A Novel Recycling Route for Spent Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
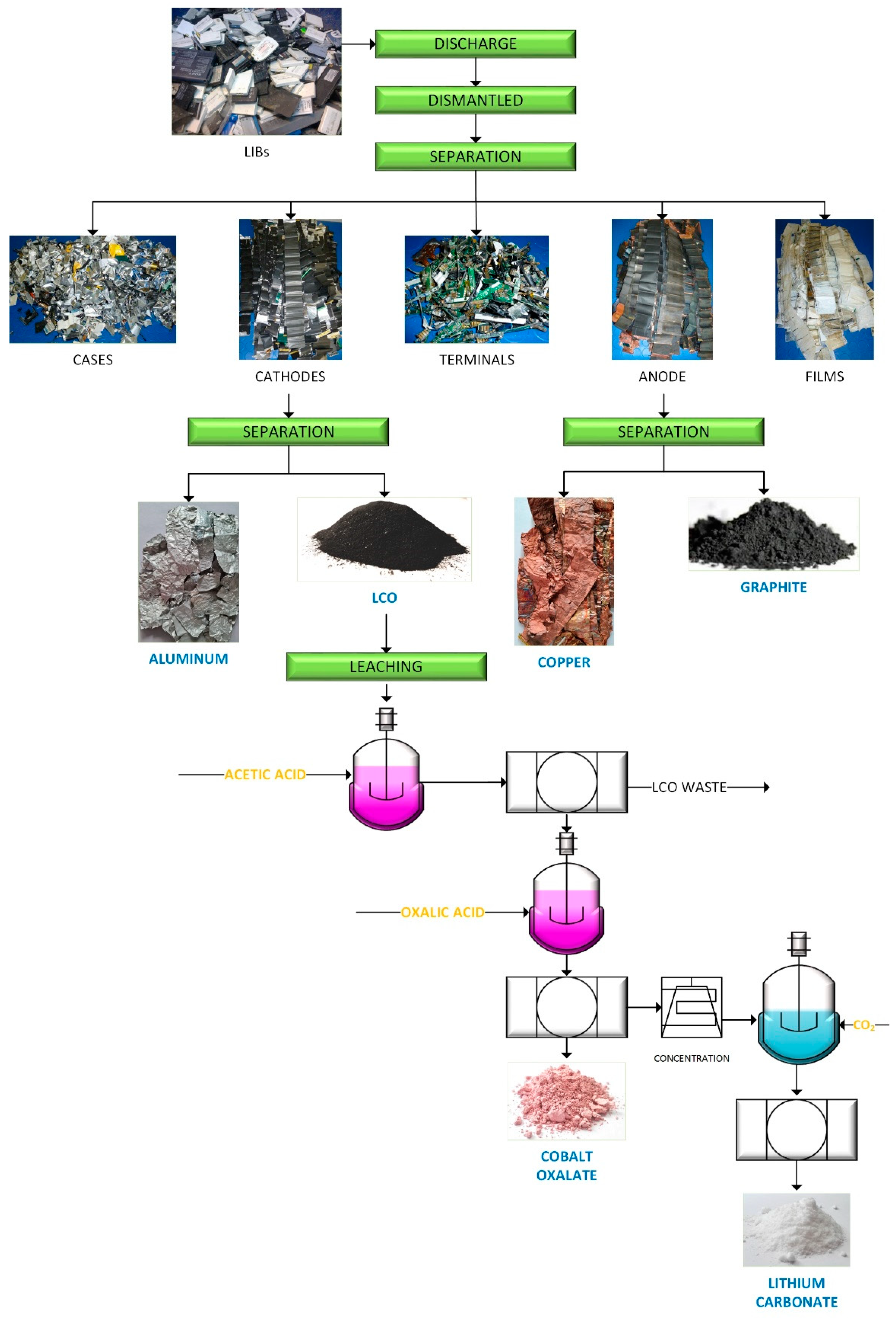
2.2. Dismantling of the Batteries
2.3. Leaching Procedure
2.4. Precipitation Procedure
2.5. Analytical Methods
3. Results and Discussion
3.1. Recycling of the Anode
3.2. Recycling of the Cathode
3.2.1. Thermochemical Analysis of the Leaching of Waste LCO
3.2.2. Analysis of the Dissolution of the LCO
Effect of Solid/Liquid Ratio
Effect of Leaching Agent Concentration
Effect of H2O2 Concentration
Effect of Temperature
Effect of Reaction Time
Effect of Stirring Speed
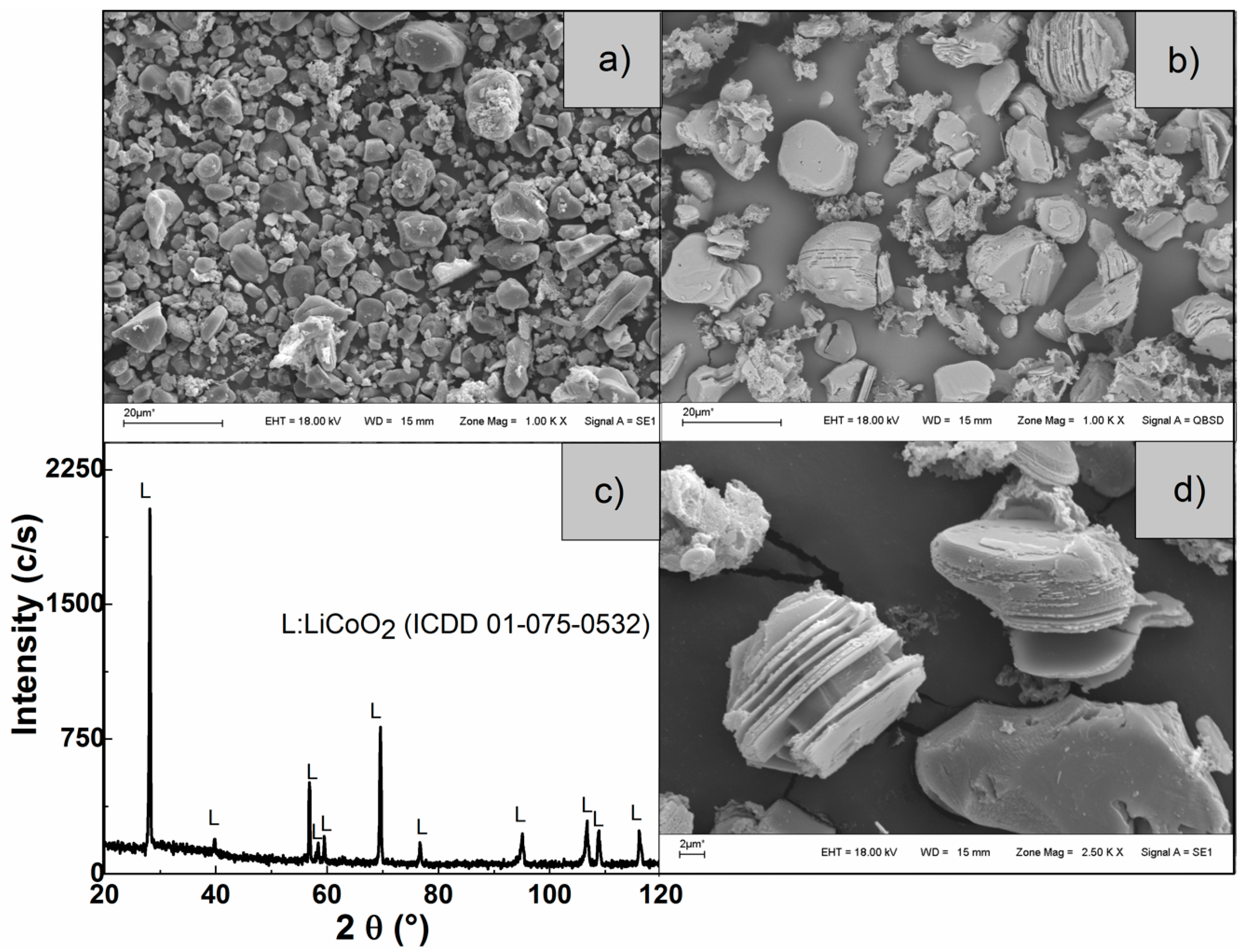
3.2.3. Characterization of the Solid Residues
3.2.4. Recovery of Metals
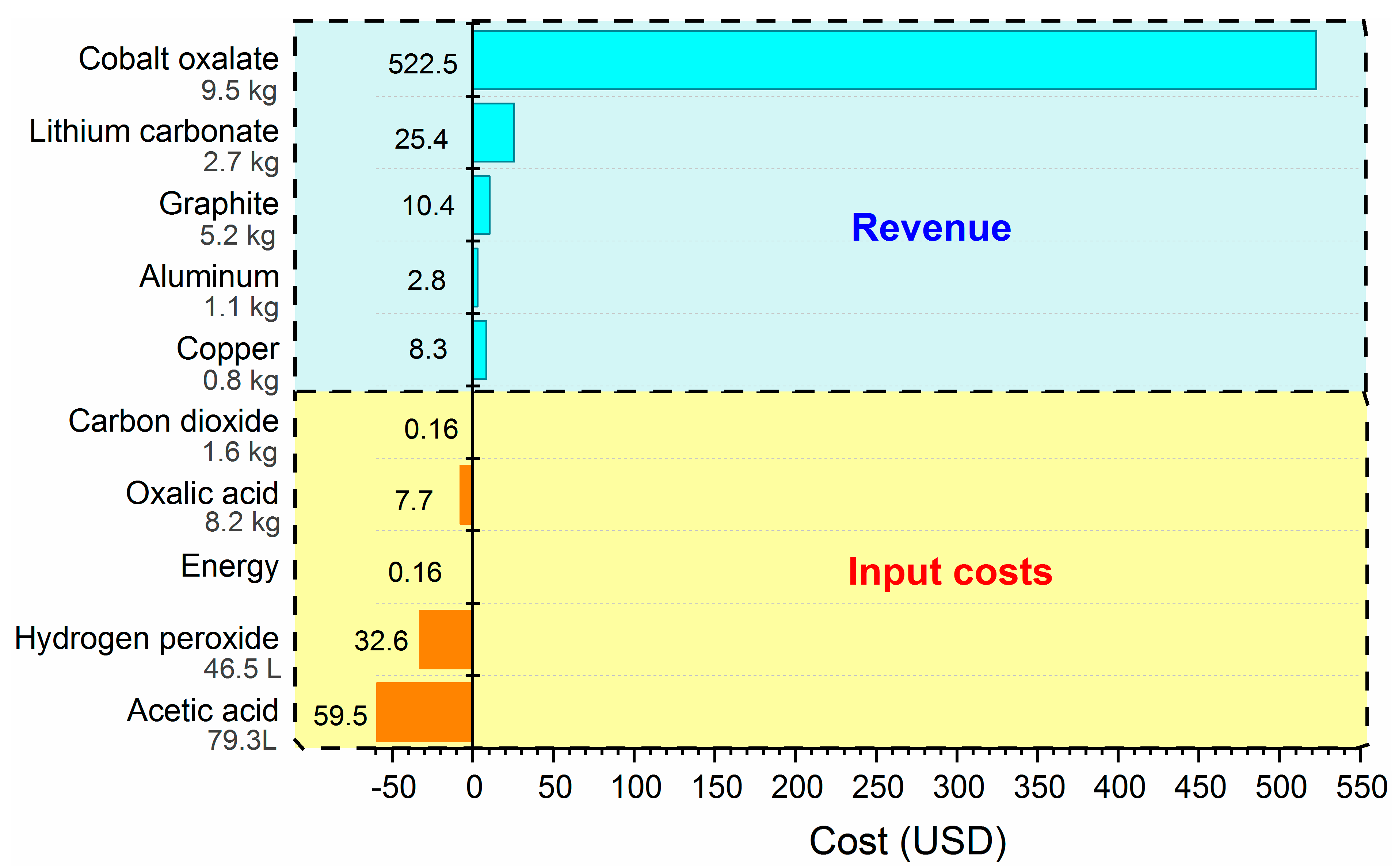
3.3. Preliminary Economic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaikh, S.; Thomas, K.; Zuhair, S. An exploratory study of e-waste creation and disposal: Upstream considerations. Resour. Conserv. Recycl. 2020, 155, 104662. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z.H. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Ministerio de Ambiente y Desarrollo Sostenible de la Nación. Manual: Gestión Integral de RAEE. Los Residuos de Aparatos Eléctricos y Electrónicos, una Fuente de Trabajo Decente Para Avanzar Hacia la Economía Circular. 2020. Available online: https://www.argentina.gob.ar/sites/default/files/manual_raee.pdf (accessed on 16 December 2021).
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. Observatorio Mundial de los Residuos Electrónicos, Universidad de las Naciones Unidas (UNU), Unión Internacional de Telecomunicaciones (UIT) y Asociación Internacional de Residuos Sólidos (ISWA), Bonn/Ginebra/Viena. 2020. Available online: https://www.itu.int/en/ITU-D/Climate-Change/Documents/GEM%202017/GEM%202017-S.pdf (accessed on 16 December 2021).
- Pinna, E.G.; Ruiz, M.C.; Ojeda, M.W.; Rodriguez, M.H. Cathodes of spent Li-ion batteries: Dissolution with phosphoric acid and recovery of lithium and cobalt from leach liquors. Hydrometallurgy 2017, 167, 66–71. [Google Scholar] [CrossRef]
- Pinna, E.G.; Martínez, A.A.; Tunez, F.M.; Drajlin, D.S.; Rodriguez, M.H. Acid Leaching acid of the LiCoO2 from LIBs: Thermodynamic study and reducing agent effect. Rev. Mex Ing. Quím. 2019, 18, 441–449. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Suarez, D.S.; Pinna, E.G.; Zeballos, C. Method for Acid Dissolution of LiCoO2 Contained in Spent Lithium-Ion Batteries. Patent No. US 0309174 A1, 25 October 2018. [Google Scholar]
- Suarez, D.S.; Pinna, E.G.; Rosales, G.D.; Rodriguez, M.H. Synthesis of lithium fluoride from spent lithium ion batteries. Minerals 2017, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.; Lin, Y.C.; Wu, S.H. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab J. Chem. 2017, 10, S3632–S3639. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef] [PubMed]
- Arambarri, J.; Hayden, J.; Elkurdy, M.; Meyers, B.; Hamatteh, Z.; Abbassi, B. Lithium ion car batteries: Present analysis and future predictions. Environ. Eng. Res. 2019, 24, 699–710. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Castillo, S.; Ansart, F.; Laberty-Robert, C.; Portal, J. Advances in the recovery of spent lithium battery compounds. J. Power Sources 2002, 112, 247–254. [Google Scholar] [CrossRef]
- Lee, C.; Rhee, K. Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and lithium recovery from active mass of spent Li-ion batteries: Theoretical and experimental approach. Hydrometallurgy 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Chen, X.; Cao, L.; Kang, D.; Li, J.; Zhou, T.; Ma, H. Recovery of valuable metals from mixed types of spent lithium ion batteries. Part II: Selective extraction of lithium. Waste Manag. 2018, 80, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Pinna, E.G.; Drajlin, D.S.; Toro, N.; Rodriguez, M.H. Kinetic modeling of the leaching of LiCoO2 with phosphoric acid. J. Mater. Res. Technol. 2020, 9, 14017–14028. [Google Scholar] [CrossRef]
- Shuva, M.A.H.; Kurny, A.S.W. Dissolution kinetics of cathode of spent lithium ion battery in hydrochloric acid solutions. J. Inst. Eng. India Ser. D 2013, 94, 13–16. [Google Scholar] [CrossRef]
- Lorenzo, M.; Hat, S.; Bertrams, T.; Murray, P.; Raval, R.; Baddeley, J. Creating chiral surfaces for enantioselective heterogeneous catalysis: R,R-tartaric acid un Cu (110). J. Phys. Chem. B 1999, 103, 10661–10669. [Google Scholar] [CrossRef]
- Meshram, P.; Mishra, A.; Abhilash, A.; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids- A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Chen, R.; Wu, F.; Chen, S.; Zhang, X. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Manag. 2010, 30, 2615–2621. [Google Scholar] [CrossRef]
- Zheng, Y.; Long, H.L.; Zhou, L.; Wu, Z.S.; You, L.; Yang, Y. Leaching procedure and kinetic studies of cobalt in cathode materials from spent lithium ion batteries using organic citric acid as leachant. Int. J. Environ. Res. 2016, 10, 159–168. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Roshanfar, M.; Golmohammadzadeh, R.; Rashchi, F. An environmentally friendly method for recovery of lithium and cobalt from spent lithium-ion batteries using gluconic and lactic acids. J. Environ. Chem. Eng. 2019, 7, 102794. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, W.; Mo, W.; Zhou, L.; Liu, J. Lithium fluoride recovery from cathode material of spent lithium-ion battery. RSC Adv. 2018, 8, 890–899. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Manjanna, J.; Pai, K.V.; Vadavi, R.; Keny, S.J.; Tripathi, V.S. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.; Zhang, Y.; Dong, P.; Wang, D.; Zhou, Z.; Duan, J.; Li, X.; Lin, Y.; Meng, Q.; Pai, K.; et al. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J. Environ. Chem. Eng. 2018, 7, 102854. [Google Scholar] [CrossRef]
- He, L.; Sun, S.; Mu, Y.; Song, X.; Yu, J. Recovery of lithium, nickel, cobalt, and manganese from spent lithium-ion batteries using L-tartaric acid as a leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Dai, Q.; Spangenberger, J.; Ahmed, S.; Gaines, L.; Kelly, J.C.; Wang, M. EverBatt: A Closed-Loop Battery Recycling Cost and Environmental Impacts Model. 2018. Available online: https://publications.anl.gov/anlpubs/2019/07/153050.pdf (accessed on 16 December 2021).
- Li, L.; Bian, Y.; Zhang, X.X.; Xue, Q.; Fan, E.; Wu, F. Economical recycling process for spent lithium-ion batteries and macro-and micro-scale mechanistic study. J. Power Sources 2018, 377, 70–79. [Google Scholar] [CrossRef]




| Elements (%) | Li | Co | Mn | Ni | C | Al | Fe | Cu |
|---|---|---|---|---|---|---|---|---|
| Cathode active materials | 7.34 | 54.56 | 2.07 | 1.78 | 1.02 | 0.29 | 0.14 | --- |
| Anode active materials | 1.70 | ----- | ----- | ----- | 97.30 | ----- | ----- | 1.00 |
| Proposed Equations | ΔG | |||
|---|---|---|---|---|
| 298 K | 323 K | 348 K | 363 K | |
| 4LiCoO2 + 12CH3COOH + 2H2O2 = 4Li+ + 4Co2+ + 8H2O + 2O2(g) + 12CH3COO− | −109.3 | −102.9 | −95.4 | −90.3 |
| 4LiCoO2 + 12CH3COOH = 4Li+ + 4Co2+ + 6H2O + O2(g) + 12CH3COO− | −60.0 | −53.2 | −45.3 | −39.9 |
| Compounds | Purity | % Atomic | |||||
|---|---|---|---|---|---|---|---|
| Mn | Ni | Fe | Co | C | O | ||
| CoC2O4 | 98.3% | 1.7 | -- | -- | 31.08 | 31.40 | 35.81 |
| Li2CO3 | 99.6% | -- | -- | -- | -- | 18.21 | 62.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, E.G.; Toro, N.; Gallegos, S.; Rodriguez, M.H. A Novel Recycling Route for Spent Li-Ion Batteries. Materials 2022, 15, 44. https://doi.org/10.3390/ma15010044
Pinna EG, Toro N, Gallegos S, Rodriguez MH. A Novel Recycling Route for Spent Li-Ion Batteries. Materials. 2022; 15(1):44. https://doi.org/10.3390/ma15010044
Chicago/Turabian StylePinna, Eliana G., Norman Toro, Sandra Gallegos, and Mario H. Rodriguez. 2022. "A Novel Recycling Route for Spent Li-Ion Batteries" Materials 15, no. 1: 44. https://doi.org/10.3390/ma15010044
APA StylePinna, E. G., Toro, N., Gallegos, S., & Rodriguez, M. H. (2022). A Novel Recycling Route for Spent Li-Ion Batteries. Materials, 15(1), 44. https://doi.org/10.3390/ma15010044







