp. 1331–1334. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]
- NEN-EN 1926:2007; Natural Stone Test Methods—Determination of Uniaxial Compressive Strength. Nederlands Normalisatie-Instituut: Delft, The Netherlands, 2007.
- Mendonça Filho, F.F.; Copuroglu, O. Re-curing of calcium aluminate cements post contact with molten slag. In Proceedings of the 17th Euroseminar on Microscopy Applied to Building Materials, Toronto, ON, Canada, 20–23 May 2019; pp. 236–243. [Google Scholar]
- Holm, N.G.; Oze, C.; Mousis, O.; Waite, J.H.; Guilbert-Lepoutre, A. Serpentinization and the Formation of H2 and CH4 on Celestial Bodies (Planets, Moons, Comets). Astrobiology 2015, 15, 587–600. [Google Scholar] [CrossRef]
- Lamadrid, H.M.; Rimstidt, J.D.; Schwarzenbach, E.M.; Klein, F.; Ulrich, S.; Dolocan, A.; Bodnar, R.J. Effect of water activity on rates of serpentinization of olivine. Nat. Commun. 2017, 8, 16107. [Google Scholar] [CrossRef]
- Brindley, G.W. Kinetics and Mechanisms of Dehydration and Recrystallization of Serpentine—I. Clays Clay Miner. 2017, 12, 35–47. [Google Scholar] [CrossRef]
- Cruciani, G.; Gualtieri, A. Dehydration dynamics of analcime by in situ synchrotron powder diffraction. Am. Mineral. 1999, 84, 112–119. [Google Scholar] [CrossRef]
- Massonne, H.J.; Willner, A.P. Phase relations and dehydration behaviour of psammopelite and mid-ocean ridge basalt at very-low-grade to low-grade metamorphic conditions. Eur. J. Mineral. 2008, 20, 867–879. [Google Scholar] [CrossRef]
- Steudel, A.; Kleeberg, R.; Koch, C.B.; Friedrich, F.; Emmerich, K. Thermal behavior of chlorites of the clinochlore-chamosite solid solution series: Oxidation of structural iron, hydrogen release and dehydroxylation. Appl. Clay Sci. 2016, 132–133, 626–634. [Google Scholar] [CrossRef]
- Lafay, R.; Montes-Hernandez, G.; Janots, E.; Chiriac, R.; Findling, N.; Toche, F. Mineral replacement rate of olivine by chrysotile and brucite under high alkaline conditions. J. Cryst. Growth 2012, 347, 62–72. [Google Scholar] [CrossRef]
- Kuzielová, E.; Žemlička, M.; Másilko, J.; Hudec, P.; Palou, M.T. Influence of hydrothermal treatment parameters on the phase composition of zeolites. J. Therm. Anal. Calorim. 2020, 1, 37–50. [Google Scholar] [CrossRef]
- Giampaolo, C.; Lombardi, G. Thermal behaviour of analcimes from two different genetic environments. Eur. J. Mineral. 1994, 6, 285–290. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Magyar Állami Földtani Intézet alkalmi kiadványa, Geological Institute of Hungary: Budapest, Hungary, 2011. [Google Scholar]
- MacKenzie, W.; Donaldson, C.; Guilford, C. Atlas of Igneous Rocks and Their Textures; Longman: London, UK, 1982. [Google Scholar]
- MacKenzie, W.; MacKenzie, W.; Guilford, C. Atlas of Rock-Forming Minerals in Thin Section; A Halsted Press Book, Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Boudier, F.; Baronnet, A.; Mainprice, D. Serpentine Mineral Replacements of Natural Olivine and their Seismic Implications: Oceanic Lizardite versus Subduction-Related Antigorite. J. Petrol. 2009, 51, 495–512. [Google Scholar] [CrossRef]
- Bron, V.A.; Stepanova, I.A.; Kudryavtseva, T.N. Effect of degree of serpentine formation in dunite on its properties and structure change during heating. Refractories 1967, 8, 553–558. [Google Scholar] [CrossRef]
- Camprubí, A.; Canet, C. Berthierine and chamosite hydrothermal: Genetic guides in the Peña Colorada magnetite-bearing ore deposit, Mexico. Earth Planets Space 2009, 61, 291–295. [Google Scholar] [CrossRef][Green Version]
- Thompson, A.J.B.; Thompson, J.F.H. Atlas of Alteration: A Field and Petrographic Guide to Hydrothermal Alteration Minerals; Geological Association of Canada, Mineral Deposits Division, Special Publication, 6; Geological Association of Canada, Mineral Deposits Division: St. John’s, CA, USA, 1996. [Google Scholar]
- Jambor, J.L.; Bladh, K.W.; Ercit, T.S.; Grice, J.D.; Grew, E.S. New Mineral Names. Am. Mineral. 1988, 73, 927–935. [Google Scholar]
- Deer, W.; Howie, R.; Zussman, J. Rock-Forming Minerals: Orthosilicates, Volume 1A; Rock-Forming Minerals; Geological Society of London: London, UK, 1982. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 25477, Chrysotile. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chrysotile (accessed on 3 March 2022).
- Faure, G.; Mensing, T. Introduction to Planetary Science: The Geological Perspective; Springer: Delft, The Netherlands, 2007. [Google Scholar]
- Schumann, W. Gemstones of the World; Sterling: New York, NY, USA, 2009. [Google Scholar]
- Sadler, B. Light Metals 2013; The Minerals, Metals & Materials Series; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Manghnani, M.; Akimoto, S. High-Pressure Research: Applications in Geophysics; Elsevier Science: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Pohl, W. Economic Geology: Principles and Practice; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Haynes, W. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Uygunoğlu, T.; Topçu, İ.B. Thermal expansion of self-consolidating normal and lightweight aggregate concrete at elevated temperature. Constr. Build. Mater. 2009, 23, 3063–3069. [Google Scholar] [CrossRef]
- Hager, I. Behaviour of cement concrete at high temperature. Bull. Pol. Acad. Sci. Tech. Sci. 2013, 61, 145–154. [Google Scholar] [CrossRef]
- Riecker, R.E.; Rooney, T.P. Weakening of Dunite by Serpentine Dehydration. Science 1966, 152, 196–198. [Google Scholar] [CrossRef] [PubMed]
- E French, M.; Hirth, G.; Okazaki, K. Fracture-induced pore fluid pressure weakening and dehydration of serpentinite. Tectonophysics 2019, 767, 228168. [Google Scholar] [CrossRef]
- Datta, A.; Mathur, B.; Samantaray, B.; Bhattacherjee, S. Dehydration and phase transformation in chrysotile asbestos—A radial distribution analysis study. Bull. Mater. Sci. 1987, 9, 103–110. [Google Scholar] [CrossRef]
- Caillére, S.; Hénin, S. Relation entre la constitution cristallochimique des phyllites et leur temperature de deshydratation application au cas des chlorites. Bulletin de la Société Française de Céramique 1960, 48, 63–67. [Google Scholar]
- Kloprogge, J.; Frost, R. Thermal decomposition of Ferrian chamosite: An infrared emission spectroscopic study. Contrib. Mineral. Petrol. 2000, 138, 59–67. [Google Scholar] [CrossRef]
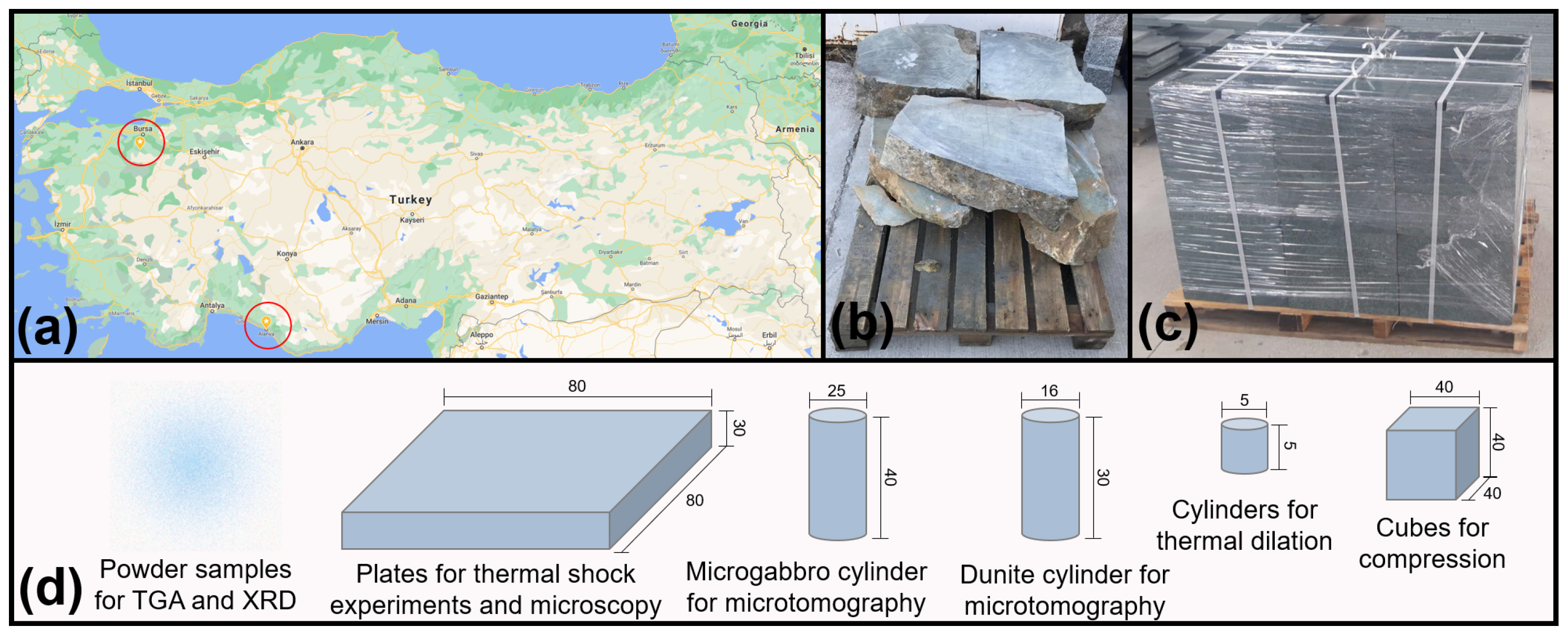
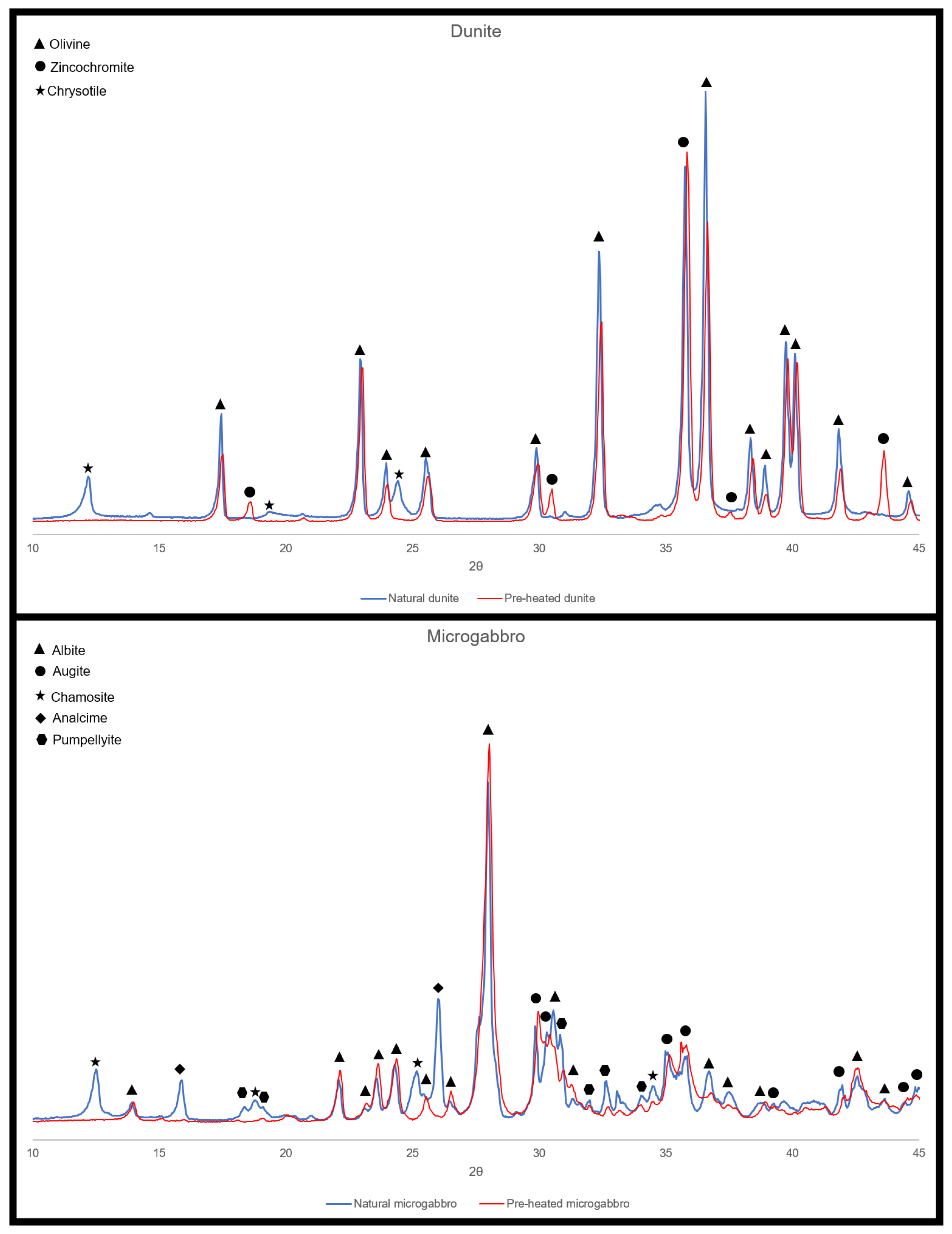
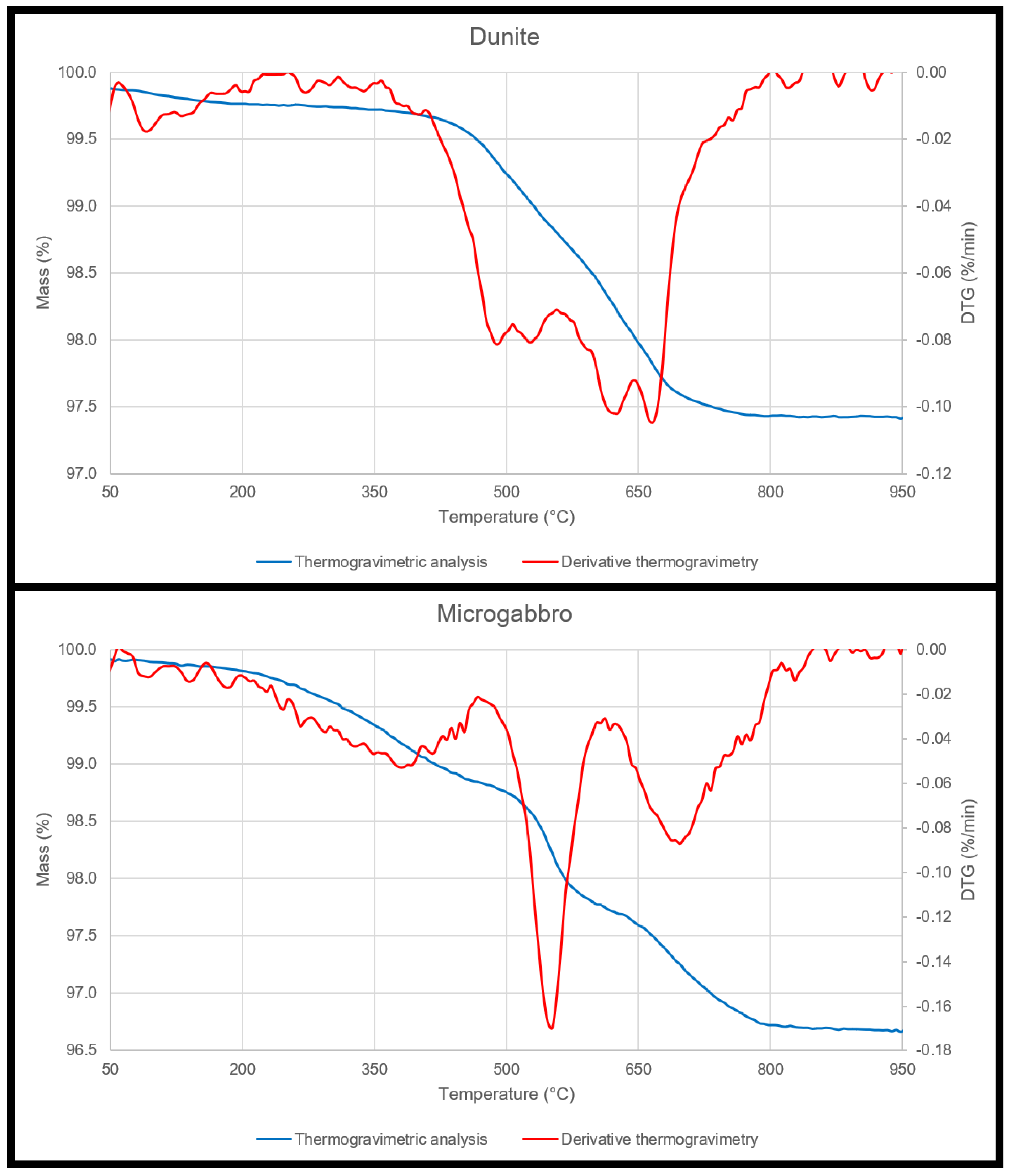
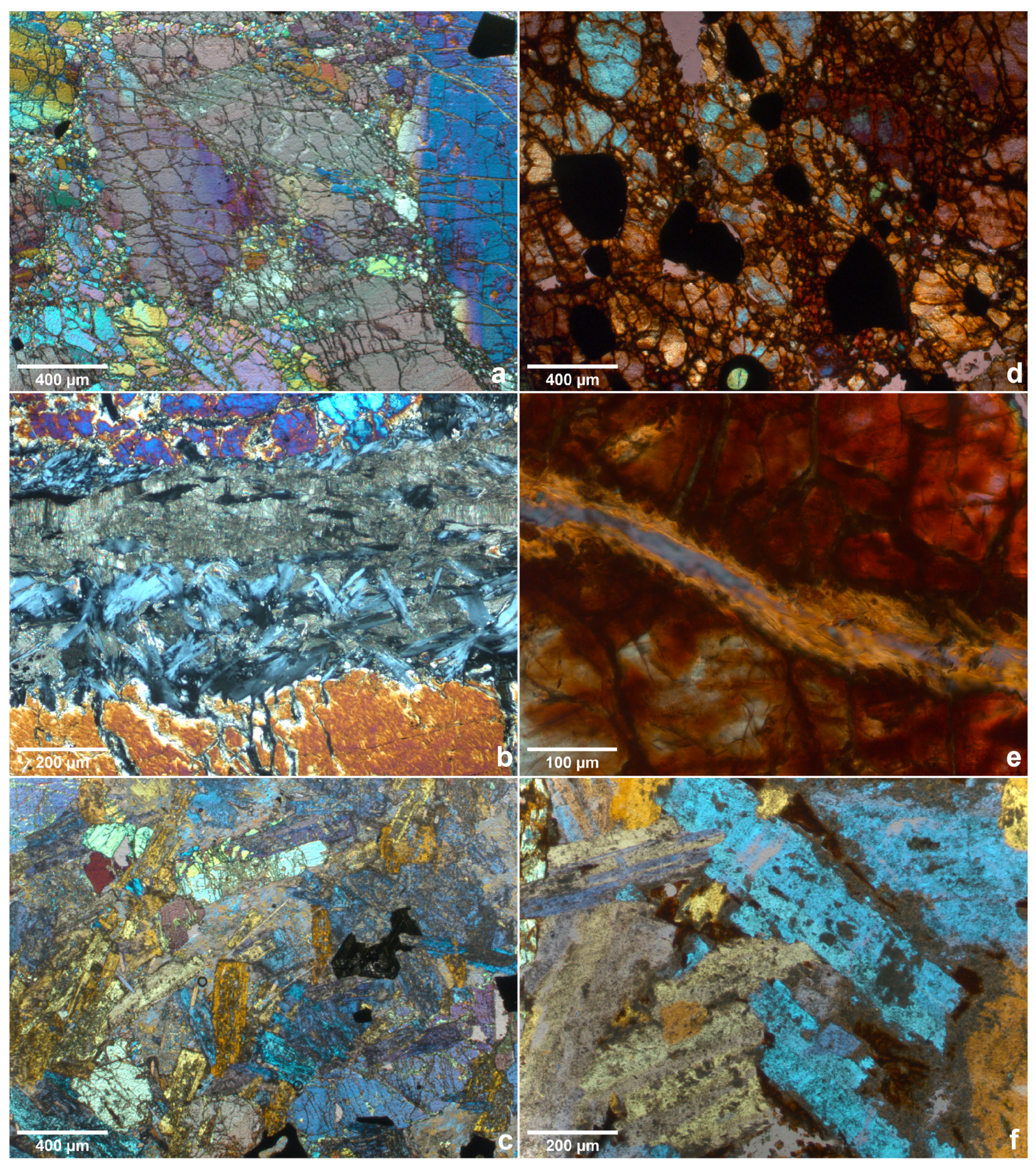

| Analysis Method | Sample Shape | Sample Dimensions (mm) |
|---|---|---|
| X-ray diffraction | Powder | <0.01 |
| Thermogravimetric analysis | Powder | <0.01 |
| Optical microscopy | Rectangle mounted in glass | 30 × 45 × 0.022 |
| Compressive strength | Cube | 40 × 40 × 40 |
| Thermal dilation | Cylinder | 5 × 5 |
| Micro tomography (dunite) | Cylinder | 16 × 30 |
| Micro tomography (microgabbro) | Cylinder | 25 × 40 |
| Phase | Density (g/cm) | Volume before Heating (%) | Volume after Heating (%) |
|---|---|---|---|
| Zincochromite | 5.434 [51] | 0.66 | 0.69 |
| Olivine | 3.287 [52] | 86.44 | 88.77 |
| Chrysotile | 2.56 [53] | 11.53 | 9.33 |
| Augite | 3.4 [54] | 07.76 | 07.37 |
| Pumpellyite | 3.18 [55] | ||
| Chamosite | 3.13 [56] | 31.98 | 29.27 |
| Albite | 2.4 [57] | ||
| Analcime | 2.3 [58] | 60.26 | 63.36 |
| Sample | Compressive Strength (MPa) | Standard Deviation (MPa) |
|---|---|---|
| Dunite | 84 | 8.58 |
| Dunite (heated) | 149 | 22.46 |
| Microgabbro | 193 | 4.31 |
| Microgabbro (heated) | 166 | 2.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mendonça Filho, F.F.; Romero Rodriguez, C.; Schlangen, E.; Çopuroğlu, O. Plutonic Rocks as Protection Layers to Concrete Exposed to Ultra-High Temperature. Materials 2022, 15, 3490. https://doi.org/10.3390/ma15103490
de Mendonça Filho FF, Romero Rodriguez C, Schlangen E, Çopuroğlu O. Plutonic Rocks as Protection Layers to Concrete Exposed to Ultra-High Temperature. Materials. 2022; 15(10):3490. https://doi.org/10.3390/ma15103490
Chicago/Turabian Stylede Mendonça Filho, Fernando França, Cláudia Romero Rodriguez, Erik Schlangen, and Oğuzhan Çopuroğlu. 2022. "Plutonic Rocks as Protection Layers to Concrete Exposed to Ultra-High Temperature" Materials 15, no. 10: 3490. https://doi.org/10.3390/ma15103490
APA Stylede Mendonça Filho, F. F., Romero Rodriguez, C., Schlangen, E., & Çopuroğlu, O. (2022). Plutonic Rocks as Protection Layers to Concrete Exposed to Ultra-High Temperature. Materials, 15(10), 3490. https://doi.org/10.3390/ma15103490









