Impact of Methanol Concentration on Properties of Ultra-Nanocrystalline Diamond Films Grown by Hot-Filament Chemical Vapour Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. HF-CVD Growth
2.2. Material Characterisation
3. Results
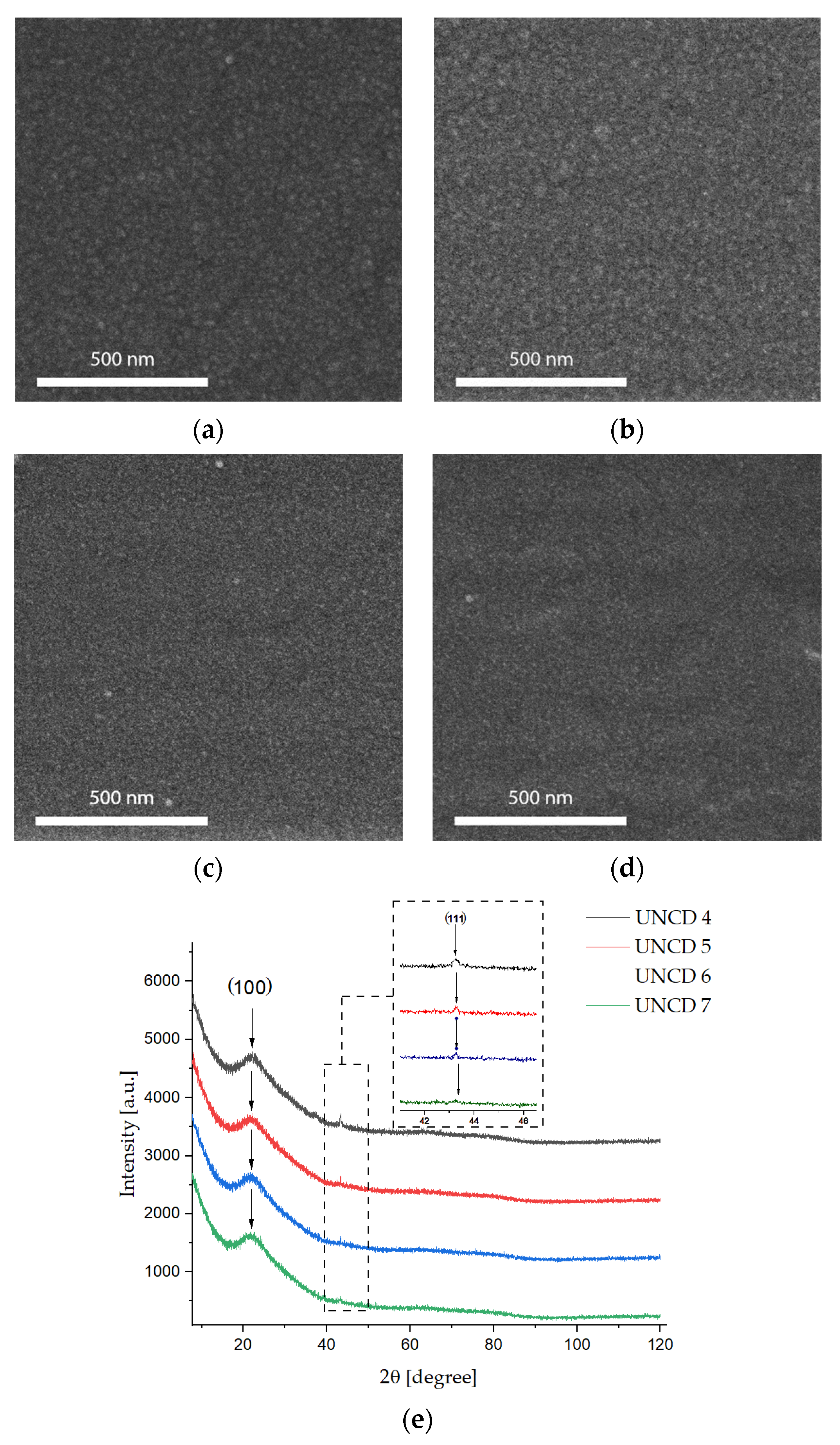
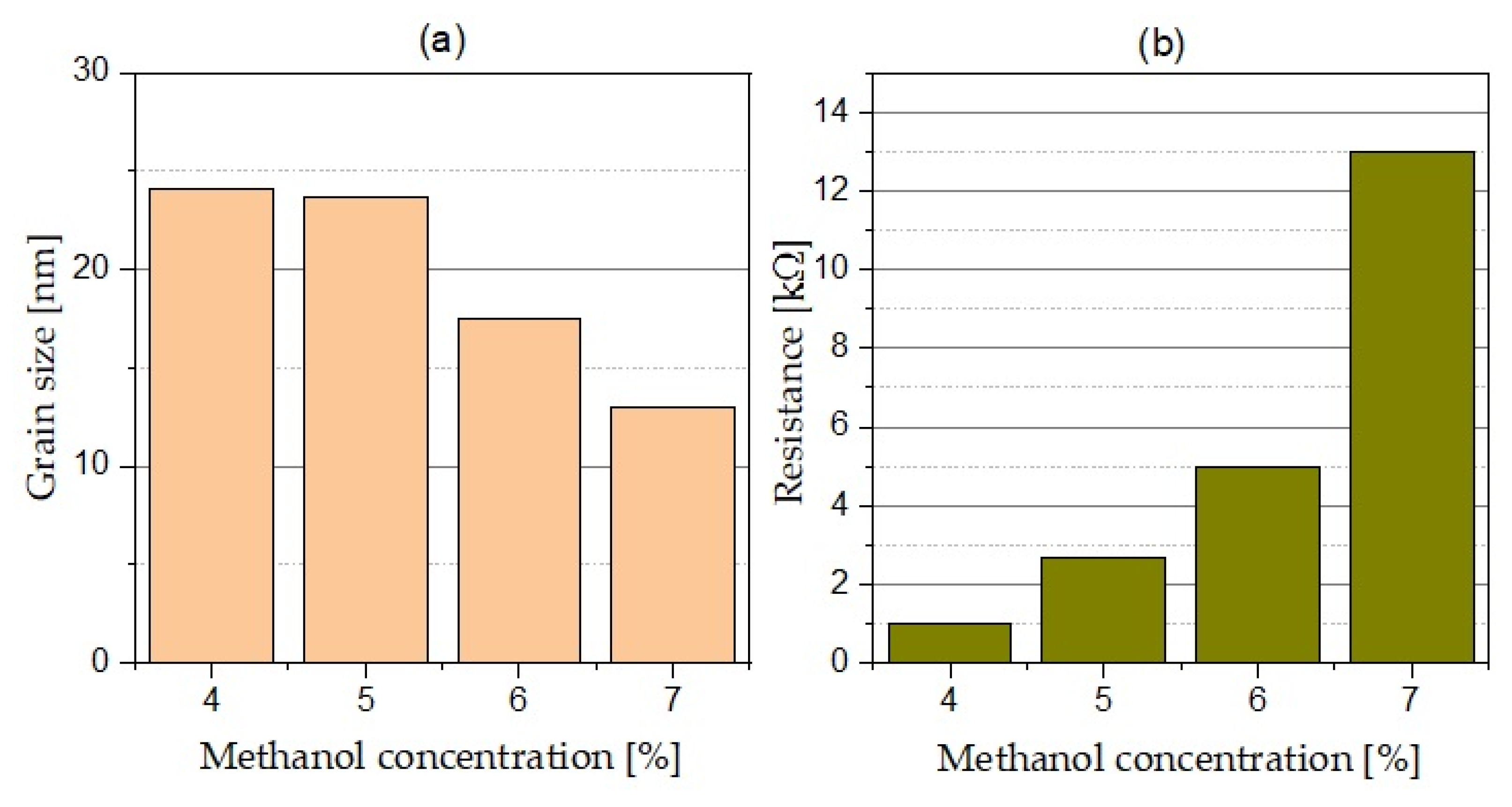
3.1. Scanning Electron Microscopy, X-ray Diffraction, and Atomic Force Microscopy
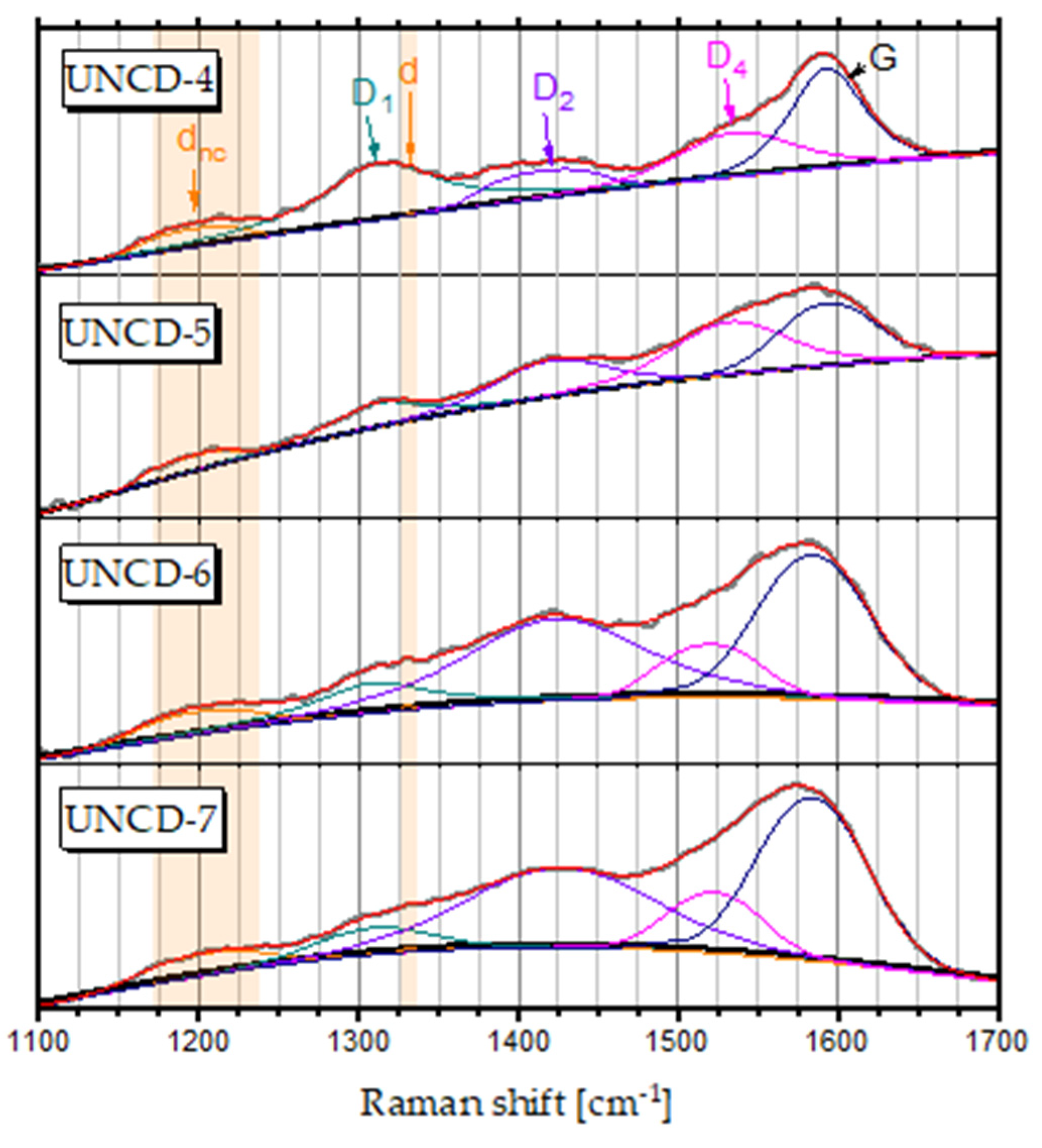
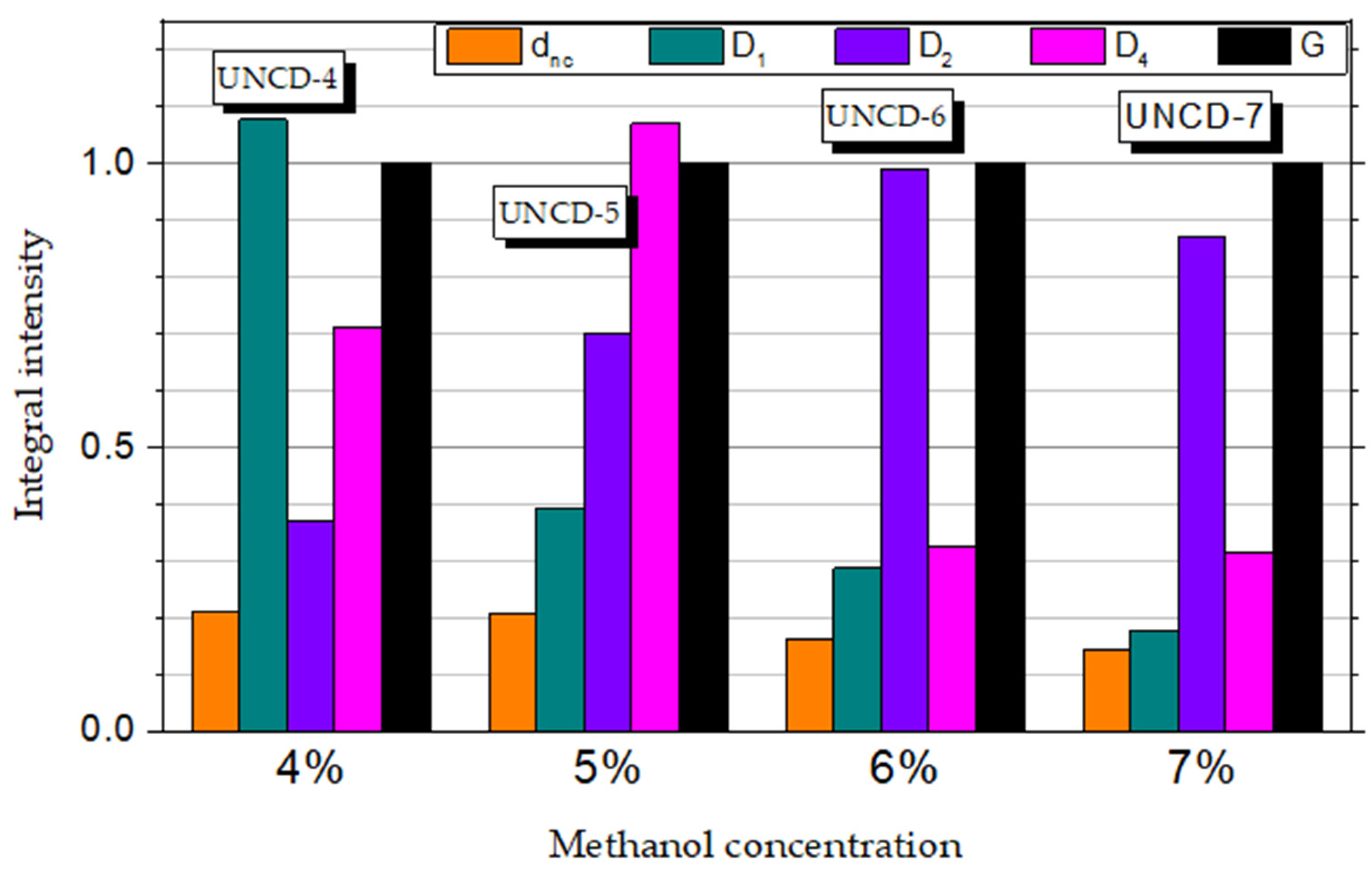
3.2. Raman Spectroscopy
3.3. X-ray Photoelectron Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bala, W.; Zorenko, Y.; Savchyn, V.; Voznyak, T.; Paprocki, K.; Popielarski, P.; Szybowicz, M. Optical and electrical properties of zno thin films grown by sol-gel method. In Solid State Phenomena; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2013; pp. 14–21. [Google Scholar]
- Popielarski, P.; Mosinska, L.; Bala, W.; Paprocki, K.; Zorenko, Y.; Zorenko, T.; Sypniewska, M. Persistent photoconductivity in ZnO thin films grown on Si substrate by spin coating method. Opt. Mater. 2019, 97, 109343. [Google Scholar] [CrossRef]
- Popielarski, P.; Bala, W.; Paprocki, K.; Mosinska, K.; Kowalska, M.; Szybowicz, M.; Makowiecki, J. Admittance spectroscopy of CuPC-Si and CoPC-Si heterostructures. Electrochim. Acta 2013, 104, 496–504. [Google Scholar] [CrossRef]
- Sun, J.; Ge, J.; Liu, W.; Lan, M.; Zhang, H.; Wang, P.; Wang, Y.; Niu, Z. Multi-enzyme co-embedded organic–inorganic hybrid nanoflowers: Synthesis and application as a colorimetric sensor. Nanoscale 2014, 6, 255–262. [Google Scholar] [CrossRef]
- Riedinger, A.; Pernia Leal, M.; Deka, S.R.; George, C.; Franchini, I.R.; Falqui, A.; Cingolani, R.; Pellegrino, T. “Nanohybrids” based on pH-responsive hydrogels and inorganic nanoparticles for drug delivery and sensor applications. Nano Lett. 2011, 11, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Choi, H.; SunPark, J.; Lee, H.J.; Kim, S.O.; Kim, J.Y.; Song, M.H. Surface modification of metal oxide using ionic liquid molecules in hybrid organic–inorganic optoelectronic devices. J. Mater. Chem. 2011, 21, 2051–2053. [Google Scholar] [CrossRef]
- Švorc, Ľ.; Stanković, D.M.; Kalcher, K. Boron-doped diamond electrochemical sensor for sensitive determination of nicotine in tobacco products and anti-smoking pharmaceuticals. Diam. Relat. Mater. 2014, 42, 1–7. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, X.; Cui, Z.; Xu, C.; Sun, Z.; Li, J.; Liu, J.; Tian, Y.; Li, H. A Nanometer-Sized Graphite/Boron-Doped Diamond Electrochemical Sensor for Sensitive Detection of Acetaminophen. ACS Omega 2021, 6, 6326–6334. [Google Scholar] [CrossRef]
- Michnik, A.; Kłos, A.; Drzazga, Z. The influence of radio-frequency radiation on thermal stability of bovine serum albumin in aqueous solution. J. Therm. Anal. Calorim. 2004, 77, 269–277. [Google Scholar] [CrossRef]
- Klos-Witkowska, A. Influence of fractional electromagnetic radiation doses on biosensor matrix component stability. Acta Phys. Pol. A 2018, 133, 101–104. [Google Scholar] [CrossRef]
- Mosińska, L.; Popielarski, P.; Fabisiak, K.; Dychalska, A. Effects of hydrogen termination of CVD diamond layers. Opt. Mater. 2020, 101, 109676. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lee, D.-C.; Tsai, T.-Y.; Hsiao, C.-Y.; Liu, J.-W.; Kao, C.-Y.; Lin, H.-K.; Chen, H.-C.; Palathinkal, T.J.; Pong, W.-F.; et al. Induction and regulation of differentiation in neural stem cells on ultra-nanocrystalline diamond films. Biomaterials 2010, 31, 5575–5587. [Google Scholar] [CrossRef]
- Chen, X.; Mohr, M.; Brühne, K.; Fecht, H.-J. Highly Conductive Nanocrystalline Diamond Films and Electronic Metallization Scheme. Materials 2021, 14, 4484. [Google Scholar] [CrossRef]
- Zhou, A.; Pacheco, E.; Zhou, B.; Feng, P. Size-Dependent Electrical Transport Properties in Conducting Diamond Nanostripes. Nanomaterials 2021, 11, 1765. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, D.; Duan, S.; Wang, Q.; Liu, C. Review on the Properties of Boron-Doped Diamond and One-Dimensional-Metal-Oxide Based PN Heterojunction. Molecules 2021, 26, 71. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zuo, X.; Guo, P.; Li, X.; Ke, P.; Wang, A. Role of deposition temperature on the mechanical and tribological properties of Cu and Cr co-doped diamond-like carbon films. Thin Solid Films 2019, 678, 16–25. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Sobaszek, M.; Sawczak, M.; Grigorian, G.; Ficek, M.; Caban, P.; Herman, A.; Cenian, A. Enhanced boron doping of thin diamond films grown in deuterium-rich microwave plasma. Diam. Relat. Mater. 2019, 96, 198–206. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Booth, L.; Catledge, S.A.; Nolen, D.; Thompson, R.G.; Vohra, Y.K. Synthesis and characterization of multilayered diamond coatings for biomedical implants. Materials 2011, 4, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Puttichaem, C.; Souza, G.; Ruthe, K.; Chainok, K. Characterization of Ultra-Thin Diamond-Like Carbon Films by SEM/EDX. Coatings 2021, 11, 729. [Google Scholar] [CrossRef]
- Surovtsev, N.V.; Kupriyanov, I.N. Effect of nitrogen impurities on the Raman line width in diamond, revisited. Crystals 2017, 7, 239. [Google Scholar] [CrossRef] [Green Version]
- Granger, M.; Xu, J.; Strojek, J.W.; Swain, G. Polycrystalline diamond electrodes: Basic properties and applications as amperometric detectors in flow injection analysis and liquid chromatography. Anal. Chim. Acta 1999, 397, 145–161. [Google Scholar] [CrossRef]
- Ramos, S.C.; Azevedo, A.F.; Baldan, M.R.; Ferreira, N.G. Effect of methane addition on ultrananocrystalline diamond formation: Morphology changes and induced stress. J. Vac. Sci. Technol. A Vac. Surf. Films 2010, 28, 27–32. [Google Scholar] [CrossRef]
- Shroder, R.E.; Nemanich, R.J.; Glass, J.T. Analysis of the composite structures in diamond thin films by Raman spectroscopy. Phys. Rev. B 1990, 41, 3738. [Google Scholar] [CrossRef]
- Prawer, S.; Nugent, K.; Jamieson, D.; Orwa, J.; Bursill, L.; Peng, J. The Raman spectrum of nanocrystalline diamond. Chem. Phys. Lett. 2000, 332, 93–97. [Google Scholar] [CrossRef]
- Prawer, S.; Nemanich, R.J. Raman spectroscopy of diamond and doped diamond. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2537–2565. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Ho, K.M. Structure, dynamics, and electronic properties of diamondlike amorphous carbon. Phys. Rev. Lett. 1993, 71, 1184. [Google Scholar] [CrossRef] [PubMed]
- Lin-Chung, P. Local vibrational modes of impurities in diamond. Phys. Rev. B 1994, 50, 16905. [Google Scholar] [CrossRef]
- Bosak, A.; Krisch, M. Phonon density of states probed by inelastic X-ray scattering. Phys. Rev. B 2005, 72, 224305. [Google Scholar] [CrossRef]
- Klauser, F.; Steinmüller-Nethl, D.; Kaindl, R.; Bertel, E.; Memmel, N. Raman studies of nano-and ultra-nanocrystalline diamond films grown by hot-filament CVD. Chem. Vap. Depos. 2010, 16, 127–135. [Google Scholar] [CrossRef]
- Dychalska, A.; Koczorowski, W.; Trzcinski, M.; Mosińska, L.; Szybowicz, M. The Effect of Surface Treatment on Structural Properties of CVD Diamond Layers with Different Grain Sizes Studied by Raman Spectroscopy. Materials 2021, 14, 1301. [Google Scholar] [CrossRef] [PubMed]
- Vecera, P.; Chacón-Torres, J.C.; Pichler, T.; Reich, S.; Soni, H.R.; Görling, A.; Edelthalhammer, K.; Peterlik, H.; Hauke, F.; Hirsch, A. The First Precise Determination of Graphene Functionalisation by in situ Raman Spectroscopy. arXiv 2017, arXiv:1703.02498. [Google Scholar]
- Chu, P.K.; Li, L. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006, 96, 253–277. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosińska, L.; Szczęsny, R.; Trzcinski, M.; Naparty, M.K. Impact of Methanol Concentration on Properties of Ultra-Nanocrystalline Diamond Films Grown by Hot-Filament Chemical Vapour Deposition. Materials 2022, 15, 5. https://doi.org/10.3390/ma15010005
Mosińska L, Szczęsny R, Trzcinski M, Naparty MK. Impact of Methanol Concentration on Properties of Ultra-Nanocrystalline Diamond Films Grown by Hot-Filament Chemical Vapour Deposition. Materials. 2022; 15(1):5. https://doi.org/10.3390/ma15010005
Chicago/Turabian StyleMosińska, Lidia, Robert Szczęsny, Marek Trzcinski, and Mieczysław Karol Naparty. 2022. "Impact of Methanol Concentration on Properties of Ultra-Nanocrystalline Diamond Films Grown by Hot-Filament Chemical Vapour Deposition" Materials 15, no. 1: 5. https://doi.org/10.3390/ma15010005
APA StyleMosińska, L., Szczęsny, R., Trzcinski, M., & Naparty, M. K. (2022). Impact of Methanol Concentration on Properties of Ultra-Nanocrystalline Diamond Films Grown by Hot-Filament Chemical Vapour Deposition. Materials, 15(1), 5. https://doi.org/10.3390/ma15010005





