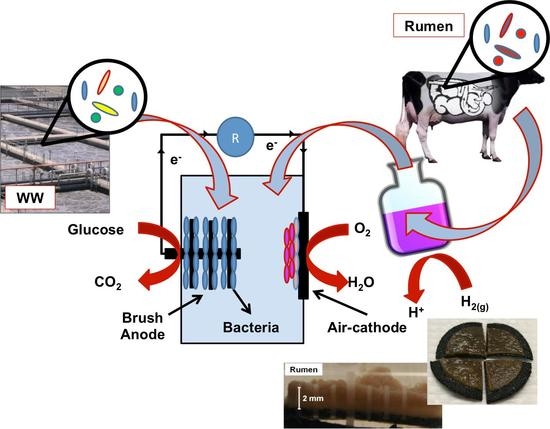
Rumen Inoculum Enhances Cathode Performance in Single-Chamber Air-Cathode Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. MFC Construction and Operation
2.2. Analyses
2.3. Bacterial Community Analysis
3. Results and Discussion
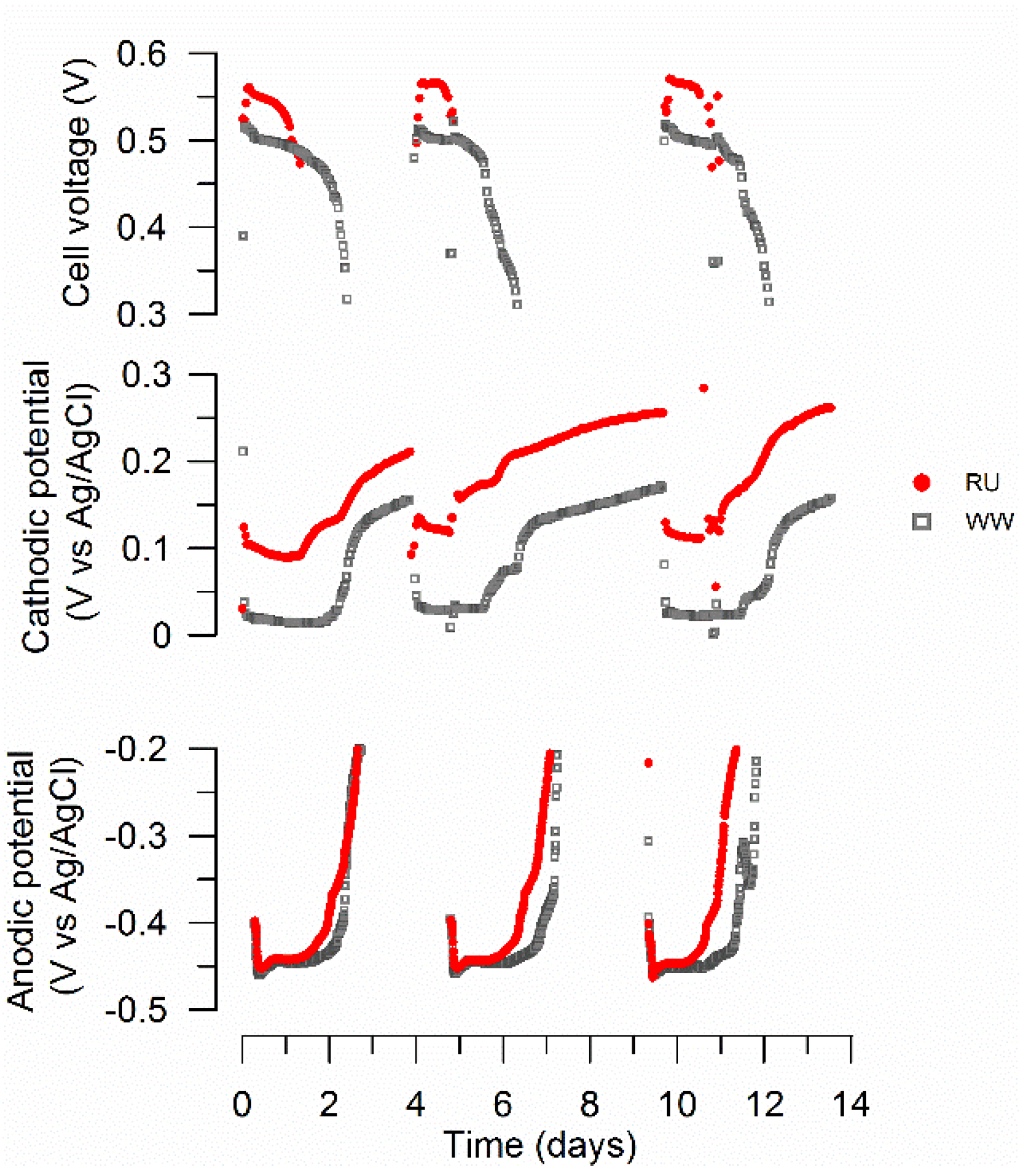
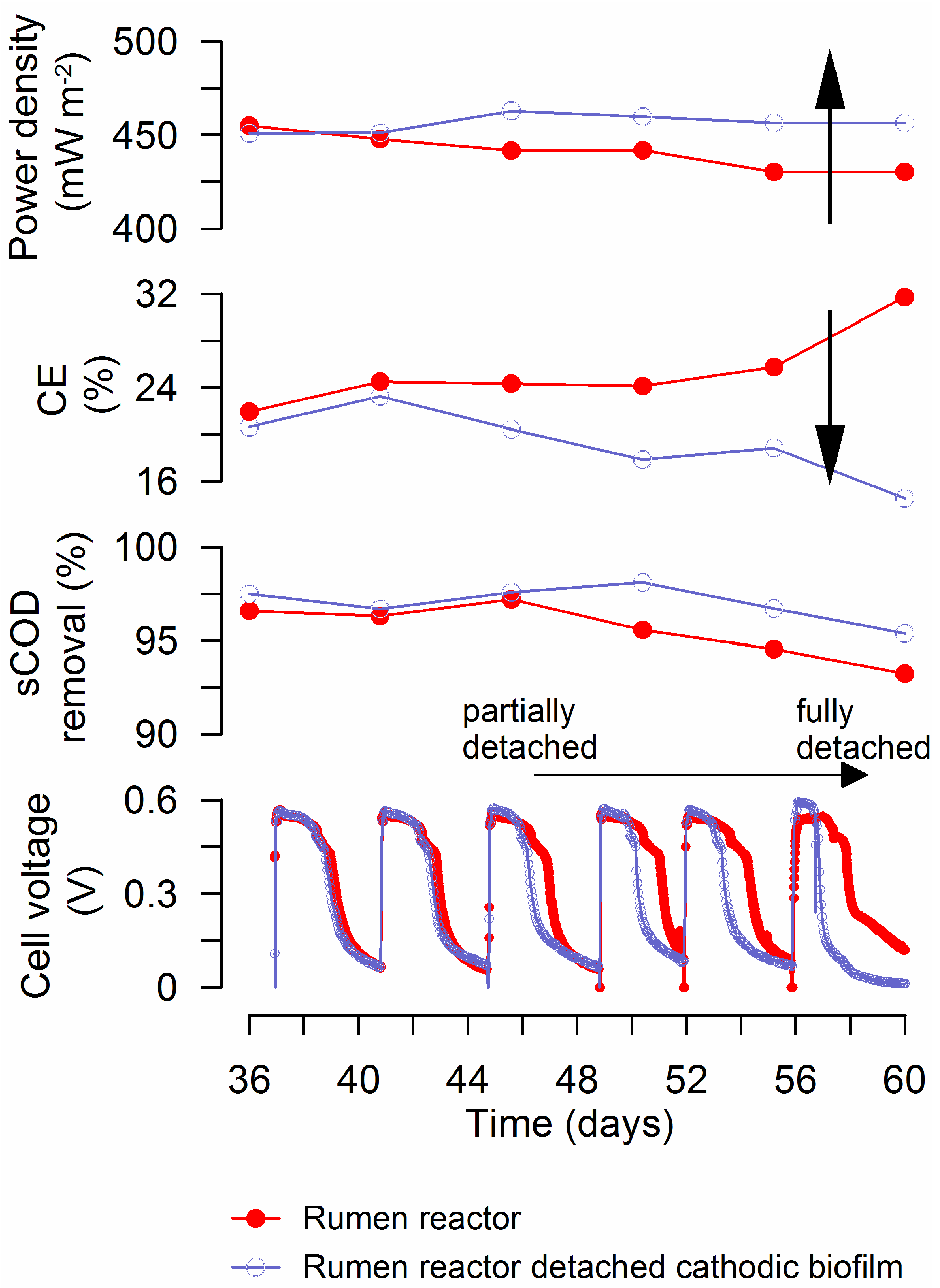
3.1. MFC Performance
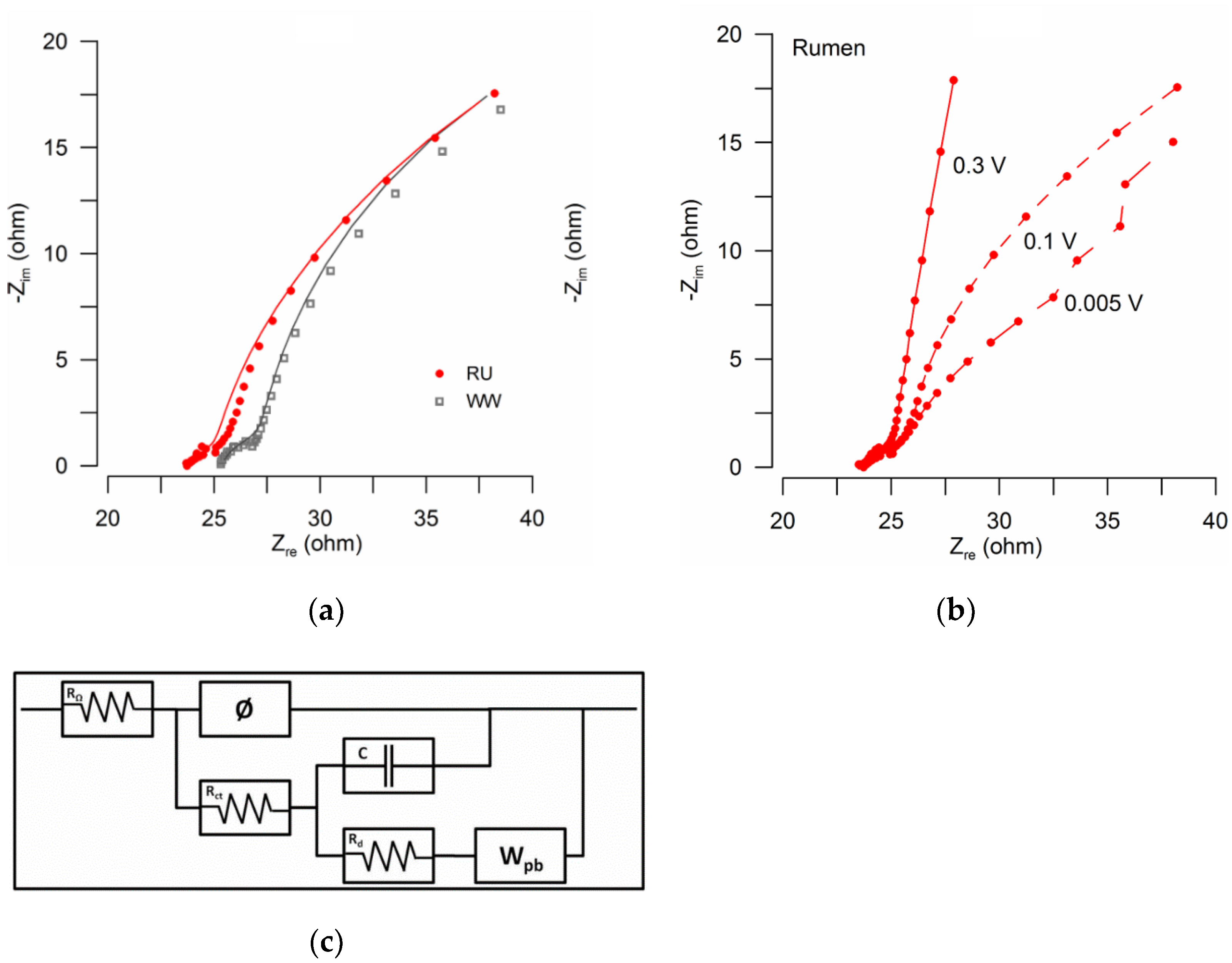
3.2. Analysis of Electrochemical Impedances
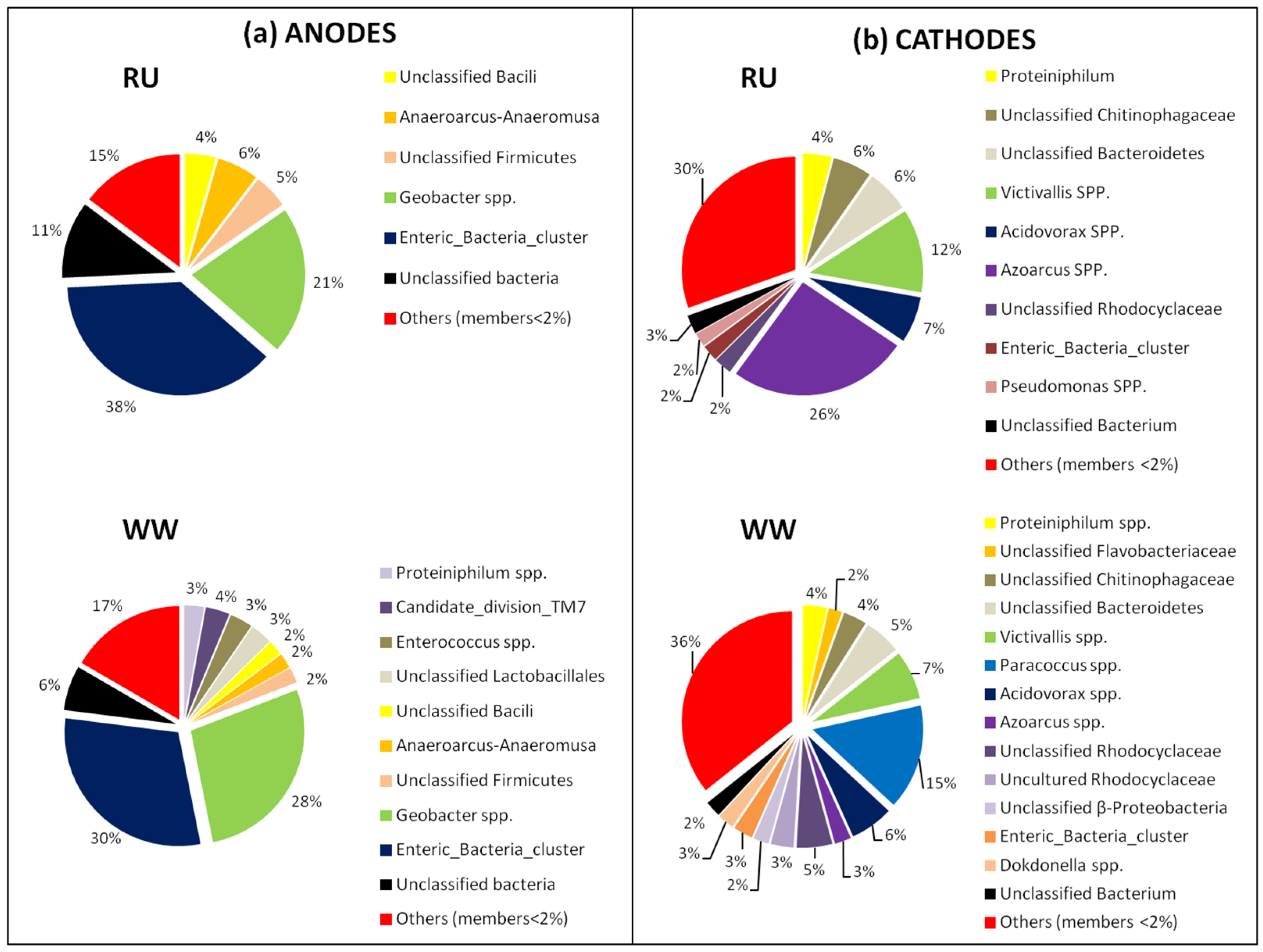
3.3. Bacterial Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2020, 754, 142429. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.S.; Akhter, F.; Mazari, S.A.; Sabzoi, N.; Aziz, S.; Soomro, S.A.; Mubarak, N.M.; Baloch, H.; Memon, A.Q.; Ahmed, S. Advanced microbial fuel cell for waste water treatment—A review. Environ. Sci. Pollut. Res. 2020, 28, 5005–5019. [Google Scholar] [CrossRef]
- Flimban, S.G.; Ismail, I.M.; Kim, T.; Oh, S.E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: Design, major elements, and scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef] [Green Version]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2018, 101, 60–81. [Google Scholar] [CrossRef]
- Dopson, M.; Ni, G.; Sleutels, T.H. Possibilities for extremophilic microorganisms in microbial electrochemical systems. FEMS Microbiol. Rev. 2015, 40, 164–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Hu, Y.; Zhang, L.; Huang, W.; Sun, J. Bacterial community shift and improved performance induced by in situ preparing dual graphene modified bioelectrode in microbial fuel cell. Bioresour. Technol. 2017, 238, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Ren, Y.; Li, X.; Wang, X. Effect of short-term alkaline intervention on the performance of buffer-free single-chamber microbial fuel cell. Bioelectrochemistry 2017, 115, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Phan, T.; Wanger, G.; Nealson, K.H.; Sekiguchi, Y.; Gorby, Y.A.; Bretschger, O. Microbial population and functional dynamics associated with surface potential and carbon metabolism. ISME J. 2013, 8, 963–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miceli, J.F.; Parameswaran, P.; Kang, D.-W.; Krajmalnik-Brown, R.; Torres, C.I. Enrichment and Analysis of Anode-Respiring Bacteria from Diverse Anaerobic Inocula. Environ. Sci. Technol. 2012, 46, 10349–10355. [Google Scholar] [CrossRef] [PubMed]
- Kouzuma, A.; Kasai, T.; Nakagawa, G.; Yamamuro, A.; Abe, T.; Watanabe, K. Comparative Metagenomics of Anode-Associated Microbiomes Developed in Rice Paddy-Field Microbial Fuel Cells. PLoS ONE 2013, 8, e77443. [Google Scholar] [CrossRef]
- Dunaj, S.; Vallino, J.; Hines, M.E.; Gay, M.; Kobyljanec, C.; Rooney-Varga, J.N. Relationships between Soil Organic Matter, Nutrients, Bacterial Community Structure, And the Performance of Microbial Fuel Cells. Environ. Sci. Technol. 2012, 46, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Chin-Tsan, W.; Che-Ming, Y.; Zih-Sheng, C.; Yao-Cheng, L. Performance of straw-fed microbial fuel cells with mixed rumen microorganisms by using different catholytes. Biomass-Bioenergy 2013, 59, 412–417. [Google Scholar] [CrossRef]
- Chung, R.; Kang, E.Y.; Shin, Y.J.; Park, J.J.; Park, P.S.; Han, C.H.; Kim, B.; Moon, S.I.; Park, J.; Chung, P.S. Development of a Consolidated Anaerobic Digester and Microbial Fuel Cell to Produce Biomethane and Electricity from Cellulosic Biomass Using Bovine Rumen Microorganisms. J. Sustain. Bioenergy Syst. 2019, 9, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Rismani-Yazdi, H.; Christy, A.D.; Dehority, B.A.; Morrison, M.; Yu, Z.; Tuovinen, O.H. Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol. Bioeng. 2007, 97, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hong, Q.; Shu, M.; Chen, Y.; Dong, L.; Ming, Y. Rumen bacteria convert cellulose into electricity in two-chamber microbial fuel cell. J. Chem. Pharm. Res. 2014, 6, 727–732. [Google Scholar]
- Zang, G.-L.; Sheng, G.-P.; Tong, Z.-H.; Liu, X.-W.; Teng, S.-X.; Li, W.-W.; Yu, H.-Q. Direct Electricity Recovery from Canna indica by an Air-Cathode Microbial Fuel Cell Inoculated with Rumen Microorganisms. Environ. Sci. Technol. 2010, 44, 2715–2720. [Google Scholar] [CrossRef]
- Rousseau, R.; Dominguez-Benetton, X.; Délia, M.-L.; Bergel, A. Microbial bioanodes with high salinity tolerance for microbial fuel cells and microbial electrolysis cells. Electrochem. Commun. 2013, 33, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Pierra, M.; Carmona-Martínez, A.A.; Trably, E.; Godon, J.-J.; Bernet, N. Specific and efficient electrochemical selection of Geoalkalibacter subterraneus and Desulfuromonas acetoxidans in high current-producing biofilms. Bioelectrochemistry 2015, 106, 221–225. [Google Scholar] [CrossRef]
- Salvin, P.; Roos, C.; Robert, F. Tropical mangrove sediments as a natural inoculum for efficient electroactive biofilms. Bioresour. Technol. 2012, 120, 45–51. [Google Scholar] [CrossRef]
- Leiva-Aravena, E.; Leiva, E.; Zamorano, V.; Rojas, C.; Regan, J.M.; Vargas, I.T. Organotrophic acid-tolerant microorganisms enriched from an acid mine drainage affected environment as inoculum for microbial fuel cells. Sci. Total Environ. 2019, 678, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W.; Hai, F. Microbial fuel cell is emerging as a versatile technology: A review on its possible applications, challenges and strategies to improve the performances. Int. J. Energy Res. 2017, 42, 369–394. [Google Scholar] [CrossRef]
- Qu, Y.; Feng, Y.; Wang, X.; Logan, B.E. Use of a Coculture to Enable Current Production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 2012, 78, 3484–3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Lawati, M.J.; Jafary, T.; Baawain, M.S.; Al-Mamun, A. A mini review on biofouling on air cathode of single chamber microbial fuel cell; prevention and mitigation strategies. Biocatal. Agric. Biotechnol. 2019, 22, 101370. [Google Scholar] [CrossRef]
- Kiely, P.D.; Rader, G.; Regan, J.M.; Logan, B.E. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Bioresour. Technol. 2011, 102, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Rossi, R.; Tian, Y.; Kim, K.-Y.; Logan, B.E. Mitigating external and internal cathode fouling using a polymer bonded separator in microbial fuel cells. Bioresour. Technol. 2017, 249, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, T.G. Microbiology of the rumen. In Rumenology; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Gregoire, K.; Becker, J. Design and characterization of a microbial fuel cell for the conversion of a lignocellulosic crop residue to electricity. Bioresour. Technol. 2012, 119, 208–215. [Google Scholar] [CrossRef]
- Tsan, W.C.; Ming, Y.C.; Sheng, C.Z.; Shuai, T. Effect of biometric flow channel on the power generation at different Reynolds numbers in the single chamber of rumen microbial fuel cells (RMFCs). Int. J. Hydrog. Energy 2011, 36, 9242–9251. [Google Scholar] [CrossRef]
- Wang, C.T.; Yang, C.M.; Chen, Z.S. Method of Correlation Among Fermentation Acids and Reducing Power from Substrate Degradation Applied to Investigate the Electrochemistry Process of Rumen Microbial Fuel Cells (RMFCs). J. Chin. Soc. Mech. Eng. 2012, 33, 249–255. [Google Scholar]
- Wang, C.-T.; Yang, C.-M.J.; Chen, Z.-S. Rumen microbial volatile fatty acids in relation to oxidation reduction potential and electricity generation from straw in microbial fuel cells. Biomass Bioenergy 2012, 37, 318–329. [Google Scholar] [CrossRef]
- Chang, S.; Shin, M.J.; Kim, Y.; Jang, S.J.; Moon, S.I.; Jeong, S.; Shim, R.; Shen, F.; Kang, S.; Chung, P.S. Influence of Red Pepper (Capsicum annuum) Addition on Bioenergy Production in Microbial Fuel Cells. J. Sustain. Bioenergy Syst. 2019, 9, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kiely, P.D.; Logan, B.E. A monetary comparison of energy recovered from microbial fuel cells and microbial electrolysis cells fed winery or domestic wastewaters. Int. J. Hydrog. Energy 2010, 35, 8855–8861. [Google Scholar] [CrossRef]
- Ueki, T.; Lovley, D.R. Genome-wide gene regulation of biosynthesis and energy generation by a novel transcriptional repressor in Geobacter species. Nucleic Acids Res. 2009, 38, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Kiely, P.D.; Call, D.F.; Yates, M.D.; Regan, J.M.; Logan, B.E. Anodic biofilms in microbial fuel cells harbor low numbers of higher-power-producing bacteria than abundant genera. Appl. Microbiol. Biotechnol. 2010, 88, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Jung, S.; Regan, J.M.; Logan, B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 2007, 98, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.D.; Cusick, R.; Call, D.; Selembo, P.A.; Regan, J.M.; Logan, B.E. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresour. Technol. 2011, 102, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Vargas, I.T.; Albert, I.U.; Regan, J.M. Spatial distribution of bacterial communities on volumetric and planar anodes in single-chamber air-cathode microbial fuel cells. Biotechnol. Bioeng. 2013, 110, 3059–3062. [Google Scholar] [CrossRef] [PubMed]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, K.; Rossi, R.; Regan, J.M.; Logan, B.E. Impact of cathodic electron acceptor on microbial fuel cell internal resistance. Bioresour. Technol. 2020, 316, 123919. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Fujiki, I.; Okabe, S. Effect of formation of biofilms and chemical scale on the cathode electrode on the performance of a continuous two-chamber microbial fuel cell. Bioresour. Technol. 2011, 102, 355–360. [Google Scholar] [CrossRef]
- Parameswaran, P.; Torres, C.I.; Lee, H.-S.; Krajmalnik-Brown, R.; Rittmann, B.E. Syntrophic interactions among anode respiring bacteria (ARB) and Non-ARB in a biofilm anode: Electron balances. Biotechnol. Bioeng. 2009, 103, 513–523. [Google Scholar] [CrossRef] [PubMed]
- You, S.-J.; Ren, N.-Q.; Zhao, Q.-L.; Wang, J.-Y.; Yang, F.-L. Power Generation and Electrochemical Analysis of Biocathode Microbial Fuel Cell Using Graphite Fibre Brush as Cathode Material. Fuel Cells 2009, 9, 588–596. [Google Scholar] [CrossRef]
- Kim, J.R.; Premier, G.C.; Hawkes, F.R.; Dinsdale, R.M.; Guwy, A.J. Development of a tubular microbial fuel cell (MFC) employing a membrane electrode assembly cathode. J. Power Sources 2009, 187, 393–399. [Google Scholar] [CrossRef]
- Jung, S.; Mench, M.M.; Regan, J.M. Impedance Characteristics and Polarization Behavior of a Microbial Fuel Cell in Response to Short-Term Changes in Medium pH. Environ. Sci. Technol. 2011, 45, 9069–9074. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J. An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements; University Science Books: Sausalito, CA, USA, 1997; p. 268. [Google Scholar]
- Springer, T.E.; Zawodzinski, T.A.; Wilson, M.S.; Gottesfeld, S. Characterization of Polymer Electrolyte Fuel Cells Using AC Impedance Spectroscopy. J. Electrochem. Soc. 1996, 143, 587–599. [Google Scholar] [CrossRef]
- Jothinathan, D.; Wilson, R.T. Comparative analysis of power production of pure, coculture, and mixed culture in a microbial fuel cell. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 520–527. [Google Scholar] [CrossRef]
- Xie, D.; Yu, H.; Li, C.; Ren, Y.; Wei, C.; Feng, C. Competitive microbial reduction of perchlorate and nitrate with a cathode directly serving as the electron donor. Electrochim. Acta 2014, 133, 217–223. [Google Scholar] [CrossRef]
- Montpart, N.; Rago, L.; Baeza, J.A.; Guisasola, A. Oxygen barrier and catalytic effect of the cathodic biofilm in single chamber microbial fuel cells. J. Chem. Technol. Biotechnol. 2018, 93, 2199–2207. [Google Scholar] [CrossRef]
- Rago, L.; Cristiani, P.; Villa, F.; Zecchin, S.; Colombo, A.; Cavalca, L.; Schievano, A. Influences of dissolved oxygen concentration on biocathodic microbial communities in microbial fuel cells. Bioelectrochemistry 2017, 116, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Dowd, S.E.; Edrington, T.S.; Anderson, R.C.; Krueger, N.; Bauer, N.; Kononoff, P.J.; Nisbet, D.J. Evaluation of bacterial diversity in the rumen and feces of cattle fed different levels of dried distillers grains plus solubles using bacterial tag-encoded FLX amplicon pyrosequencing. J. Anim. Sci. 2010, 88, 3977–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoetendal, E.G.; Plugge, C.M.; Akkermans, A.D.L.; de Vos, W.M. Victivallis vadensis gen. nov., sp. nov., a sugar-fermenting anaerobe from human faeces. Int. J. Syst. Evol. Microbiol. 2003, 53, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-S.; Guo, Y.; Yang, P.; Li, C.-M.; Gao, H.; Feng, L.; Zhang, Y. Performance evaluation and bacteria analysis of AFB-MFC enriched with high-strength synthetic wastewater. Water Sci. Technol. 2013, 69, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Lee, Y.-J. Optimization of culture conditions and electricity generation using Geobacter sulfurreducens in a dual-chambered microbial fuel-cell. Int. J. Hydrog. Energy 2010, 35, 13028–13034. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Qu, Y.; Feng, Y. Enhanced Shewanella oneidensis MR-1 anode performance by adding fumarate in microbial fuel cell. Chem. Eng. J. 2017, 328, 697–702. [Google Scholar] [CrossRef]
- Cournet, A.; Délia, M.-L.; Bergel, A.; Roques, C.; Bergé, M. Electrochemical reduction of oxygen catalyzed by a wide range of bacteria including Gram-positive. Electrochem. Commun. 2010, 12, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Zhang, L.; Li, D.; Zhan, G.; Qian, J.; Tao, Y. Dissimilatory nitrate reduction by Pseudomonas alcaliphila with an electrode as the sole electron donor. Biotechnol. Bioeng. 2012, 109, 2904–2910. [Google Scholar] [CrossRef] [PubMed]



| Reactor | sCOD Removal (%) | CH4 * (%) | CO2 * (%) | CE (%) |
|---|---|---|---|---|
| Rumen plus WW (RU) | 96.1 (± 0.5) | 14.9 (± 1.3) | 7.1 (± 1.4) | 22.8 (± 0.9) |
| Wastewater (WW) | 91.7 (± 4.1) | 14.4 (± 2.1) | 5.9 (± 1.4) | 23.0 (± 0.4) |
| Rumen plus WW + fumarate (RF) | 95.6 (± 0.2) | 12.3 (± 1.1) | 5.8 (± 0.6) | 22.3 (± 1.1) |
| Parameter | Rumen | WW |
|---|---|---|
| Rohm (Ω) | 23.9 | 25.3 |
| Rct (Ω) | 3.6 | 4.1 |
| Rd (Ω) | 69.2 | 79.5 |
| Goodness (χ2) | 1.77 × 10−4 | 2.00 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, I.T.; Tapia, N.; Regan, J.M. Rumen Inoculum Enhances Cathode Performance in Single-Chamber Air-Cathode Microbial Fuel Cells. Materials 2022, 15, 379. https://doi.org/10.3390/ma15010379
Vargas IT, Tapia N, Regan JM. Rumen Inoculum Enhances Cathode Performance in Single-Chamber Air-Cathode Microbial Fuel Cells. Materials. 2022; 15(1):379. https://doi.org/10.3390/ma15010379
Chicago/Turabian StyleVargas, Ignacio T., Natalia Tapia, and John M. Regan. 2022. "Rumen Inoculum Enhances Cathode Performance in Single-Chamber Air-Cathode Microbial Fuel Cells" Materials 15, no. 1: 379. https://doi.org/10.3390/ma15010379
APA StyleVargas, I. T., Tapia, N., & Regan, J. M. (2022). Rumen Inoculum Enhances Cathode Performance in Single-Chamber Air-Cathode Microbial Fuel Cells. Materials, 15(1), 379. https://doi.org/10.3390/ma15010379