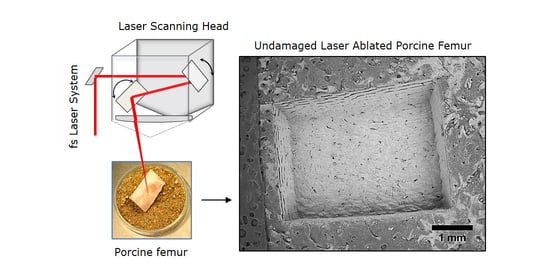
Ablation of Bone Tissue by Femtosecond Laser: A Path to High-Resolution Bone Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biological Samples of Porcine Femur
2.2. Experimental Setup and Laser Processing Station
2.3. Protocols for Sample Characterization after Laser Processing
- Optical microscopy (MF-B1010C, Mitutoyo, Kanagawa, Japan) analyses were carried out to evaluate the ablation depth. Five measurements were carried out on each ablation cavity in order to have statistically valid values and compensate for possible ablation inhomogeneities on the bottom of the cavities. The standard deviation was calculated to be between ±0.02 mm and ±0.09 mm for all measurements in all regimes of ablation. For all ablated samples, the ablation rate was calculated as the ratio between the ablated volume (mm3) and the processing time (s).
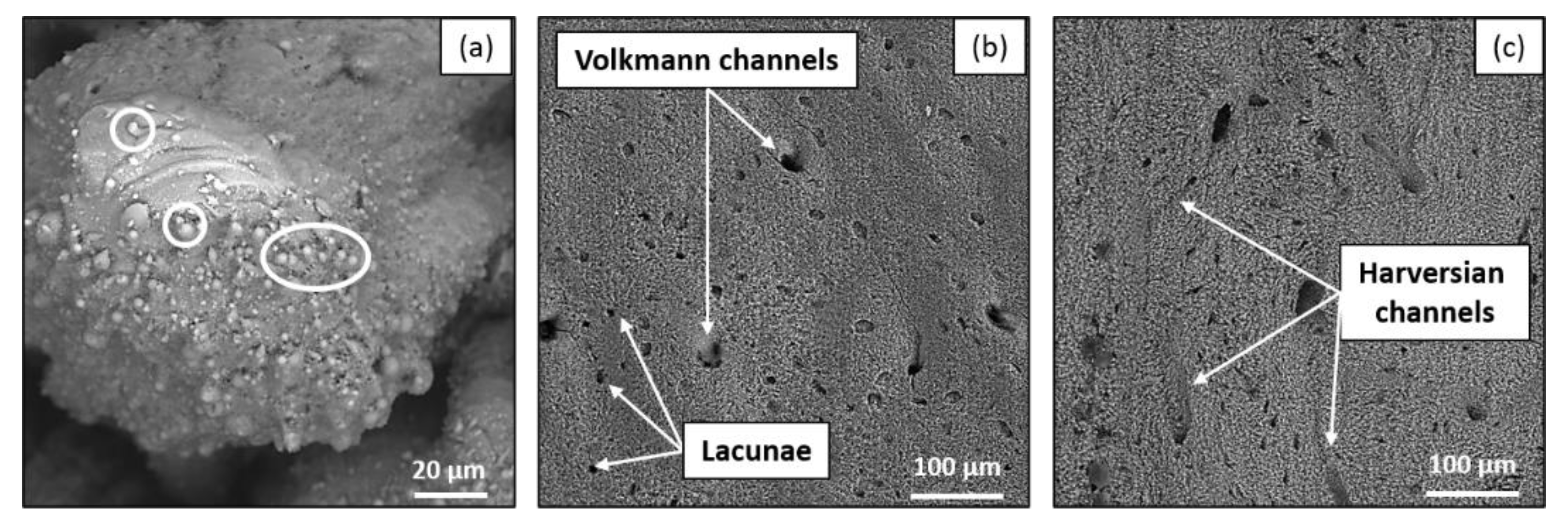
- SEM (Vega3, Tescan, Brno, Czech Republic) observations were carried to analyze the quality of ablation and the overall aspect of the bottom of the cavities in order to define a possible correlation between the observed morphology and the calcination state of the sample.
- EDX (Bruker US Quantax, Billerica, MA, USA) measurements were carried out on all processed samples in order to quantify the laser-induced variation in atomic% of the main bone tissue components, such as C, O, Ca, Mg and P. To account for the semi-qualitative nature of the EDX analyses, all measured values were normalized with respect to the reference value of the same element measured on a non-laser-processed area of the same sample. For each ablated cavity, three measurements were carried out for statistical purposes.
3. Results
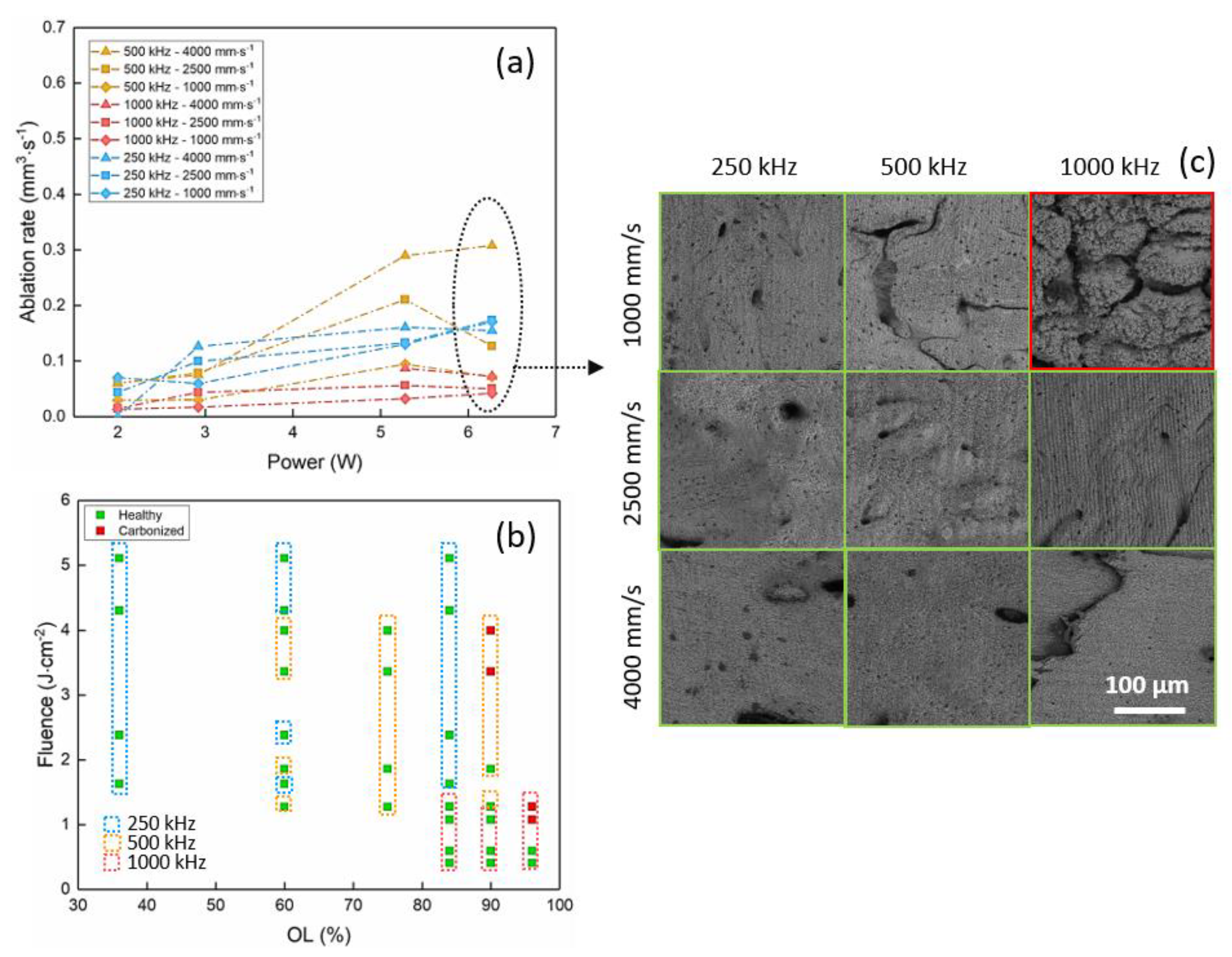
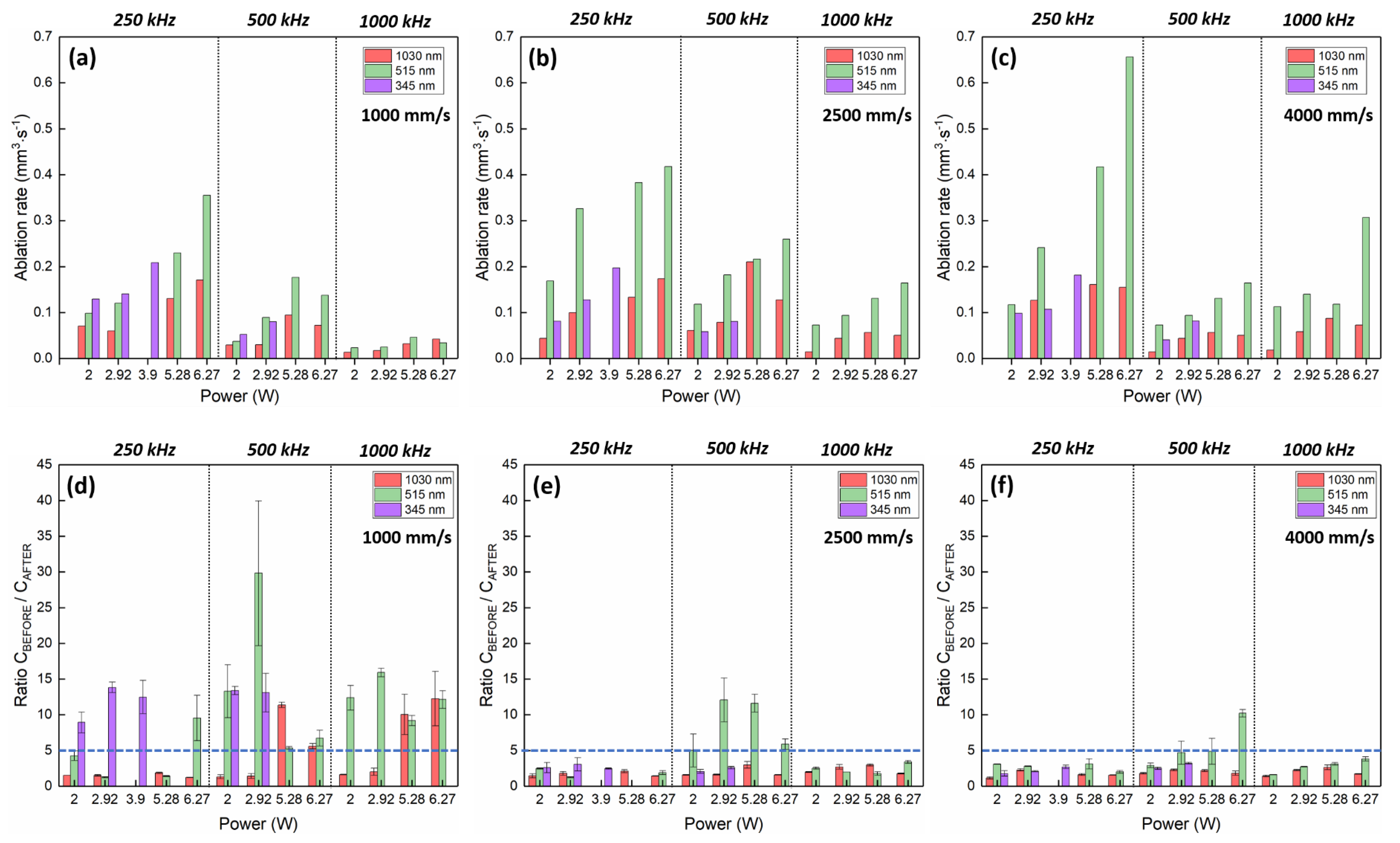
3.1. Porcine Femur Laser Ablation in IR Regime (1030 nm)
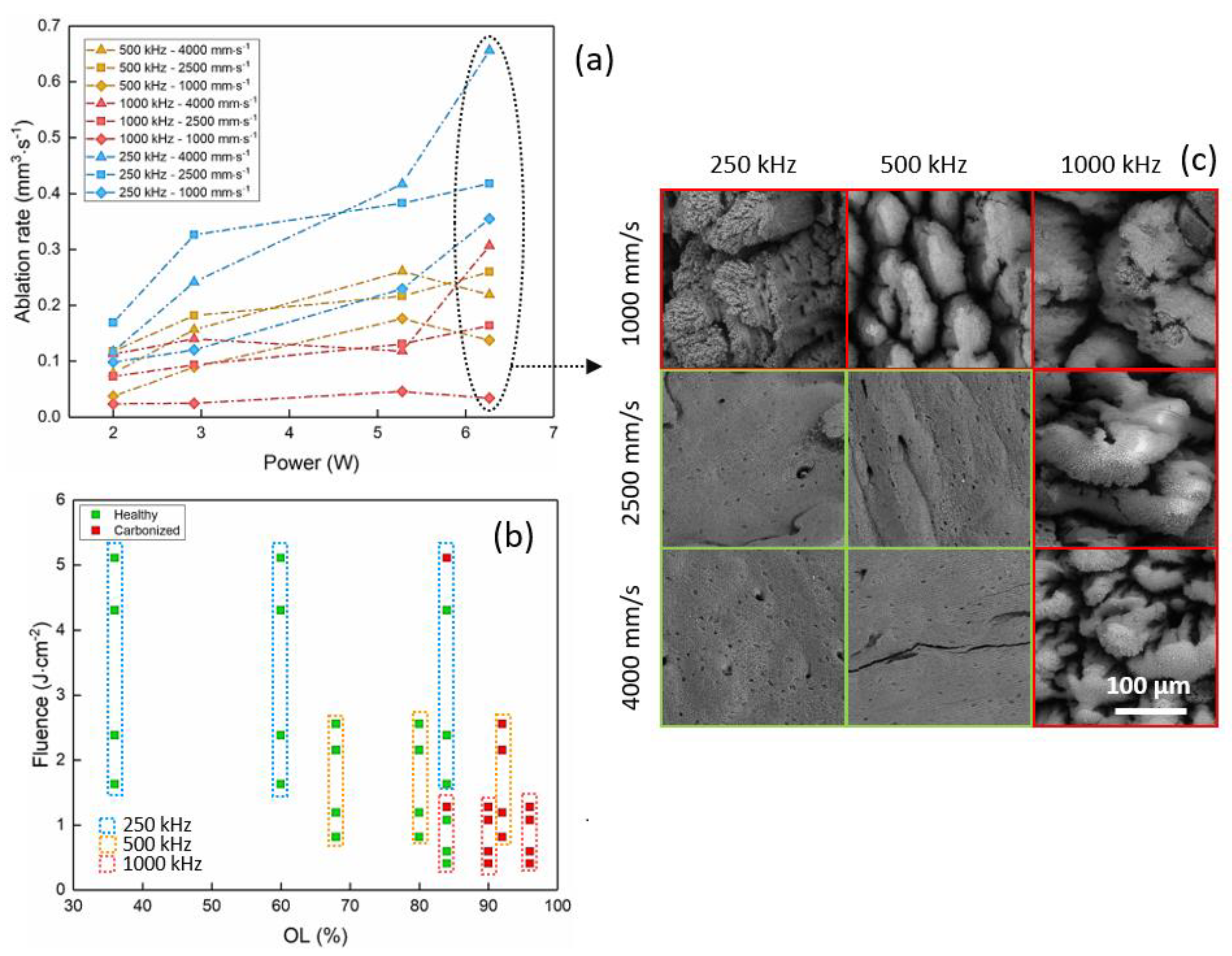
3.2. Porcine Femur Laser Ablation in Visible Regime (515 nm)
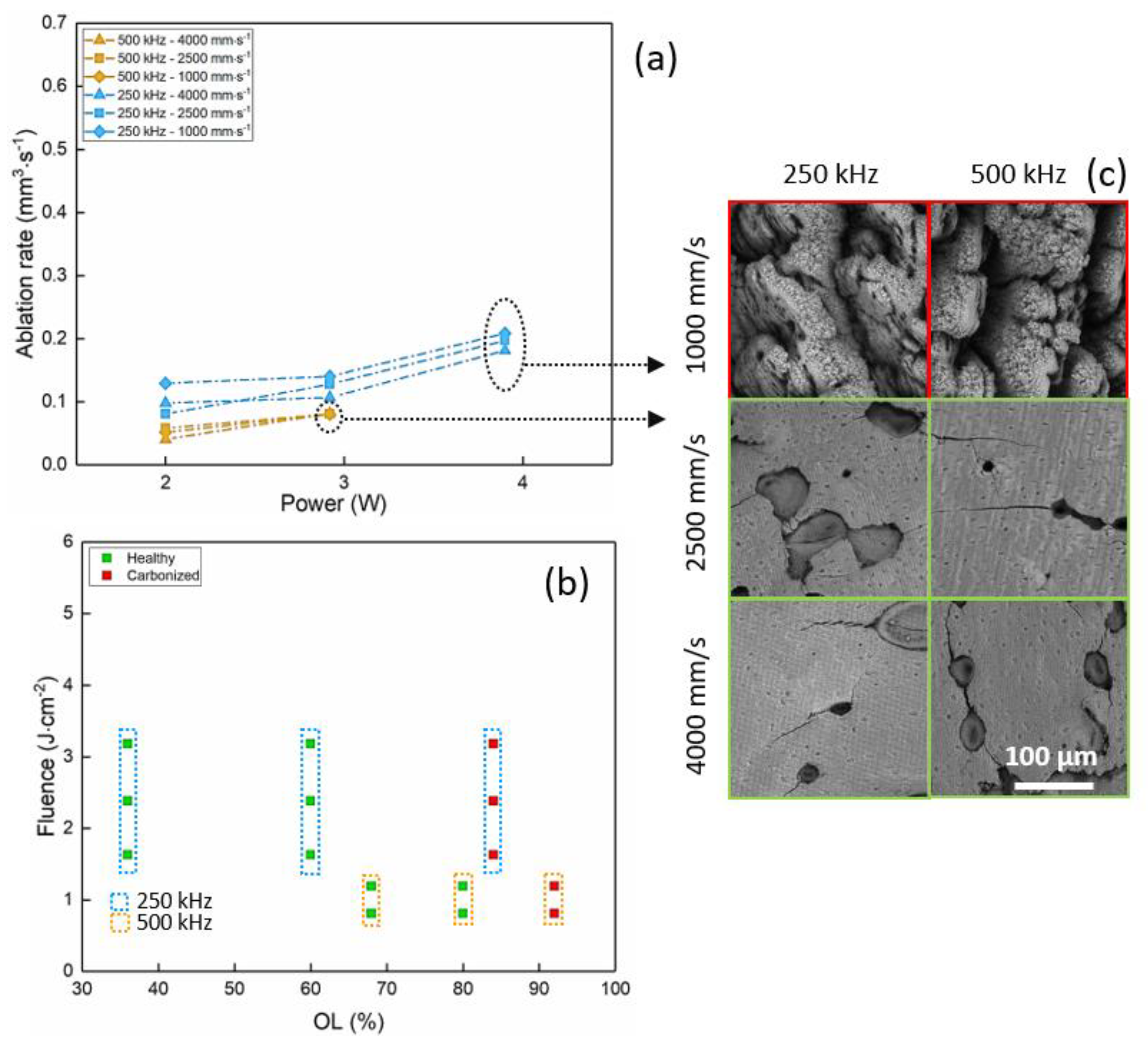
3.3. Porcine Femur Laser Ablation in UV Regime (343 nm)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meijer, J.; Du, K.; Gillner, A.; Hoffmann, D.; Kovalenko, V.; Masuzawa, T.; Ostendorf, A.; Poprawe, R.; Schulz, W. Laser machining by short and ultrashort pulses, state of the art and new opportunities in the age of the photons. CIRP Ann. 2002, 51, 531–550. [Google Scholar] [CrossRef]
- Eilzer, S.; Funck, M.C.; Wedel, B. Industrial Fiber Beam Delivery System for Ultrafast Lasers: Applications and Recent Advances; Dorsch, F., Kaierle, S., Eds.; SPIE: San Francisco, CA, USA, 2016; p. 974103. [Google Scholar]
- Shah, R. History and results; Indications and contraindications of SMILE, compared with LASIK. Asia-Pac. J. Ophthalmol. 2019, 8, 371–376. [Google Scholar] [CrossRef]
- Photocoagulation for Diabetic Macular Edema. Early Treatment Diabetic Retinopathy Study Report Number 1. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Boord, M. Laser in dermatology. Clin. Tech. Small Anim. Pract. 2006, 21, 145–149. [Google Scholar] [CrossRef]
- Plötz, C.; Schelle, F.; Bourauel, C.; Frentzen, M.; Meister, J. Ablation of porcine bone tissue with an ultrashort pulsed laser (USPL) system. Lasers Med. Sci. 2014, 30, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Niemz, M. Ablation of femural bone with femtosecond laser pulses—A feasibility study. Lasers Med. Sci. 2007, 22, 171–174. [Google Scholar] [CrossRef]
- Cangueiro, L.T.; Vilar, R.; Botelho do Rego, A.M.; Muralha, V.S.F. Femtosecond laser ablation of bovine cortical bone. J. Biomed. Opt. 2012, 17, 125005. [Google Scholar] [CrossRef]
- Aljekhedab, F.; Zhang, W.; Favero, J.; Haugen, H.K.; Wohl, G.R.; Fang, Q. Bovine cortical bone ablation by femtosecond laser (Conference Presentation). In Lasers in Dentistry XXIV; Rechmann, P., Fried, D., Eds.; SPIE: San Francisco, CA, USA, 14 March 2018; p. 14. [Google Scholar]
- Serebryakov, V.A.; Boĭko, É.V.; Petrishchev, N.N.; Yan, A.V. Medical applications of mid-IR lasers Problems and prospects. J. Opt. Technol. 2010, 77, 6–17. [Google Scholar] [CrossRef]
- Cangueiro, L.; Vilar, R. Influence of the pulse frequency and water cooling on the femtosecond laser ablation of bovine cortical bone. Appl. Surf. Sci. 2013, 283, 1012–1017. [Google Scholar] [CrossRef]
- Luke, A.M.; Mathew, S.; Altawash, M.M.; Madan, B.M. Lasers: A Review With Their Applications in Oral Medicine. J. Lasers Med Sci. 2019, 10, 324–329. [Google Scholar] [CrossRef]
- Markolf, H.N. Laser-Tissue Interactions Fundamentas and Applications, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Agbeboh, N.; Oladele, I.; Daramola, O.; Adediran, A.; Olasukanmi, O.; Tanimola, M. Environmentally sustainable processes for the synthesis of hydroxyapatite. Heliyon 2020, 6, e03765. [Google Scholar] [CrossRef]
- Romeo, U.; Del Vecchio, A.; Palata, G.; Tenore, G.; Visca, P.; Maggiore, C. Bone damage induced by different cutting instruments: An in vitro study. Braz. Dent. J. 2009, 20, 162–168. [Google Scholar] [CrossRef]
- Girard, B.; Yu, D.; Armstrong, M.; Wilson, B.; Clokie, C.; Miller, R.D. Effects of femtosecond laser irradiation on osseous tissues. Lasers Surg. Med. 2007, 39, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.D.; Mackanos, M.A.; Chung, M.T.; Hyun, J.S.; Montoro, D.T.; Grova, M.; Liu, C.; Wang, J.; Palanker, D.; Connolly, A.J.; et al. Femtosecond plasma mediated laser ablation has advantages over mechanical osteotomy of cranial bone. Lasers Surg. Med. 2012, 44, 805–814. [Google Scholar] [CrossRef]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies Differences in Bone Composition, Density, and Quality: Potential Implications for in Vivo Bone Research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Legros, R.; Balmain, N.; Bonel, G. Age-related changes in mineral of rat and bovine cortical bone. Calcif. Tissue Int. 1987, 41, 137–144. [Google Scholar] [CrossRef]
- Rico, M.; Jubera, M.; Blas, A.S.; Roso, L.; Lazkoz, A.; Arregui, J. Improvements on Characterization of the Threshold and Productivity in Femtosecond Laser Ablation of Bone. In Proceedings of the 2019 Conference on Lasers and Electro-Optics Europe & European Quantum Electronics Conference (CLEO/Europe-EQEC), Munich, Germany, 23–27 June 2019; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2019; p. 1. [Google Scholar]
- Zhang, J.; Guan, K.; Zhang, Z.; Guan, Y. In vitro evaluation of ultrafast laser drilling large-size holes on sheepshank bone. Opt. Express 2020, 28, 25528. [Google Scholar] [CrossRef] [PubMed]
- Tulea, C.-A.; Caron, J.; Gehlich, N.; Lenenbach, A.; Noll, R.; Loosen, P. Laser cutting of bone tissue under bulk water with a pulsed ps-laser at 532 nm. J. Biomed. Opt. 2015, 20, 105007. [Google Scholar] [CrossRef]
- Canteli, D.; Muñoz-García, C.; Morales, M.; Márquez, A.; Lauzurica, S.; Arregui, J.; Lazkoz, A.; Molpeceres, C. Thermal effects in the ablation of bovine cortical bone with pulsed laser sources. Materials 2019, 12, 2916. [Google Scholar] [CrossRef]
- Liebschner, M.A. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 2004, 25, 1697–1714. [Google Scholar] [CrossRef]
- Cone, S.G.; Warren, P.B.; Fisher, M.B. Rise of the Pigs: Utilization of the porcine model to study musculoskeletal biomechanics and tissue engineering during skeletal growth. Tissue Eng. Part C Methods 2017, 23, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Hillier, M.L.; Bell, L.S. Differentiating human bone from animal bone: A review of histological methods. J. Forensic Sci. 2007, 52, 249–263. [Google Scholar] [CrossRef]
- Joly, D.; March, R.J.; Martinez, G. Les os brûlés de Paso Otero 5: Un témoignage possible de l’utilisation de l’os comme combustible par des chasseurs-cueilleurs de la fin du Pléistocène en Argentine. ArchéoSciences 2005, 29, 83–93. [Google Scholar] [CrossRef]
- Krap, T.; Ruijter, J.M.; Nota, K.; Karel, J.; Burgers, A.L.; Aalders, M.C.G.; Oostra, R.-J.; Duijst, W. Colourimetric analysis of thermally altered human bone samples. Sci. Rep. 2019, 9, 8923. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Ancona, A.; Döring, S.; Jauregui, C.; Röser, F.; Limpert, J.; Nolte, S.; Tünnermann, A. Femtosecond and picosecond laser drilling of metals at high repetition rates and average powers. Opt. Lett. 2009, 34, 3304–3306. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, B.; Jaeggi, B.; Schmid, M.; Rouffiange, V.; Martin, P.-E. Optimization of the Volume Ablation Rate for Metals at Different Laser Pulse-Durations from Ps to Fs; SPIE: San Francisco, CA, USA, 2012; p. 824307. [Google Scholar]
- Kramer, T.; Remund, S.; Jäggi, B.; Schmid, M.; Neuenschwander, B. Ablation dynamics—From absorption to heat accumulation/ultra-fast laser matter interaction. Adv. Opt. Technol. 2018, 7, 129–144. [Google Scholar] [CrossRef]
- Hutson, M.S.; Hauger, S.A.; Edwards, G. Thermal diffusion and chemical kinetics in laminar biomaterial due to heating by a free-electron laser. Phys. Rev. E 2002, 65, 061906. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Li, N.; Zhou, S.N.; Huang, Z.T.; Zhuang, Z.F. Study of hemoglobin response to mid-ultraviolet (UVB) radiation using micro-Raman spectroscopy. Appl. Phys. A 2017, 123, 237. [Google Scholar] [CrossRef]
- Wood, B.R.; Hammer, L.; Davis, L.; McNaughton, D. Raman microspectroscopy and imaging provides insights into heme aggregation and denaturation within human erythrocytes. J. Biomed. Opt. 2005, 10, 014005–01400513. [Google Scholar] [CrossRef]
- Donnelly, E.; Meredith, D.S.; Nguyen, J.T.; Boskey, A.L. Bone tissue composition varies across anatomic sites in the proximal femur and the iliac crest: Bone mineral properties vary across anatomic sites. J. Orthop. Res. 2011, 30, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.J.; Prantner, V.; Jurvelin, J.S.; Kröger, H.; Isaksson, H. Composition and microarchitecture of human trabecular bone change with age and differ between anatomical locations. Bone 2013, 54, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.A.; Dhami, L.D. Overview of lasers. Indian J. Plast. Surg. 2008, 41, S101–S113. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; Nishioka, N.; Mikic, B. Thermodynamic response of soft biological tissues to pulsed infrared-laser irradiation. Biophys. J. 1996, 70, 2981–2993. [Google Scholar] [CrossRef][Green Version]
- Vogel, A.A.; Venugopalan, V. Mechanisms of Pulsed Laser Ablation of Biological Tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef] [PubMed]
- Bernal, L.M.B.; Schmidt, I.T.; Vulin, N.; Widmer, J.; Snedeker, J.G.; Cattin, P.C.; Zam, A.; Rauter, G. Optimizing controlled laser cutting of hard tissue (bone). at-Automatisierungstechnik 2018, 66, 1072–1082. [Google Scholar] [CrossRef]
- Leung, B.Y.; Webster, P.J.; Fraser, J.M.; Yang, V.X. Real-time guidance of thermal and ultrashort pulsed laser ablation in hard tissue using inline coherent imaging. Lasers Surg. Med. 2012, 44, 249–256. [Google Scholar] [CrossRef]
- Abbasi, H.; Rauter, G.; Guzman, R.; Cattin, P.C.; Zam, A. Differentiation of femur bone from surrounding soft tissue using laser-induced breakdown spectroscopy as a feedback system for smart laserosteotomy. In Proceedings of the SPIE Biophotonics: Photonic Solutions Better Health Care VI, Strasbourg, France, 23–26 April 2018; p. 1068519. [Google Scholar] [CrossRef]

| Ref. | Bone Type | Wavelength (nm) | Pulse Duration | Repetition Rate (kHz) | Average Power (W) | Ablation Rate (mm3/s) |
|---|---|---|---|---|---|---|
| [9] | Bovine | 800 | 210 fs | 1 | 0.2 | 0.2 × 10−3 |
| [11] | Bovine | 1030 | 500 fs | 3 | 1 | 20 × 10−3 |
| [7] | Porcine femur | 1030 | 900 fs | 20 | 5 | 0.15 |
| [20] | NA | 1030 | 240 fs | 60 | NA | 0.05 |
| [21] | Sheep shank | 1030 | 230 fs | 300 | 20 | 0.99 |
| [6] | Porcine ribs | 1064 | 8 ps | 500 | 9 | 0.09 |
| [22] | Bovine femur | 532 | 25 ps | 100 | 20 | 0.19 |
| [23] | Bovine femur | 1064 | 12 ps | 600 | NA | 0.15 |
| Repetition Rate RR (kHz) | Average Power P (W) | Scanning Speed v (mm/s) |
|---|---|---|
| IR regime (1030 nm) | ||
| 250 | 2–2.92–5.28–6.27 | 1000–2500–4000 |
| 500 | ||
| 1000 | ||
| Visible regime (515 nm) | ||
| 250 | 2–2.92–5.28–6.27 | 1000–2500–4000 |
| 500 | ||
| 1000 | ||
| UV regime (343 nm) | ||
| 250 | 2–2.92–3.9 | 1000–2500–4000 |
| Regime | P (W) | RR (kHz) | v (mm/s) | OL (%) | Ablation Rate (mm3/s) | Ratio CBEFORE/CAFTER |
|---|---|---|---|---|---|---|
| IR—1030 nm | 6.27 | 500 | 4000 | 60 | 0.30 | 1.74 |
| Visible—515 nm | 6.27 | 250 | 4000 | 36 | 0.66 | 2.02 |
| UV—343 nm | 3.9 | 250 | 2500 | 60 | 0.20 | 2.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemini, L.; Al-Bourgol, S.; Machinet, G.; Bakkali, A.; Faucon, M.; Kling, R. Ablation of Bone Tissue by Femtosecond Laser: A Path to High-Resolution Bone Surgery. Materials 2021, 14, 2429. https://doi.org/10.3390/ma14092429
Gemini L, Al-Bourgol S, Machinet G, Bakkali A, Faucon M, Kling R. Ablation of Bone Tissue by Femtosecond Laser: A Path to High-Resolution Bone Surgery. Materials. 2021; 14(9):2429. https://doi.org/10.3390/ma14092429
Chicago/Turabian StyleGemini, Laura, Samy Al-Bourgol, Guillaume Machinet, Aboubakr Bakkali, Marc Faucon, and Rainer Kling. 2021. "Ablation of Bone Tissue by Femtosecond Laser: A Path to High-Resolution Bone Surgery" Materials 14, no. 9: 2429. https://doi.org/10.3390/ma14092429
APA StyleGemini, L., Al-Bourgol, S., Machinet, G., Bakkali, A., Faucon, M., & Kling, R. (2021). Ablation of Bone Tissue by Femtosecond Laser: A Path to High-Resolution Bone Surgery. Materials, 14(9), 2429. https://doi.org/10.3390/ma14092429