Evaluation of Radiation Resistance of Polystyrene Using Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Average Molecular Weight Sample Preparation and γ Radiation Experiments
2.3. Determination of Average Molecular Weight
3. Theoretical Study of Scissionning Reactions
3.1. Modeling
3.1.1. Polystyrene
3.1.2. Fluorinated Polystyrene
3.2. Reactive Molecular Dynamics Simulation
4. Results
4.1. Simulation Results
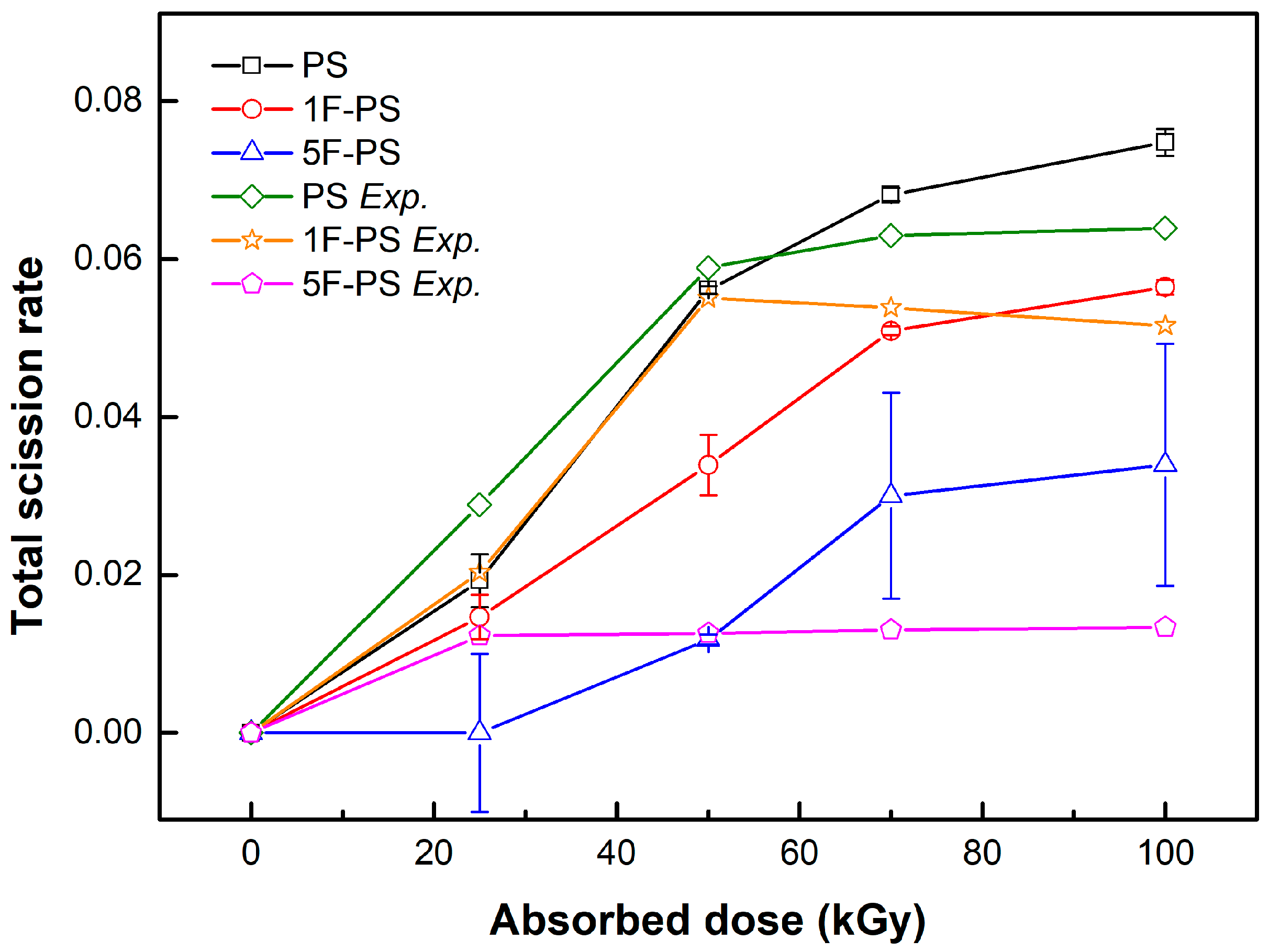
4.2. Comparison with Experiment Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, D.; Austerlitz, C.; Benhabib, S.; Mota, H.; Allison, R.R.; Campos, D. Study of a spherical phantom for Gamma knife dosimetry. J. Appl. Clin. Med. Phys. 2010, 11, 222–229. [Google Scholar] [CrossRef]
- Gould, R.F. Irradiation of Polymers, Copyright, Advances in Chemistry Series, FOREWORD; American Chemical Society: Washington, DC, USA, 1967; pp. i–iv. [Google Scholar]
- Onyiriuka, E.C.; Hersh, L.S.; Hertl, W. Surface modification of polystyrene by gamma-radiation. Appl. Spectrosc. 1990, 44, 808–811. [Google Scholar] [CrossRef]
- Onyiriuka, E.C. The effects of high-energy radiation on the surface chemistry of polystyrene: A mechanistic study. J. Appl. Polym. Sci. 1993, 47, 2187–2194. [Google Scholar] [CrossRef]
- Florin, R.E.; Wall, L.A. Gamma irradiation of fluorocarbon polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1961, 65, 375. [Google Scholar] [CrossRef]
- Carswell-Pomerantz, T.; Babanalbandi, A.; Dong, L.; Hill, D.J.; Perera, M.C.; Pomery, P.J.; Saadat, G.; Whittaker, A.K. Stability and Stabilization of Polymers under Irradiation; IAEA: Vienna, Austria, 1999; p. 111. [Google Scholar]
- Von White, G., II; Tandon, R.; Serna, L.M.; Celina, M.C.; Bernstein, R. An Overview of Basic Radiation Effects on Polymers and Glasses. 2013. Available online: https://www.osti.gov/servlets/purl/1671997 (accessed on 21 November 2021).
- Fuse, N.; Homma, H.; Okamoto, T. Remaining issues of the degradation models of polymeric insulation used in nuclear power plant safety cables. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 571–581. [Google Scholar] [CrossRef]
- Gillen, K.T.; Clough, R.L. Predictive aging results in radiation environments. Radiat. Phys. Chem. 1993, 41, 803–815. [Google Scholar] [CrossRef]
- Manas, D.; Ovsik, M.; Mizera, A.; Manas, M.; Hylova, L.; Bednarik, M.; Stanek, M. The effect of irradiation on mechanical and thermal properties of selected types of polymers. Polymers 2018, 10, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polvi, J.; Luukkonen, P.; Nordlund, K.; Järvi, T.T.; Kemper, T.W.; Sinnott, S.B. Primary radiation defect production in polyethylene and cellulose. J. Phys. Chem. B 2012, 116, 13932–13938. [Google Scholar] [CrossRef]
- Bowmer, T.N.; Cowen, L.K.; O’donnell, J.H.; Winzor, D.J. Degradation of polystyrene by gamma irradiation: Effect of air on the radiation-induced changes in mechanical and molecular properties. J. Appl. Polym. Sci. 1979, 24, 425–439. [Google Scholar] [CrossRef]
- Shimizu, Y.; Mitsui, H. γ-radiation-induced crosslinking of polystyrene. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 2307–2316. [Google Scholar] [CrossRef]
- Wall, L.A.; Brown, D.W. Gamma irradiation of polymethyl methacrylate and polystyrene. J. Phys. Chem. 1957, 61, 129–136. [Google Scholar] [CrossRef]
- Wackerly, J.W.; Dunne, J.F. Synthesis of polystyrene and molecular weight determination by 1H NMR end-group analysis. J. Chem. Educ. 2017, 94, 1790–1793. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comp. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Brenner, D.W. Empirical potential for hydrocarbons for use in simulating the chemical vapor deposition of diamond films. Phys. Rev. B 1990, 42, 9458. [Google Scholar] [CrossRef]
- Van Duin, A.C.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef] [Green Version]
- Rahnamoun, A.; Van Duin, A.C.T. Reactive molecular dynamics simulation on the disintegration of Kapton, POSS polyimide, amorphous silica, and teflon during atomic oxygen impact using the ReaxFF reactive force-field method. J. Phys. Chem. A 2014, 118, 2780–2787. [Google Scholar] [CrossRef]
- Beardmore, K.; Smith, R. Ion bombardment of polyethylene. Nucl. Instrum. Meth. B 1995, 102, 223–227. [Google Scholar] [CrossRef]
- Nordlund, K.; Ghaly, M.; Averback, R.S.; Caturla, M.; de La Rubia, T.D.; Tarus, J. Defect production in collision cascades in elemental semiconductors and fcc metals. Phys. Rev. B 1998, 57, 7556. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.T. Basic physics of radiation damage production. J. Nucl. Mater. 1994, 216, 1–28. [Google Scholar] [CrossRef]
- Polvi, J.; Nordlund, K. Irradiation effects in high-density polyethylene. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 312, 54–59. [Google Scholar] [CrossRef]
- Oshima, A.; Horiuchi, H.; Nakamura, A.; Kobayashi, S.; Terui, A.; Mino, A.; Shimura, R.; Washio, M. Trapped radical behavior of electron beam irradiated polytetrafluoroethylene fine powder at various temperatures. Sci. Rep. 2021, 11, 10907. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Florin, R.E.; Wall, L.A. Stress relaxation of γ-irradiated fluorocarbon elastomers. J. Polym. Sci. Part A Gen. Pap. 1965, 3, 1685–1712. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Westlake, J.F.; Sharma, S.C. Molecular weight distribution in radiation-induced polymerization. I. γ-radiation-induced free-radical polymerization of liquid styrene. J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 1729–1747. [Google Scholar] [CrossRef]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]


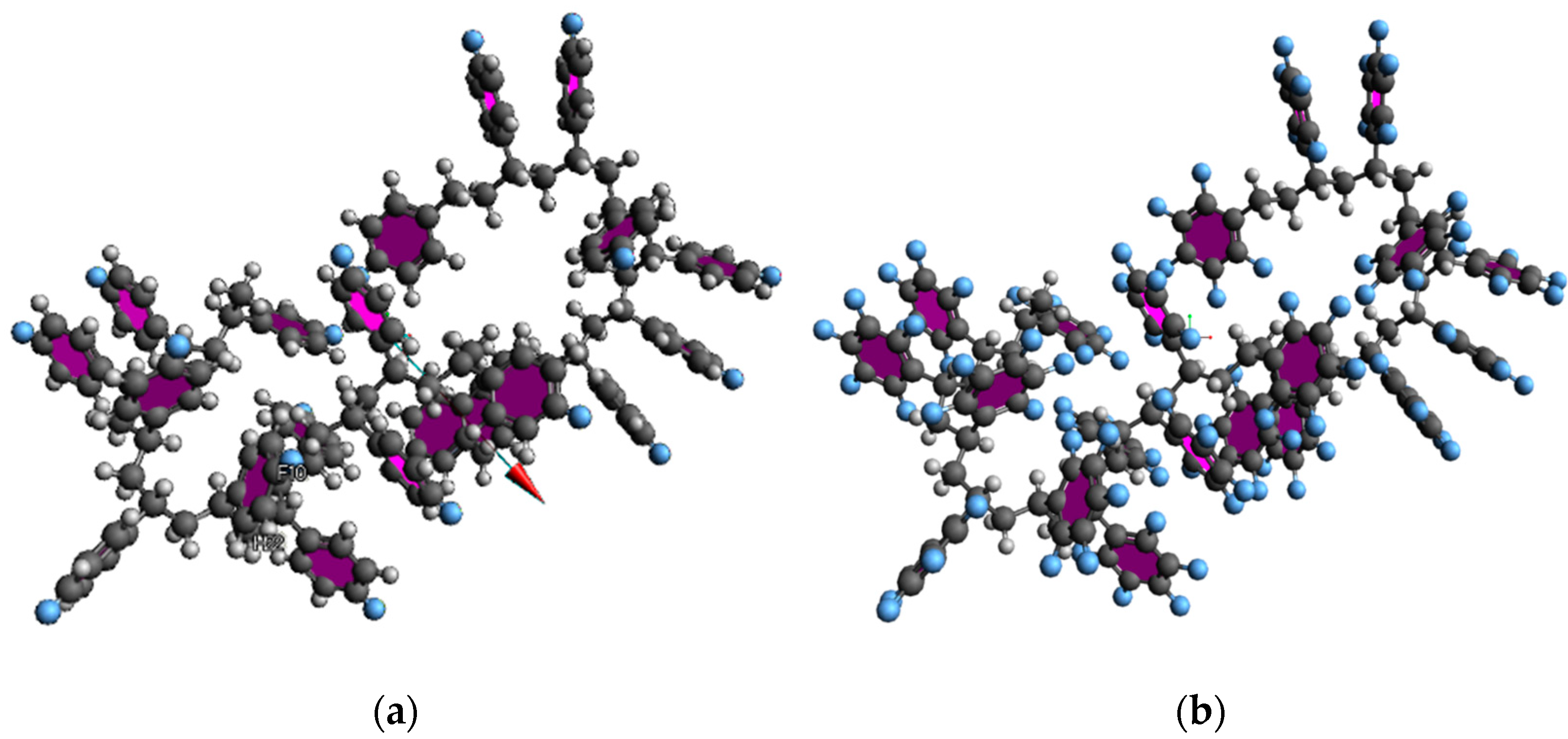


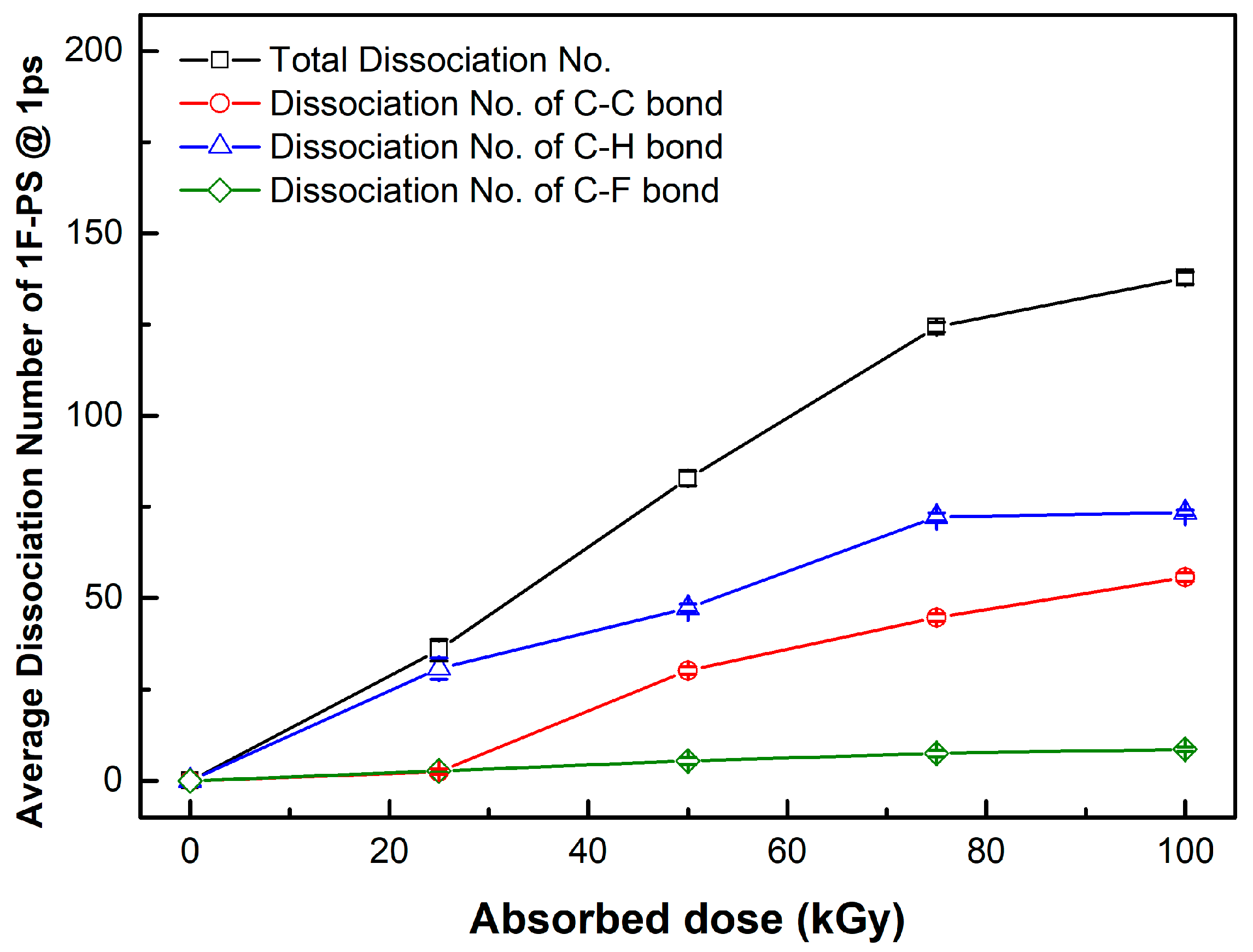
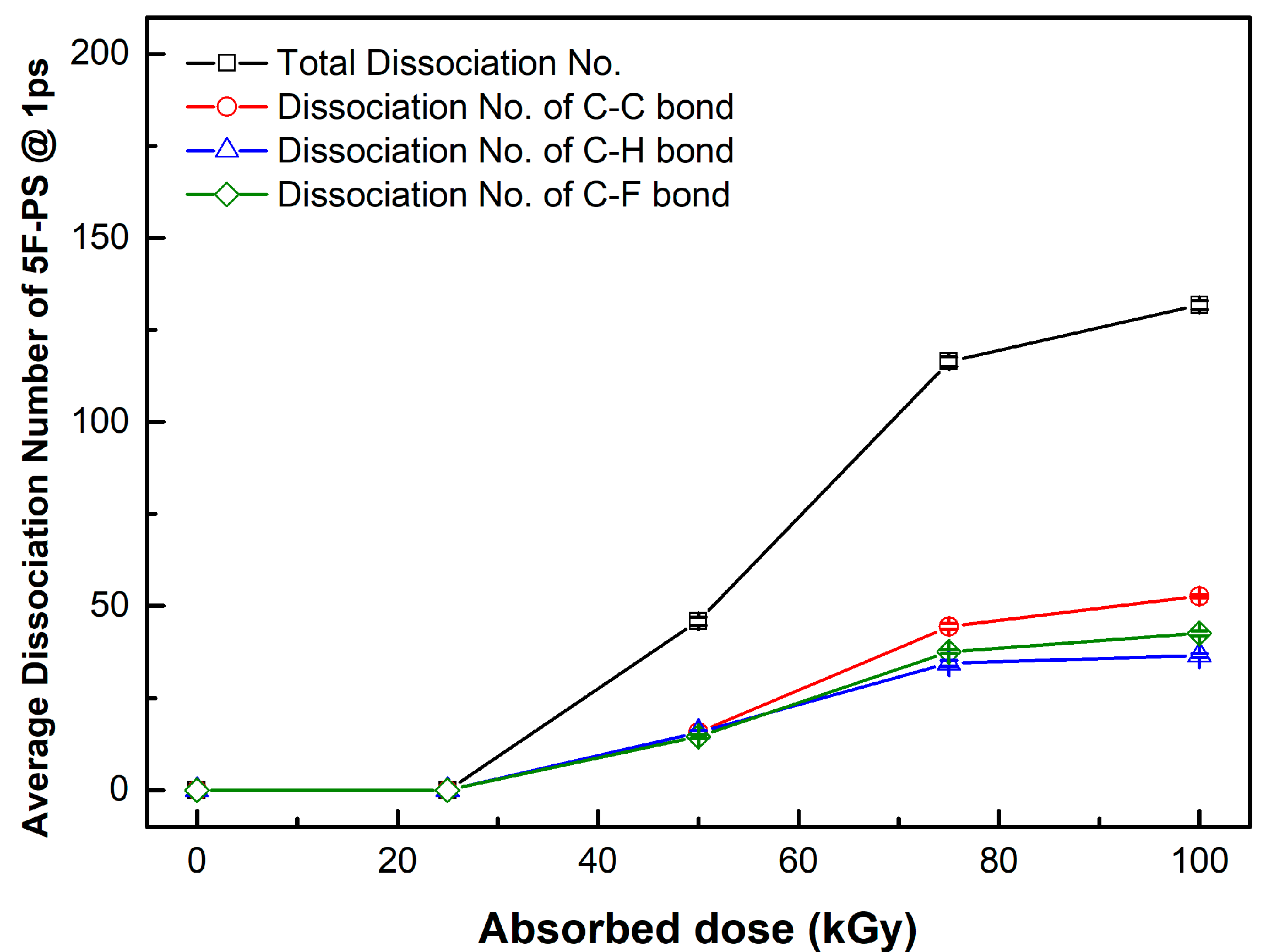
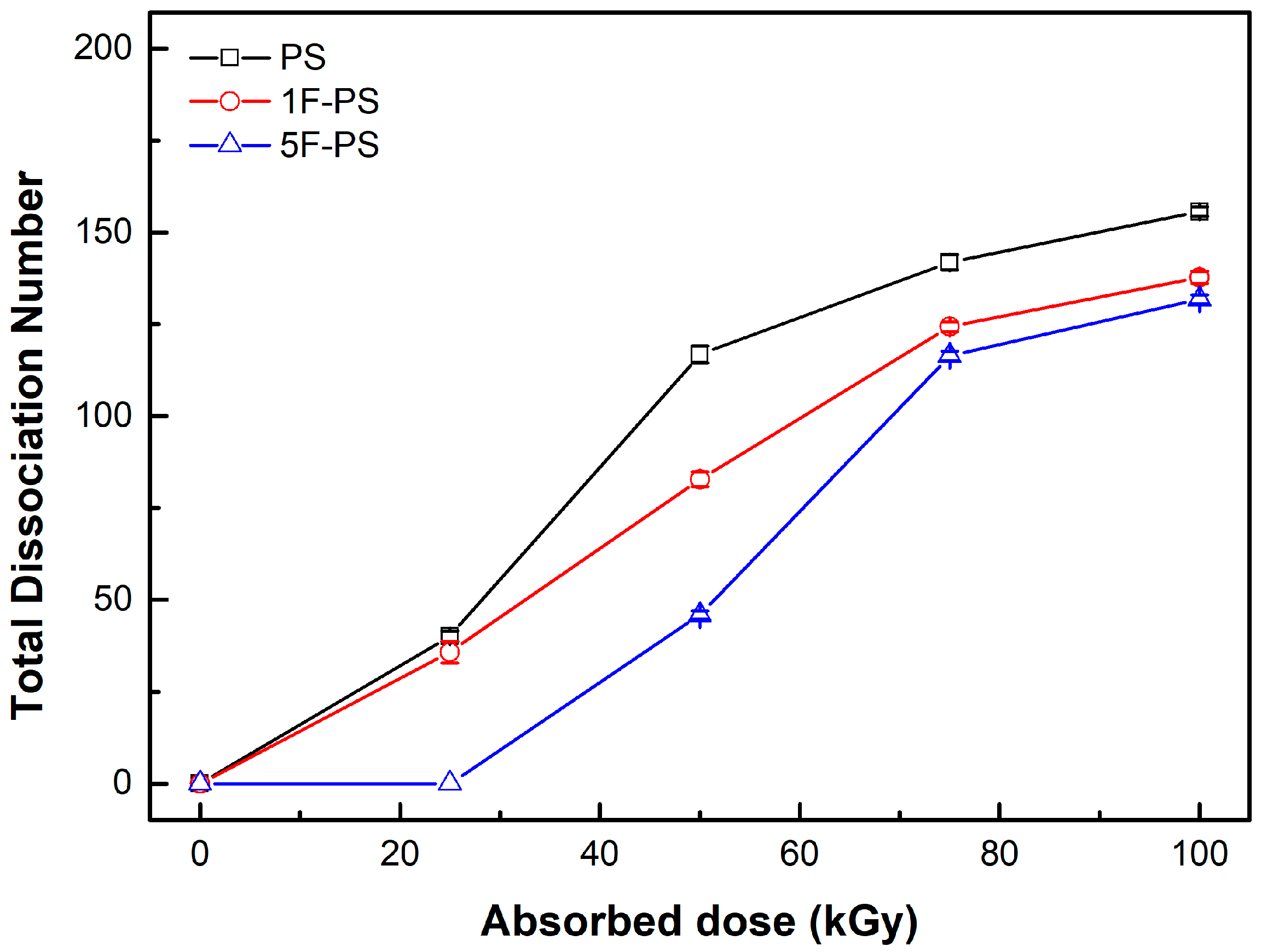

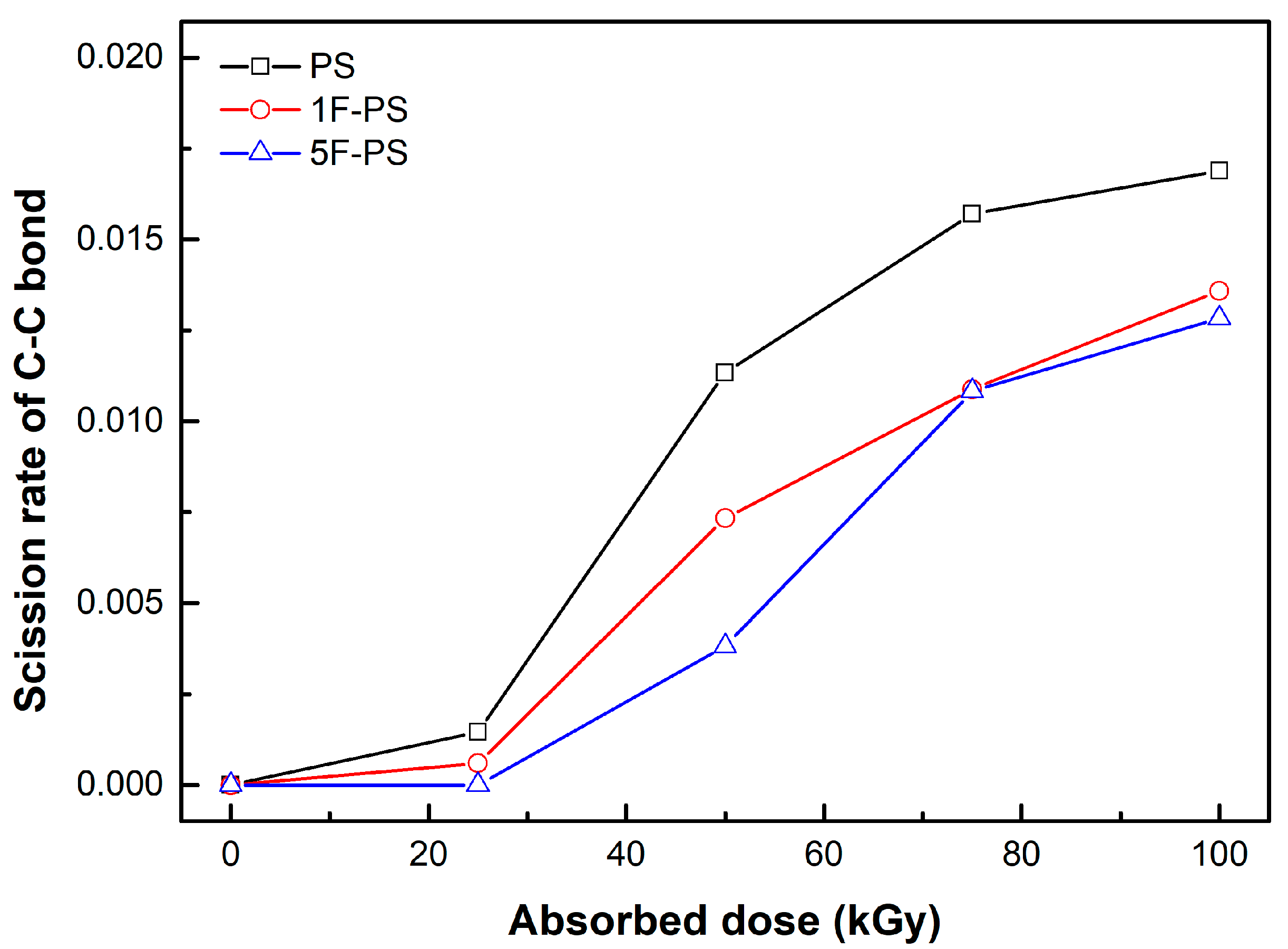
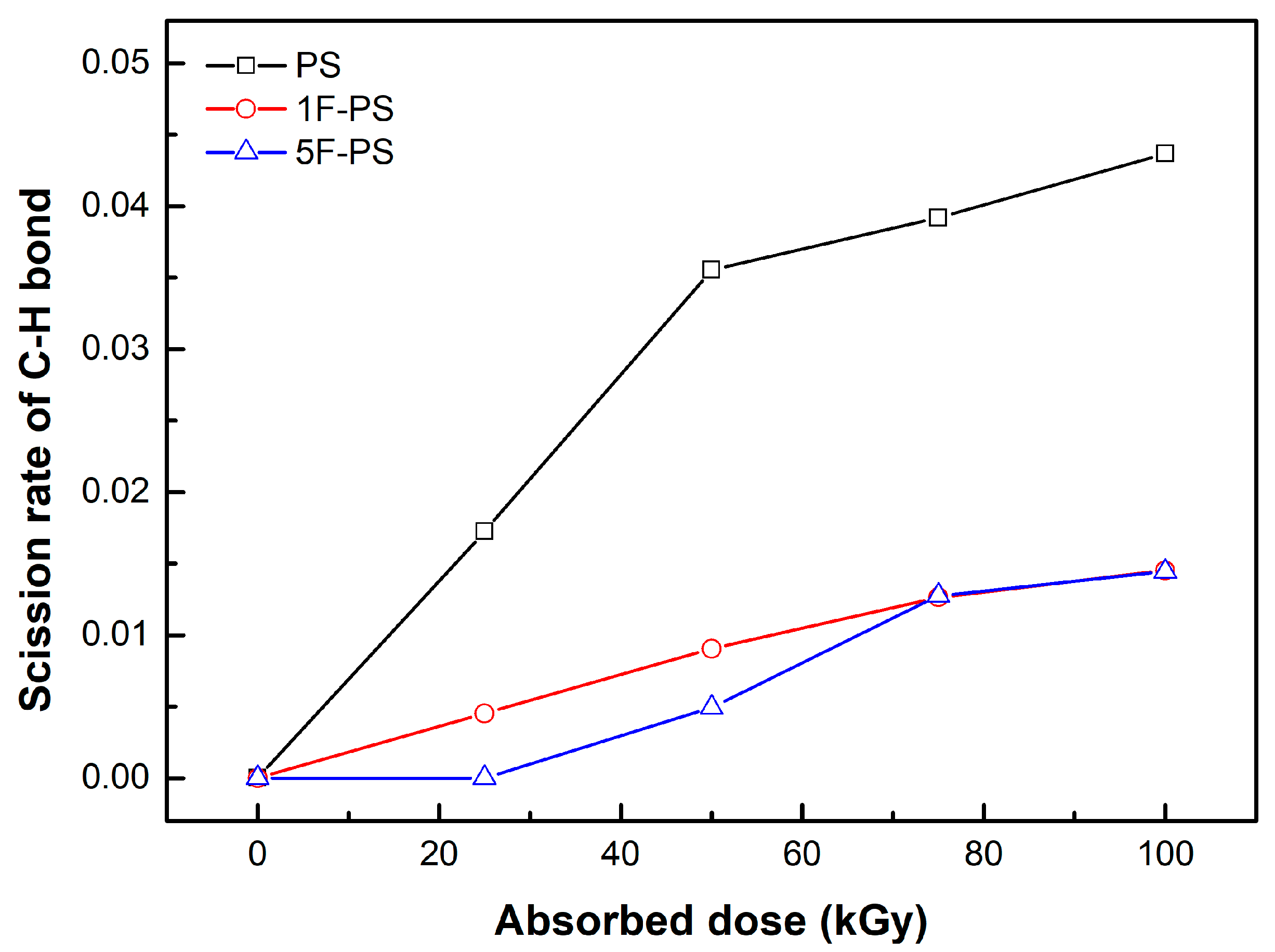

| PKA Radius (Å) | Absorbed Dose (kGy) | No. of Simulations | Maximum Dissociation Number of C-C Bond | Maximum Dissociation Number of C-H Bond | Total Dissociation Number | Dissociation Rate (#/kGy) |
|---|---|---|---|---|---|---|
| 1.6 | 25 | 10 | 7 | 35 | 42 | 1.68 |
| 2.3 | 50 | 10 | 50 | 71 | 117 | 3 |
| 2.8 | 75 | 10 | 67 | 78 | 140 | 0.92 |
| 3.2 | 100 | 10 | 70 | 87 | 157 | 0.68 |
| PKA Radius (Å) | Absorbed Dose (kGy) | No. of Simulations | Maximum Dissociation Number of C-C Bond | Maximum Dissociation Number of C-F Bond | Maximum Dissociation Number of C-H Bond | Total Dissociation Number | Dissociation Rate (#/kGy) |
|---|---|---|---|---|---|---|---|
| 1.6 | 25 | 10 | 3 | 3 | 30 | 36 | 1.44 |
| 2.3 | 50 | 10 | 31 | 6 | 43 | 85 | 1.96 |
| 2.8 | 75 | 10 | 46 | 8 | 73 | 127 | 1.68 |
| 3.2 | 100 | 10 | 57 | 9 | 74 | 140 | 0.52 |
| PKA Radius (Å) | Absorbed Dose (kGy) | No. of Simulations | Maximum Dissociation Number of C-C Bond | Maximum Dissociation Number of C-F Bond | Maximum Dissociation Number of C-H Bond | Total Dissociation Number | Dissociation Rate (#/kGy) |
|---|---|---|---|---|---|---|---|
| 1.6 | 25 | 10 | 0 | 0 | 0 | 0 | 0 |
| 2.3 | 50 | 10 | 16 | 15 | 15 | 47 | 1.88 |
| 2.8 | 75 | 10 | 45 | 38 | 35 | 118 | 2.84 |
| 3.2 | 100 | 10 | 53 | 43 | 37 | 133 | 0.6 |
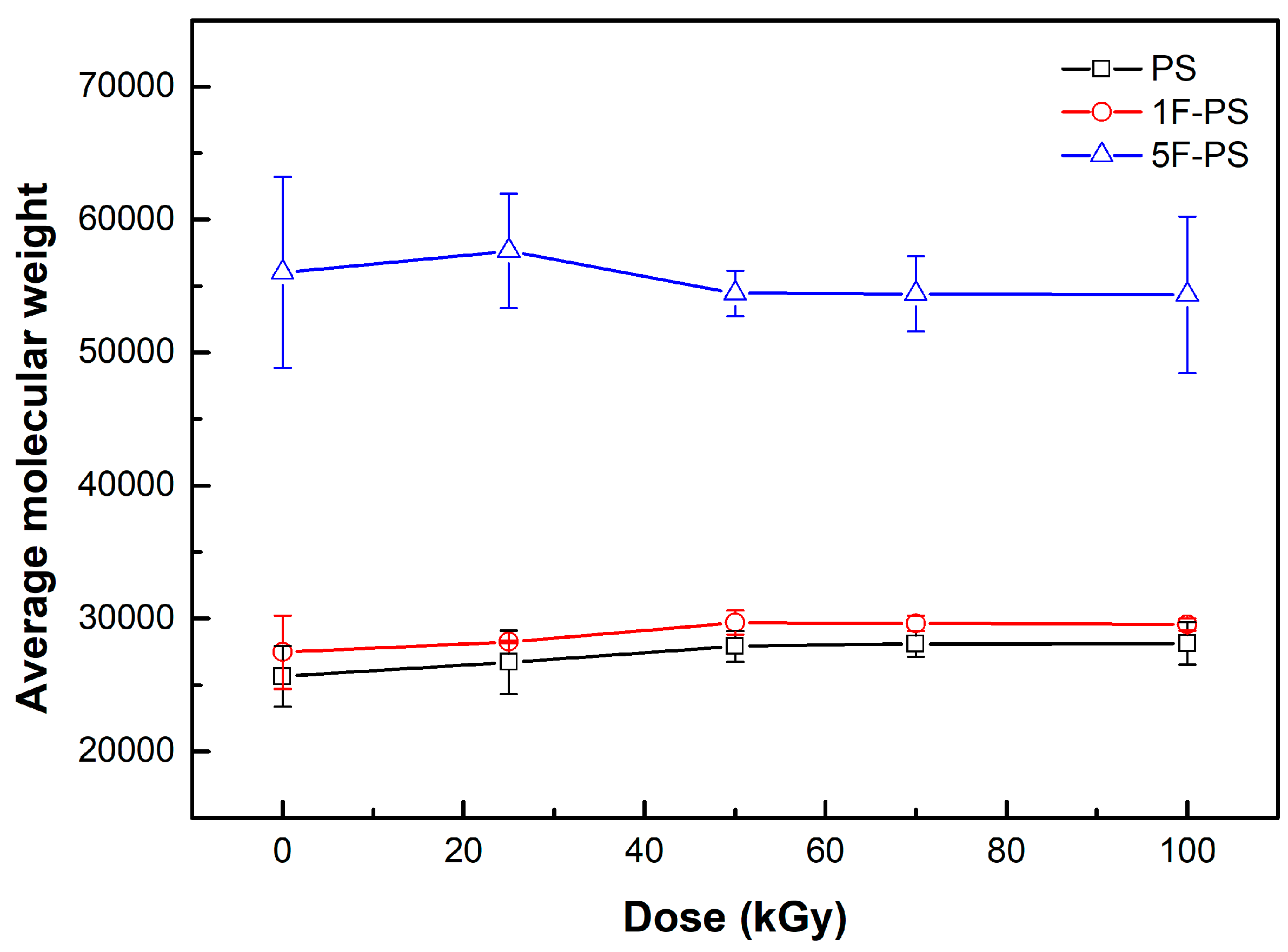
| Absorbed Dose (kGy) | Mn of PS | Mn of 1F-PS | Mn of 5F-PS | The Ratio of Differential Mn (PS) | The Ratio of Differential Mn (1F-PS) | The Ratio of Differential Mn (5F-PS) |
|---|---|---|---|---|---|---|
| 0 | 25,651.0 | 27,468.3 | 56,011.0 | 0.00000 | 0.00000 | 0.00000 |
| 25 | 26,714.3 | 28,249.0 | 57,630.0 | 0.02887 | 0.02032 | 0.01229 |
| 50 | 27,917.6 | 29,691.5 | 54,441.0 | 0.05890 | 0.05505 | 0.01261 |
| 75 | 28,089.0 | 29,639.0 | 54,391.0 | 0.06296 | 0.05384 | 0.01303 |
| 100 | 28,129.0 | 29,540.0 | 54,345.5 | 0.06391 | 0.05156 | 0.01340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeon, Y.-H.; Shim, H.-E.; Park, J.-H.; Lee, N.-H.; Park, J.-Y.; Chae, M.-S.; Mun, J.-H.; Lee, J.-H.; Gwon, H.-J. Evaluation of Radiation Resistance of Polystyrene Using Molecular Dynamics Simulation. Materials 2022, 15, 346. https://doi.org/10.3390/ma15010346
Yeon Y-H, Shim H-E, Park J-H, Lee N-H, Park J-Y, Chae M-S, Mun J-H, Lee J-H, Gwon H-J. Evaluation of Radiation Resistance of Polystyrene Using Molecular Dynamics Simulation. Materials. 2022; 15(1):346. https://doi.org/10.3390/ma15010346
Chicago/Turabian StyleYeon, Yeong-Heum, Ha-Eun Shim, Jin-Hyung Park, Nam-Ho Lee, Jae-Yeon Park, Moon-Sik Chae, Jung-Ho Mun, Jae-Hyun Lee, and Hui-Jeong Gwon. 2022. "Evaluation of Radiation Resistance of Polystyrene Using Molecular Dynamics Simulation" Materials 15, no. 1: 346. https://doi.org/10.3390/ma15010346
APA StyleYeon, Y.-H., Shim, H.-E., Park, J.-H., Lee, N.-H., Park, J.-Y., Chae, M.-S., Mun, J.-H., Lee, J.-H., & Gwon, H.-J. (2022). Evaluation of Radiation Resistance of Polystyrene Using Molecular Dynamics Simulation. Materials, 15(1), 346. https://doi.org/10.3390/ma15010346







