Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc
Abstract
1. Introduction
2. Materials and Methods
3. Results
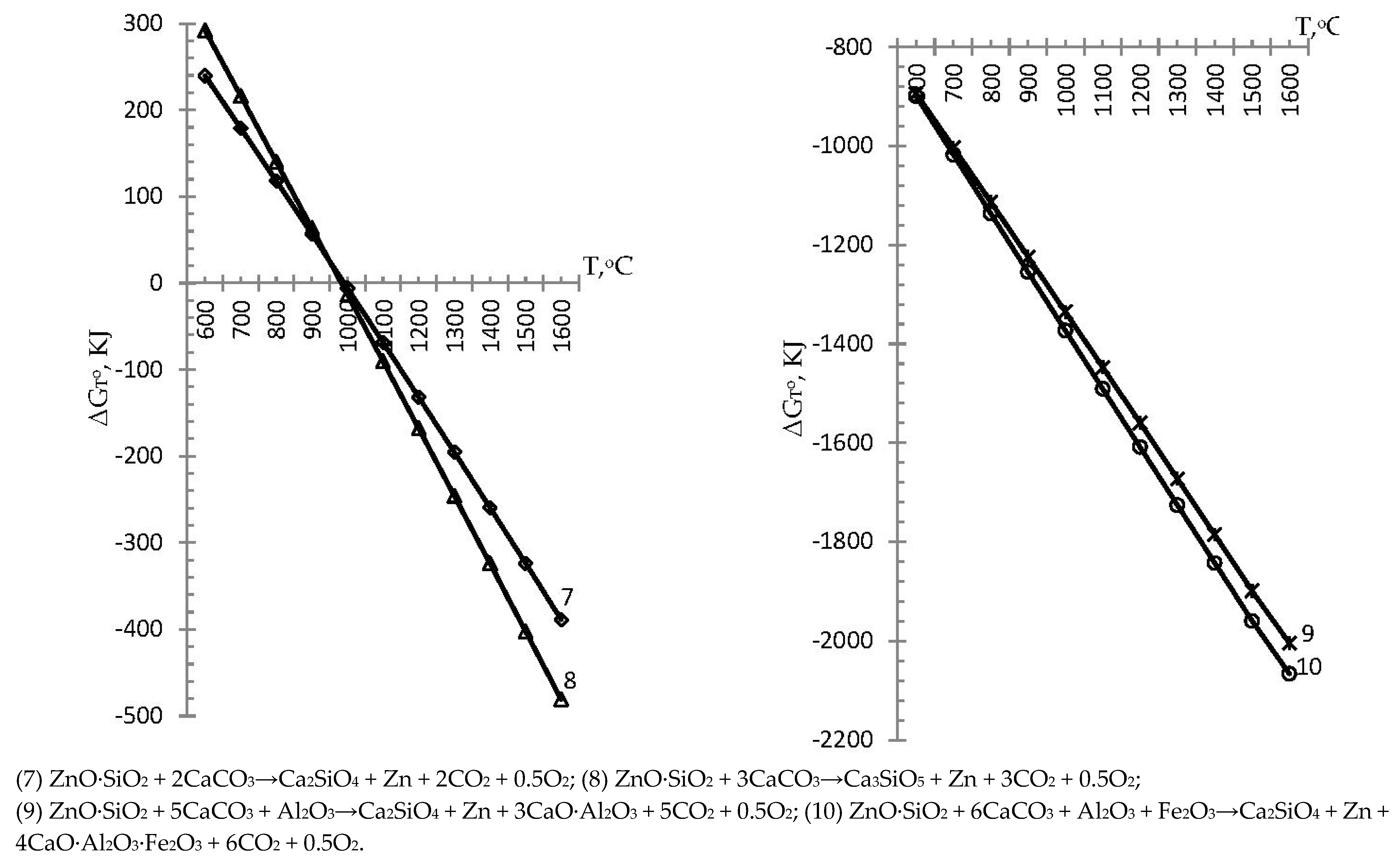
→ Ca2SiO4 + Zn↑ + 3CaO∙Al2O3 + 5CO2↑ + 0.5O2↑
→ Ca2SiO4 + Zn↑ + 4CaO∙Al2O3∙Fe2O3 + 6CO2↑ + 0.5O2↑
4. Discussion
5. Conclusions
- -
- the formation of alite during the course of reaction (8) was found to be possible in the temperature range from 982.9 to 1500 °C with Gibbs energies of −0.05 and −402.1 kJ, respectively, better than the standard process (reaction (4)) at −11.4 kJ;
- -
- the formation of tricalcium aluminate during reaction (9) was found to be thermodynamically possible in the temperature range from 600 °C at ΔGTo = −893.8 kJ to 1500 °C at ΔGTo = −1899.3 kJ, better than the standard process (reaction (5)) at −1570.1 kJ;
- -
- the formation of four calcium alumoferrite (4CaO∙Al2O3∙Fe2O3) during reaction (10) was found to be possible in the temperature range from 600 °C at ΔGTo = −898.9 kJ to 1500 °C at ΔGTo = −1959.3 kJ, better than the standard process (reaction (5)) at −1570.2 kJ;
- -
- the formation of the main minerals of cement clinker in reaction group II at a temperature of 1500 °C, depending on the Gibbs energy, was found to be represented by the following series 4CaO∙Al2O3∙Fe2O3 > 3CaO∙Al2O3 > Ca3SiO5 > Ca2SiO4.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrenko, E.S.; Vechkinzova, E.A.; Urazbekov, A.K. Context analysis and prospects of development of the mining and metallurgical industry of Kazakhstan. Ekonom. Otnosh. 2019, 9, 2661–2676. [Google Scholar] [CrossRef]
- Satbaev, B.N.; Koketaev, A.I.; Aimbetova, E.O.; Shalabaev, N.T.; Satbaev, A.B. Environmental technology for the integrated disposal of man-made wastes of the metallurgical industry: Self-curing, chemically resistant refractory mass. Refract. Ind. Ceram. 2019, 60, 318–322. [Google Scholar] [CrossRef]
- Kazakhstan in a New Reality: Time for Action. President of Kazakhstan Kassym-Jomart Tokayev’s State of the Nation Address. Available online: https://www.akorda.kz/en/president-kassym-jomart-tokayev-delivers-his-state-of-the-nation-address-to-the-people-of-kazakhstan-28624 (accessed on 1 September 2020).
- The Strategic Plan for Development of the Republic of Kazakhstan until the Year 2025, Has Been Approved by the Decree of the President of the Republic of Kazakhstan No. 636. Available online: https://adilet.zan.kz/rus/docs/U1800000636 (accessed on 15 February 2018).
- Khoroshavin, L.B.; Perepelitsyn, V.A.; Kochkin, D.K. Problems of technogenic resources. Refract. Ind. Ceram. 1998, 39, 366–368. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Kenzhibaeva, G.S.; Botabaev, N.E.; Kutzhanova, A.N.; Iztleuov, G.M.; Suigenbaeva, A.Z.; Ashirbaev, K.A.; Kolesnikova, O.G. Thermodynamic Modeling of Chemical and Phase Transformations in a Waelz Process-Slag—Carbon System. Refract. Ind. Ceram. 2020, 61, 289–292. [Google Scholar] [CrossRef]
- Vasilieva, N.V.; Fedorova, E.R. Process control quality analysis. Tsvetnye Met. 2020, 10, 70–76. [Google Scholar] [CrossRef]
- Ferreira, W.L.; Reis, É.L.; Lima, R.M. Incorporation of residues from the minero-metallurgical industry in the production of clay–lime brick. J. Clean. Prod. 2015, 87, 505–510. [Google Scholar] [CrossRef][Green Version]
- Kolesnikov, A.S.; Naraev, V.N.; Natorkhin, M.I.; Saipov, A.A.; Kolesnikova, O.G. Review of the processing of minerals and technogenic sulfide raw material with the extraction of metals and recovering elemental sulfur by electrochemical methods. Rasayan J. Chem. 2020, 13, 2420–2428. [Google Scholar] [CrossRef]
- Peng, Z.; Gregurek, D.; Wenzl, C.; White, J.F. White Slag Metallurgy and Metallurgical Waste Recycling. J. Metall. 2016, 68, 2313–2315. [Google Scholar] [CrossRef]
- Efremova, S. Scientific and technical solutions to the problem of utilization of waste from plant- and mineral-based industries. Russ. J. Gen. Chem. 2012, 82, 963–968. [Google Scholar] [CrossRef]
- Mamyrbekova, A.; Mamitova, A.D.; Mamyrbekova, A. Electrochemical Behavior of Sulfur in Aqueous Alkaline Solutions. Russ. J. Phys. Chem. A 2018, 92, 582–586. [Google Scholar] [CrossRef]
- Nadirov, K.S.; Zhantasov, M.K.; Sakybayev, B.A. The study of the gossypol resin impact on adhesive properties of the intermediate layer of the pipeline three-layer rust protection coating. Inter. J. Adhes. Adhesiv. 2017, 78, 195–199. [Google Scholar] [CrossRef]
- Myrzabekov, B.E.; Bayeshov, A.B.; Makhanbetov, A.B.; Mishra, B.; Baigenzhenov, O.S. Dissolution of Platinum in Hydrochloric Acid Under Industrial-Scale Alternating Current Polarization. Metal. Mat. Trans. B Proc. Metal. Mat. Proc. Sci. 2018, 49, 23–27. [Google Scholar] [CrossRef]
- Reznichenko, V.A.; Lipikhina, M.S.; Morozov, A.A. Complex Use of Ores and Concentrates; Nauka: Moscow, Russia, 1989; pp. 1–72. [Google Scholar]
- Lis, T.; Nowacki, K.; Żelichowska, M.; Kania, H. Innovation in metallurgical waste management. Metal.-Sisak Zagreb. 2015, 54, 283–285. [Google Scholar]
- Borisov, D.; Stefanov, B.; Stoyanov, S.K. An algorithm for metallurgical waste minimization. J. Chem. Technol. Metal. 2014, 49, 99–105. [Google Scholar]
- Zharmenov, A.A. Complex Processing of Mineral Raw Materials in Kazakhstan; Folio: Astana, Kazakhstan, 2003; pp. 1–272. [Google Scholar]
- Alshanov, R.A. Kazakhstan on the World Mineral Resource Market: Problems and Their Solution; LLP «Print-S»: Almaty, Kazakhstan, 2004; pp. 1–220. [Google Scholar]
- Kolesnikov, A.S.; Zhakipbaev, B.Y.; Zhanikulov, N.N.; Kolesnikova, O.G.; Akhmetova, E.K.; Kuraev, R.M.; Shal, A.L. Review of technogenic waste and methods of its processing for the purpose of complex utilization of tailings from the enrichment of non-ferrous metal ores as a component of the raw material mixture in the production of cement clinker. Rasayan J. Chem. 2021, 14, 997–1005. [Google Scholar] [CrossRef]
- Fechet, R.; Zlagnean, M.; Moanta, A.; Ciobanu, L. Mining wastes—Sampling, processing and using in anufacture portland cement. Roman. J. Miner. Depos. 2010, 84, 67–70. [Google Scholar]
- Aghazadeh-Ghomi, M.; Pourabbas, Z. Rapid synthesis of zinc oxide nanoparticles from an alkaline zinc solution via direct precipitation. J. Mat. Sci. Mat. Electron. 2021, 32, 24363–24368. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Serikbaev, B.E.; Zolkin, A.L.; Kenzhibaeva, G.S. Processing of Non-Ferrous Metallurgy Waste Slag for its Complex Recovery as a Secondary Mineral Raw Material. Refract. Ind. Ceram. 2021, 62, 375–380. [Google Scholar] [CrossRef]
- Satbaev, B.; Yefremova, S.; Zharmenov, A.; Kablanbekov, A.; Yermishin, S.; Shalabaev, N.; Satbaev, A.; Khen, V. Rice Husk Research: From Environmental Pollutant to a Promising Source of Organo-Mineral Raw Materials. Materials 2021, 14, 4119. [Google Scholar] [CrossRef]
- Khudyakova, T.M.; Kenzhibaeva, G.S.; Kutzhanova, A.N.; Iztleuov, G.M.; Mynbaeva, E. Optimization of Raw Material Mixes in Studying Mixed Cements and Their Physicomechanical Properties. Refract. Ind. Ceram. 2019, 60, 76–81. [Google Scholar] [CrossRef]
- Babushkin, V.I.; Matveyev, G.M.; Mchedlov-Petrosyan, O.P. Thermodynamics of Silicates; Stroyizdat: Moscow, Russia, 1986; 408p. [Google Scholar]
- Roine, A. Outokumpu HSC Chemistry for Windows. Chemical Reaction and Equilibrium Loftware with Extensive Thermochemical Database; Outokumpu Research OY: Pori, Finland, 2002; 268p. [Google Scholar]
- Zeleznik, F.J.; Gordon, S. Calculation of complex chemical equilibria. Ind. Eng. Chem. 1968, 60, 27–57. [Google Scholar] [CrossRef]
- Rog, G.; Kozlowska-Rog, A.; Zakula-Sokol, K. Thermodynamic functions of calcium aluminate. J. Chem. Thermodyn. 1993, 25, 807–810. [Google Scholar]
- Zhanikulov, N.N.; Khudyakova, T.M.; Taimasov, B.T. Receiving portland cement from technogenic raw materials of South Kazakhstan portlandcement. Eurasian Chem. Technol. J. 2019, 21, 334–340. [Google Scholar] [CrossRef]
- Demidov, A.I. Thermodynamic characteristics of a quasi-binary system CaO—Si1/2O in a solid state. Sci. Technol. Ved. SPbPU 2018, 24, 134–139. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Mochalov, A.V.; Bituev, A.V.; Zayakhanov, M.E. Structuring Behavior of Composite Materials Based on Cement, Limestone, and Acidic Ash. Inorg. Mat. 2019, 55, 1079–1085. [Google Scholar] [CrossRef]
- Pratskova, S.E.; Burmistrov, V.A.; Starikova, A.A. Thermodynamic modeling of oxide melts of CaO—Al2O3—SiO2: Systems. Russ. J. Chem. Chem. Tech. 2020, 63, 45–50. (In Russian) [Google Scholar] [CrossRef]
- Tennis, P.D.; Jennings, H.M. A model for two types of calcium silicate hydrate in the microstructure of Portland cement pastes. Cem. Concr. Res. 2000, 30, 855–863. [Google Scholar] [CrossRef]
- Taimasov, B.T.; Sarsenbayev, B.K.; Khudyakova, T.M. Development and testing of low-energy-intensive technology of receiving sulphate-resistant and road Portland cement. Eurasian Chem. Technol. J. 2017, 19, 347–355. [Google Scholar] [CrossRef]
- Loganina, V.I.; Fediuk, R.S. Thermodynamic Approach to Assessing the Curing of Protective and Decorative Coatings of Exterior Walls of Buildings. Mater. Sci. Forum. 2020, 974, 3–8. [Google Scholar] [CrossRef]
- Fukuda, K. Crystal Chemistry of Cement-Clinker Minerals and Melt Differentiation Reaction of Interstitial Melt. Nihon Kessho Gakkaishi 2011, 53, 81–85. [Google Scholar] [CrossRef][Green Version]
- Hanein, T.; Glasser, F.; Bannerman, M. Thermodynamic data for cement clinkering. Cem. Concr. Res. 2020, 132, 106043. [Google Scholar] [CrossRef]
- Zaleska, M.; Pavlik, Z.; Pavlikova, M.; Scheinherrova, L.; Pokorny, J.; Trnik, A.; Svora, P.; Fort, J.; Jankovsky, O.; Suchorab, Z. Biomass ash-based mineral admixture prepared from municipal sewage sludge andits application in cement composites. Clean Technol. Environ. Policy 2018, 20, 159–171. [Google Scholar] [CrossRef]
- Jankovsky, O.; Pavlikova, M.; Sedmidubskz, D.; Bousa, D.; Lojka, M.; Pokorny, J.; Zaleska, M.; Pavlik, Z. Study on pozzolana activity of wheat straw ash as potential admixture for blended cements. Ceram.-Silik. 2017, 61, 327–339. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Zhang, L.; Yang, K.; Guan, X.; Zhao, R. Insights on Substitution Preference of Pb Ions in Sulfoaluminate Cement Clinker Phases. Materials 2021, 14, 44. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, K.; Chen, Y.; Fan, G.; Zhang, L.; Guo, B.; Guan, X.; Zhao, R. Revealing the substitution preference of zinc in ordinary Portland cement clinker phases: A study from experiments and DFT calculations. J. Hazard. Mater. 2021, 409, 124504. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnikov, A.; Fediuk, R.; Kolesnikova, O.; Zhanikulov, N.; Zhakipbayev, B.; Kuraev, R.; Akhmetova, E.; Shal, A. Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials 2022, 15, 324. https://doi.org/10.3390/ma15010324
Kolesnikov A, Fediuk R, Kolesnikova O, Zhanikulov N, Zhakipbayev B, Kuraev R, Akhmetova E, Shal A. Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials. 2022; 15(1):324. https://doi.org/10.3390/ma15010324
Chicago/Turabian StyleKolesnikov, Alexandr, Roman Fediuk, Olga Kolesnikova, Nurgali Zhanikulov, Bibol Zhakipbayev, Rasim Kuraev, Elmira Akhmetova, and Aizhan Shal. 2022. "Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc" Materials 15, no. 1: 324. https://doi.org/10.3390/ma15010324
APA StyleKolesnikov, A., Fediuk, R., Kolesnikova, O., Zhanikulov, N., Zhakipbayev, B., Kuraev, R., Akhmetova, E., & Shal, A. (2022). Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials, 15(1), 324. https://doi.org/10.3390/ma15010324







