Effect of Oxalic Acid Treatment on Conductive Coatings Formed by Ni@Ag Core–Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
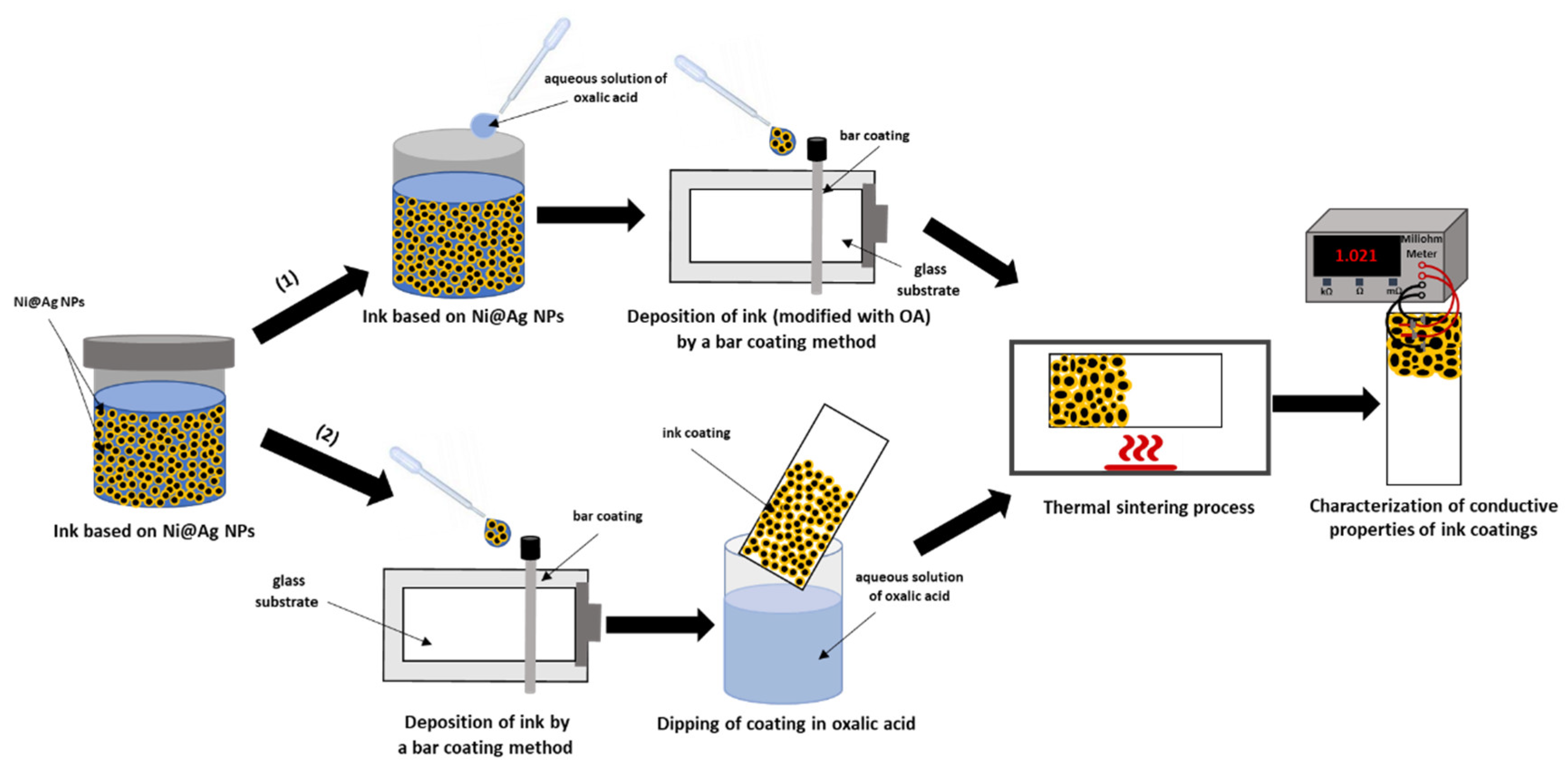
2.2. Fabrication of Ni@Ag NPs Based Ink
2.3. Metallic Coating Fabrication
2.4. Characterization
3. Results and Discussion
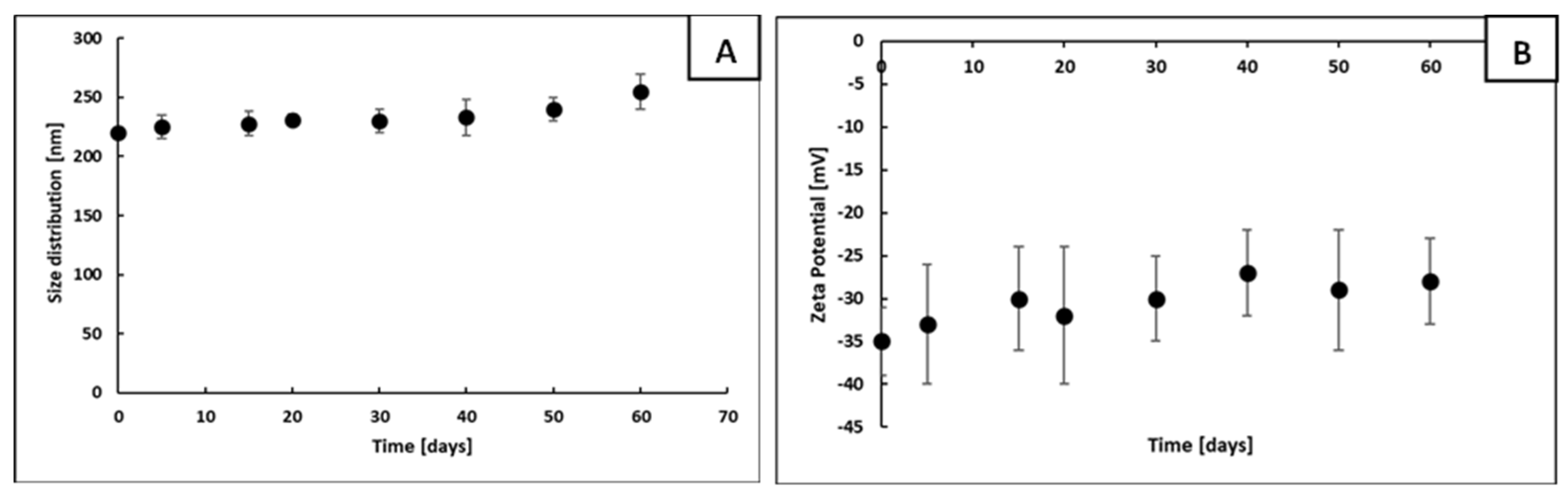
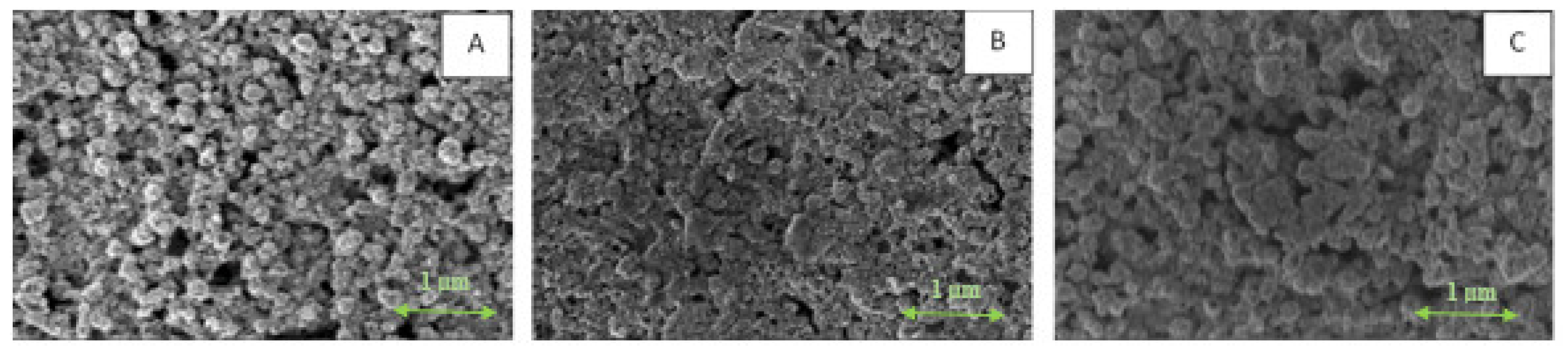
3.1. Fabrication of Ni@Ag NP-Based Ink
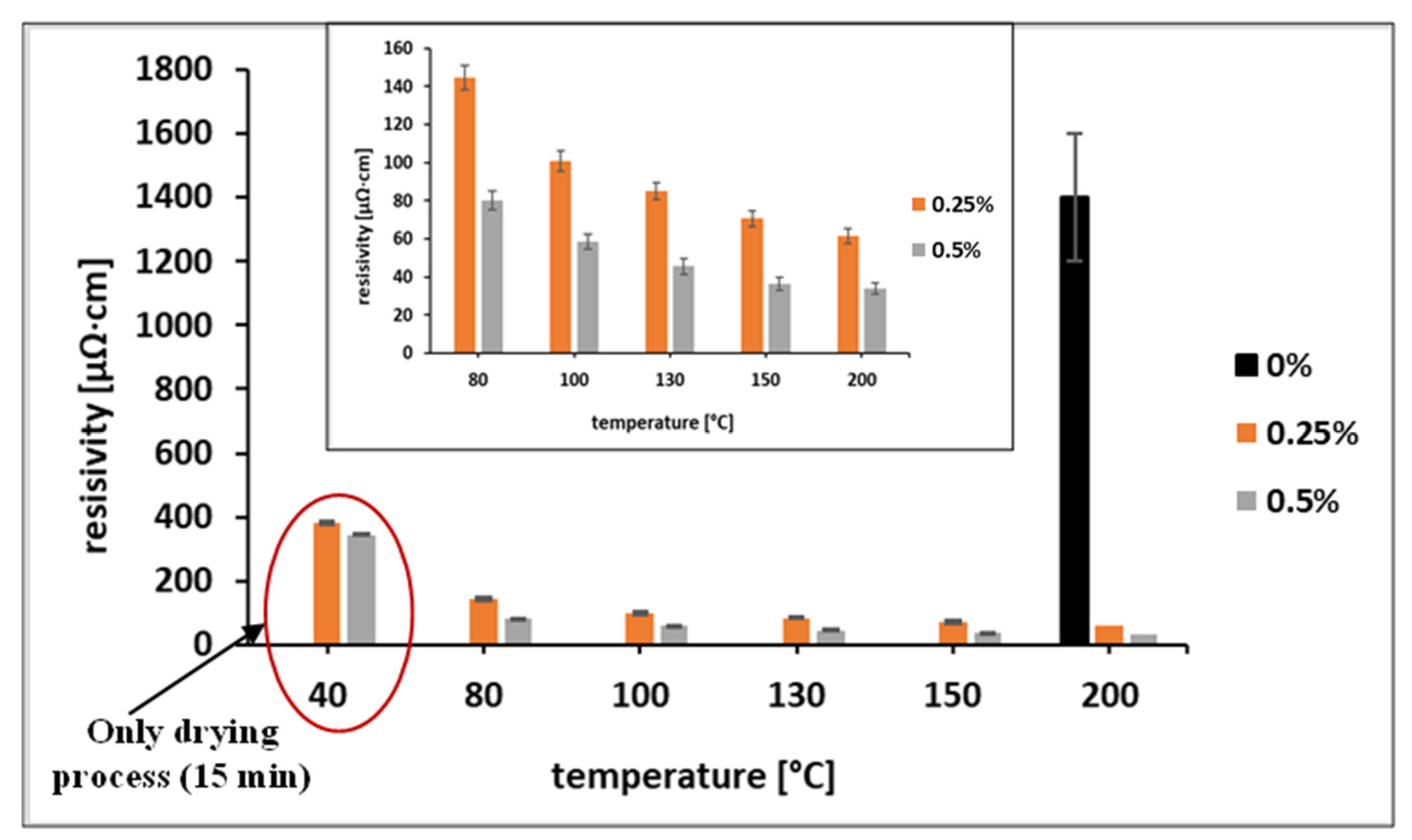
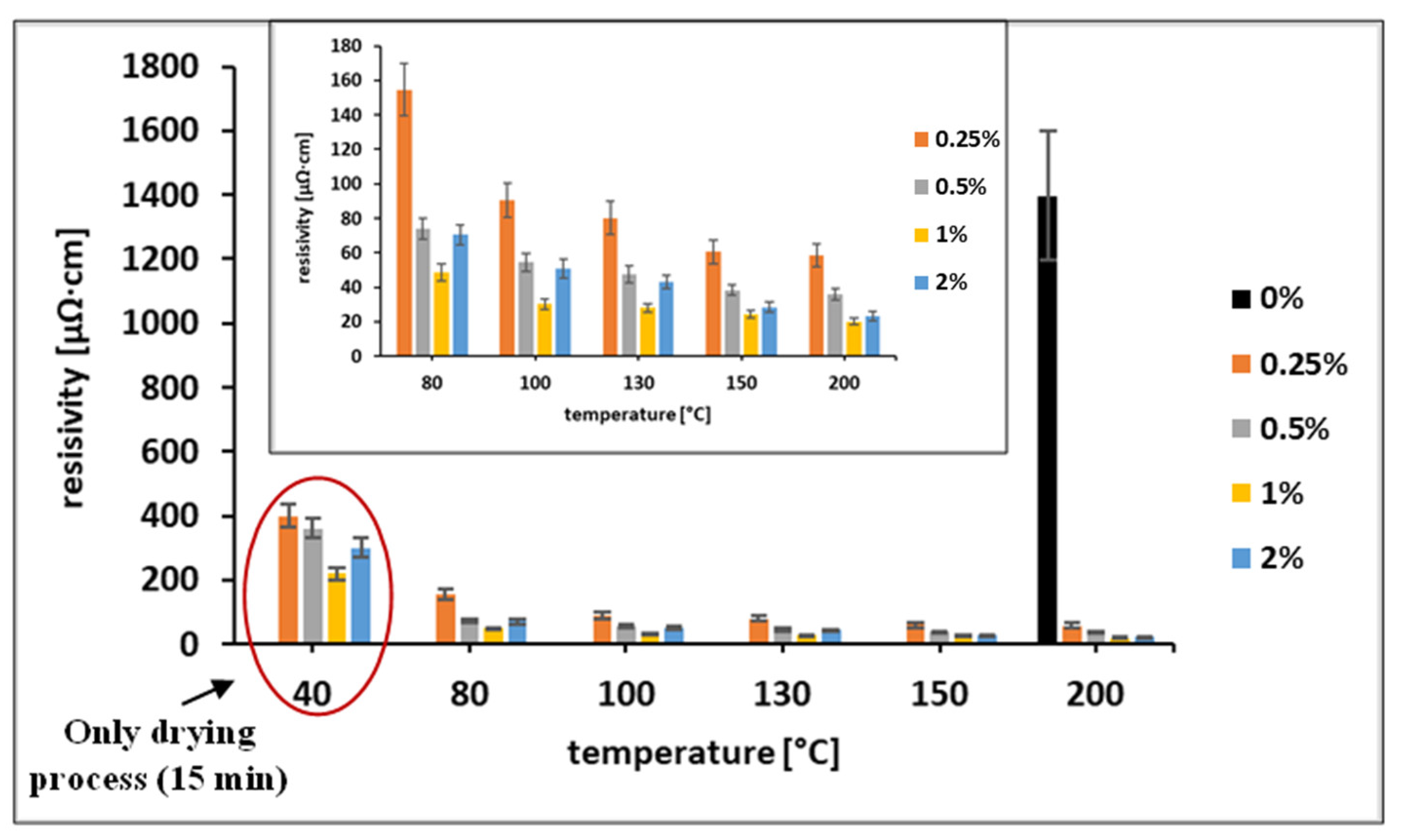
3.2. Effect of OA Addition on the Properties of Ni-Ag-Based Ink
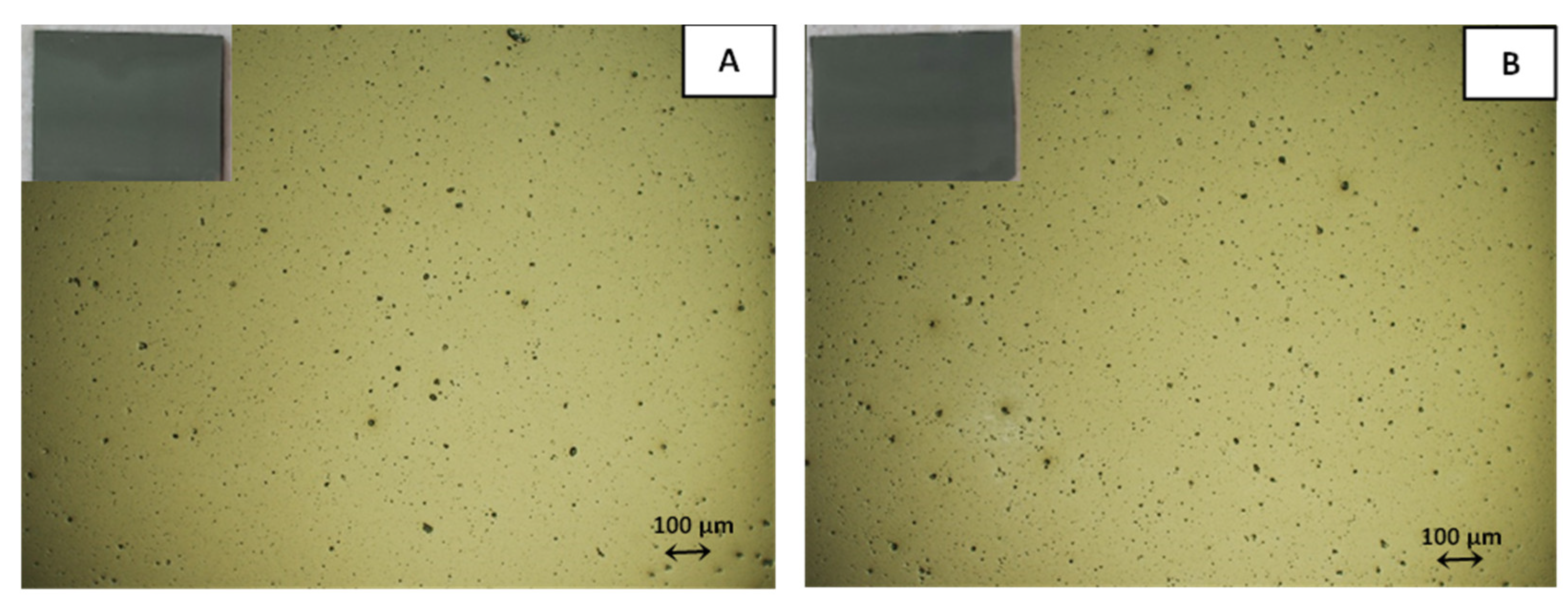
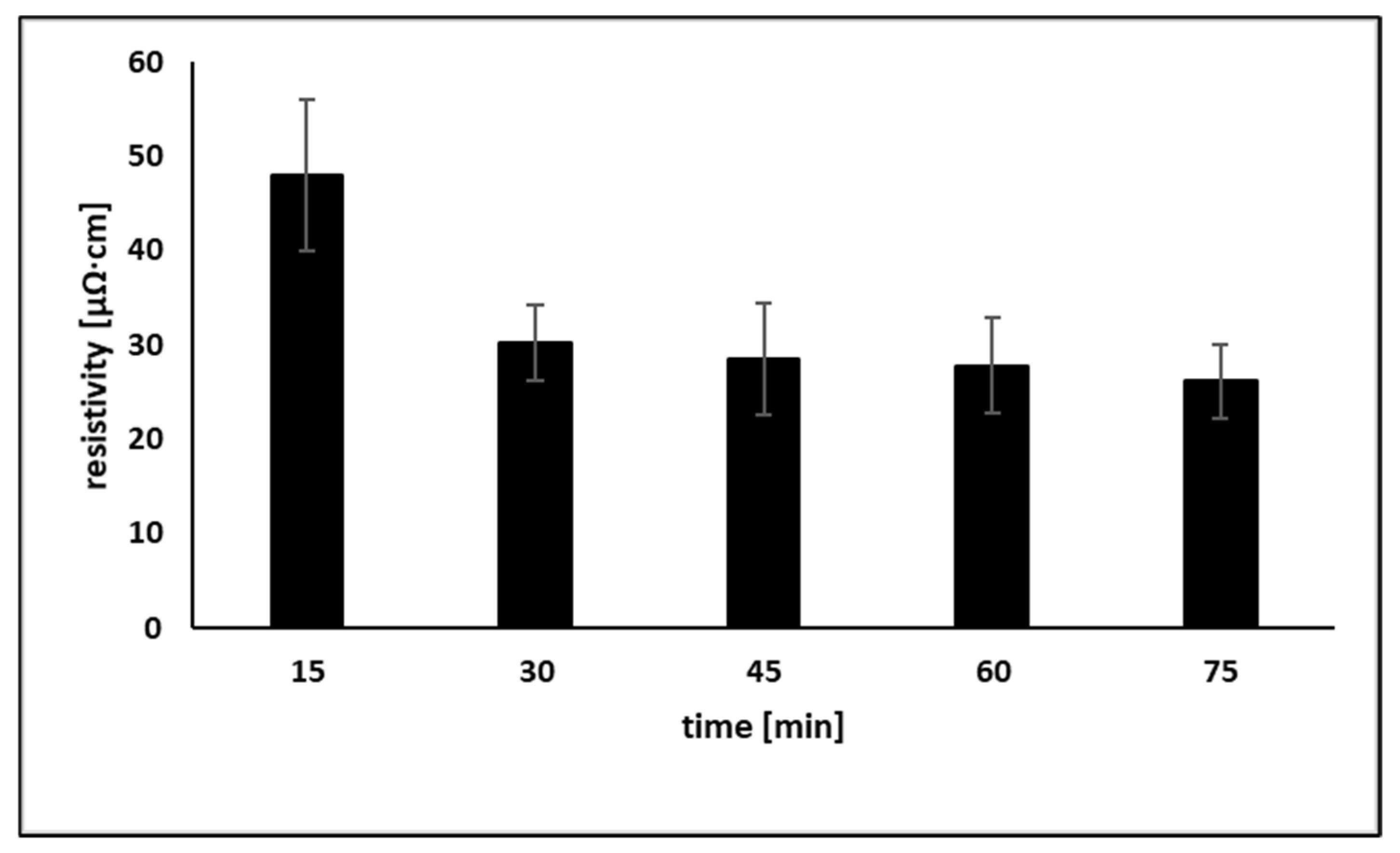
3.3. Post-Printing Treatment with OA
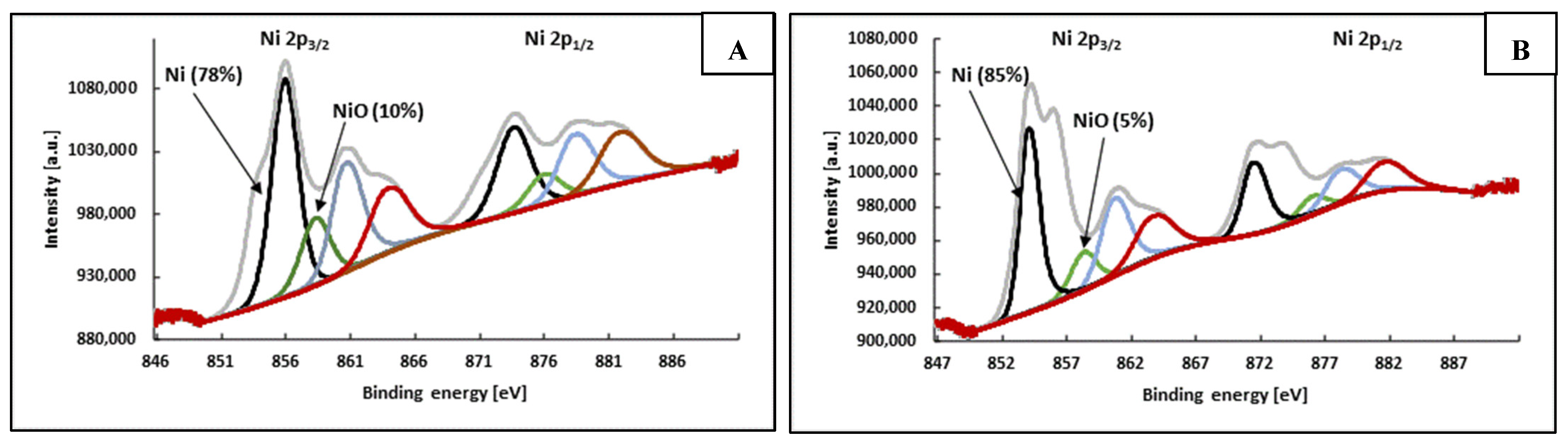
3.4. Mechanism of OA Action
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamyshny, A.; Magdassi, S. Conductive nanomaterials for 2D and 3D printed flexible electronics. Chem. Rev. 2019, 48, 1712–1740. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Matallic nanoinks for inkjet printing of conductive 2D and 3D structures. In Nanomaterials for 2D and 3D Printing; Kamyshny, A., Magdassi, S., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 119–160. [Google Scholar]
- Kamyshny, A.; Magdassi, S. Conductive nanomaterials for printed electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.D.; Elias, A.L.; Chung, H.-J. Flexible electronics under strain: A review of mechanical characterization and durability enhancement strategies. J. Mater. Sci. 2016, 51, 2771–2805. [Google Scholar] [CrossRef]
- Wünscher, S.; Abbel, R.; Perelaer, J.; Schubert, U.S. Progress of alternative sintering approaches of inkjet-printed metal inks and their application for manufacturing of flexible electronic devices. J. Mater. Chem. C 2014, 2, 10232–10261. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Socha, R.; Pawlowski, R.; Warszyński, P.; Szczepanowicz, K. Application of metallic inks based on nickel-silver core-shell nanoparticles for fabrication of conductive films. Nanotechnology 2019, 30, 225301. [Google Scholar] [CrossRef] [PubMed]
- Pajor-Świerzy, A.; Gaweł, D.; Drzymała, E.; Socha, R.; Parlińska-Wojtan, M.; Szczepanowicz, K.; Warszynski, P. The optimization of methods of synthesis of nickel-silver core-shell nanoparticles for conductive coatings. Nanotechnology 2019, 30, 015601. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Pawłowski, R.; Warszynski, P.; Szczepanowicz, K. The conductive properties of ink coating based on Ni-Ag core-shell nanoparticles with the bimodal size distribution. J. Mater. Sci. Mater. Electron. 2020, 31, 12991–12999. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Szendera, F.; Pawłowski, R.; Szczepanowicz, K. Nanocomposite inks based on nickel-silver core-shell and silver nanoparticles for fabrication conductive coatings at low-temperature sintering. Colloids Interfaces 2021, 5, 15. [Google Scholar] [CrossRef]
- Jing, J.J.; Xie, J.; Chen, G.Y.; Li, W.H.; Zhang, M.M. Preparation of nickel-silver core-shell nanoparticles by liquid-phase reduction for use in conductive paste. J. Exp. Nanosci. 2015, 10, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Nayak, L.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. A review on inkjet printing of nanoparticle inks for flexible electronics. J. Mater. Chem. C 2019, 7, 8771–8795. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y. Printing conductive nanomaterials for flexible and stretchable electronics: A review of materials, processes, and applications. Adv. Mater. Technol. 2019, 4, 1–41. [Google Scholar] [CrossRef]
- Grouchko, M.; Kamyshny, A.; Mihailescu, C.F.; Anghel, D.F.; Magdassi, S. Conductive inks with a “built-in” mechanism that enables sintering at room temperature. ACS Nano 2011, 5, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Corsino, D.C.; Balela, M.D.L. Room temperature sintering of printer silver nanoparticle conductive ink. Mater. Sci. Eng. 2017, 264, 012020. [Google Scholar] [CrossRef]
- Noha, J.; Hab, J.; Kima, D. Femtosecond and nanosecond laser sintering of silver nanoparticles on a flexible substrate. Appl. Surf. Sci. 2020, 511, 145574. [Google Scholar] [CrossRef]
- Zhai, D.; Zhang, T.; Guo, J.; Fang, X.; Wei, J. Water-based ultraviolet curable conductive inkjet ink containing silver nano-colloids for flexible electronics. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 1–9. [Google Scholar] [CrossRef]
- Deng, D.; Cheng, Y.; Jin, Y.; Qi, T.; Xiao, F. Antioxidative effect of lactic acid-stabilized copper nanoparticles prepared in aqueous solution. J. Mater. Chem. 2012, 22, 23989–23995. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Kim, I.; Kim, J. The effect of reduction atmospheres on the sintering behaviors of inkjet printed Cu interconnectors. J. Appl. Phys. 2010, 108, 102807. [Google Scholar] [CrossRef]
- Woo, K.; Kim, Y.; Lee, B.; Kim, J.; Moon, J. Effect of carboxylic acid on sintering of inkjet-printed copper nanoparticulate films. ACS Appl. Mater. Interfaces 2011, 3, 2377–2382. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Farraj, Y.; Kamyshny, A.; Magdassi, S. Effect of carboxylic acids on conductivity of metallic films formed by inks based on copper@silver core-shell particles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 320–327. [Google Scholar] [CrossRef]
- Zaheer Rafiuddin, Z. Silver nanoparticles to self-assembled films: Green synthesis and characterization. Colloids Surf. B 2012, 90, 48–52. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, A.; Zhao, X.; Pan, X.; Han, Y.; Suo, N.; Yu, L.; Zuo, Z. Structural and magnetic properties of NieCueCo ferrites prepared from sol-gel auto combustion method with different complexing agents. J. Alloys Compd. 2020, 816, 152501. [Google Scholar] [CrossRef]
- Bayri, B.; Rosset, R.; Desbarres, J.; Jardy, A.; Noel, D.; Kerrec, O.; Lantes, B. Complexing properties of the man organic acids used in decontamination solutions for nuclear power plants and reactions involved in their degradation or elimination. Nucl. Eng. Des. 1996, 160, 159–170. [Google Scholar] [CrossRef]
- Penuela, J.; Martinez, J.D.; Araujo, M.L.; Brito, F.; Lubes, G.; Rodriguez, M.; Lubes, V. Speciation of the nickel (II) complexes with oxalic and malonic acids studied in 1.0 mol·dm−3 NaCl at 25 °C. J. Coord. Chem. 2011, 64, 2698–2705. [Google Scholar] [CrossRef]
- Kanzaki, M.; Kawaguchi, Y.; Kawasaki, H. Fabrication of conductive copper films on flexible polymer substrates by low-temperature sintering of composite Cu ink in air. ACS Appl. Mater. Interfaces 2017, 9, 20852–20858. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Peng, Y.; Zhang, Y.; Cheng, H.; Chen, M. Cu-Cu bonding enhancement at low temperature by using carboxylic acid surface-modified Cu nanoparticles. Mater. Lett. 2018, 227, 179–183. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Staśko, D.; Pawłowski, R.; Mordarski, G.; Kamyshny, A.; Szczepanowicz, K. Polydispersity vs. monodispersity. How the properties of Ni-Ag core-shell nanoparticles affect the conductivity of ink coatings. Materials 2021, 14, 2304. [Google Scholar] [CrossRef]
- K Hand Coater, Pre-Press Equipment, RK Print Coat Instruments. Available online: https://www.rkprint.com/products/k-hand-coater/ (accessed on 25 October 2021).
- Extech Instruments, Milliohm Meters. Available online: http://www.extech.com/products/resources/380560_380562_UM-en.pdf (accessed on 25 October 2021).
- Kamyshny, A.; Steinke, J.; Magdassi, S. Metal-based inkjet inks for printed electronics. Open Appl. Phys. J. 2011, 4, 19–36. [Google Scholar] [CrossRef]
- Song, J.; Birbach, N.L.; Hinestroza, J.P. Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellulose 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Figueroa, A.C.; Sileo, E.E.; Morando, P.J.; Blesa, A.M. Dissolution of Nickel Oxide in Oxalic Acid Aqueous Solutions. J. Colloid Interface Sci. 2001, 244, 353–358. [Google Scholar] [CrossRef]
- Ingham, B.; Lim, T.H.; Dotzler, C.J.; Henning, A.; Toney, M.F.; Tilley, R.D. How nanoparticles coalesce: An in situ study of Au nanoparticle aggregation and grain growth. Chem. Mater. 2011, 23, 3312–3317. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajor-Świerzy, A.; Pawłowski, R.; Sobik, P.; Kamyshny, A.; Szczepanowicz, K. Effect of Oxalic Acid Treatment on Conductive Coatings Formed by Ni@Ag Core–Shell Nanoparticles. Materials 2022, 15, 305. https://doi.org/10.3390/ma15010305
Pajor-Świerzy A, Pawłowski R, Sobik P, Kamyshny A, Szczepanowicz K. Effect of Oxalic Acid Treatment on Conductive Coatings Formed by Ni@Ag Core–Shell Nanoparticles. Materials. 2022; 15(1):305. https://doi.org/10.3390/ma15010305
Chicago/Turabian StylePajor-Świerzy, Anna, Radosław Pawłowski, Piotr Sobik, Alexander Kamyshny, and Krzysztof Szczepanowicz. 2022. "Effect of Oxalic Acid Treatment on Conductive Coatings Formed by Ni@Ag Core–Shell Nanoparticles" Materials 15, no. 1: 305. https://doi.org/10.3390/ma15010305
APA StylePajor-Świerzy, A., Pawłowski, R., Sobik, P., Kamyshny, A., & Szczepanowicz, K. (2022). Effect of Oxalic Acid Treatment on Conductive Coatings Formed by Ni@Ag Core–Shell Nanoparticles. Materials, 15(1), 305. https://doi.org/10.3390/ma15010305






