Internal Dynamics of Ionic Liquids over a Broad Temperature Range—The Role of the Cation Structure
Abstract
:1. Introduction
2. Theory
3. Materials and Methods
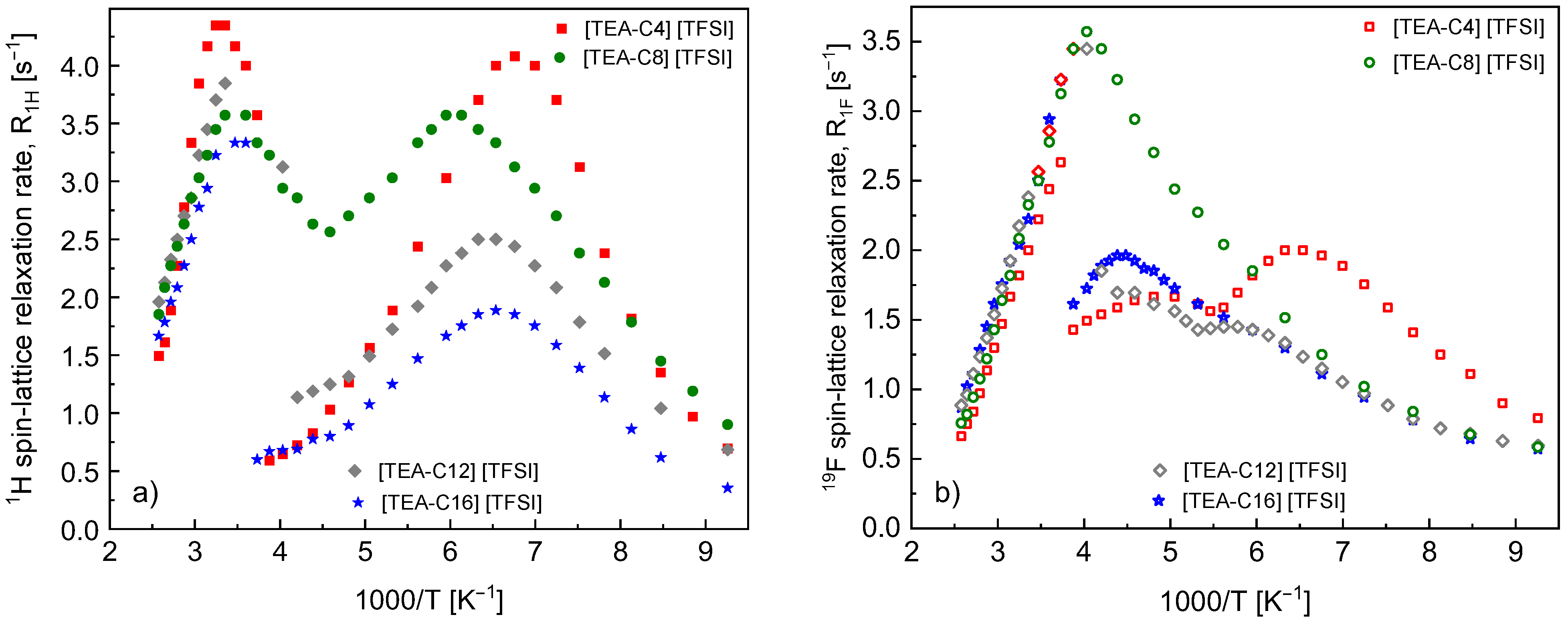
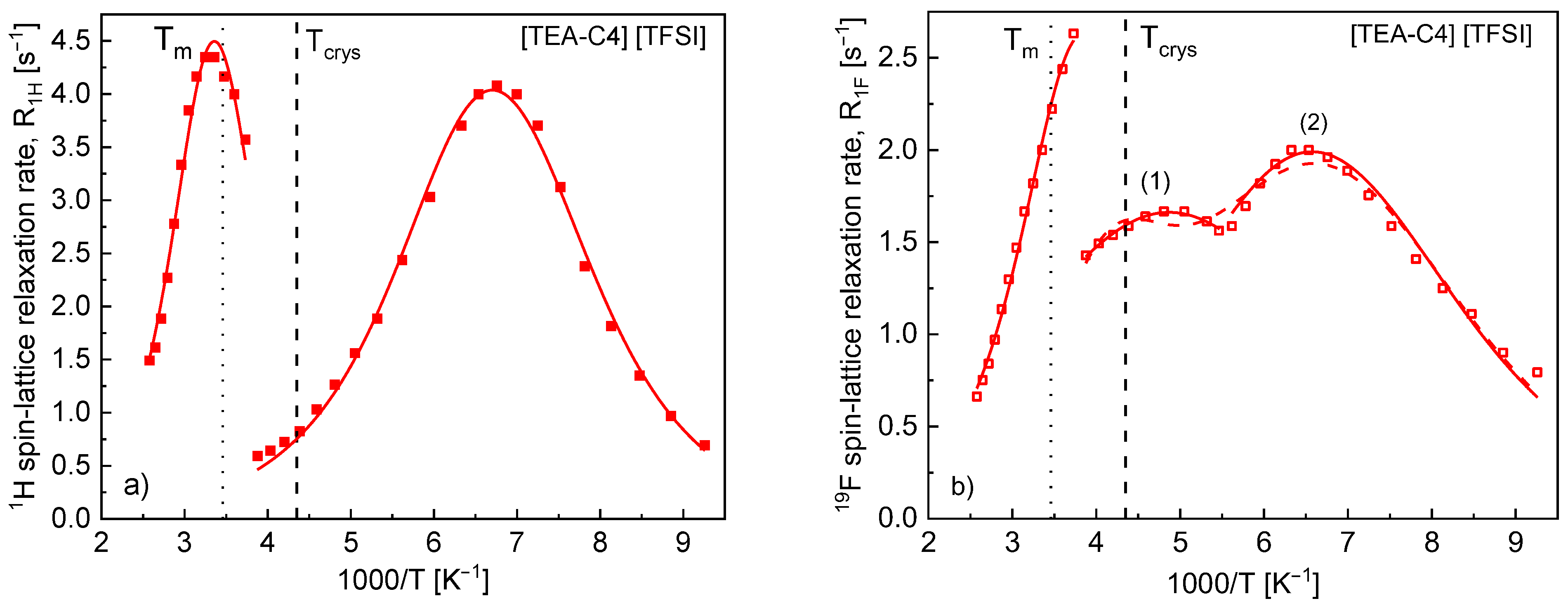
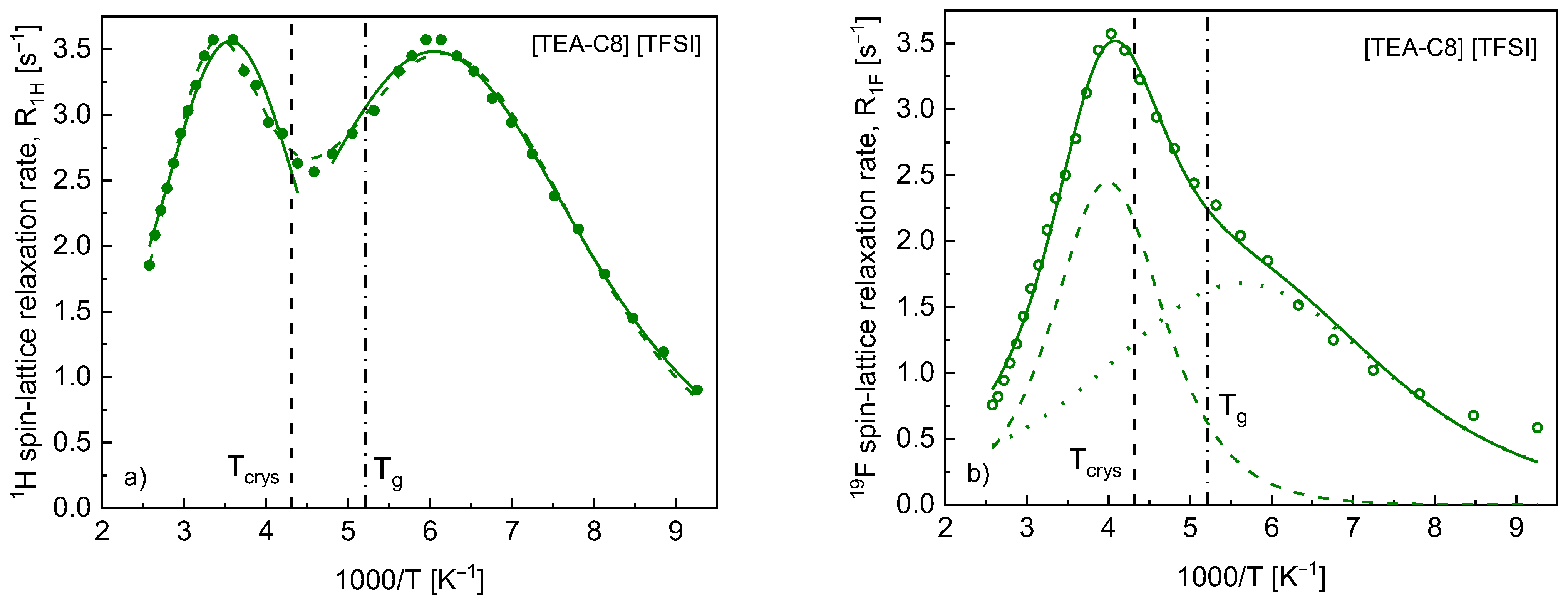
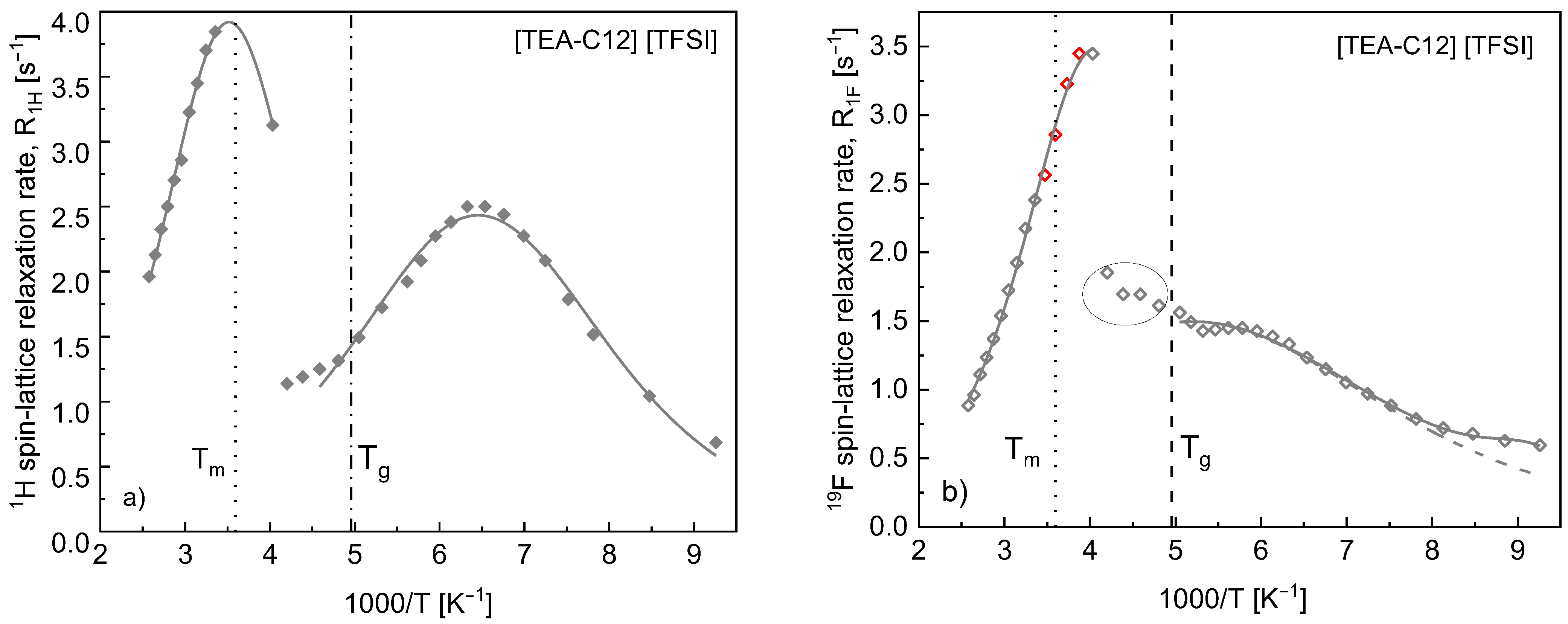
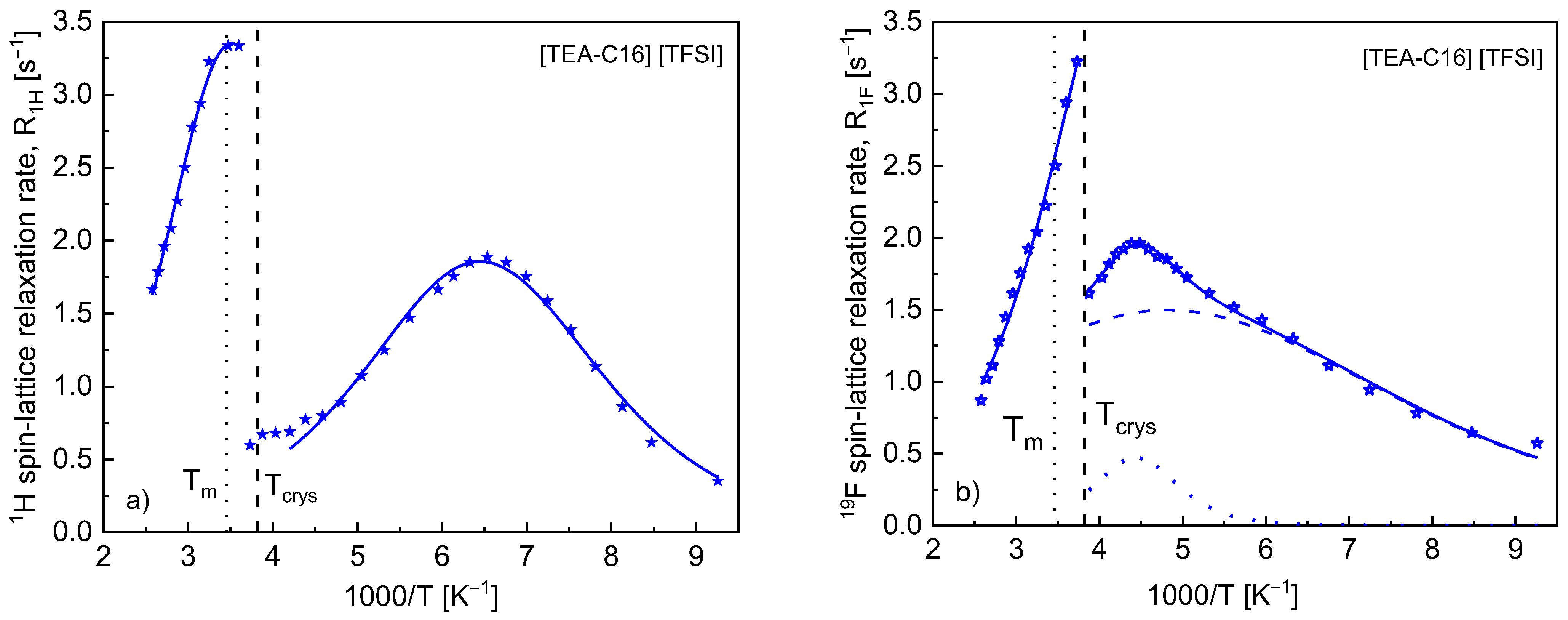
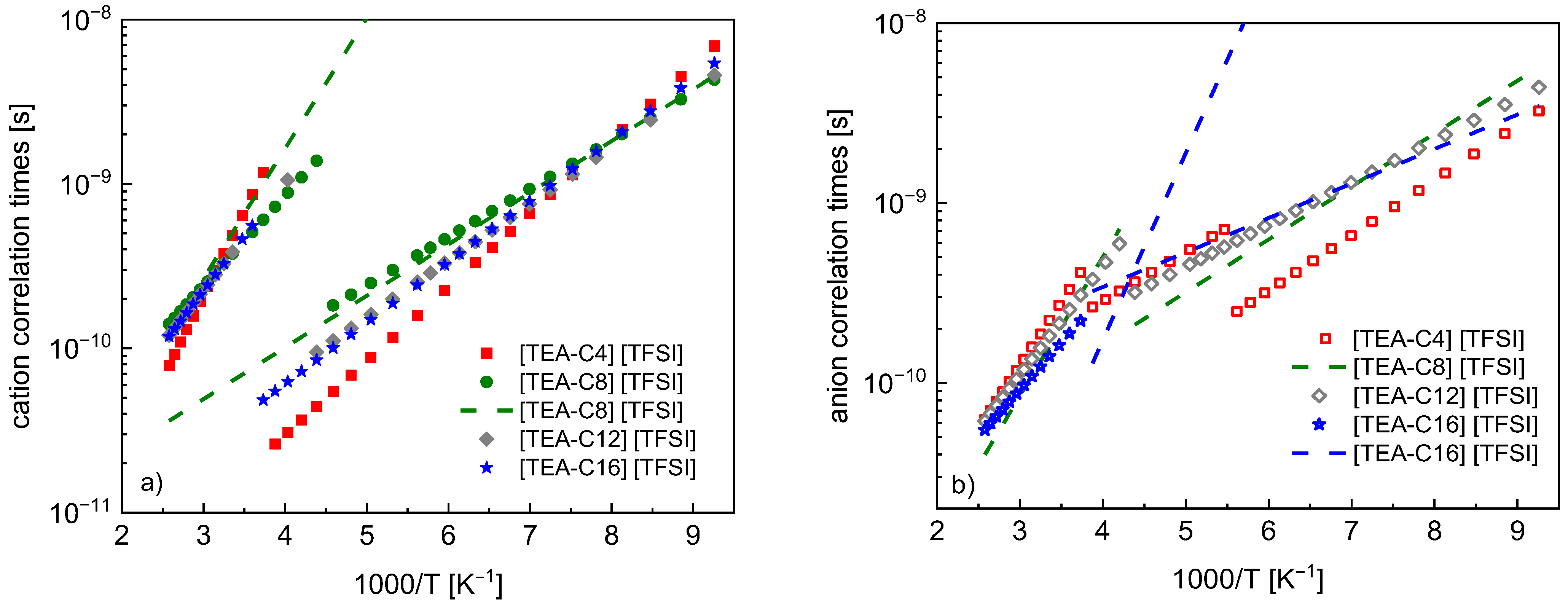
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weingärtner, H. NMR studies of ionic liquids: Structure and dynamics. Curr. Opin. Colloid Interface Sci. 2013, 18, 183–189. [Google Scholar] [CrossRef]
- Turguła, A.; Graś, M.; Gabryelczyk, A.; Lota, G.; Pernak, J. Long-Chain Ionic Liquids Based on Monoquaternary DABCO Cations and TFSI Anions: Towards Stable Electrolytes for Electrochemical Capacitors. Chempluschem 2020, 85, 2679–2688. [Google Scholar] [CrossRef]
- Peng, H.; Kubo, M.; Shiba, H. Molecular dynamics study of mesophase transitions upon annealing of imidazolium-based ionic liquids with long-alkyl chains. Phys. Chem. Chem. Phys. 2018, 20, 9796–9805. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Bartlewicz, O.; Dąbek, I.; Szymańska, A.; Maciejewski, H. Heterogeneous Catalysis with the Participation of Ionic Liquids. Catalysts 2020, 10, 1227. [Google Scholar] [CrossRef]
- Md Moshikur, R.; Chowdhury, M.R.; Moniruzzaman, M.; Goto, M. Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem. 2020, 22, 8116–8139. [Google Scholar] [CrossRef]
- Kordala-Markiewicz, R.; Rodak, H.; Markiewicz, B.; Walkiewicz, F.; Sznajdrowska, A.; Materna, K.; Marcinkowska, K.; Praczyk, T.; Pernak, J. Phenoxy herbicidal ammonium ionic liquids. Tetrahedron 2014, 70, 4784–4789. [Google Scholar] [CrossRef]
- Price, W.S. NMR Diffusometry. In Modern Magnetic Resonance; Springer: Dordrecht, The Netherlands, 2008; pp. 109–115. ISBN 978-1-4020-3910-2. [Google Scholar]
- Price, W.S. NMR Studies of Translational Motion; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Kruk, D.; Meier, R.; Rachocki, A.; Korpała, A.; Singh, R.K.; Rössler, E.A. Determining diffusion coefficients of ionic liquids by means of field cycling nuclear magnetic resonance relaxometry. J. Chem. Phys. 2014, 140, 244509. [Google Scholar] [CrossRef] [PubMed]
- Seyedlar, A.O.; Stapf, S.; Mattea, C. Dynamics of the ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethylsulphonyl)imide studied by nuclear magnetic resonance dispersion and diffusion. Phys. Chem. Chem. Phys. 2014, 17, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Kruk, D.; Wojciechowski, M.; Brym, S.; Singh, R.K. Dynamics of ionic liquids in bulk and in confinement by means of 1H NMR relaxometry—BMIM-OcSO4 in an SiO2 matrix as an example. Phys. Chem. Chem. Phys. 2016, 18, 23184–23194. [Google Scholar] [CrossRef]
- Kruk, D.; Wojciechowski, M.; Verma, Y.L.; Chaurasia, S.K.; Singh, R.K. Dynamical properties of EMIM-SCN confined in a SiO2 matrix by means of 1H NMR relaxometry. Phys. Chem. Chem. Phys. 2017, 19, 32605–32616. [Google Scholar] [CrossRef]
- Wencka, M.; Apih, T.; Korošec, R.C.; Jenczyk, J.; Jarek, M.; Szutkowski, K.; Jurga, S.; Dolinšek, J. Molecular dynamics of 1-ethyl-3-methylimidazolium triflate ionic liquid studied by 1H and 19F nuclear magnetic resonances. Phys. Chem. Chem. Phys. 2017, 19, 15368–15376. [Google Scholar] [CrossRef]
- Pilar, K.; Rua, A.; Suarez, S.N.; Mallia, C.; Lai, S.; Jayakody, J.; Hatcher, J.L.; Wishart, J.F.; Greenbaum, S. Investigation of dynamics in BMIM TFSA ionic liquid through variable temperature and pressure NMR relaxometry and diffusometry. J. Electrochem. Soc. 2017, 164, H5189–H5196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordikhani, A.; Stapf, S.; Mattea, C. Nuclear magnetic relaxation and diffusion study of the ionic liquids 1-ethyl- and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide confined in porous glass. Magn. Reson. Chem. 2019, 57, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, N.K.; Fraenza, C.C.; Greenbaum, S.G.; Ashby, D.; Dunn, B.S. NMR Relaxometry and Diffusometry Analysis of Dynamics in Ionic Liquids and Ionogels for Use in Lithium-Ion Batteries. J. Phys. Chem. B 2020, 124, 6843–6856. [Google Scholar] [CrossRef]
- Kruk, D.; Wojciechowski, M.; Florek-Wojciechowska, M.; Singh, R.K. Dynamics of Ionic Liquids in Confinement by Means of NMR Relaxometry—EMIM-FSI in a Silica Matrix as an Example. Materials 2020, 13, 4351. [Google Scholar] [CrossRef]
- Kruk, D.; Masiewicz, E.; Lotarska, S.; Markiewicz, R.; Jurga, S. Correlated Dynamics in Ionic Liquids by Means of NMR Relaxometry: Butyltriethylammonium bis(Trifluoromethanesulfonyl)imide as an Example. Int. J. Mol. Sci. 2021, 22, 9117. [Google Scholar] [CrossRef]
- Kimmich, R.; Anoardo, E. Field-Cycling NMR Relaxometry. ChemInform 2004, 35, 257–320. [Google Scholar] [CrossRef]
- Fujara, F.; Kruk, D.; Privalov, A.F. Solid state Field-Cycling NMR relaxometry: Instrumental improvements and new applications. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 82, 39–69. [Google Scholar] [CrossRef]
- Daniel, C. Introduction: General Theory of Nuclear Relaxation. Adv. Inorg. Chem. 2005, 57, 3–40. [Google Scholar] [CrossRef]
- Slichter, C.P. Principles of Magnetic Resonance; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 1990; ISBN 978-3-540-50157-2. [Google Scholar]
- Kowalewski, J.; Mäler, L. Nuclear Spin Relaxation in Liquids: Theory, Experiments, and Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–372. [Google Scholar] [CrossRef]
- Kruk, D. Understanding Spin Dynamics; Pan Stanford Publishing: Singapore, 2015; ISBN 9789814463492. [Google Scholar]
- Markiewicz, R.; Klimaszyk, A.; Jarek, M.; Taube, M.; Florczak, P.; Kempka, M.; Fojud, Z.; Jurga, S. Influence of Alkyl Chain Length on Thermal Properties, Structure, and Self-Diffusion Coefficients of Alkyltriethylammonium-Based Ionic Liquids. Int. J. Mol. Sci. 2021, 22, 5935. [Google Scholar] [CrossRef]
- Davidson, D.W.; Cole, R.H. Dielectric relaxation in glycerol, propylene glycol, and n-propanol. J. Chem. Phys. 1951, 19, 1484–1490. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Kahlau, R.; Kruk, D.; Blochowicz, T.; Novikov, V.N.; Rössler, E.A. Generalization of the Cole–Davidson and Kohlrausch functions to describe the primaryresponse of glass-forming systems. J. Phys. Condens. Matter 2010, 22, 365101. [Google Scholar] [CrossRef]
- Rault, J. Origin of the Vogel–Fulcher–Tammann law in glass-forming materials: The α–β bifurcation. J. Non-Cryst. Solids 2000, 271, 177–217. [Google Scholar] [CrossRef]
- Kubica-Misztal, A.; Rochowski, P.; Florek-Wojciechowska, M.; Kruk, D. Dynamics of solid alanine by means of nuclear magnetic resonance relaxometry. J. Chem. Phys. 2017, 146, 164501. [Google Scholar] [CrossRef] [PubMed]

| [TEA-C4][TFSI] | ||||||
|---|---|---|---|---|---|---|
| 1H | [Hz2] | [s] | (kJ/moL) | (Hz2) | (s) | (kJ/moL) |
| 3.96 × 109 (±7.7 × 107) | 1.87 × 10−13 (±5.8 × 10−14) | 19.49 (±0.82) | 3.56 × 109 (±4.0 × 107) | 4.73 × 10−13 (±5.2 × 10−14) | 8.61 (±0.14) | |
| 19F | [Hz2] | [s] | (kJ/moL) | (Hz2) | (s) | (kJ/moL) |
| (Hz2) | (s) | (kJ/moL) | ||||
| 2.32 × 109 (±4.9 × 107) | 9.36 × 10−13 (±1.6 × 10−13) | 13.54 (±0.51) | 1.46 × 109 (±5.4 × 106) | 2.34 × 10−11 (±2.2 × 10−12) | 5.20 (±0.17) | |
| 1.75 × 109 (±2.9 × 107) | 4.76 × 10−12 (±7.6 × 10−13) | 5.86 (±0.18) | ||||
| [TEA-C8][TFSI] | ||||||
|---|---|---|---|---|---|---|
| 1H | [Hz2] | (s) | (kJ/moL) | (Hz2) | (s) | (kJ/moL) |
| 3.14 × 109 (±4.5 × 107) | 5.38 × 10−12 (±8.6 × 10−13) | 10.52 (±0.39) | 3.07 × 109 (±1.9 × 107) | 8.16 × 10−12 (±6.1 × 10−13) | 5.63 (±0.09) | |
| [Hz2] | (s) | (kJ/moL) | (Hz2) | (s) | (kJ/moL) | |
| 2.30 × 109 (±4.1 × 107) | 1.12 × 10−12 (±2.1 × 10−13) | 15.16 (±0.48) | 3.03 × 109 (±2.2 × 107) | 5.62 × 10−12 (±5.8 × 10−13) | 6.01 (±0.12) | |
| 19F | [Hz2] | (s) | (kJ/moL) | (Hz2) | (s) | (kJ/moL) |
| 2.16 × 109 (±2.7 × 108) | 4.10 × 10−13 (±3.7 × 10−14) | 14.77 (±1.91) | 1.48 × 109 (±1.6 × 108) | 1.08 × 10−11 (±6.0 × 10−12) | 5.63 (±0.52) | |
| [TEA-C12][TFSI] | ||||||
|---|---|---|---|---|---|---|
| 1H | (Hz2) | (s) | (kJ/moL) | (Hz2) | (s) | (kJ/moL) |
| 3.45 × 109 (±3.1 × 107) | 2.56 × 10−12 (±2.3 × 10−13) | 12.42 (±0.22) | 2.14 × 109 (±2.8 × 107) | 2.88 × 10−12 (±4.0 × 10−13) | 6.62 (±0.18) | |
| 19F | (Hz2) | [s] | (kJ/moL) | (Hz2) | (s) | (kJ/moL) |
| (Hz2) | (s) | (kJ/moL) | ||||
| 3.07 × 109 (±3.8 × 107) | 1.68 × 10−12 (±1.8 × 10−13) | 11.61 (±0.30) | 1.90 × 108 (±4.4 × 107) | 1.17 × 10−17 (±4.1 × 10−18) | 15.82 (±2.40) | |
| 1.32 × 109 (±1.8 × 107) | 3.0 × 10−11 (±1.4 × 10−11) | 4.48 (±0.63) | ||||
| [TEA-C16][TFSI] | ||||||
|---|---|---|---|---|---|---|
| 1H | [Hz2] | (s) | (kJ/moL) | (Hz2) | (s) | (kJ/moL) |
| 2.95 × 109 (±2.3 × 107) | 2.35 × 10−12 (±3.6 × 10−13) | 12.64 (±0.44) | 1.63 × 109 (±2.3 × 107) | 1.99 × 10−12 (±2.6 × 10−13) | 7.10 (±0.17) | |
| 19F | [Hz2] | (s) | (kJ/moL) | (Hz2) | (s) | (kJ/moL) |
| (Hz2) | (s) | (kJ/moL) | ||||
| 3.64 × 109 (±6.2 × 108) | 2.42 × 10−12 (±3.9 × 10−13) | 10.06 (±0.84) | 1.32 × 109 (±1.4 × 108) | 5.84 × 10−11 (±1.9 × 10−11) | 3.67 (±0.25) | |
| 4.15 × 108 (±1.3 × 108) | 1.22 × 10−14 (±5.9 × 10−11) | 19.87 (±8.69) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, D.; Jancelewicz, M.; Klimaszyk, A.; Markiewicz, R.; Fojud, Z.; Jurga, S. Internal Dynamics of Ionic Liquids over a Broad Temperature Range—The Role of the Cation Structure. Materials 2022, 15, 216. https://doi.org/10.3390/ma15010216
Kruk D, Jancelewicz M, Klimaszyk A, Markiewicz R, Fojud Z, Jurga S. Internal Dynamics of Ionic Liquids over a Broad Temperature Range—The Role of the Cation Structure. Materials. 2022; 15(1):216. https://doi.org/10.3390/ma15010216
Chicago/Turabian StyleKruk, Danuta, Mariusz Jancelewicz, Adam Klimaszyk, Roksana Markiewicz, Zbigniew Fojud, and Stefan Jurga. 2022. "Internal Dynamics of Ionic Liquids over a Broad Temperature Range—The Role of the Cation Structure" Materials 15, no. 1: 216. https://doi.org/10.3390/ma15010216
APA StyleKruk, D., Jancelewicz, M., Klimaszyk, A., Markiewicz, R., Fojud, Z., & Jurga, S. (2022). Internal Dynamics of Ionic Liquids over a Broad Temperature Range—The Role of the Cation Structure. Materials, 15(1), 216. https://doi.org/10.3390/ma15010216








