A Nanoindentation Approach for Time-Dependent Evaluation of Surface Free Energy in Micro- and Nano-Structured Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Scanning Electron Microscopy
2.4. Atomic Force Microscopy
2.5. Roughness
2.6. Nanoindentation Measurements
3. Results
3.1. Topography Analysis
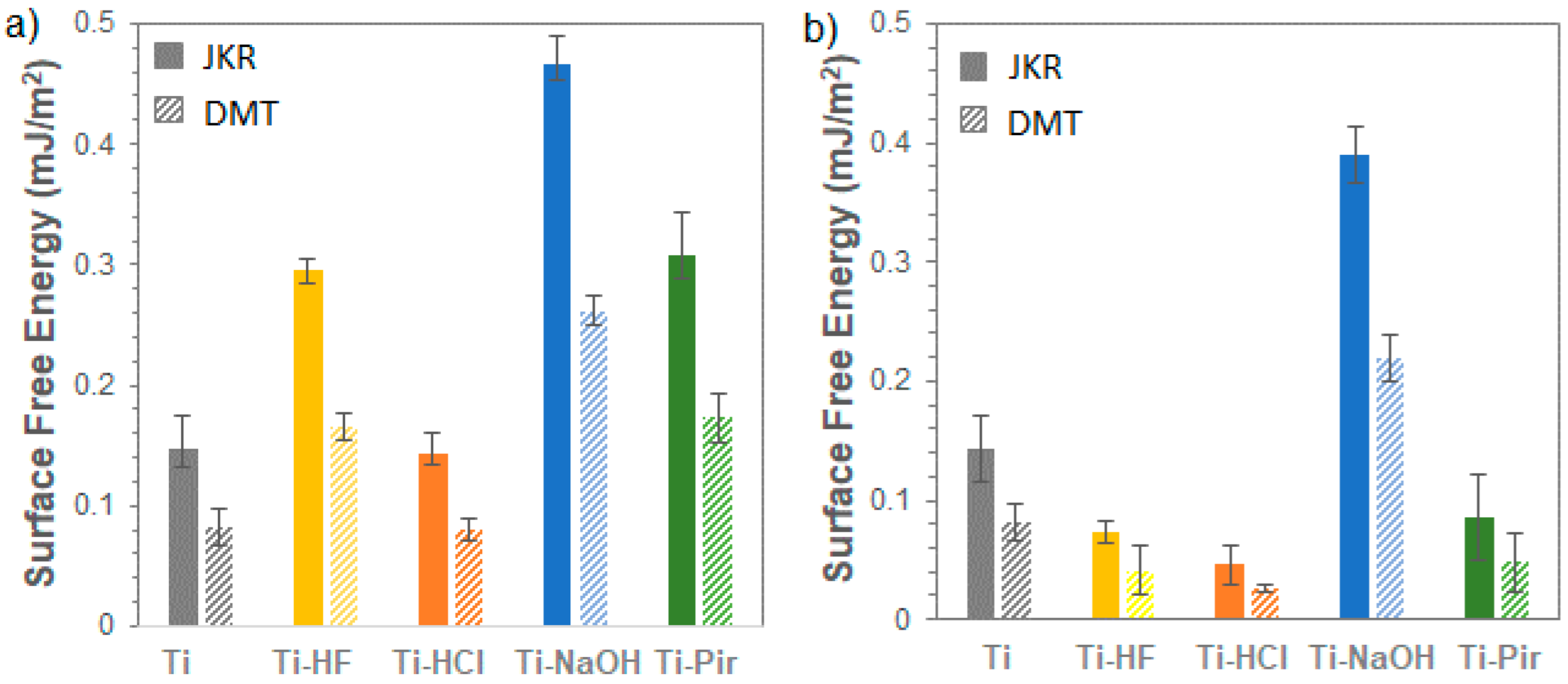
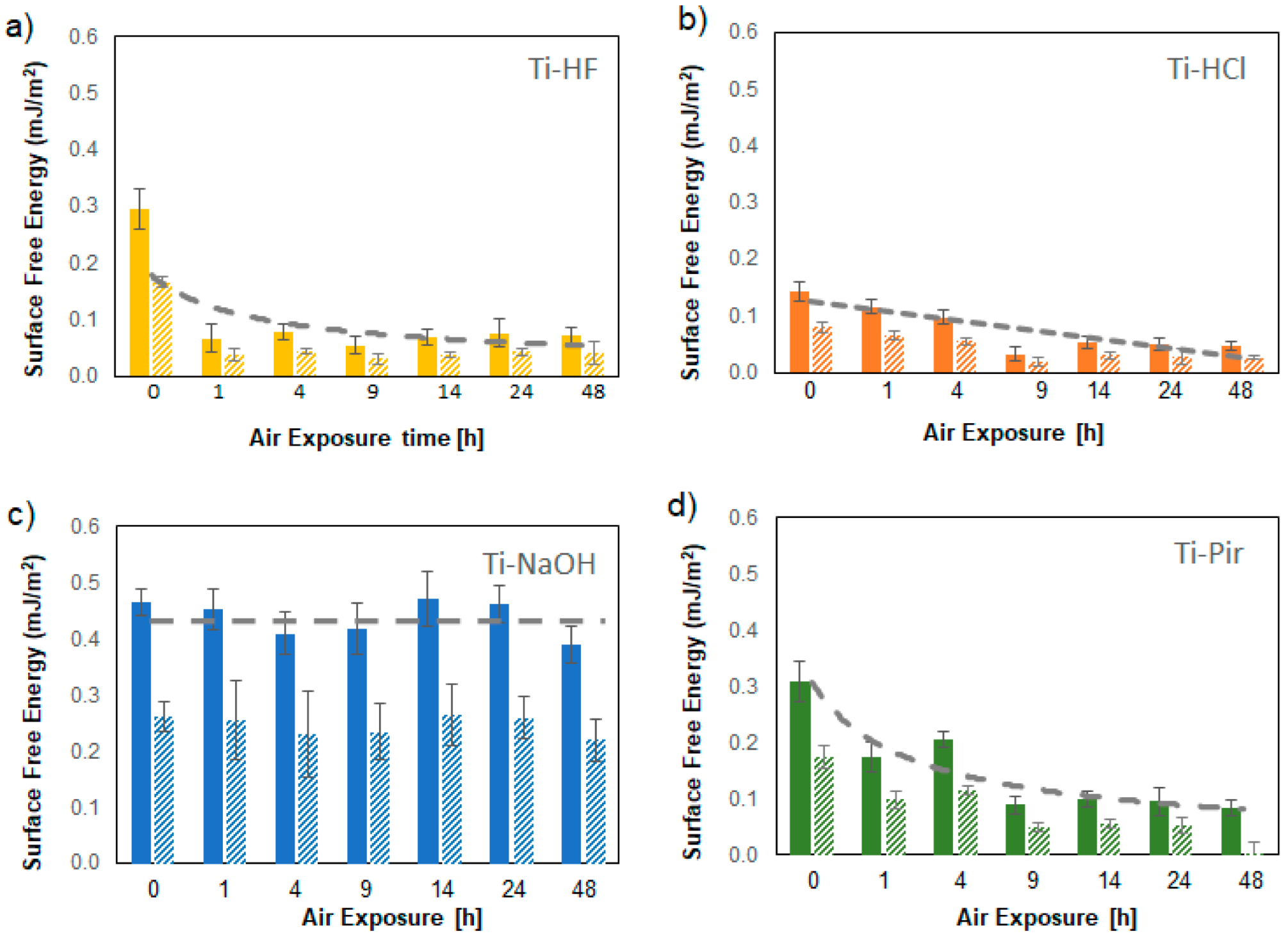
3.2. Nanoindentation SFE Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Santis, S.; Sotgiu, G.; Porcelli, F.; Marsotto, M.; Iucci, G.; Orsini, M. A Simple Cerium Coating Strategy for Titanium Oxide Nano-tubes’ Bioactivity Enhancement. Nanomaterials 2021, 11, 445. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Battocchio, C.; Concolato, S.; De Santis, S.; Fahlman, M.; Iucci, G.; Santi, M.; Sotgiu, G.; Orsini, M. Chitosan functionalization of titanium and Ti6Al4V alloy with chloroacetic acid as linker agent. Mater. Sci. Eng. C 2019, 99, 1133–1140. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2009, 7, S93–S105. [Google Scholar] [CrossRef] [Green Version]
- Novelli, F.; De Santis, S.; Punzi, P.; Giordano, C.; Scipioni, A.; Masci, G. Self-assembly and drug release study of linear l, d-oligopeptide-poly(ethylene glycol) conjugates. New Biotechnol. 2017, 37, 99–107. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, T.; Miyaji, F.; Kim, H.-M.; Nakamura, T. Spontaneous Formation of Bonelike Apatite Layer on Chemically Treated Titanium Metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. [Google Scholar] [CrossRef]
- Li, B.E.; Li, Y.; Min, Y.; Hao, J.Z.; Liang, C.Y.; Li, H.P.; Wang, G.C.; Liu, S.M.; Wang, H.S. Synergistic effects of hierarchical hybrid micro/nanostructures on the biological properties of titanium orthopaedic implants. RSC Adv. 2015, 5, 49552–49558. [Google Scholar] [CrossRef]
- Warcaba, M.; Kowalski, K.; Kopia, A.; Moskalewicz, T. Impact of Surface Topography, Chemistry and Properties on the Adhesion of Sodium Alginate Coatings Electrophoretically Deposited on Titanium Biomaterials. Met. Mater. Trans. A 2021, 52, 4454–4467. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Batishcheva, K.; Islamova, A. Investigation of free surface energy of rough aluminum alloy substrate. AIP Conf. Proc. 2019, 2135, 020011. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Islamova, A.; Orlova, E.; Ivashutenko, A.; Shanenkov, I.; Zykov, I.; Feoktistov, D. Influence of roughness on polar and dispersed components of surface free energy and wettability properties of copper and steel surfaces. Surf. Coat. Technol. 2021, 422, 127518. [Google Scholar] [CrossRef]
- Miyajima, H.; Awadzi, G.; Ozer, F.; Mante, F.K. Effect of surface physico-chemico-biological modifications of titanium on critical and theoretical surface free energy. Appl. Surf. Sci. 2019, 470, 386–394. [Google Scholar] [CrossRef]
- Vishnu, J.; Manivasagam, G. High-Surface-Energy Nanostructured Surface on Low-Modulus Beta Titanium Alloy for Orthopedic Implant Applications. J. Mater. Eng. Perform. 2021, 30, 4370–4379. [Google Scholar] [CrossRef]
- Kumar, S.S.; Hiremath, S.S. Effect of surface roughness and surface topography on wettability of machined biomaterials using flexible viscoelastic polymer abrasive media. Surf. Topogr. Metrol. Prop. 2018, 7, 015004. [Google Scholar] [CrossRef]
- Yan, Y.; Chibowski, E.; Szcześ, A. Surface properties of Ti-6Al-4V alloy part I: Surface roughness and apparent surface free energy. Mater. Sci. Eng. C 2017, 70, 207–215. [Google Scholar] [CrossRef]
- Echeverry-Rendón, M.; Galvis, O.; Aguirre, R.; Robledo, S.; Castaño, J.G.; Echeverria, F. Modification of titanium alloys surface properties by plasma electrolytic oxidation (PEO) and influence on biological response. J. Mater. Sci. Mater. Med. 2017, 28, 169. [Google Scholar] [CrossRef]
- Carrier, O.; Bonn, D. Contact Angles and the Surface Free Energy of Solids in Droplet Wetting Evaporation; Academic Press: Cambridge, MA, USA, 2015; pp. 15–23. [Google Scholar] [CrossRef]
- Cardellini, A.; Bellussi, F.M.; Rossi, E.; Chiavarini, L.; Becker, C.; Cant, D.; Asinari, P.; Sebastiani, M. Integrated molecular dynamics and experimental approach to characterize low-free-energy perfluoro-decyl-acrylate (PFDA) coated silicon. Mater. Des. 2021, 208, 109902. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [Green Version]
- Whyman, G.; Bormashenko, E.; Stein, T. The rigorous derivation of Young, Cassie–Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 2008, 450, 355–359. [Google Scholar] [CrossRef]
- Schuster, J.M.; Schvezov, C.E.; Rosenberger, M.R. Analysis of the Results of Surface Free Energy Measurement of Ti6Al4V by Different Methods. Procedia Mater. Sci. 2015, 8, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Lilli, M.; Rossi, E.; Tirillò, J.; Sarasini, F.; Di Fausto, L.; Valente, T.; González, C.; Fernández, A.; Lopes, C.S.; Moscatelli, R.; et al. Quantitative multi-scale characterization of single basalt fibres: Insights into strength loss mechanisms after thermal conditioning. Mater. Sci. Eng. A 2020, 797, 139963. [Google Scholar] [CrossRef]
- Rossi, E.; Bauer, J.; Sebastiani, M. Humidity-dependent flaw sensitivity in the crack propagation resistance of 3D-printed nano-ceramics. Scr. Mater. 2021, 194, 113684. [Google Scholar] [CrossRef]
- Rossi, E.M.; Phani, P.S.; Guillemet, R.; Cholet, J.; Jussey, D.; Oliver, W.C.; Sebastiani, M. A novel nanoindentation protocol to characterize surface free energy of superhydrophobic nanopatterned materials. J. Mater. Res. 2021, 36, 2357–2370. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Muller, V.; Yushchenko, V.; Derjaguin, B. On the influence of molecular forces on the deformation of an elastic sphere and its sticking to a rigid plane. J. Colloid Interface Sci. 1980, 77, 91–101. [Google Scholar] [CrossRef]
- Tabor, D. Surface forces and surface interactions. J. Colloid Interface Sci. 1977, 58, 2–13. [Google Scholar] [CrossRef]
- Mazzola, L.; Sebastiani, M.M.; Bemporad, E.E.; Carassiti, F.F. An Innovative Non-contact Method to Determine Surface Free Energy on Micro-areas. J. Adhes. Sci. Technol. 2012, 26, 131–150. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; De Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. Part A 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Abe, Y.; Yoshida, Y.; Nakayama, Y.; Okazaki, M.; Akagawa, Y. Acid pretreatment of titanium implants. Biomaterials 2003, 24, 1821–1827. [Google Scholar] [CrossRef]
- Nagassa, M.E.; Daw, A.E.; Rowe, W.G.; Carley, A.; Thomas, D.W.; Moseley, R. Optimisation of the hydrogen peroxide pre-treatment of titanium: Surface characterisation and protein adsorption. Clin. Oral Implant. Res. 2008, 19, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, L.; Zhang, R.; Tian, Y.; Liu, S.; He, M.; Huang, C.; Tian, W. A novel method to fabricate organic-free superhydrophobic surface on titanium substrates by removal of surface hydroxyl groups. Appl. Surf. Sci. 2019, 479, 1089–1097. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Zhang, X.; Li, Y. Enhancing the photoelectrochemical water splitting activity of rutile nanorods by removal of surface hydroxyl groups. Catal. Today 2016, 259, 360–367. [Google Scholar] [CrossRef]
- Sotgiu, G.; Orsini, M.; Porcelli, F.; de Santis, S.; Petrucci, E. Wettability of Micro and Nanostructured Surface of Titanium Based Electrodes: Influence of Chemical and Electrochemical Etching. Chem. Eng. Trans. 2021, 86, 1417–1422. [Google Scholar]
- Camargo, W.A.; Takemoto, S.; Hoekstra, J.W.; Leeuwenburgh, S.; Jansen, J.A.; Beucken, J.J.V.D.; Alghamdi, H.S. Effect of surface alkali-based treatment of titanium implants on ability to promote in vitro mineralization and in vivo bone formation. Acta Biomater. 2017, 57, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Raines, A.; Wieland, M.; Schwartz, Z.; Boyan, B. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007, 28, 2821–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
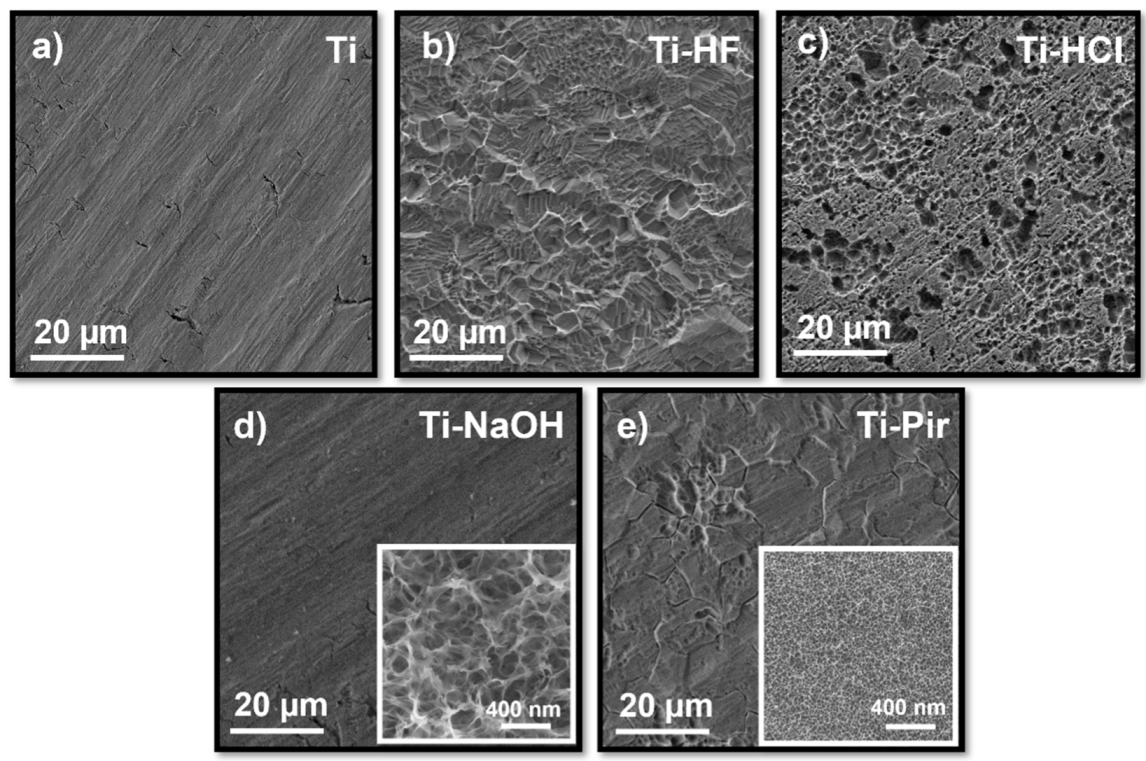
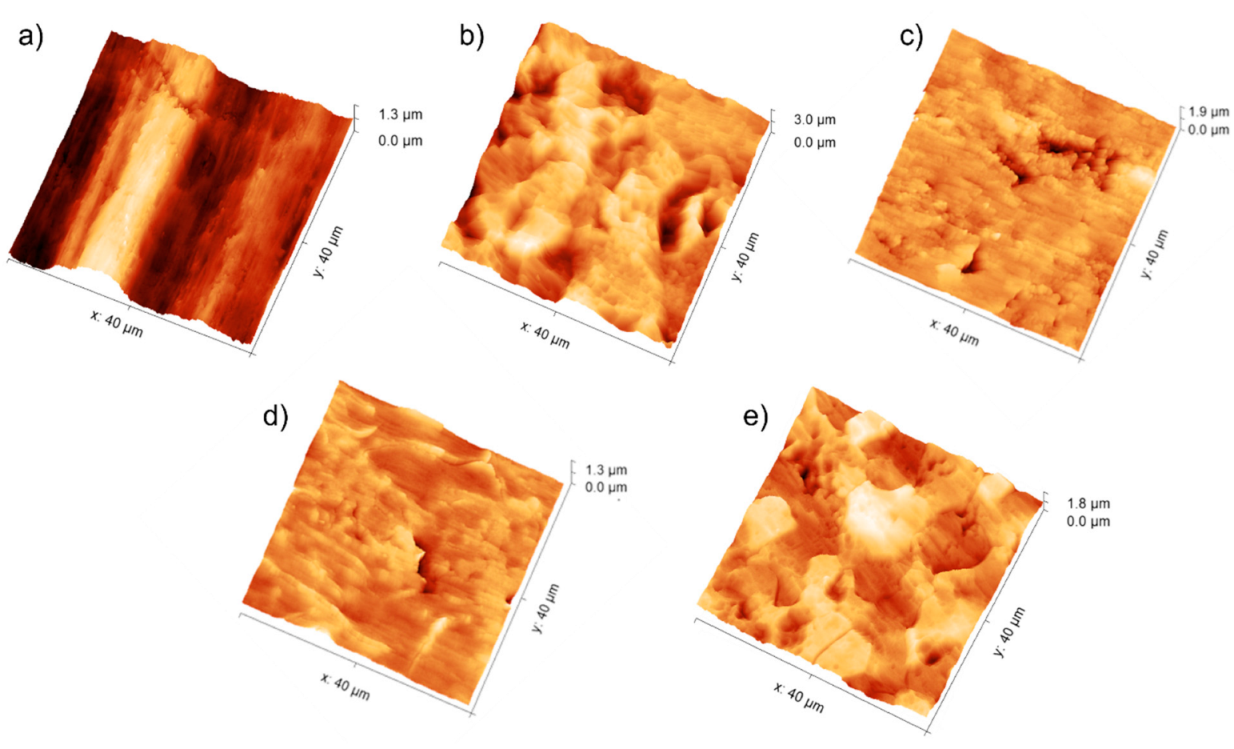

| Sample | Ra (µm) | Rq (µm) | Rp (µm) | Rv (µm) | Rt (µm) | Rsk | Rku | Rw |
|---|---|---|---|---|---|---|---|---|
| Ti | 0.21 | 0.26 | 0.93 | 0.80 | 1.73 | 0.68 | −0.29 | 1.06 |
| Ti-HF | 0.33 | 0.41 | 1.25 | 1.75 | 3.01 | −0.48 | 0.18 | 1.15 |
| Ti-HCl | 0.09 | 0.12 | 0.79 | 0.72 | 1.51 | −0.73 | 2.72 | 1.12 |
| Ti-NaOH | 0.09 | 0.11 | 0.59 | 0.50 | 1.09 | 0.41 | 0.16 | 1.11 |
| Ti-Pir | 0.20 | 0.25 | 0.73 | 1.07 | 1.79 | 0.19 | −0.41 | 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, S.; Rossi, E.; Sebastiani, M.; Sennato, S.; Bemporad, E.; Orsini, M. A Nanoindentation Approach for Time-Dependent Evaluation of Surface Free Energy in Micro- and Nano-Structured Titanium. Materials 2022, 15, 287. https://doi.org/10.3390/ma15010287
De Santis S, Rossi E, Sebastiani M, Sennato S, Bemporad E, Orsini M. A Nanoindentation Approach for Time-Dependent Evaluation of Surface Free Energy in Micro- and Nano-Structured Titanium. Materials. 2022; 15(1):287. https://doi.org/10.3390/ma15010287
Chicago/Turabian StyleDe Santis, Serena, Edoardo Rossi, Marco Sebastiani, Simona Sennato, Edoardo Bemporad, and Monica Orsini. 2022. "A Nanoindentation Approach for Time-Dependent Evaluation of Surface Free Energy in Micro- and Nano-Structured Titanium" Materials 15, no. 1: 287. https://doi.org/10.3390/ma15010287
APA StyleDe Santis, S., Rossi, E., Sebastiani, M., Sennato, S., Bemporad, E., & Orsini, M. (2022). A Nanoindentation Approach for Time-Dependent Evaluation of Surface Free Energy in Micro- and Nano-Structured Titanium. Materials, 15(1), 287. https://doi.org/10.3390/ma15010287










