Corrosion Behavior and Susceptibility to Stress Corrosion Cracking of Leaded and Lead-Free Brasses in Simulated Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Aggressive Environments
- -
- 100 ppm Cl− (0.0028 M NaCl) + 400 ppm SO42− (0.0042 M Na2SO4) + 50 ppm NO3− (0.00081 M NaNO3);
- -
- 400 ppm Cl− (0.0112 M NaCl) + 400 ppm SO42− (0.0042 M Na2SO4) + 50 ppm NO3− (0.00081 M NaNO3);
- -
- 700 ppm Cl− (0.0196 M NaCl) + 400 ppm SO42− (0.0042 M Na2SO4) + 50 ppm NO3− (0.00081 M NaNO3).
2.2. SEM-EDS Surface Analyses
2.3. Electrochemical Tests
2.4. Slow Strain Rate Tests (SSRT)
3. Results
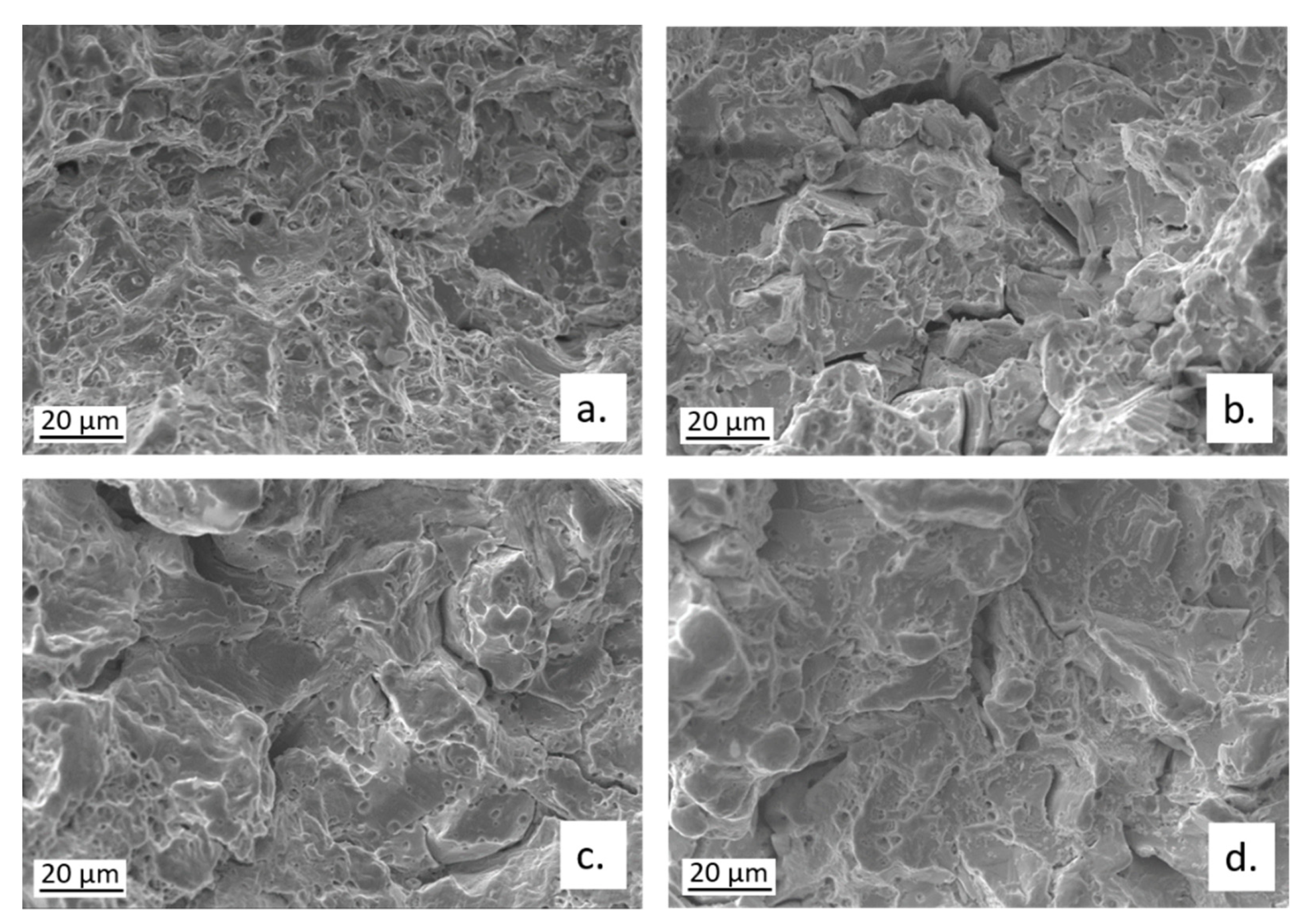
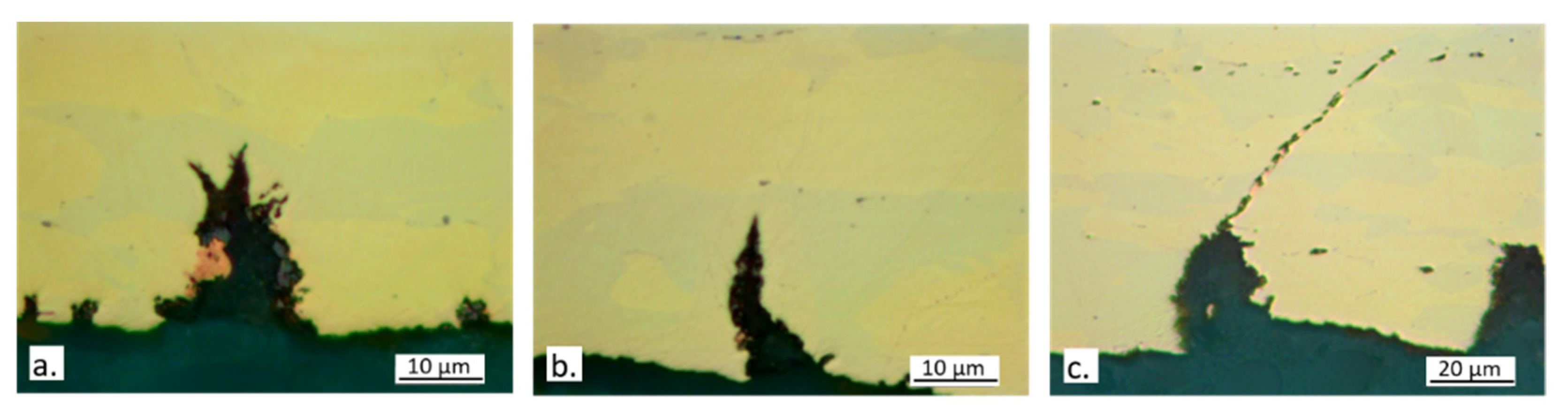
3.1. SEM-EDS Surface Analyses
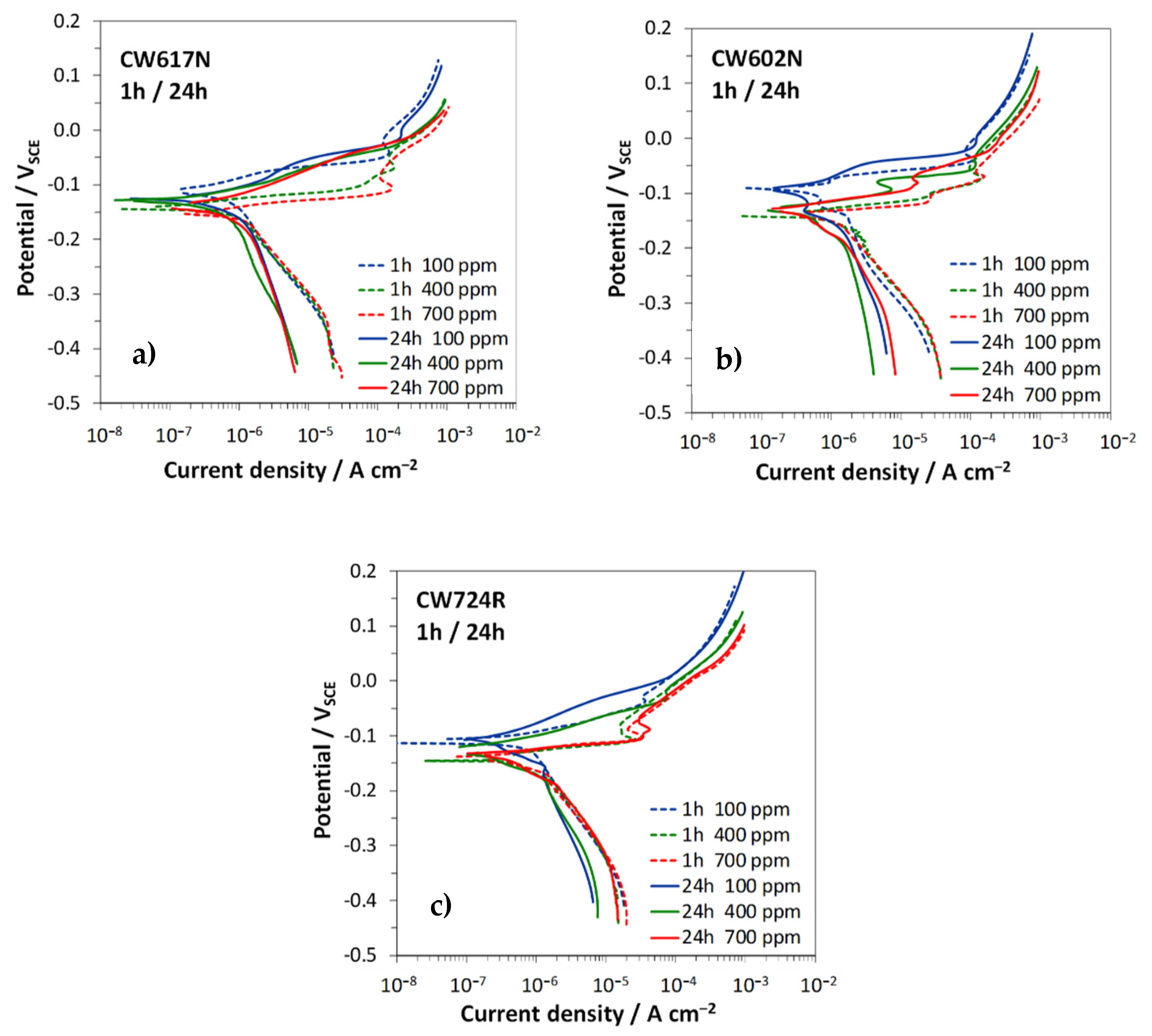
3.2. Polarization Curves
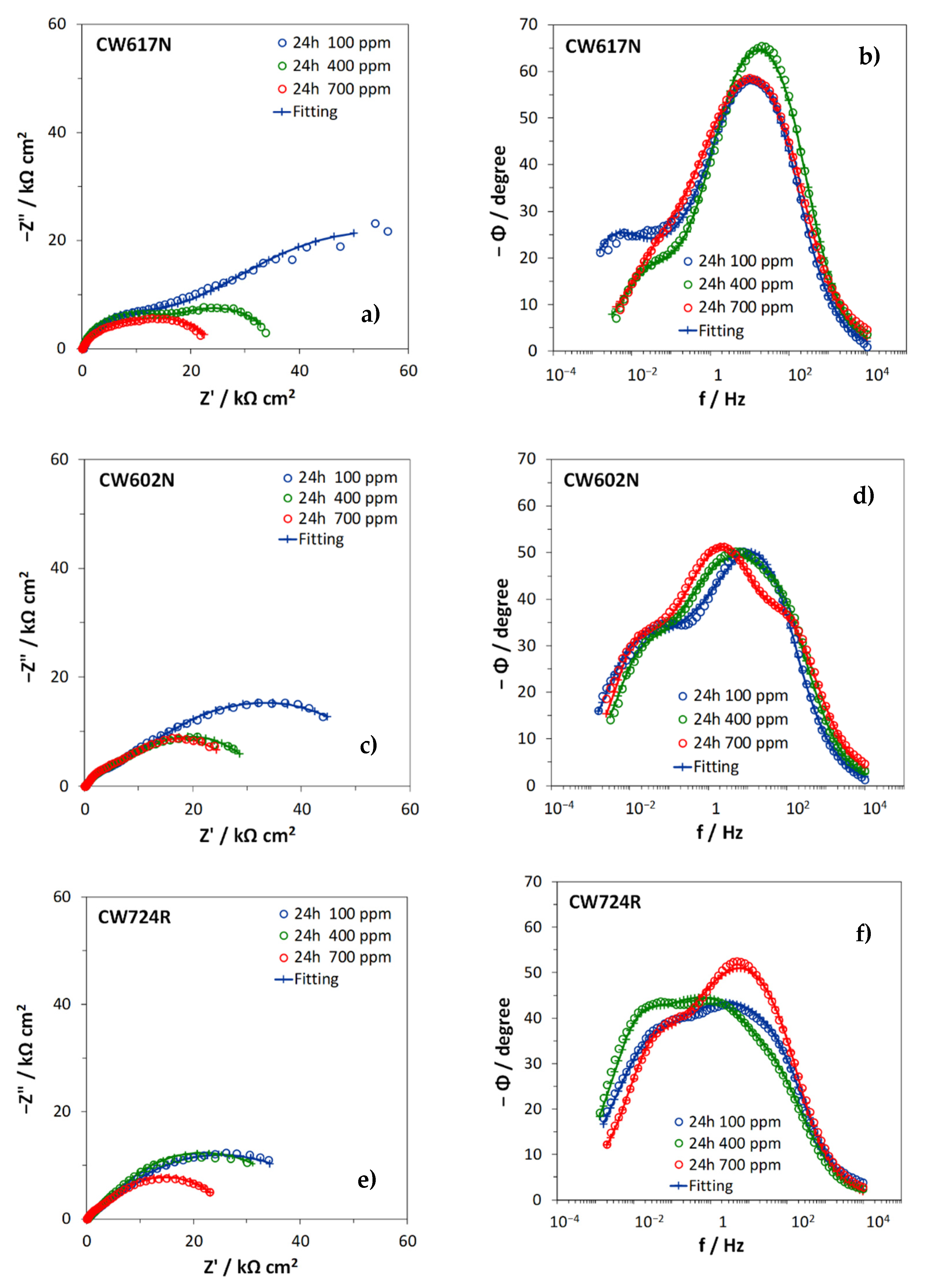
3.3. Electrochemical Impedance Spectroscopy Tests
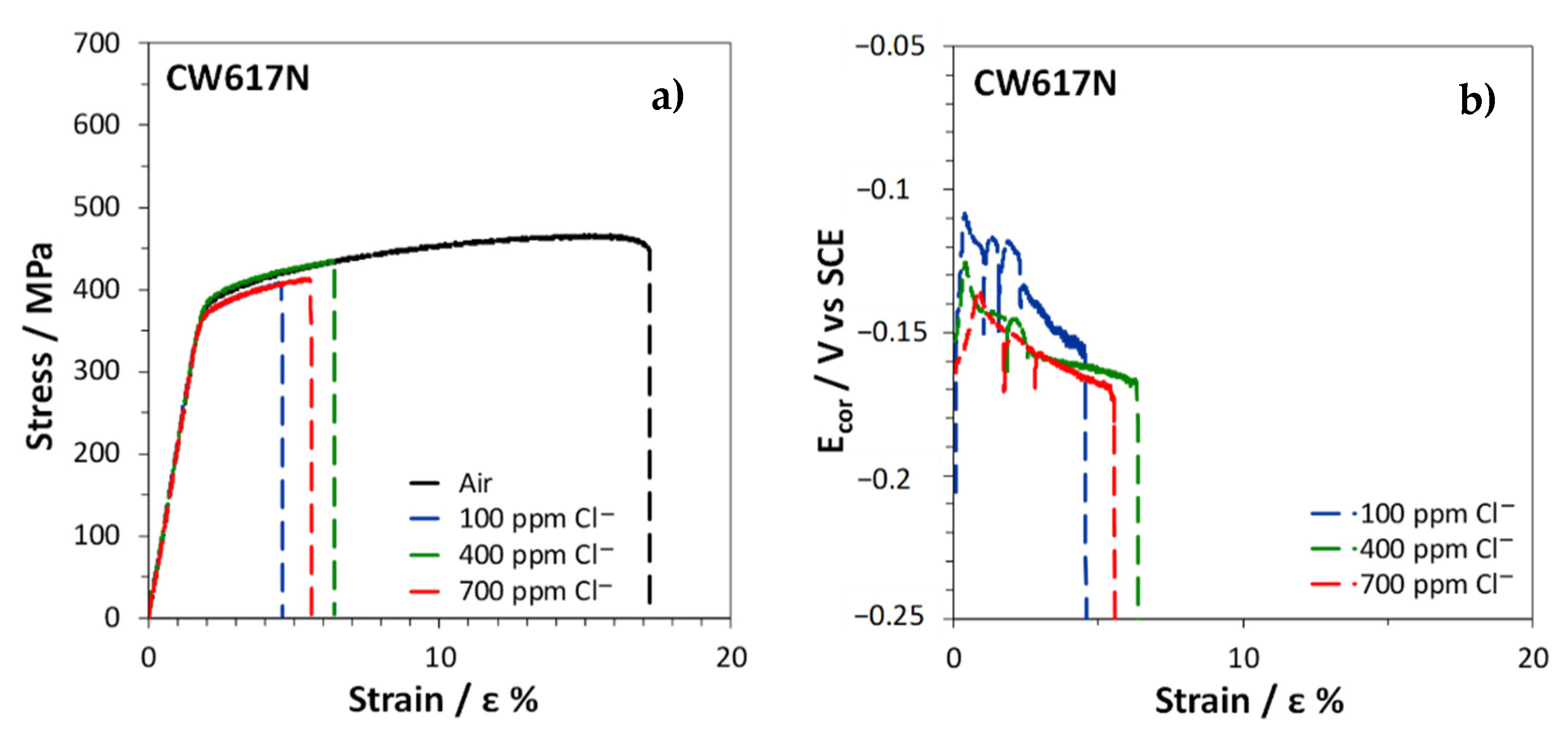
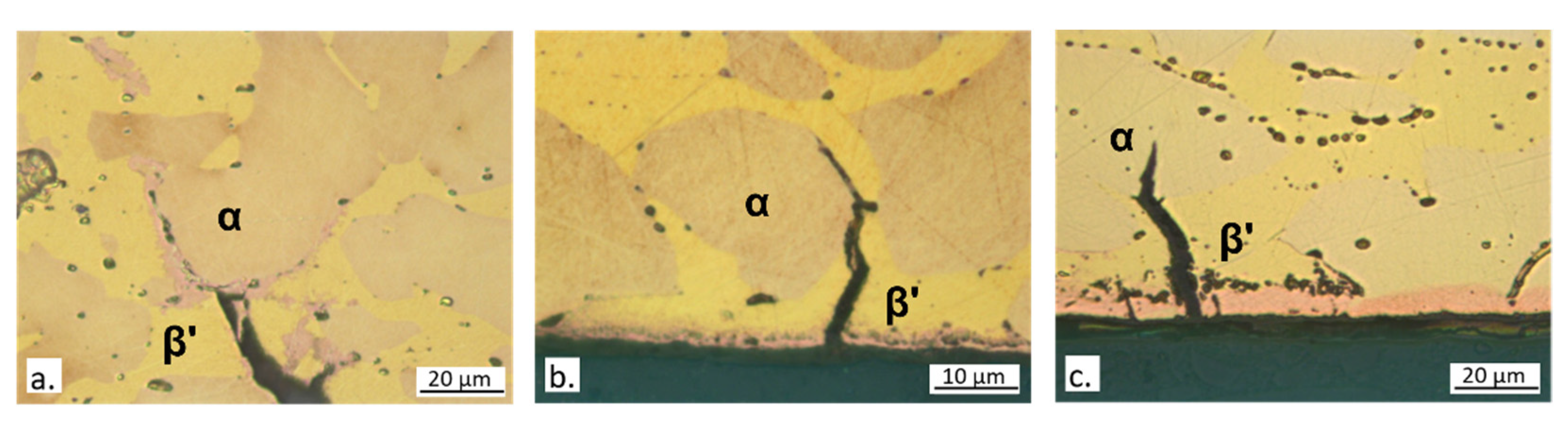
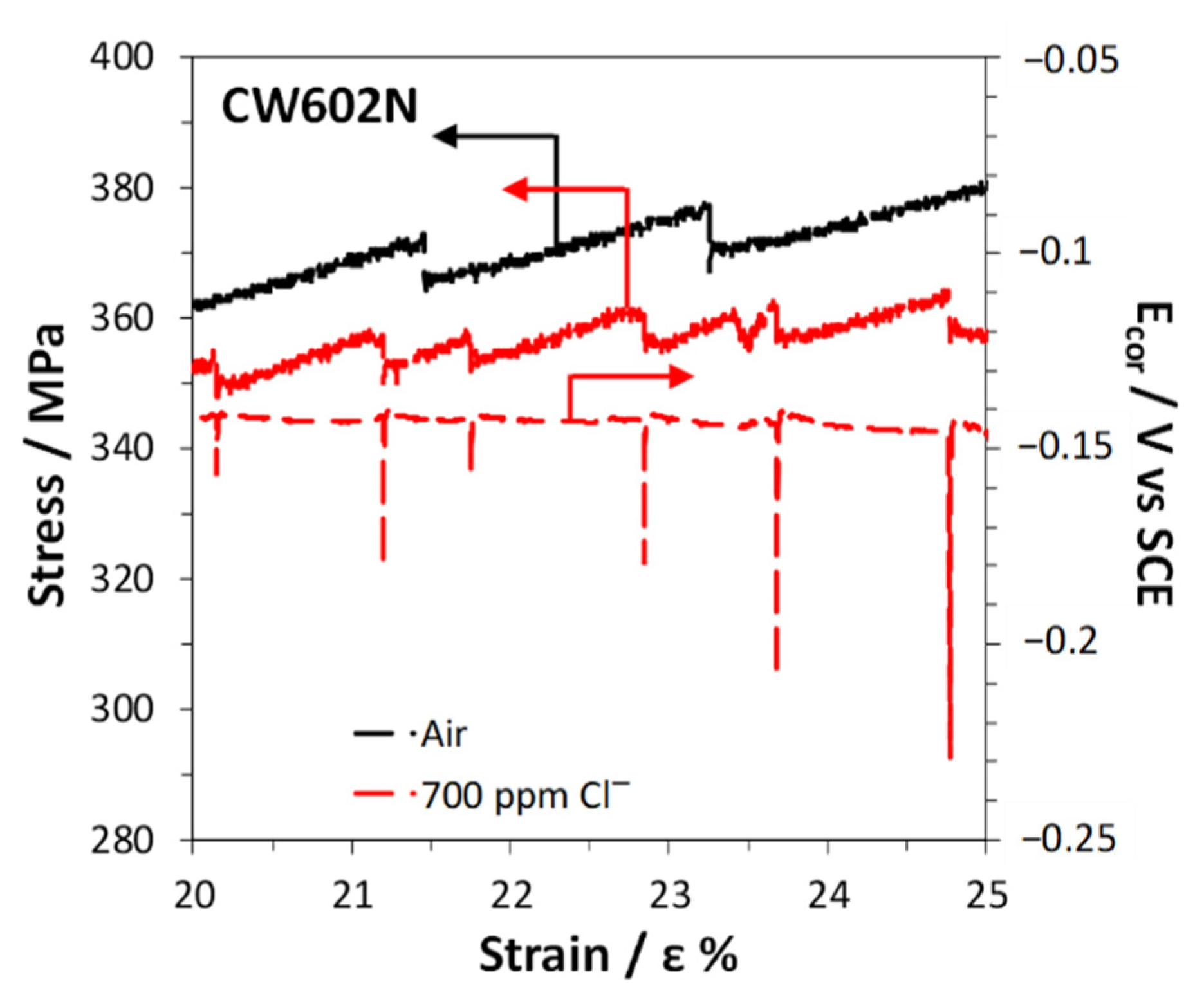
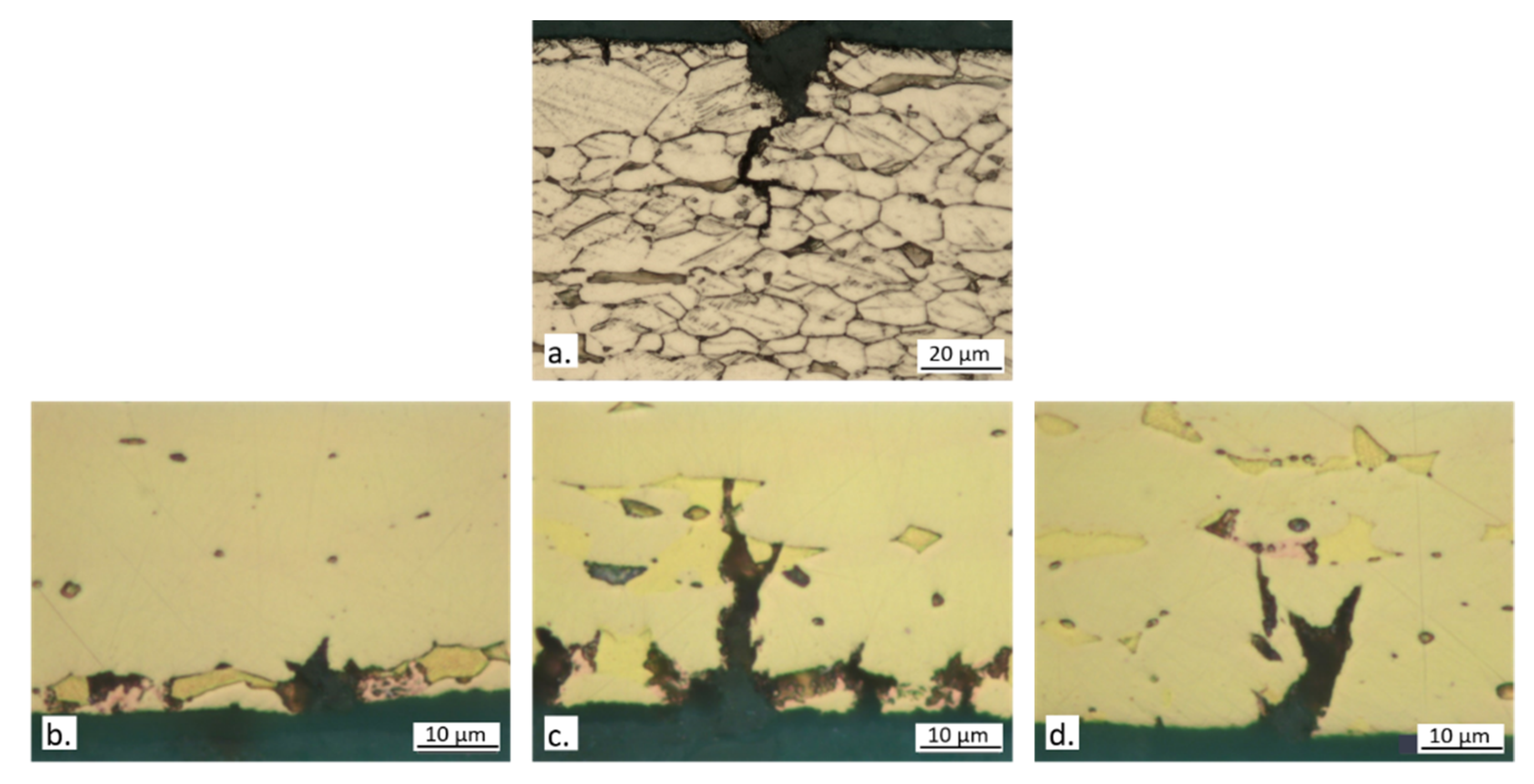
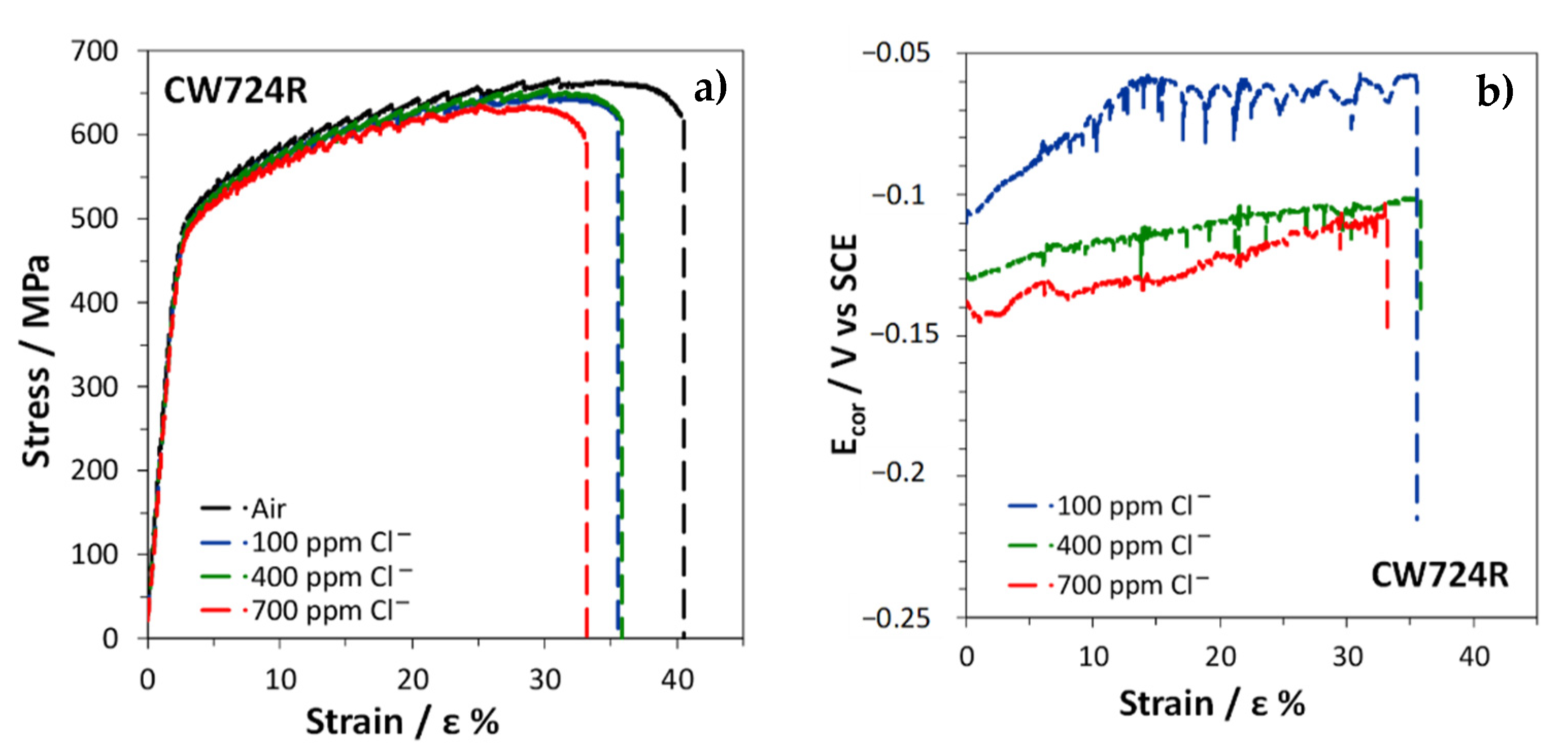
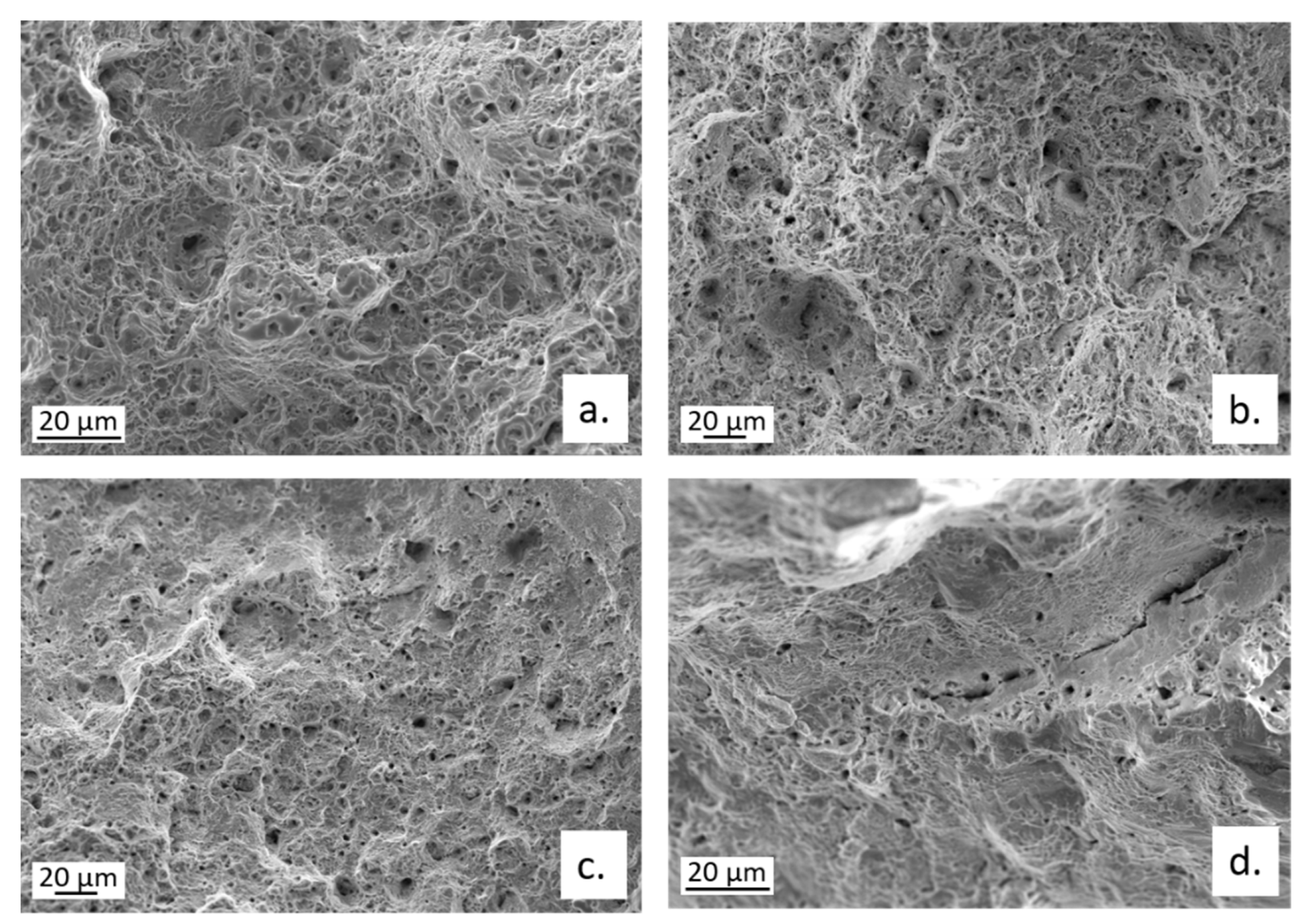
3.4. Slow Strain Rate Tests
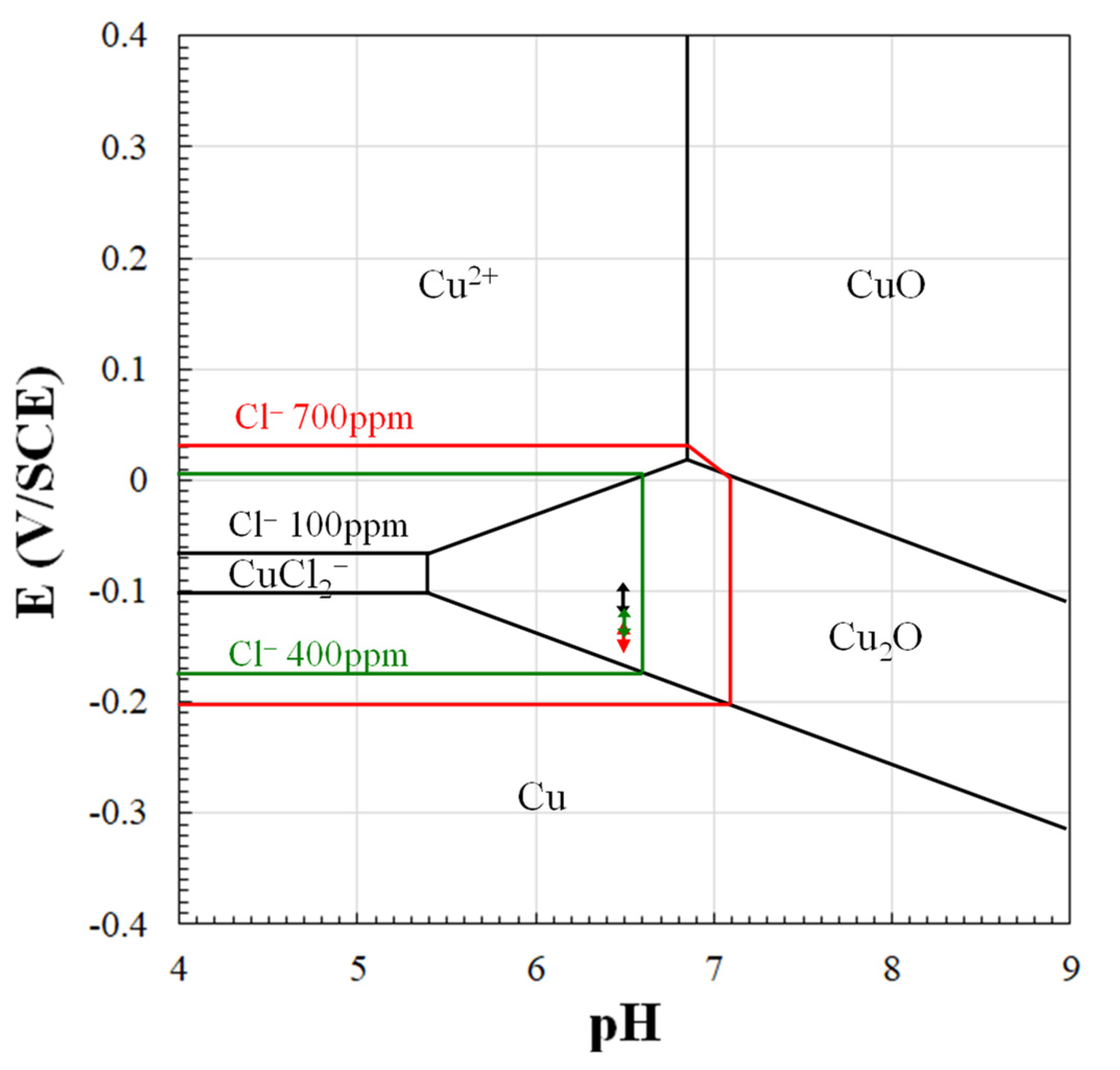
4. Discussion
CuClads + Cl− ⇄ CuCl2−
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pickering, H.W. Electrolytic dissolution of binary alloys containing a noble metal. J. Electrochem. Soc. 1967, 144, 698–706. [Google Scholar] [CrossRef]
- Assouli, B.; Srhiri, A.; Idrissi, H. Characterization and control of selective corrosion of α, β’-brass by acoustic emission. NDT E Int. 2003, 36, 117–126. [Google Scholar] [CrossRef]
- Jagodzinski, Y.; Aaltonen, P.; Smuk, S.; Tarasenko, O.; Hänninen, H. Internal friction study of environmental effects on metals and alloys. J. Alloys Compd. 2000, 310, 256. [Google Scholar] [CrossRef]
- Ahmed, N.; Tinker, E.B. Kinetic studies of the dissolution of brass. Can. J. Chem. Eng. 1967, 45, 110–114. [Google Scholar] [CrossRef]
- Horton, R.M. New metallographic evidence for dezincification of brass by redisposition of copper. Corrosion 1970, 26, 160–163. [Google Scholar] [CrossRef]
- Sitnikov, A.D.; Pchel’nikov, A.P.; Marshakov, I.K.; Losev, V.V. Laws of dezincing of alpha-brasses during corrosion in chloride solutions. Prot. Met. 1979, 15, 24–27. [Google Scholar]
- Polunin, A.V.; Pchel’nikov, A.P.; Losev, V.V.; Marshakov, I.K. Electrochemical studies of the kinetics and mechanism of brass dezincification. Electrochim. Acta 1982, 27, 467–475. [Google Scholar] [CrossRef]
- Zhou, P.; Hutchison, M.J.; Erning, J.W.; Scully, J.R.; Ogle, K. An in situ kinetic study of brass dezincification and corrosion. Electrochim. Acta 2017, 229, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Heidersbach, R.H., Jr.; Verink, E.D., Jr. The Dezincification of Alpha and Beta Brasses. Corrosion 1972, 28, 397–418. [Google Scholar] [CrossRef]
- Zhou, P.; Erning, J.W.; Ogle, K. Interactions between elemental components during the dealloying of Cu-Zn alloys. Electrochim. Acta 2019, 293, 290–298. [Google Scholar] [CrossRef]
- Oishi, K.; Sasaki, I.; Otani, J. Effect of silicon addition on grain refinement of copper alloys. Mater. Lett. 2003, 57, 2280–2286. [Google Scholar] [CrossRef]
- Eremiáš, B.; Převorovský, D.; Janík, V.; Faltus, J. Chemical composition of new copper alloys for machining and its effect on their susceptibility to corrosion cracking. Mater. Corros. 2007, 58, 681–686. [Google Scholar] [CrossRef]
- Seuss, F.; Gaag, N.; Virtanen, S. Corrosion mechanism of CuZn21Si3P in aggressive tap water. Mater. Corros. 2016, 68, 42–49. [Google Scholar] [CrossRef]
- Choucri, J.; Zanotto, F.; Grassi, V.; Balbo, A.; Ebn Touhami, M.; Mansouri, I.; Monticelli, C. Corrosion behavior of different brass alloys for drinking water distribution systems. Metals 2019, 9, 649. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathi, A.; Polan, N.W. Stress Corrosion Cracking of Cu/Zn and Cu/Zn/Ni Alloys: The Role of Dealloying. Metall. Mater. Trans. A 1982, 13, 2027–2033. [Google Scholar] [CrossRef]
- Guo, X.Z.; Gao, K.W.; Qiao, L.J.; Chu, W.Y. Stress corrosion cracking relation with dezincification layer-induced stress. Metall. Mater. Trans. A 2001, 32, 1309–1312. [Google Scholar] [CrossRef]
- Guo, X.J.; Gao, K.W.; Qiao, L.J.; Chu, W.Y. The correspondence between susceptibility to SCC of brass and corrosion-induced tensile stress with various pH values. Corros. Sci. 2002, 44, 2367–2378. [Google Scholar] [CrossRef]
- Gao, K.W.; Chu, W.Y.; Li, H.L.; Liu, Y.P.; Qiao, L.J. Correspondence between hydrogen enhancing dezincification layer-induced stress and susceptibility to SCC of brass. Mater. Sci. Eng. A 2004, 371, 51–56. [Google Scholar] [CrossRef]
- Brandl, E.; Malke, R.; Beck, T.; Wanner, A.; Hack, T. Stress corrosion cracking and selective corrosion of copper-zinc alloys for the drinking water installation. Mater. Corros. 2009, 60, 251–258. [Google Scholar] [CrossRef]
- Allam, N.K.; Nazeer, A.A.; Ashou, E.A. Effect of Annealing on the Stress Corrosion Cracking of α-Brass in Aqueous Electrolytes Containing Aggressive Ions. Ind. Eng. Chem. Res. 2010, 49, 9529–9533. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Tzou, R.J.; Chang, Y.S.; Wang, L.H.; Oung, J.C.; Shih, H.C. Two distinct fracture modes of copper alloys in fluoride environments. Corros. Sci. 2005, 47, 79–93. [Google Scholar] [CrossRef]
- Allam, N.K.; Nazeer, A.A.; Ashour, E.A. Electrochemical characterization and stress corrosion cracking behavior of α-brass in molybdate-containing electrolytes. J. Solid State Electrochem. 2012, 16, 353–360. [Google Scholar] [CrossRef]
- Sieradzki, K.; Newman, R.C. Brittle behavior of ductile metals during stress-corrosion cracking. Philos. Mag. A 1985, 51, 95–132. [Google Scholar] [CrossRef]
- Sieradzki, K.; Kim, J.S.; Cole, A.T.; Newman, R.C. The Relationship between Dealloying and Transgranular Stress-Corrosion Cracking of Cu-Zn and Cu-Al Alloys. J. Electrochem. Soc. 1987, 134, 1635–1639. [Google Scholar] [CrossRef]
- Badwe, N.; Chen, X.; Schreiber, D.K.; Olszta, M.J.; Overman, N.R.; Karasz, E.K.; Tse, A.Y.; Bruemmer, S.M.; Sieradzki, K. Decoupling the role of stress and corrosion in the intergranular cracking of noble-metal alloys. Nat. Mater. 2018, 17, 887–893. [Google Scholar] [CrossRef]
- Lu, H.; Gao, K.; Chu, W. Determination of tensile stress induced by dezincification layer during corrosion for brass. Corros. Sci. 1998, 40, 1663–1670. [Google Scholar] [CrossRef]
- Galvele, J.R. Surface mobility mechanism of stress corrosion cracking. Corros. Sci. 1993, 25, 419–434. [Google Scholar] [CrossRef]
- Giordano, C.M.; Duffo, G.S.; Galvele, J.R. The effect of Cu2+ concentration on the stress corrosion cracking susceptibility of α-brass in cupric nitrate solutions. Corros. Sci. 1997, 39, 1915–1923. [Google Scholar]
- Hintz, M.B.; Nettleton, L.J.; Heldt, L.A. Stress Corrosion Cracking of Alpha-Beta brass in distilled water and sodium sulfate solutions. Metall. Trans. A 1985, 16, 971–978. [Google Scholar] [CrossRef]
- Monticelli, C.; Balbo, A.; Esvan, J.; Chiavari, C.; Martini, C.; Zanotto, F.; Marvelli, L.; Robbiola, L. Evaluation of 2-(salicylideneimino) thiophenol and other Schiff bases as bronze corrosion inhibitors by electrochemical techniques and surface analysis. Corros. Sci. 2019, 148, 144–158. [Google Scholar] [CrossRef]
- Jie, H.; Xu, Q.; Wei, L.; Min, Y.L. Etching and heating treatment combined approach for superhydrophobic surface on brass substrates and the consequent corrosion resistance. Corros. Sci. 2016, 102, 251–258. [Google Scholar] [CrossRef]
- Rochdi, A.; Kassou, O.; Dkhireche, N.; Touir, R.; El Bakri, M.; Ebn Touhami, M.; Sfaira, M.; Mernari, B.; Hammouti, B. Inhibitive properties of 2,5-bis(n-methylphenyl)-1,3,4-oxadiazole and biocide on corrosion, biocorrosion and scaling controls of brass in simulated cooling water. Corros. Sci. 2014, 80, 442–452. [Google Scholar] [CrossRef]
- El Bakri, M.; Touir, R.; Tazouti, A.; Dkhireche, N.; Ebn Touhami, M.; Rochdi, A.; Zarrouk, A. Corrosion Inhibition Study of Brass in Simulated Cooling Water by Triazole Derivatives, Cetyltrimethylammonium Bromide and Their Mixture. Arab. J. Sci. Eng. 2016, 41, 75–88. [Google Scholar] [CrossRef]
- Abedini, M.; Ghasemi, H.M. Corrosion behavior of Al-brass alloy during erosion—Corrosion process: Effects of jet velocity and sand concentration. Mater. Corros. 2016, 67, 513–521. [Google Scholar] [CrossRef]
- Du, X.S.; Su, Y.J.; Li, J.X.; Qiao, L.J.; Chu, W.Y. Inhibitive effects and mechanism of phosphates on the stress corrosion cracking of brass in ammonia solutions. Corros. Sci. 2012, 60, 69–75. [Google Scholar] [CrossRef]
- Aljinović, L.J.; Gudić, S.; Šmith, M. Inhibition of CuNi10Fe corrosion in seawater by sodium-diethyl-dithiocarbamate: An electrochemical and analytical study. J. Appl. Electrochem. 2000, 30, 973–979. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Rios, J.F.; Calderón, J.A.; Nogueira, R.P. Electrochemical Behavior of Copper in Drinking Water: Evaluation of Dissolution Process at Low Anodic Overpotential. J. Braz. Chem. Soc. 2011, 22, 1362–1370. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Xiao, L. On the phenomena related to the dynamic strain aging in alpha brass. Scr. Metall. 1988, 22, 179–182. [Google Scholar] [CrossRef]
- Hu, X.; Margolin, H.; Duan, X.; Nourbakhsh, S. Burst phenomena in fatigue of 70-30 alpha-brass at room temperature. Mater. Sci. Eng. A 1992, 157, 181–194. [Google Scholar] [CrossRef]
- Sharififar, M.; Akbari Mousavi, S.A.A. Tensile deformation and fracture behavior of CuZn5 brass alloy at high temperature. Mater. Sci. Eng. A 2014, 594, 118–124. [Google Scholar] [CrossRef]
- Adams, S.M. Serrated flow in alpha-beta brass. Scr. Metall. 1973, 7, 173–177. [Google Scholar] [CrossRef]
- Fernández, S.A.; Alvarez, M.G. Passivity breakdown and stress corrosion cracking of a-brass in sodium nitrate solutions. Corros. Sci. 2011, 53, 82–88. [Google Scholar] [CrossRef]
- Trojanová, Z.; Cáceres, C.H.; Lukáč, P.; Čížek, L. Serrated flow in AZ91 magnesium alloy in tension and compression. Kov. Mater. 2008, 46, 249–256. [Google Scholar]
- Johar, M.H.; Torbati-Sarraf, H.; Ahangari, M.; Saremi, M. Inhibiting effect of Benzotriazole on the stress corrosion cracking of Cu-27% Ni cupronickel and Cu-30% Zn brass in Mattsson’s solution. Mater. Lett. 2021, 293, 129735. [Google Scholar] [CrossRef]
- Nikolaychuk, P.A.; Tyurin, A.G. Thermodynamic evaluation of corrosion electrochemical behaviour of silicon brass CuZn17Si3. Inorg. Mater. 2013, 49, 457–467. [Google Scholar] [CrossRef]
- Bacarella, A.L.; Griess, J.C., Jr. The anodic dissolution of copper in flowing sodium chloride solutions between 25 and 175 °C. J. Electrochem. Soc. 1973, 120, 459–465. [Google Scholar] [CrossRef]
- Mathiyarasu, J.; Palaniswamy, N.; Muralidharan, V.S. Electrochemical behaviour of copper-nickel alloy in chloride solution. J. Chem. Sci. 1999, 111, 377–386. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Rajala, P.; Bomberg, M.; Carpén, L. EIS study on aerobic corrosion of copper in ground water: Influence of micro-organisms. Electroch. Acta 2017, 240, 163–174. [Google Scholar] [CrossRef]
- Deslouis, C.; Tribollet, B.; Mengoli, G.; Musiani, M.M. Electrochemical behaviour of copper in neutral aerated chloride solution. I. Steady-state investigation. J. Appl. Electrochem. 1988, 18, 374–383. [Google Scholar] [CrossRef]
- Sánchez, M.; Gamby, J.; Perrot, H.; Rose, D.; Vivier, V. Microelectrochemistry of copper in NaCl solution: Comparison between conventional microelectrode and microelectrochemical cell. Electrochem. Commun. 2010, 12, 1230–1232. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, W.; Li, N.; Liu, X.; Han, L.; Dai, W. Surface Characterization and Corrosion Behavior of 90/10 Copper-Nickel Alloy in Marine Environment. Materials 2019, 12, 1869. [Google Scholar] [CrossRef] [Green Version]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE International: Cebelcor, Belgium, 1974. [Google Scholar]
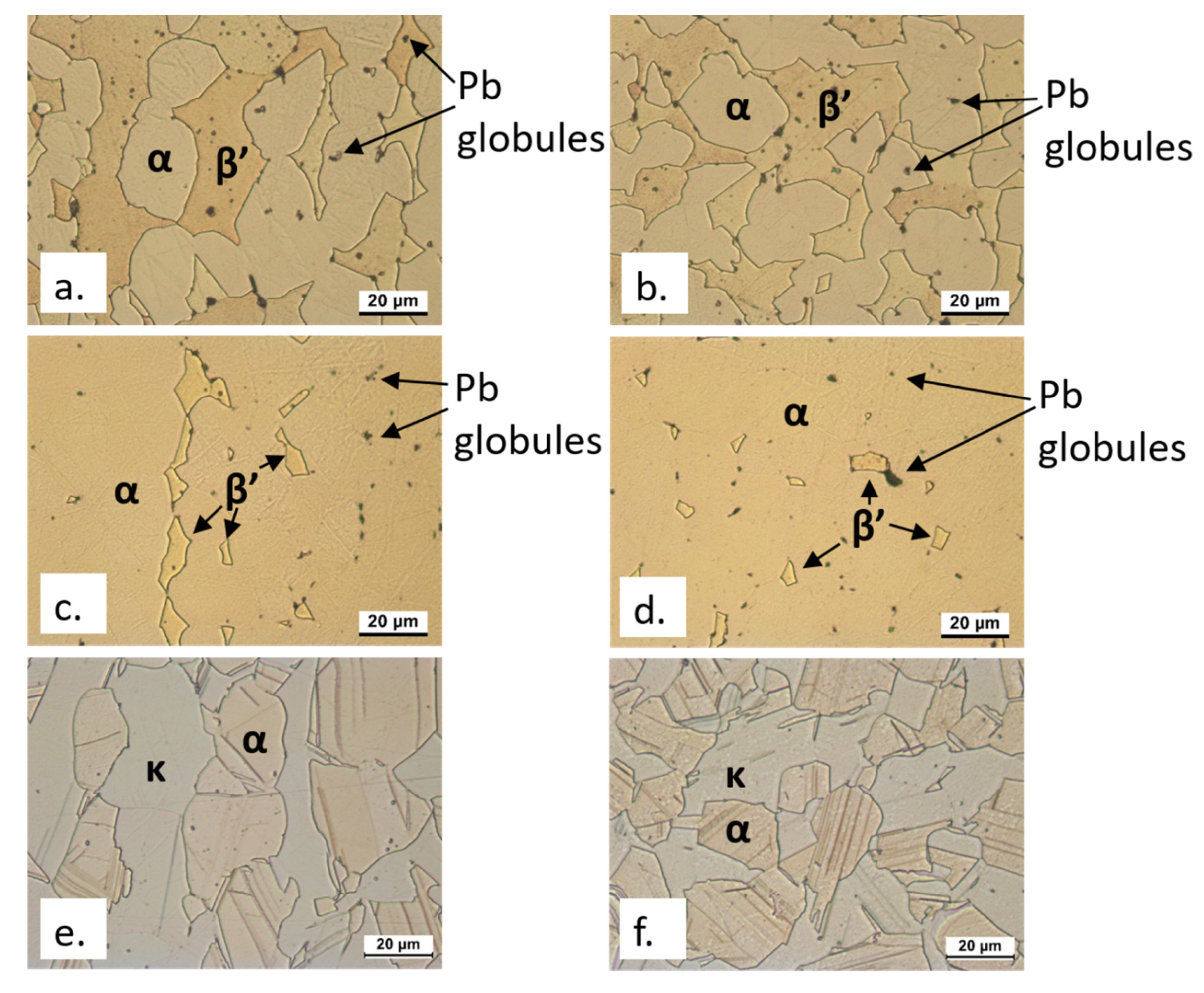
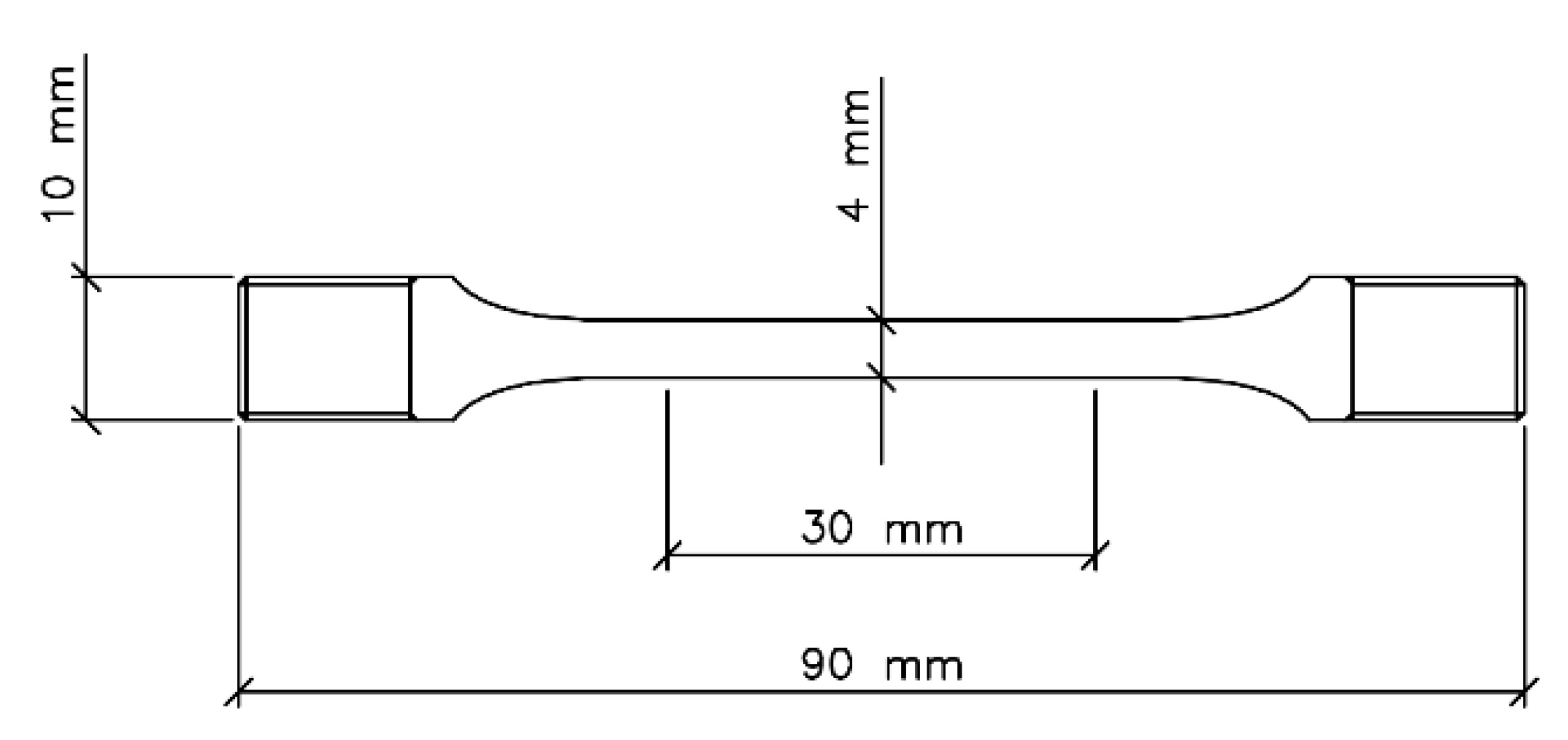



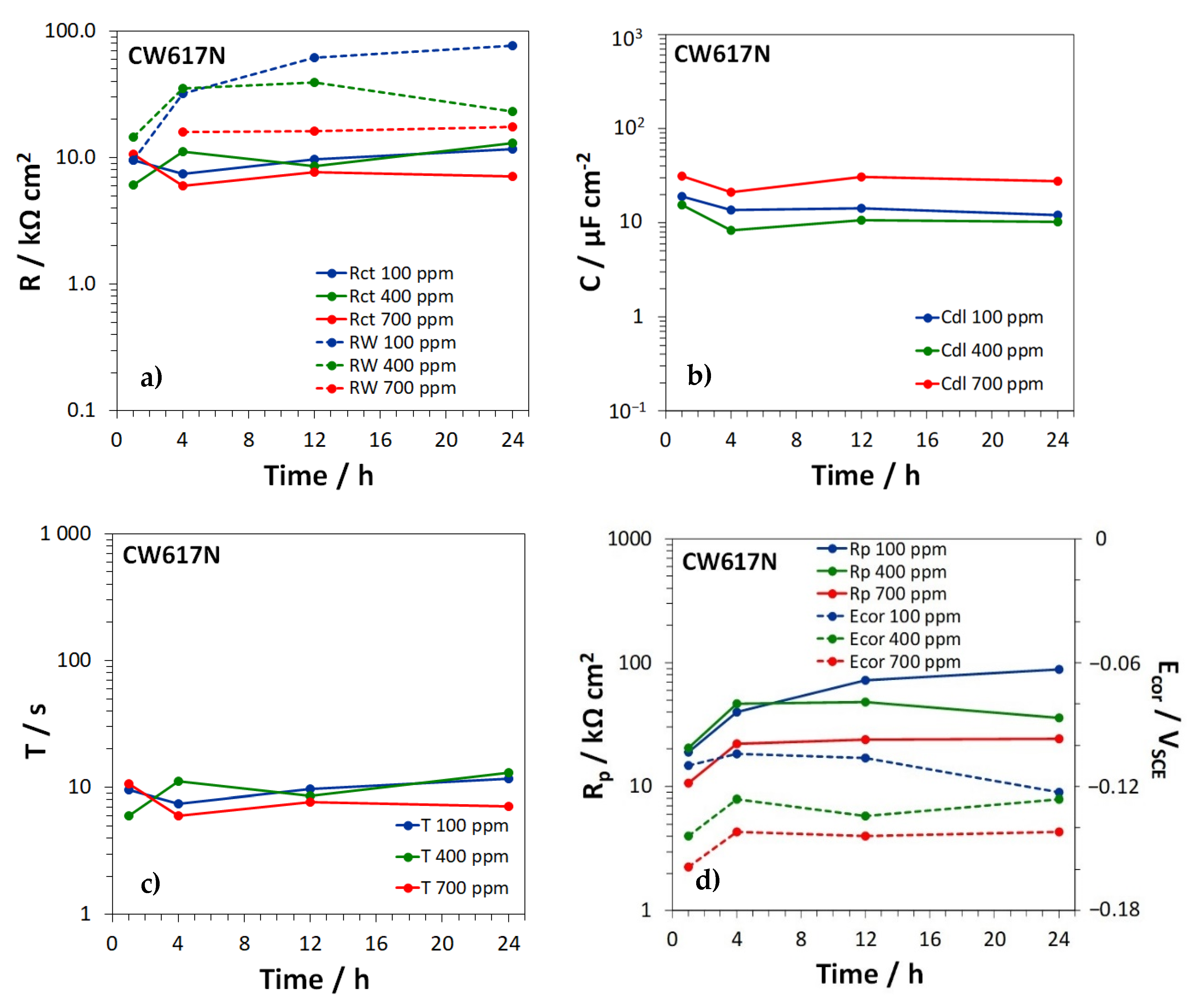
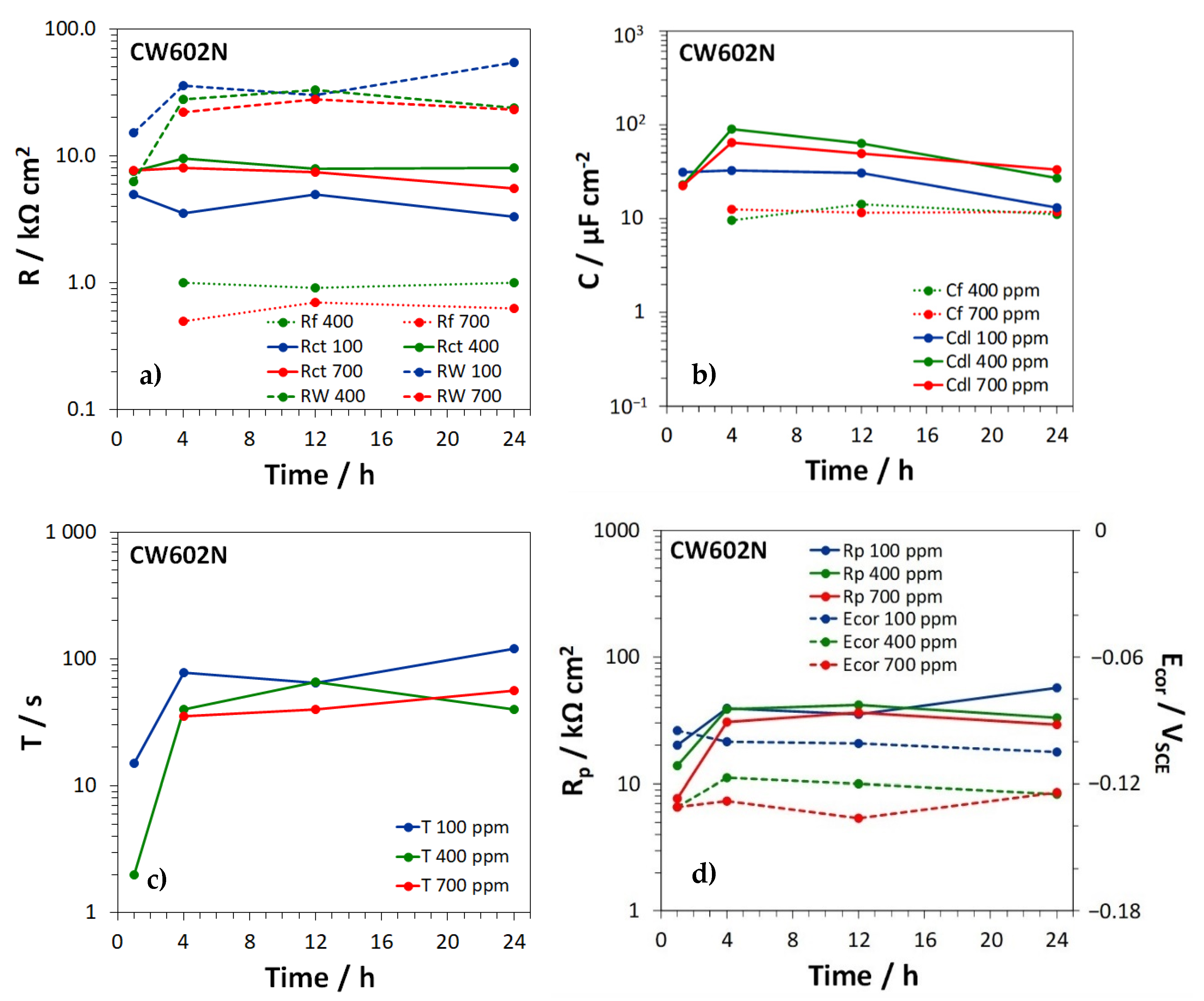
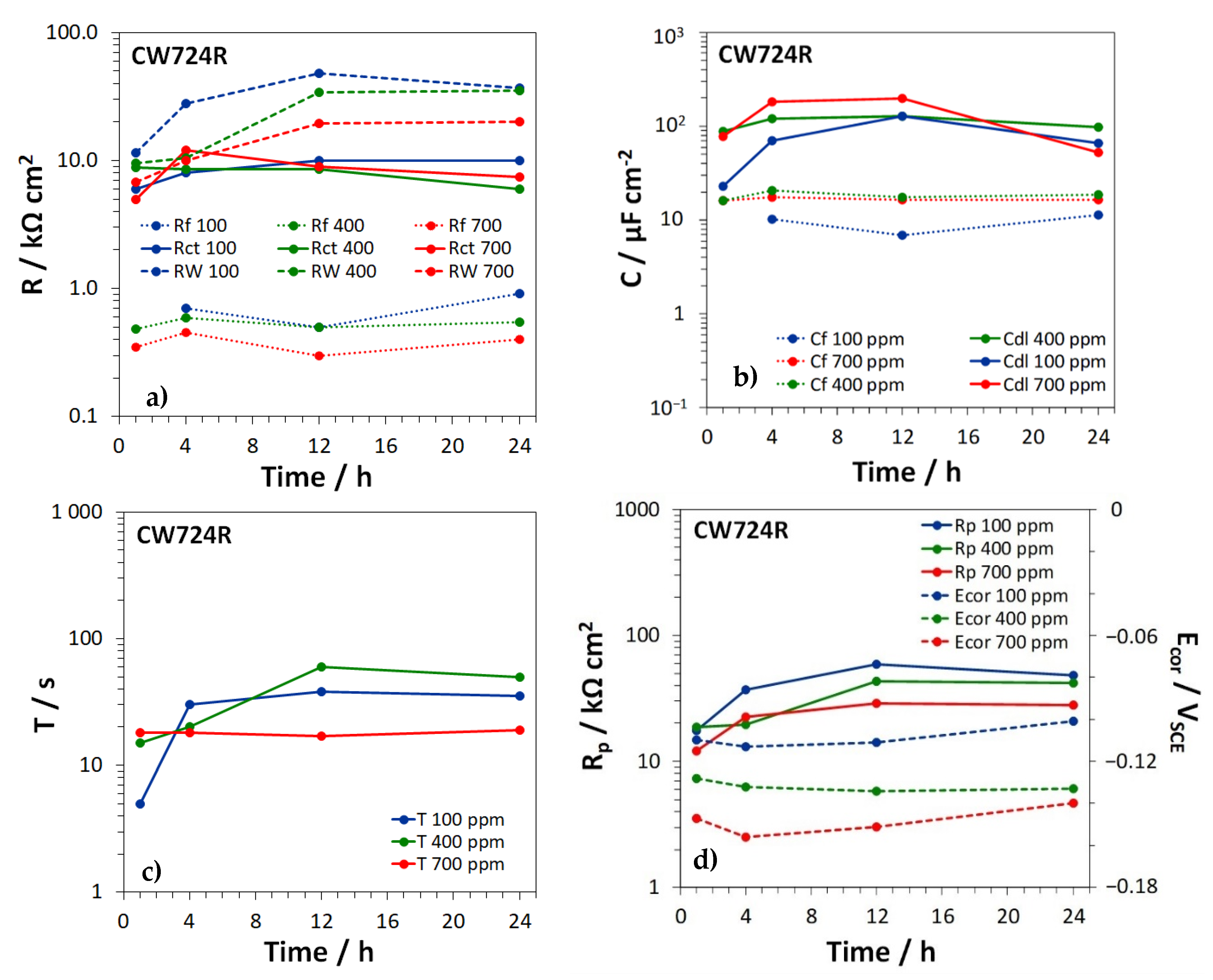


| EN Standard Designation | Cu | Zn | Pb | Sn | Fe | Ni | Al | Si | As | P | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CW617N CuZn40Pb2 | 58.50 | 38.90 | 2.0 | 0.20 | 0.30 | 0.10 | 0.00 | <0.001 | <0.10 | ||
| CW602N CuZn36Pb2As | 61.70 | 35.83 | 1.90 | 0.11 | 0.12 | 0.03 | 0.02 | <0.001 | 0.09 | <0.20 | |
| CW724R CuZn21Si3P | 77.10 | 19.52 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 3.3 | 0.00 | 0.05 | <0.10 |
| Alloy | Time h | 100 ppm Cl− | 400 ppm Cl− | 700 ppm Cl | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecor V vs. SCE | icor μA cm−2 | βa mV | |βc| mV | Ecor V vs. SCE | icor μA cm−2 | βa mV | |βc| mV | Ecor V vs. SCE | icor μA cm−2 | βa mV | |βc| mV | ||
| CW617N | 1 | −0.111 | 0.69 | 33 | 167 | −0.142 | 0.79 | 23 | 150 | −0.158 | 1.08 | 23 | 151 |
| 24 | −0.122 | 0.70 | 70 | 237 | −0.126 | 0.66 | 53 | 257 | −0.141 | 0.88 | 56 | 256 | |
| CW602N | 1 | −0.095 | 0.74 | 45 | 187 | −0.132 | 1.12 | 25 | 153 | −0.132 | 1.10 | 20 | 150 |
| 24 | −0.105 | 0.62 | 55 | 256 | −0.125 | 0.82 | 49 | 259 | −0.124 | 0.85 | 43 | 211 | |
| CW724R | 1 | −0.109 | 0.65 | 32 | 166 | −0.128 | 0.82 | 19 | 130 | −0.148 | 0.80 | 21 | 147 |
| 24 | −0.101 | 0.59 | 58 | 269 | −0.132 | 0.60 | 47 | 189 | −0.140 | 0.80 | 24 | 151 | |
| Environment (25 °C) | CW617N | CW602N | CW724R | |||
|---|---|---|---|---|---|---|
| ɛf% | R | ɛf% | R | ɛf% | R | |
| Air | 17.2 | 46.9 | 45.6 | |||
| SDW (100 ppm Cl−) | 4.6 | 0.27 | 30.4 | 0.65 | 39.9 | 0.88 |
| SDW (400 ppm Cl−) | 6.4 | 0.37 | 30.5 | 0.65 | 40.3 | 0.88 |
| SDW (700 ppm Cl−) | 5.6 | 0.32 | 28.3 | 0.60 | 37.3 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choucri, J.; Balbo, A.; Zanotto, F.; Grassi, V.; Touhami, M.E.; Mansouri, I.; Monticelli, C. Corrosion Behavior and Susceptibility to Stress Corrosion Cracking of Leaded and Lead-Free Brasses in Simulated Drinking Water. Materials 2022, 15, 144. https://doi.org/10.3390/ma15010144
Choucri J, Balbo A, Zanotto F, Grassi V, Touhami ME, Mansouri I, Monticelli C. Corrosion Behavior and Susceptibility to Stress Corrosion Cracking of Leaded and Lead-Free Brasses in Simulated Drinking Water. Materials. 2022; 15(1):144. https://doi.org/10.3390/ma15010144
Chicago/Turabian StyleChoucri, Jamal, Andrea Balbo, Federica Zanotto, Vincenzo Grassi, Mohamed Ebn Touhami, Ilyass Mansouri, and Cecilia Monticelli. 2022. "Corrosion Behavior and Susceptibility to Stress Corrosion Cracking of Leaded and Lead-Free Brasses in Simulated Drinking Water" Materials 15, no. 1: 144. https://doi.org/10.3390/ma15010144
APA StyleChoucri, J., Balbo, A., Zanotto, F., Grassi, V., Touhami, M. E., Mansouri, I., & Monticelli, C. (2022). Corrosion Behavior and Susceptibility to Stress Corrosion Cracking of Leaded and Lead-Free Brasses in Simulated Drinking Water. Materials, 15(1), 144. https://doi.org/10.3390/ma15010144







