1. Introduction
Geopolymers are inorganic polymeric materials that have an amorphous or semi-crystalline structure and are similar to zeolites in chemical composition. For the production of geopolymer materials, a precursor is used, which is aluminosilicate material with high contents of silicon and aluminium oxides and an alkaline solution of Na (sodium) or K (potassium) silicates and hydroxides. Initially, geopolymers were developed as more temperature-resistant alternatives to thermoset polymers, but they are also currently being developed as construction materials with various applications [
1].
A cement-free binder is one of their applications, in which Portland cement (PC) is replaced to manufacture a high-quality matrix for construction materials: geopolymer concretes and mortars. The use of geopolymer binders reduces CO
2 emissions associated with Portland cement production. In addition, aluminosilicate precursors, which are industrial waste [
2] such as FA (fly ash) and GGBFS (ground granulated blast furnace slag) or mining tailings or waste andesite dust [
3] are often used in the process of geopolymer synthesis.
FA is a by-product produced in coal-fired power plants. The composition of this waste material is significantly variable, depending on the coal source and burning conditions. The decisive factors that control the reactivity of the precursor and the solubility in alkaline solutions are the chemical composition, the contents of the crystalline and glass phases and the particle size distribution. The results of the research indicate that the microstructure of the geopolymer is particularly influenced by the particle fineness, the composition of the amorphous phase and the content of oxides [
4]. Blast furnace slag is formed as a by-product of iron ore smelting. It is calcium-rich raw material, used in the production of mineral binders. Ground granulated blast furnace slag is a mixture highly glassy phases with a composition close to that of gehlenite and akermanite [
4].
The properties and durability of composites based on geopolymer binders are sometimes even better than those obtained with Portland cement [
5]. High compressive strength is associated with the interconnected structure of oligomers [
6,
7].
Such materials present beneficial properties in harsh environmental conditions such as acid and sulphate attack, chloride ingress and thermal and fire conditions (T = 1000–1200 °C) [
7,
8]. Moreover, geopolymer matrix materials may undergo rapid setting, still presenting suitable long-term strength [
6].
The geopolymers show a great potential also in 3D printing manufacturing both in additive manufacturing, where specific rheological performances need to be ensured [
9,
10], but also in a powder-based 3D printer dry geopolymer-based material achieved satisfactory results [
11].
The mechanisms of geopolymer products formation are complex. According to [
12], the geopolymer synthesis process consists of the following phases: alkalinization, depolymerisation of silicates, gel formation of oligo-sialates, polycondensation, reticulation, networking and geopolymer solidification. If these reactions are to proceed correctly, they require the supply of aluminosilicate raw materials with a high content of SiO
2 and Al
2O
3 and the appropriate amount of alkali metal Na or K ions in an alkaline solution [
13]. An alkaline medium is required to enable silica and alumina dissolving and also to ensure hydrolyse of the raw material particle surfaces [
12,
14]. Scientific literature related to geopolymers and alkali-activated materials (AAM) offers a number of possibilities regarding the type of alkaline solution to be used and the proportion of alkali species such as hydroxides and/or silicates in the activating solution. The typically used solution is a mix of liquid silicates and hydroxides (solids dissolved in water) [
13]. The commonly used liquid silicate solutions have a molar modulus of about 2.5. The molar modulus (molar ratio) or silicate modulus, is the basic characteristic of aqueous solutions of sodium or potassium silicate equal to the ratio of the number of moles of silicon oxide to moles of metal oxide. The synthesis process of geopolymeric materials is much more effective when using solutions with a modulus below 2.0. Hence, it is necessary to combine silicate solutions with hydroxide. The purpose of this combination is to lower the value of the molar modulus of the alkaline mixture. In the conducted research, we used Geosil
® ready-to-use products with a molar module of 1.7, dedicated to the geopolymer binder. This solution is convenient in the application and safer for the user. It reduces the troublesome process of mixing alkaline substances and improves the process of preparing a geopolymer mix. Such technological facilitations also extend the possibilities of using geopolymer materials [
15]. As alkali-activators have been suggested to be a contributor to the environmental burdens due to the energy-demanding production process, there are studies showing the possibilities to produce alkali-activated materials from chemically modified waste-derived activators: waste glass and rice husk ash [
16] therefore, the environmental impact of geopolymers can be significantly reduced.
Another important factor in the technology of geopolymer materials is curing parameters, such as the curing temperature and moisture exchange with the surroundings. For FA-based geopolymers, thermal curing is necessary for setting and geopolymerisation to occur [
17]. This is considered an important limitation for the application of those materials. Furthermore, the need to heat the fly ash geopolymers increases the energy consumption of the entire process. Using Ca-rich raw material such as ground granulated blast-furnace slag in combination with fly ash, as the former reacts at room temperature, gives promising results in the production of calcium–aluminium–silicate–hydrate (C–A–S–H) gel.
Moreover, the reaction develops quickly, which means a rapid initial setting time. Previous results have shown that the blend of FA–GGBFS [
18,
19] reacts at room temperature. Changing the ratio between FA and GGBFS allows adjusting the setting time. This requires further research to provide more data to better understand the geopolymer binding mechanisms of such binary systems.
This research presents an investigation into how the low sodium or potassium silicate molar ratio solution affects properties of the blend of FA- and GGBFS-based geopolymer mortars. During geopolymerisation, the alkaline solution plays a crucial role in the material mechanical properties development. The most important characteristics of an alkaline solution are (1) proportions between the hydroxide and the silicate, (2) hydroxide concentration [
20], (3) type of cations [
21] and (4) the molar ratio of the alkaline solution. All these parameters affect the mechanical behaviour of geopolymers. The main goal and ambition of this study is to show the impact of the silicate solution type (sodium or potassium) on the microstructure development, porosity and mechanical properties of mortars with geopolymer matrix. A one-component alkaline solution was used in this study, which facilitated the geopolymer mixture preparation process, limiting the arduous hydroxide dissolution procedure.
2. Materials and Methods
2.1. Raw Materials
The precursor for this research was composed of fly ash, which is waste from coal combustion, containing an abundance of silica and alumina (SiO
2 and Al
2O
3). The oxide composition of FA is shown in
Table 1; the FA specific mass was 2.1 g/cm
3. Following the ASTM C618 document [
22], this fly ash can be classified as F class ash. The FA also meets the standard EN 450-1:2012 [
23] requirements for concrete additive type II.
The second component of the precursor was ground granulated blast furnace slag. This CaO-rich raw material makes it possible for the mixture to bind at ambient temperature. The chemical composition was specified by the supplier, and the detected oxides are presented in
Table 2. The GGBFS specific mass was 2.9 g/cm
3.
Commercially available liquid silicates were used as the alkali source. Sodium silicate (Na-Sil) and potassium silicate (K-Sil) were used. The molar ratio (MR) of liquid silicates is the activating solution mole ratio of silica to sodium oxide or to potassium oxide. The MR of 1.7 was the same for both solutions. Their chemical specifications are presented in
Table 3.
In this study, the ratio between the total water mass (i.e., sum of mass of water added and mass of water in the liquid silicates) and the total mass of binder is the water/binder ratio. The water/binder ratio was kept constant at 0.30 for all mortars. In these considerations the binder mass is the sum of FA and GGBFS masses. The amount of additional water necessary to obtain the workable mixture was determined based on previous research [
24] and the extra water was added directly to the liquid silicates. Quartz sand was used (d = 0/2 mm; specific mass of 2.64 g/cm
3) for preparing all the geopolymer mortars. The particle size distribution of quartz sand is given in
Table 4 corresponded to standardized curve for sands used for mortars (CEN Standard Sand according to EN 196-1 [
25].
The sand to binder mass ratio was kept constant, with a value of 1.50. For all materials, the mass ratio of the FA, GGBFS and sand was the same. Three levels of GGBFS addition were used: 10%, 30% and 50% of FA mass.
To assess the influence of the sodium or potassium silicate solution on the properties of the geopolymer mortars, three mortars were manufactured. Two types of liquid silicates were used to prepare each mortar: K-Sil and Na-Sil, which are potassium silicate solution and sodium silicate solution, respectively. The details on the mix compositions are in
Table 5.
The differences in density between FA and GGBFS required mass shares of other components to be adjusted. This enabled to obtain the same volume of mortar for different ratios of fly ash and ground granulated blast furnace slag masses.
2.2. Preparation of Mortars and Samples
Geopolymer mortars were produced according to the procedure which first involves the preparation of the binder. First, supplementary water was added to the liquid silicates, which is required to obtain the water/binder ratio. Subsequently, FA was mixed with the alkaline-activating solution. Mixing was performed in a rotary mixer for 10 min at a low speed. In the next step, ground granulated blast furnace slag was added and 5 min mixing was applied. In this way, the geopolymer binder was obtained. While mixing the binder, the rotary mixer was switched off for 1 min to remove the solids stuck to the walls of the container. Finally, sand was gradually added to the mixture for 3 min, while the rotary mixer worked at low speeds. The total mixing duration time was aprox. of 20 min it has been selected based on literature reports [
12,
26] and optimised as the result of the team’s previous experience in the preparation of geopolymer blends. This mixing time guarantees the homogenization of the mixture of precursor and activator. Shorter mixing periods should be applied when a high amount of GGBFS is used in FA blended geopolymer due to the flash-setting in mixes where a high CaO amount is provided [
27,
28].
The geopolymer mortars were moulded into prismatic 40 × 40 × 160 mm specimens. The samples were compacted on a shaking table and covered with plastic lids. Twenty-four hours after casting, the specimens were removed from moulds and stored at room temperature (18 °C ± 2 °C) and relative humidity HR = 75%, protected from water evaporation using plastic to limit moisture exchange with the environment.
2.3. Mechanical Tests
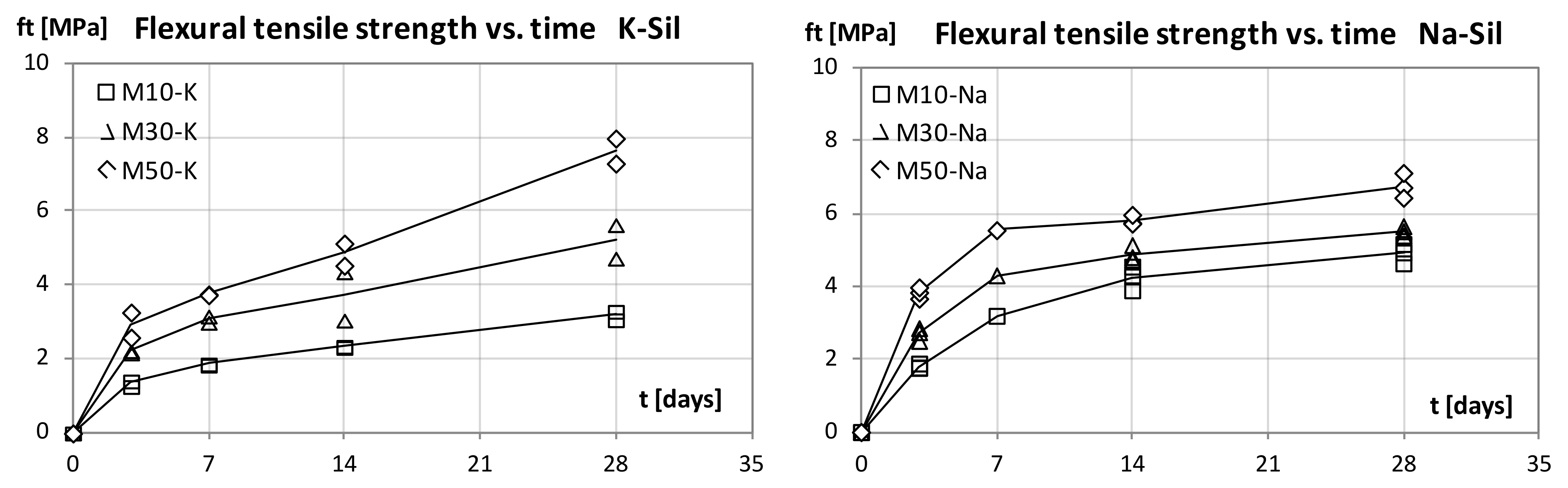
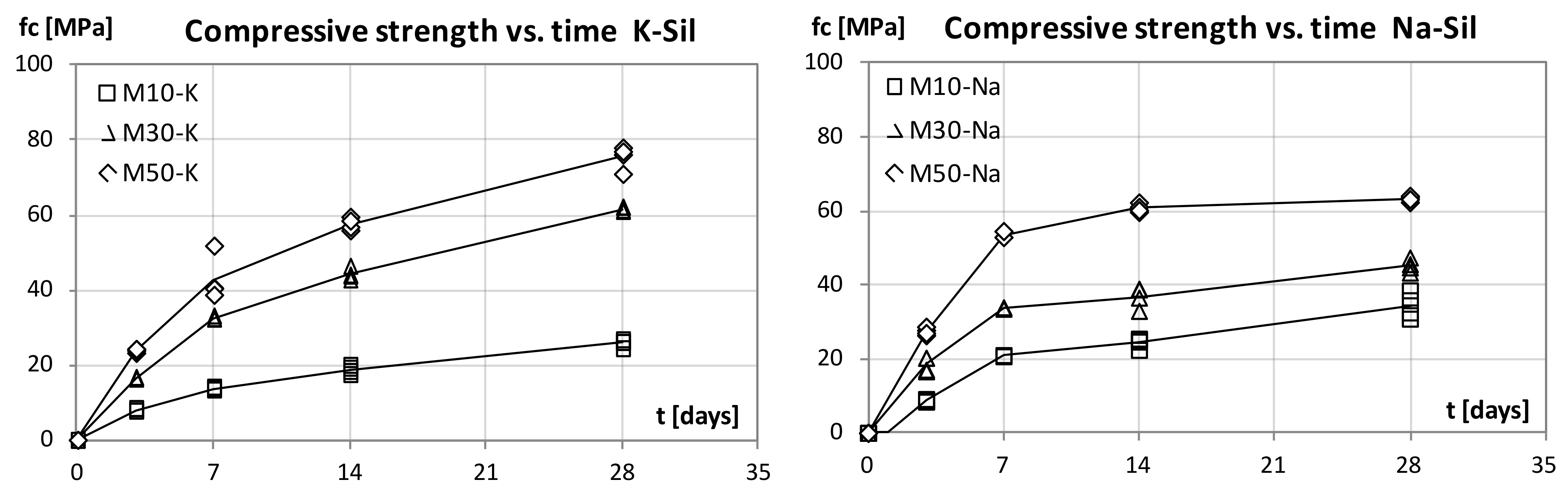
Bending and compressive strength tests were performed in a mechanical device (CONTROLS, construction materials testing equipment). For bending, two 40 × 40 × 160 mm prismatic specimens at each age (3, 7, 14 and 28 days) were tested for each mortar type. The loading rate applied was of 50 N/s, as this is recommended in PN-EN 196-1 for testing cement mortars. Apart from the bending test, compressive strength analysis was also carried out on the samples of the same age. The mortar prisms used in these tests were the ones remaining from the bending test. During the compressive tests, the loading rate was 2400 N/s, as this is also the adequate value according to PN-EN 196-1.
2.4. SEM and MIP
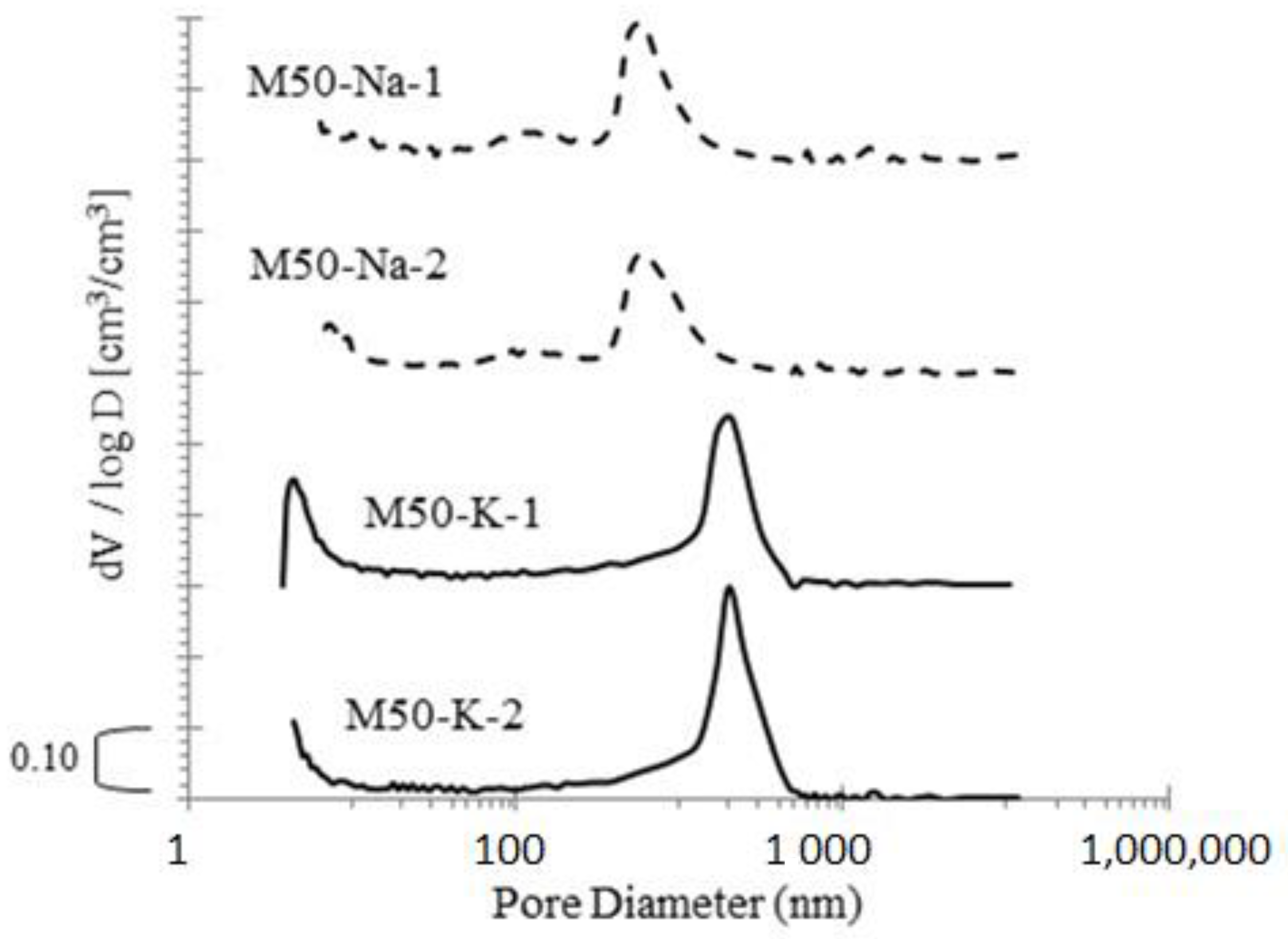
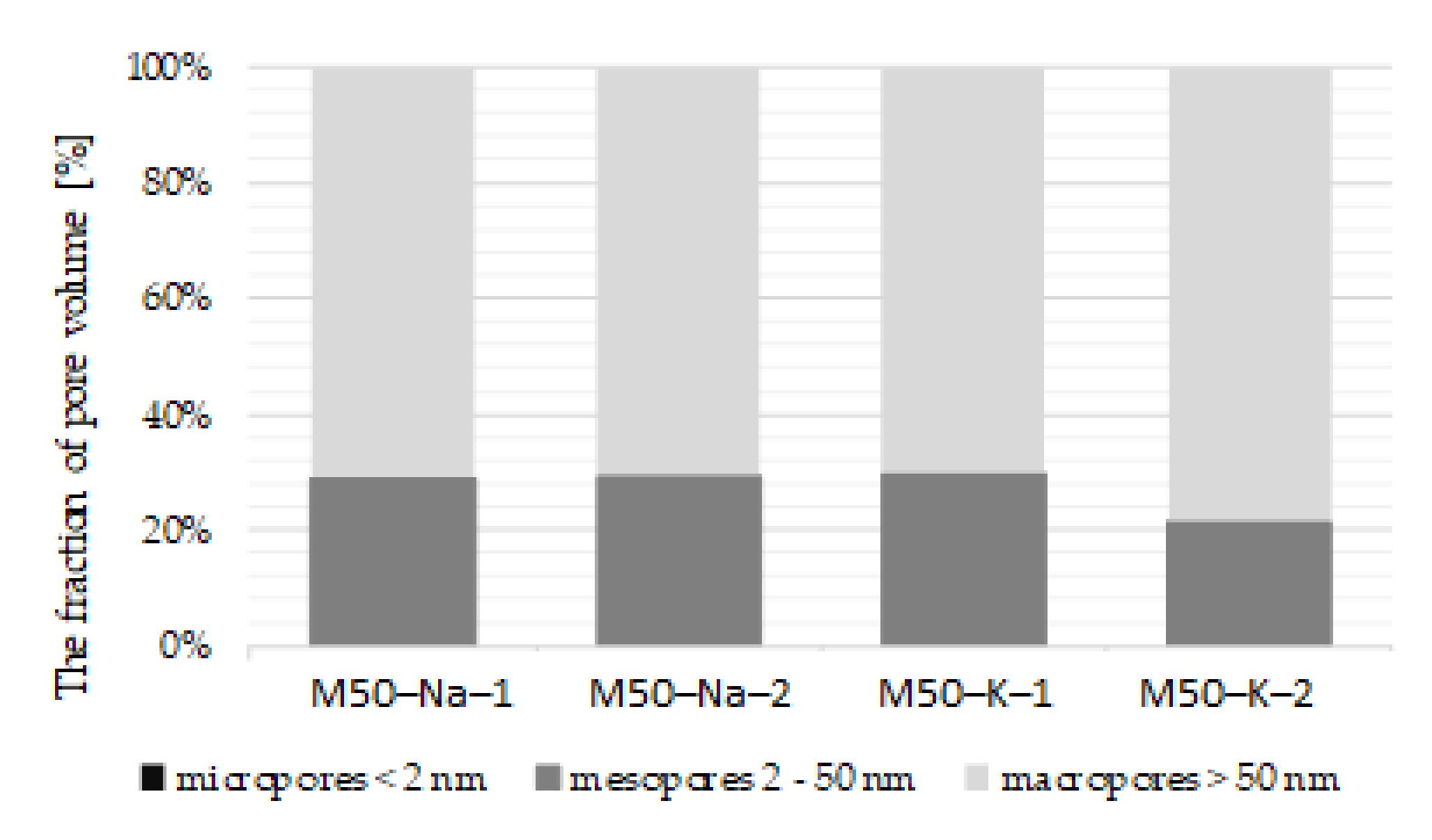
Scanning electron microscopy (SEM, Zeiss EVO-MA 10) observations and mercury intrusion porosimetry (MIP, PoreMaster 33 Automatic Pore Size Analyzer made by Quantachrome) were used for microstructure analysis and to evaluate total porosity, pore size distribution and volume of pores. SEM observations, as well as an EDS chemical analysis, and MIP were carried out for mortars with 50% FA and 50% GGBFS blended binders. After 28 days of curing, porosity (MIP) and SEM observations were conducted.
4. Conclusions
This study focused on the examination of the mechanical properties of FA–GGBFS blended geopolymer mortars. Mortars that contained three different levels of GGBFS, activated with the sodium or potassium alkaline solution, were compared. All prepared materials were set and hardened at ambient temperature with no additional heating. For the geopolymer mortars with blended binders, the compressive strength at 28 days was higher than 75 MPa for 50% FA and 50% GGBFS blended precursors with the potassium alkaline solution. We observed that, as the amount of GGBFS used increases, the strength of the material grows. Mortars with the sodium alkaline solution were characterised by a higher strength at a young age (3 and 7 days). However, the values of strength 28 days after casting were higher for geopolymer mortars with the potassium alkaline solution.
Observations of the blended FA–GGBFS geopolymer mortar microstructure indicate a high matrix heterogeneity with numerous microcracks. Matrix defects may be caused by the rapid kinetics of the material binding reaction or shrinkage associated with the drying of the material. In the analysed case, with a high content of GGBFS in the binder, there were no evidently visible differences between the alkaline solution types that we used. The structure of the material was primarily determined by the precursor that was used. The presence of phases N(K)–A–S–H and C–A–S–H in the material can be deduced from the matrix chemical analysis and EDS observations. The higher values of strength for highly dosed GGBFS were associated with the more compact and dense structure of C–A–S–H in the material.
The results of the presented research provide information on the influence of the alkaline factor on the selected properties of the geopolymeric mortar with mixed precursor (FA-GGBFS). The observations resulting from the research allow to state that the type of the alkali metal cation significantly influences the structure of the created geopolymer matrix. Smaller sodium ions can move more easily in the geopolymeric matrix and more effectively initiate the geopolymerization reaction by more intensive release of silicate and aluminate monomers. On the other hand, larger potassium ions may be responsible for the formation of a strong geopolymeric backbone in the structure of the material. The binding mechanism of aluminosilicate materials in an alkaline environment is complex and still not fully understood. The authors of the research plan to extend the work carried out and a detailed structural analysis of the developed binders.
The variety of raw materials used, as well as chemical and morphological differentiation cause difficulties in creating universal rules in geopolymer technology. For alkali activated materials it is most advantageous to use locally available waste materials. Therefore, it is very often necessary to develop technology taking into account the nature of the specific, available raw material.