Bone Regeneration Using PEVAV/β-Tricalcium Phosphate Composite Scaffolds in Standardized Calvarial Defects: Micro-Computed Tomographic Experiment in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Scaffold
2.2. Ethical Approval, Sample Size, Randomization, and Grouping of the Study Animals
2.3. General Anesthesia and Surgical Procedure
2.4. Micro-CT Analysis
2.5. Statistical Analysis
3. Results
3.1. General Observations and Scaffold Applicability during Surgeries
3.2. Micro-CT Analysis
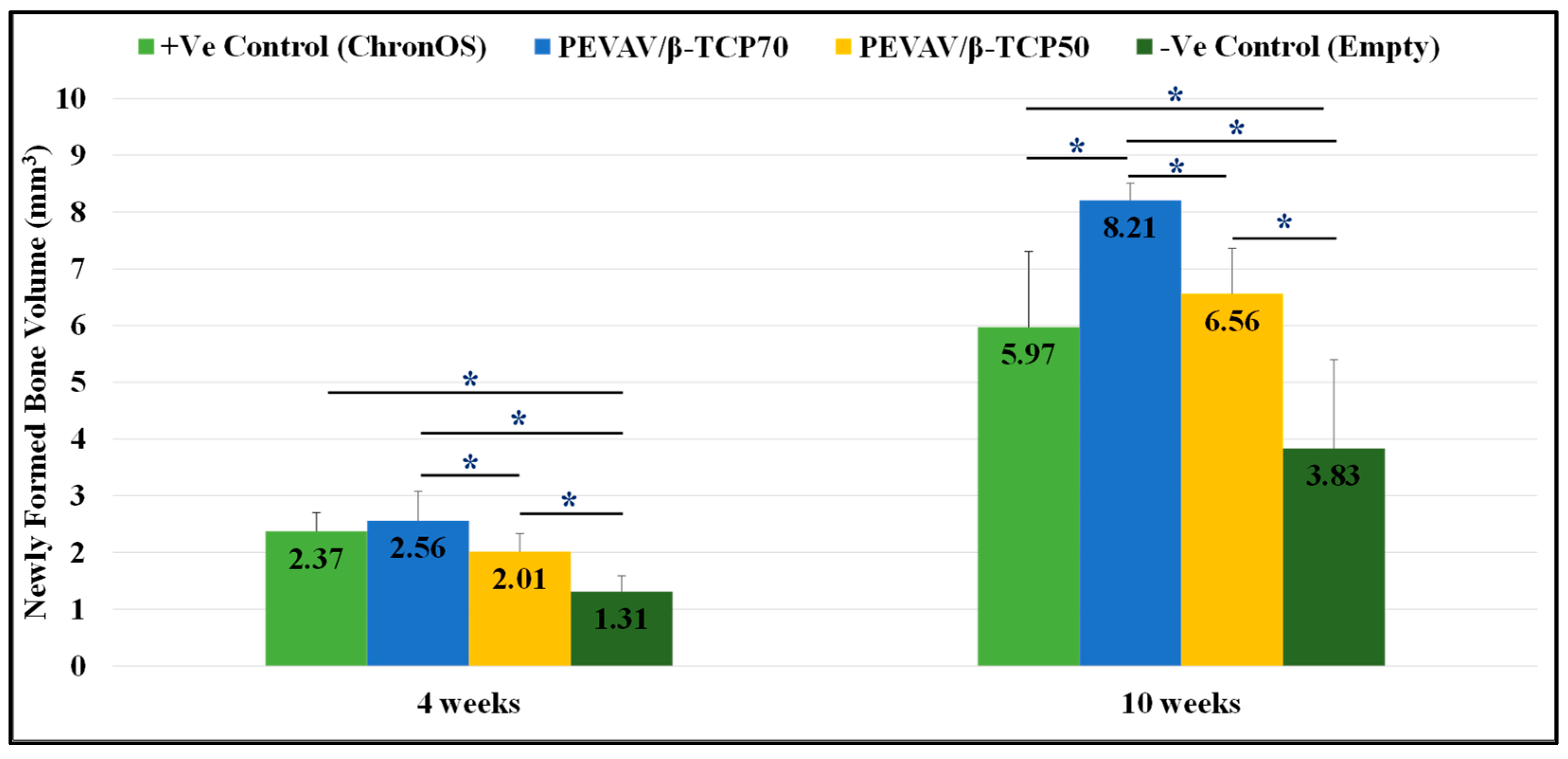
3.2.1. Comparison between the Groups in Terms of Newly Formed Bone Volume
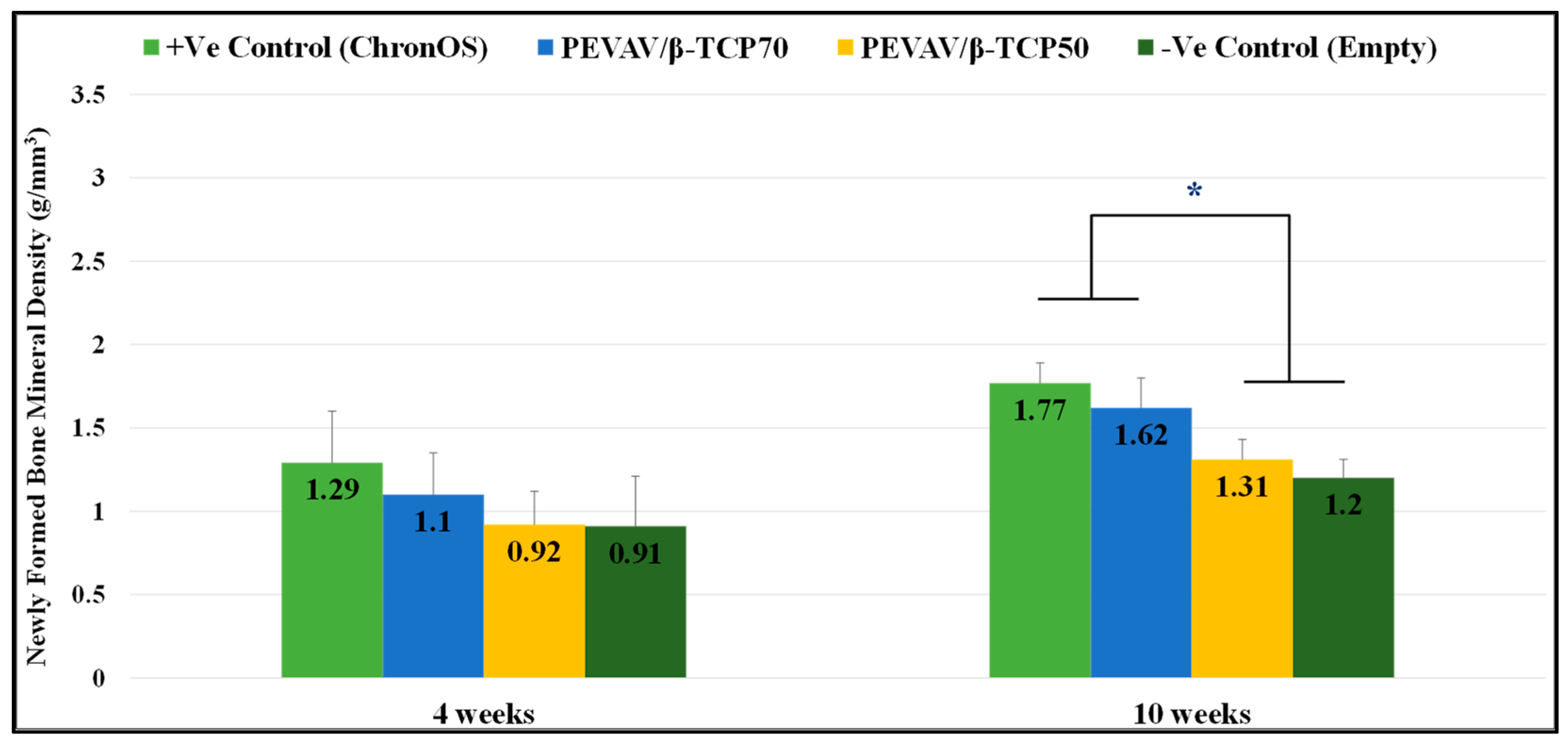
3.2.2. Comparison between the Groups in Term of Newly Formed Bone Mineral Density
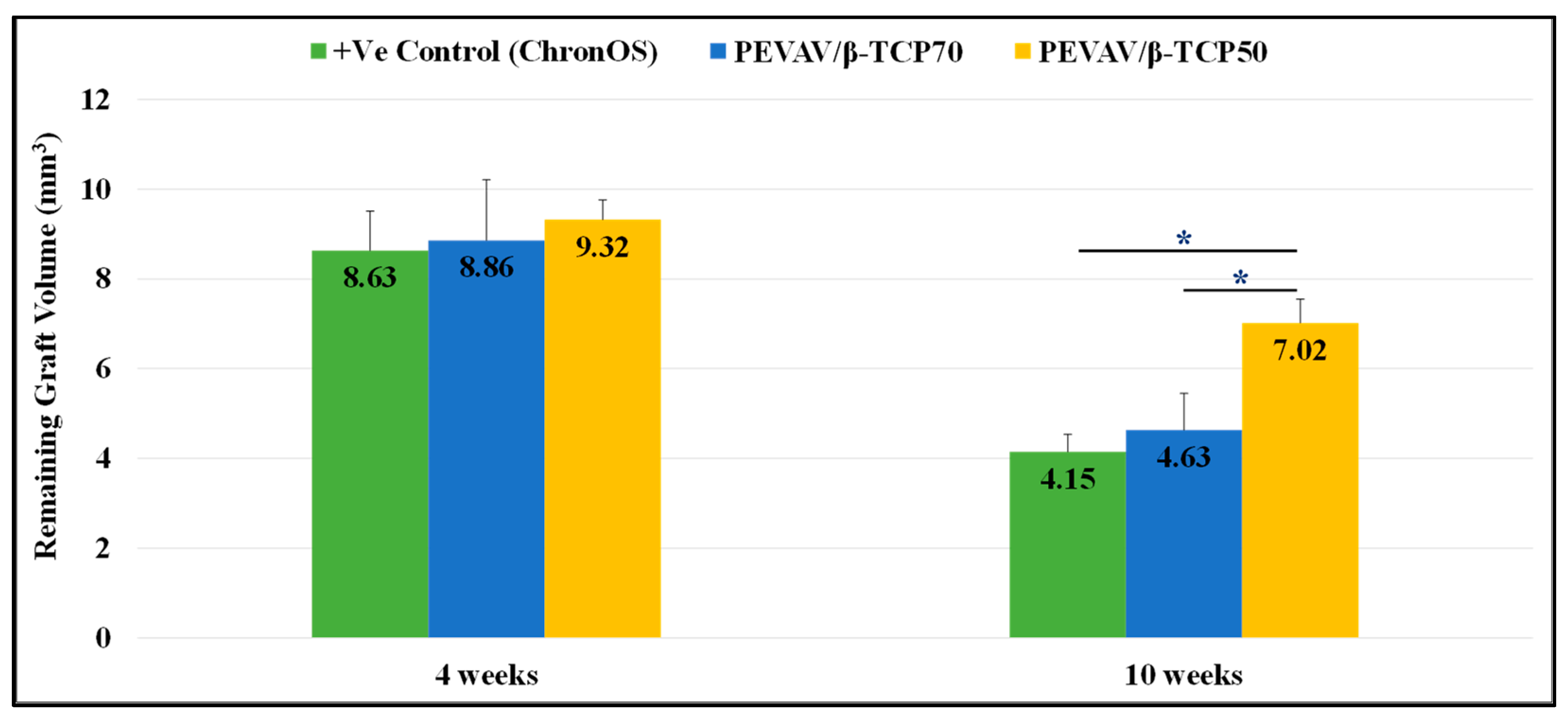
3.2.3. Comparison between the Groups in Terms of Remaining Graft Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaheen, M.Y.; Basudan, A.M.; Niazy, A.A.; van den Beucken, J.J.J.P.; Jansen, J.A.; Alghamdi, H.S. Histological and Histomorphometric Analyses of Bone Regeneration in Osteoporotic Rats Using a Xenograft Material. Materials 2021, 14, 222. [Google Scholar] [CrossRef]
- Ahmed, A.G.; Awartani, F.A.; Niazy, A.A.; Jansen, J.A.; Alghamdi, H.S.J.A.S. A Combination of Biphasic Calcium Phosphate (Maxresorb®) and Hyaluronic Acid Gel (Hyadent®) for Repairing Osseous Defects in a Rat Model. Appl. Sci. 2020, 10, 1651. [Google Scholar] [CrossRef]
- Badwelan, M.; Alkindi, M.; Ramalingam, S.; Nooh, N.; Al Hezaimi, K. The Efficacy of Recombinant Platelet-Derived Growth Factor on Beta-Tricalcium Phosphate to Regenerate Femoral Critical Sized Segmental Defects: Longitudinal In Vivo Micro-CT Study in a Rat Model. J. Investig. Surg. 2018, 33, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Nakagiri, R.; Watanabe, S.; Takabatake, K.; Tsujigiwa, H.; Watanabe, T.; Matsumoto, H.; Kimata, Y. Long-Term Effect of Honeycomb β-Tricalcium Phosphate on Zygomatic Bone Regeneration in Rats. Materials 2020, 13, 5374. [Google Scholar] [CrossRef]
- Mangano, C.; Sinjari, B.; Shibli, J.A.; Mangano, F.; Hamisch, S.; Piattelli, A.; Perrotti, V.; Iezzi, G. A Human Clinical, Histological, Histomorphometrical, and Radiographical Study on Biphasic HA-Beta-TCP 30/70 in Maxillary Sinus Augmentation. Clin. Implant. Dent. Relat. Res. 2015, 17, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, Z.; Bai, H.; Li, J.; Li, X.; Chen, G.; Lu, J. Clinical evaluation of β-TCP in the treatment of lacunar bone defects: A prospective, randomized controlled study. Mater. Sci. Eng. C 2013, 33, 1894–1899. [Google Scholar] [CrossRef]
- Sadiasa, A.; Nguyen, T.H.; Lee, B.-T. In vitro and in vivo evaluation of porous PCL-PLLA 3D polymer scaffolds fabricated via salt leaching method for bone tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2014, 25, 150–167. [Google Scholar] [CrossRef]
- Miyai, T.; Ito, A.; Tamazawa, G.; Matsuno, T.; Sogo, Y.; Nakamura, C.; Yamazaki, A.; Satoh, T. Antibiotic-loaded poly-ε-caprolactone and porous β-tricalcium phosphate composite for treating osteomyelitis. Biomaterials 2008, 29, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Layman, J.M.; Watkins, J.R.; Bowlin, G.L.; Matthews, J.A.; Simpson, D.G.; Wnek, G.E. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials 2003, 24, 907–913. [Google Scholar] [CrossRef]
- López, O.B.L.; Sierra, G.L.; Mejía, G.A.I. Biodegradability of poly (vinyl alcohol). Polym. Eng. Sci. 1999, 39, 1346–1352. [Google Scholar] [CrossRef]
- Khalil, M.; Al-Shamary, D.; Al-Deyab, S. Synthesis of poly (δ-valerolactone) by activated monomer polymerization, its characterization and potential medical application. Asian J. Biochem. Pharm. Res. 2015, 5, 137–147. [Google Scholar]
- Saeed, W.S.; Al-Odayni, A.-B.; Alghamdi, A.A.; Al-Owais, A.A.; Semlali, A.; Aouak, T. Miscibility of Poly(Ethylene-co-Vinylalcohol)/Poly(δ-Valerolactone) Blend and Tissue Engineering Scaffold Fabrication Using Naphthalene as Porogen. Polym. Plast. Technol. Eng. 2018. [Google Scholar] [CrossRef]
- Badwelan, M.; Alkindi, M.; Alghamdi, O.; Saeed, W.S.; Al-Odayni, A.-B.; Alrahlah, A.; Aouak, T.J.P. Poly (δ-Valerolactone)/Poly (ethylene-Co-Vinylalcohol)/β-Tri-Calcium Phosphate Composite as Scaffolds: Preparation, Properties, and In Vitro Amoxicillin Release. Polymers 2020, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Pihlman, H.; Keränen, P.; Paakinaho, K.; Linden, J.; Hannula, M.; Manninen, I.-K.; Hyttinen, J.; Manninen, M.; Laitinen-Vapaavuori, O. Novel osteoconductive β-tricalcium phosphate/poly(L-lactide-co-e-caprolactone) scaffold for bone regeneration: A study in a rabbit calvarial defect. J. Mater. Sci. Mater. Med. 2018, 29, 156. [Google Scholar] [CrossRef] [PubMed]
- Saeed, W.S.; Al-Odayni, A.-B.; Alrahlah, A.; Alghamdi, A.A.; Aouak, T. Preparation and Characterization of Poly(δ-Valerolactone)/TiO2 Nanohybrid Material with Pores Interconnected for Potential Use in Tissue Engineering. Materials 2019, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 2004, 4, 26. [Google Scholar] [CrossRef]
- Kim, H.C.; Song, J.M.; Kim, C.J.; Yoon, S.Y.; Kim, I.R.; Park, B.S.; Shin, S.H. Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 16. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.L.; Underwood, W.; Anthony, R.; Cartner, S.; Corey, D.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.; Meyer, R. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2013. [Google Scholar]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater. Sci. Eng. C 2016, 60, 357–364. [Google Scholar] [CrossRef]
- van der Pol, U.; Mathieu, L.; Zeiter, S.; Bourban, P.E.; Zambelli, P.Y.; Pearce, S.G.; Boure, L.P.; Pioletti, D.P. Augmentation of bone defect healing using a new biocomposite scaffold: An in vivo study in sheep. Acta Biomater. 2010, 6, 3755–3762. [Google Scholar] [CrossRef]
- Waarsing, J.H.; Day, J.S.; Weinans, H. An improved segmentation method for in vivo μCT imaging. J. Bone Miner. Res. 2004, 19, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, M.; Suchý, T.; Tonar, Z.; Foltan, R.; Kalbáčová, M.H.J.C.-S. Micro-CT in tissue engineering scaffolds designed for bone regeneration: Principles and application. Ceram. Silikáty 2018, 62, 194–199. [Google Scholar] [CrossRef]
- Al-Hezaimi, K.; Ramalingam, S.; Al-Askar, M.; ArRejaie, A.S.; Nooh, N.; Jawad, F.; Aldahmash, A.; Atteya, M.; Wang, C.-Y. Real-time-guided bone regeneration around standardized critical size calvarial defects using bone marrow-derived mesenchymal stem cells and collagen membrane with and without using tricalcium phosphate: An in vivo micro-computed tomographic and histologic experiment in rats. Int. J. Oral Sci. 2016, 8, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, H.-J.; Kim, K.-S.; Lee, S.J.; Lee, J.-T.; Kim, S.-Y.; Chang, N.-H.; Park, S.-Y.J.M. In vivo evaluation of 3D-printed polycaprolactone scaffold implantation combined with β-TCP powder for alveolar bone augmentation in a beagle defect model. Materials 2018, 11, 238. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef]
- Park, H.J.; Min, K.D.; Lee, M.C.; Kim, S.H.; Lee, O.J.; Ju, H.W.; Moon, B.M.; Lee, J.M.; Park, Y.R.; Kim, D.W.; et al. Fabrication of 3D porous SF/β-TCP hybrid scaffolds for bone tissue reconstruction. J. Biomed. Mater. Res. Part A 2016, 104, 1779–1787. [Google Scholar] [CrossRef]
- Neamat, A.; Gawish, A.; Gamal-Eldeen, A.M. β-Tricalcium phosphate promotes cell proliferation, osteogenesis and bone regeneration in intrabony defects in dogs. Arch. Oral Biol. 2009, 54, 1083–1090. [Google Scholar] [CrossRef]
- Lobo, S.E.; Arinzeh, T.L. Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.A.; Schlegel, K.; Schultze-Mosgau, S.; Kloss, F.R.; Rupprecht, S.; Kessler, P. Degradation characteristics of α and β tri-calcium-phosphate (TCP) in minipigs. J. Biomed. Mater. Res. 2002, 63, 115–121. [Google Scholar] [CrossRef]
- Takata, T.; Miyauchi, M.; Wang, H.-L. Migration of osteoblastic cells on various guided bone regeneration membranes. Clin. Oral Implant. Res. 2001, 12, 332–338. [Google Scholar] [CrossRef]
- Wang, H.-L.; Carroll, W.J. Guided bone regeneration using bone grafts and collagen membranes. Quintessence Int. 2001, 32, 504–515. [Google Scholar]
- Ramalingam, S.; Al-Rasheed, A.; ArRejaie, A.; Nooh, N.; Al-Kindi, M.; Al-Hezaimi, K. Guided bone regeneration in standardized calvarial defects using beta-tricalcium phosphate and collagen membrane: A real-time in vivo micro-computed tomographic experiment in rats. Odontology 2016, 104, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.A.; Saeed, W.S.; Al-Odayni, A.-B.; Alharthi, F.A.; Semlali, A.; Aouak, T. Poly (ethylene-co-vinylalcohol)/Poly (δ-valerolactone)/Aspirin Composite: Model for a New Drug-Carrier System. Polymers 2019, 11, 439. [Google Scholar] [CrossRef] [PubMed]

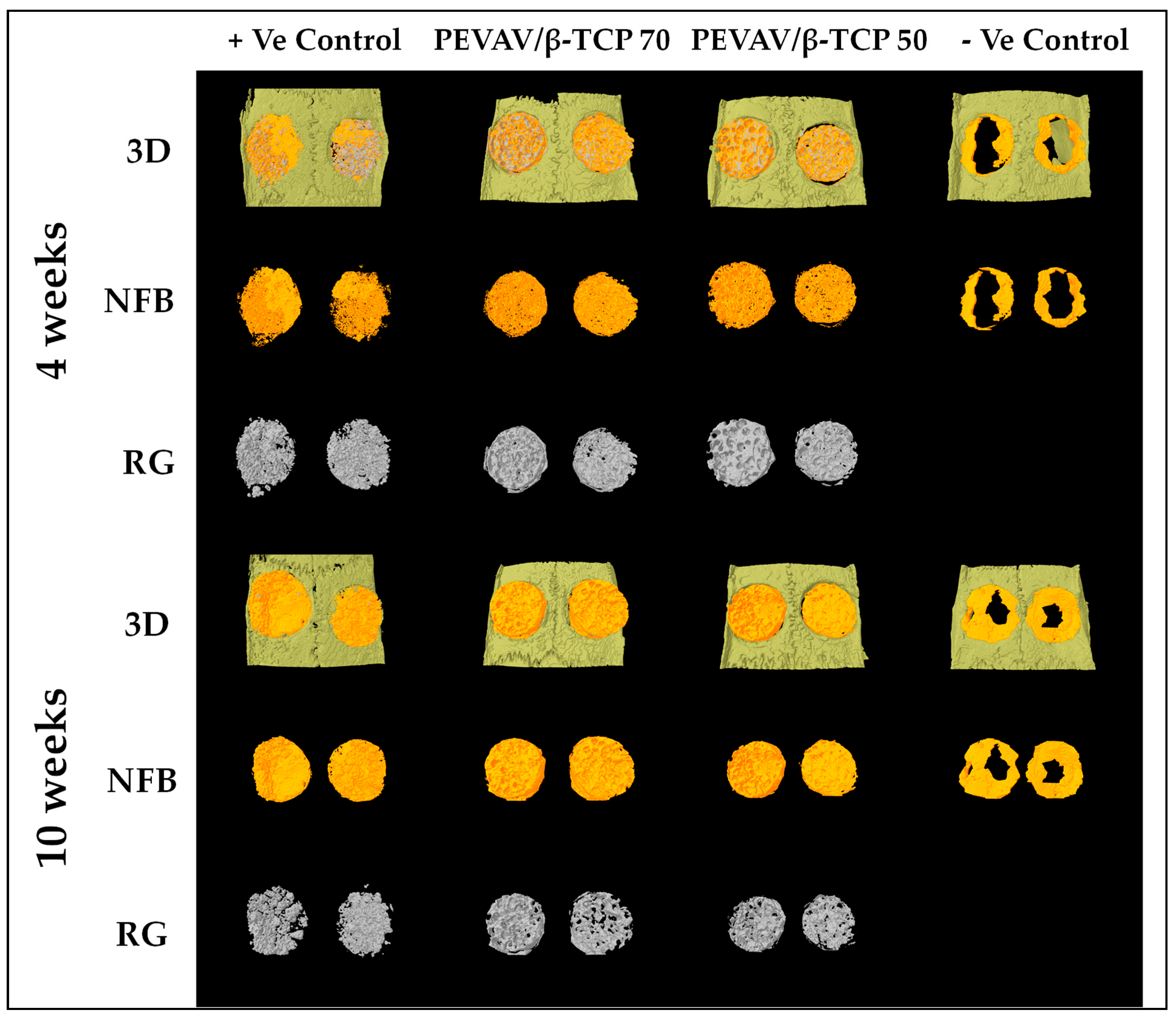
| Study Group (n = 10/group) | Biomaterial Used in Grafting the CSD |
|---|---|
| Positive control | CSD was filled with β-TCP (ChronOS®, DePuy Synthes, Addison, TX, USA) soaked in normal saline and covered by RCM (BioCollagen; 0.2 mm × 5 mm × 7.5 mm; BIOTECK S.P.A., Vicenza, Italy). |
| PEVAV/β-TCP 70 | CSD was filled with PEVAV/ β-TCP 70 biocomposite scaffold soaked in normal saline and covered by RCM (BioCollagen; 0.2 mm × 5 mm × 7.5 mm; BIOTECK S.P.A., Vicenza, Italy). |
| PEVAV/β-TCP 50 | CSD was filled with PEVAV/ β-TCP 50 biocomposite scaffold soaked in normal saline and covered by RCM (BioCollagen; 0.2 mm × 5 mm × 7.5 mm; BIOTECK S.P.A., Vicenza, Italy). |
| Negative control | CSD was kept empty, to be filled with a blood clot and covered by RCM (BioCollagen; 0.2 mm × 5 mm × 7.5 mm; BIOTECK S.P.A., Vicenza, Italy). |
| Time Point | Group (n = 10) | Newly Formed Bone Volume (mm3) | Newly Formed Bone Mineral Density (g/mm3) | Remaining Graft Volume (mm3) |
|---|---|---|---|---|
| 4 weeks | Positive Control | 2.37 ± 0.33 ″ | 1.29 ± 0.31 | 8.63 ± 0.88 |
| PEVAV/β-TCP 70 | 2.56 ± 0.52 ″§ | 1.10 ± 0.25 | 8.86 ± 1.35 | |
| PEVAV/β-TCP 50 | 2.01 ± 0.32 ″^ | 0.92 ± 0.20 | 9.32 ± 0.44 | |
| Negative Control | 1.31 ± 0.28 †^§ | 0.91 ± 0.30 | - | |
| 10 weeks | Positive Control | 5.97 ± 1.34 ″^ | 1.77 ± 0.12 §″ | 4.15 ± 0.39 § |
| PEVAV/β-TCP 70 | 8.21 ± 0.30 ″†§ | 1.62 ± 0.18 §″ | 4.63 ± 0.82 § | |
| PEVAV/β-TCP 50 | 6.56 ± 0.80 ^″ | 1.31 ± 0.12 †^ | 7.02 ± 0.53 †^ | |
| Negative Control | 3.83 ± 1.57 †^§ | 1.20 ± 0.11 †^ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badwelan, M.; Alkindi, M.; Alghamdi, O.; Ahmed, A.; Ramalingam, S.; Alrahlah, A. Bone Regeneration Using PEVAV/β-Tricalcium Phosphate Composite Scaffolds in Standardized Calvarial Defects: Micro-Computed Tomographic Experiment in Rats. Materials 2021, 14, 2384. https://doi.org/10.3390/ma14092384
Badwelan M, Alkindi M, Alghamdi O, Ahmed A, Ramalingam S, Alrahlah A. Bone Regeneration Using PEVAV/β-Tricalcium Phosphate Composite Scaffolds in Standardized Calvarial Defects: Micro-Computed Tomographic Experiment in Rats. Materials. 2021; 14(9):2384. https://doi.org/10.3390/ma14092384
Chicago/Turabian StyleBadwelan, Mohammed, Mohammed Alkindi, Osama Alghamdi, Abeer Ahmed, Sundar Ramalingam, and Ali Alrahlah. 2021. "Bone Regeneration Using PEVAV/β-Tricalcium Phosphate Composite Scaffolds in Standardized Calvarial Defects: Micro-Computed Tomographic Experiment in Rats" Materials 14, no. 9: 2384. https://doi.org/10.3390/ma14092384
APA StyleBadwelan, M., Alkindi, M., Alghamdi, O., Ahmed, A., Ramalingam, S., & Alrahlah, A. (2021). Bone Regeneration Using PEVAV/β-Tricalcium Phosphate Composite Scaffolds in Standardized Calvarial Defects: Micro-Computed Tomographic Experiment in Rats. Materials, 14(9), 2384. https://doi.org/10.3390/ma14092384







