Modified Natural Dolomite and Its Influence on the Production of Glycerol Carbonate: Effects of Structural and Basicity Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Dolomite and Various Treatments
2.2. Characterization
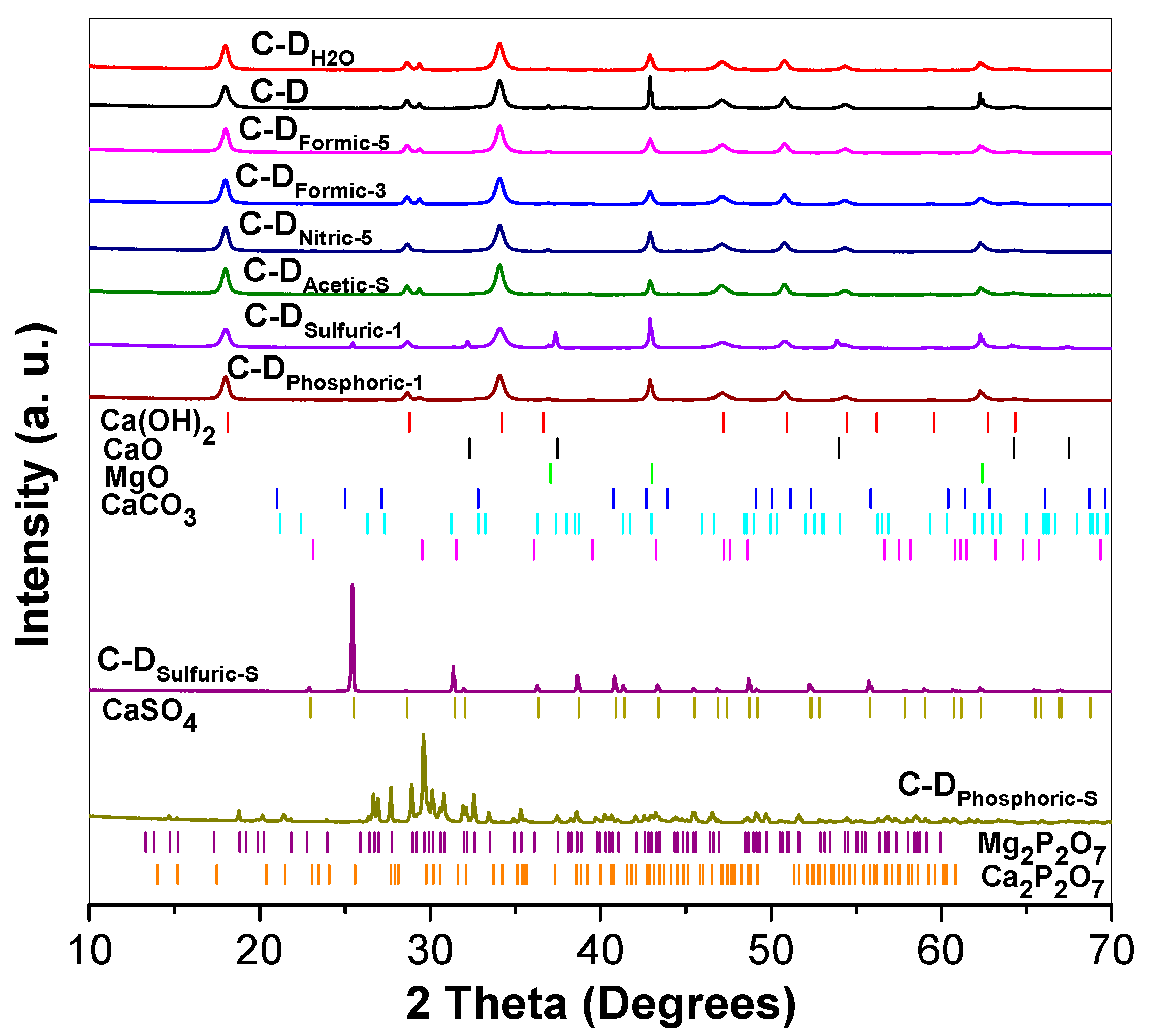
2.2.1. XRD Analysis
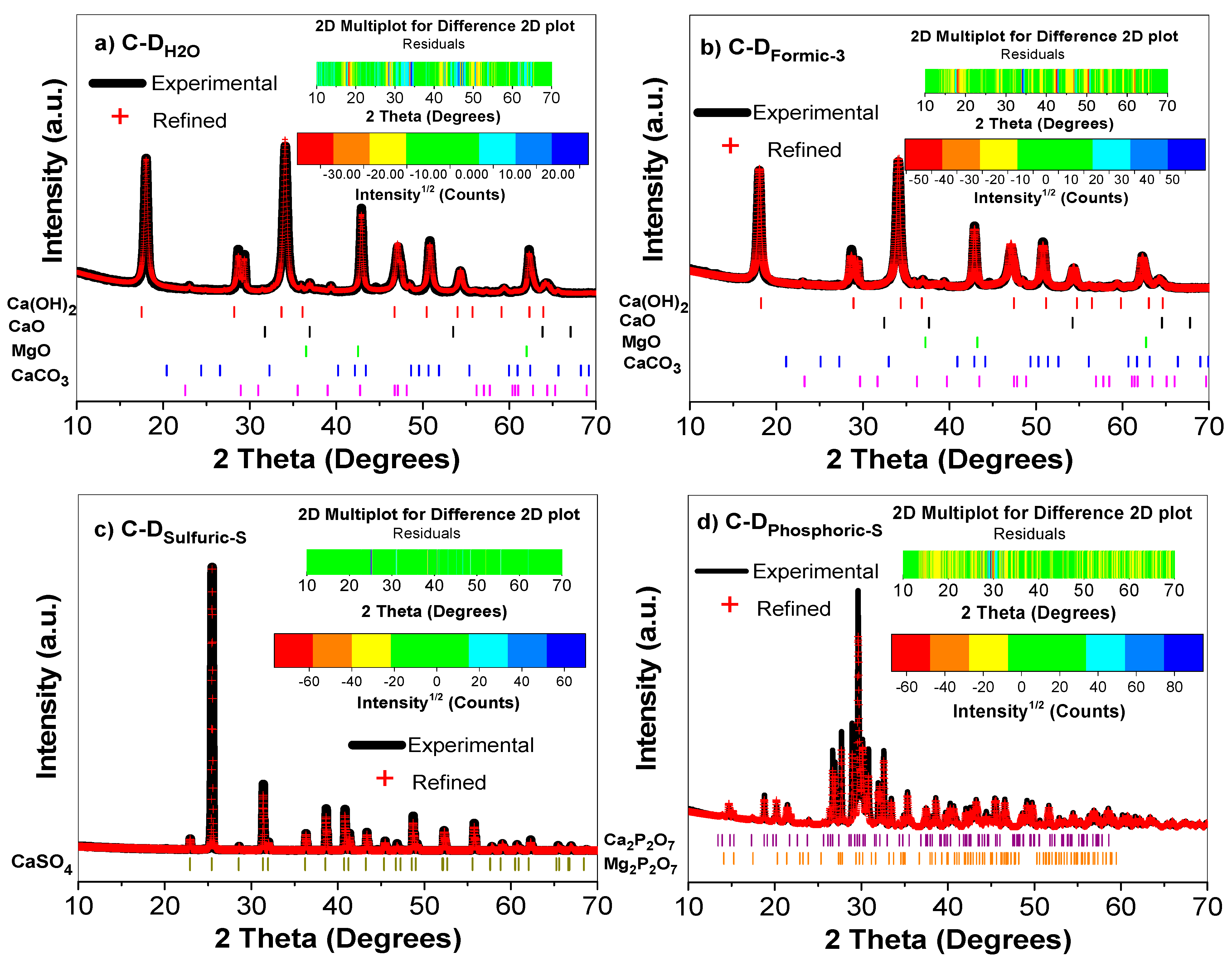
2.2.2. Rietveld Refinement
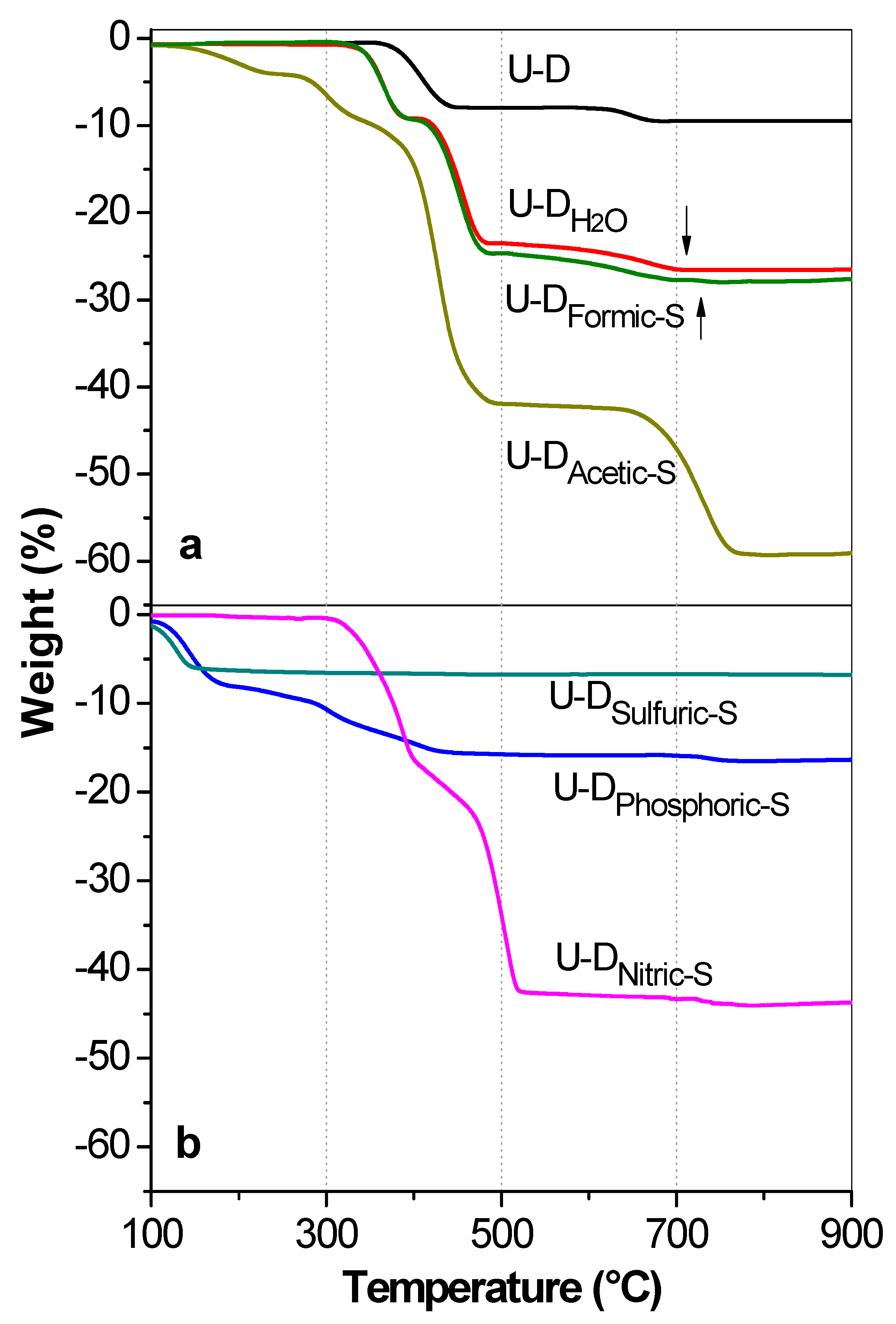
2.2.3. Thermogravimetric Analysis (TGA)
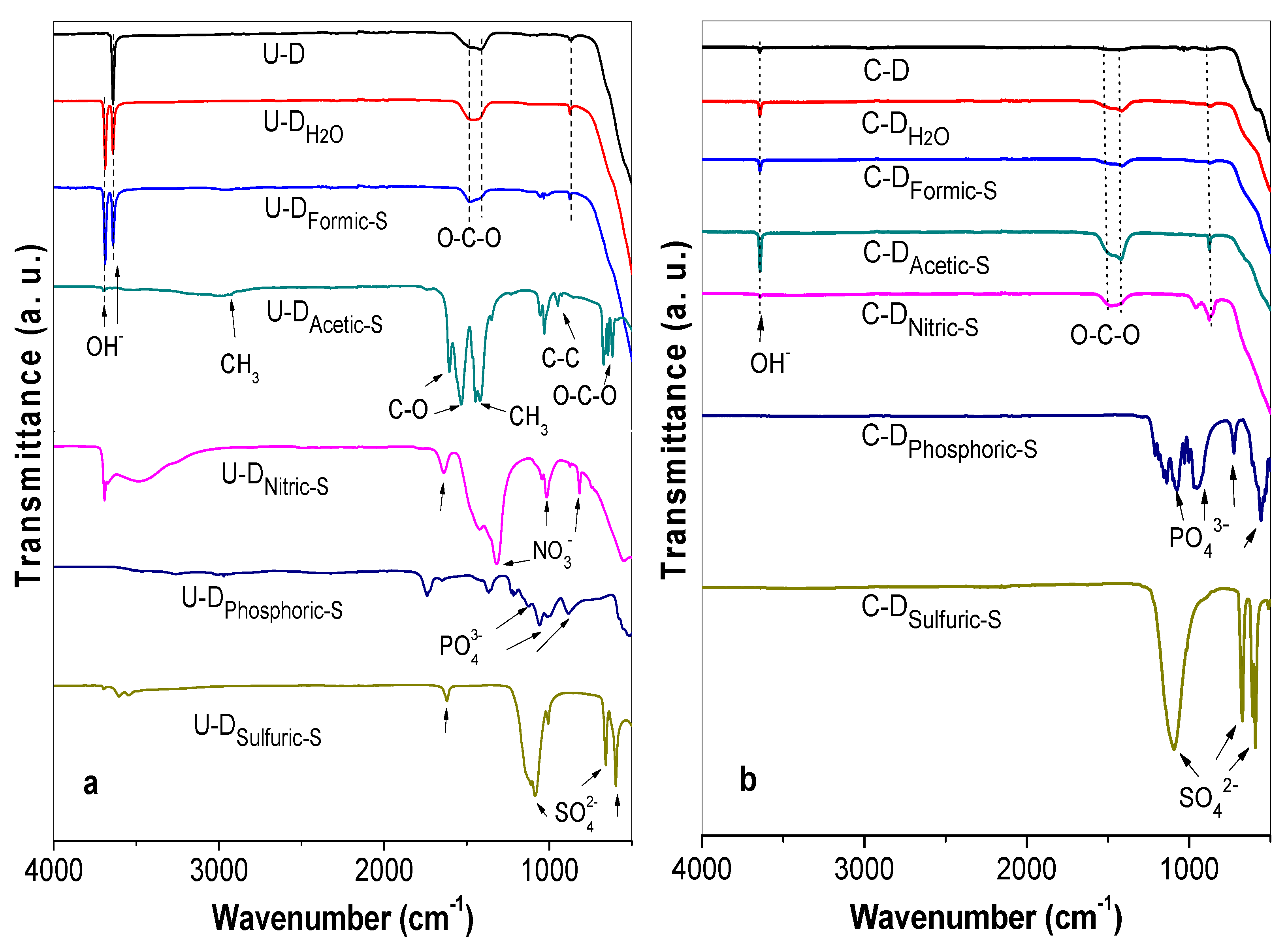
2.2.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.2.5. N2 Adsorption-Desorption Isotherms Measurements
2.2.6. CO2 Temperature-Programed Desorption (CO2-TPD)
2.3. Glycerol Carbonate Synthesis and Product Analysis
3. Results and Discussion
3.1. XRD Analysis and Rietveld Refinement
3.2. FT-IR Characterization of the Treated Samples Before Calcination
3.3. FT-IR Characterization of the Treated Samples after Calcination
3.4. Thermogravimetric Analysis (TGA) of Uncalcined Samples
3.5. Textural Properties of the Treated Samples
3.6. CO2 Adsorption and Basicity
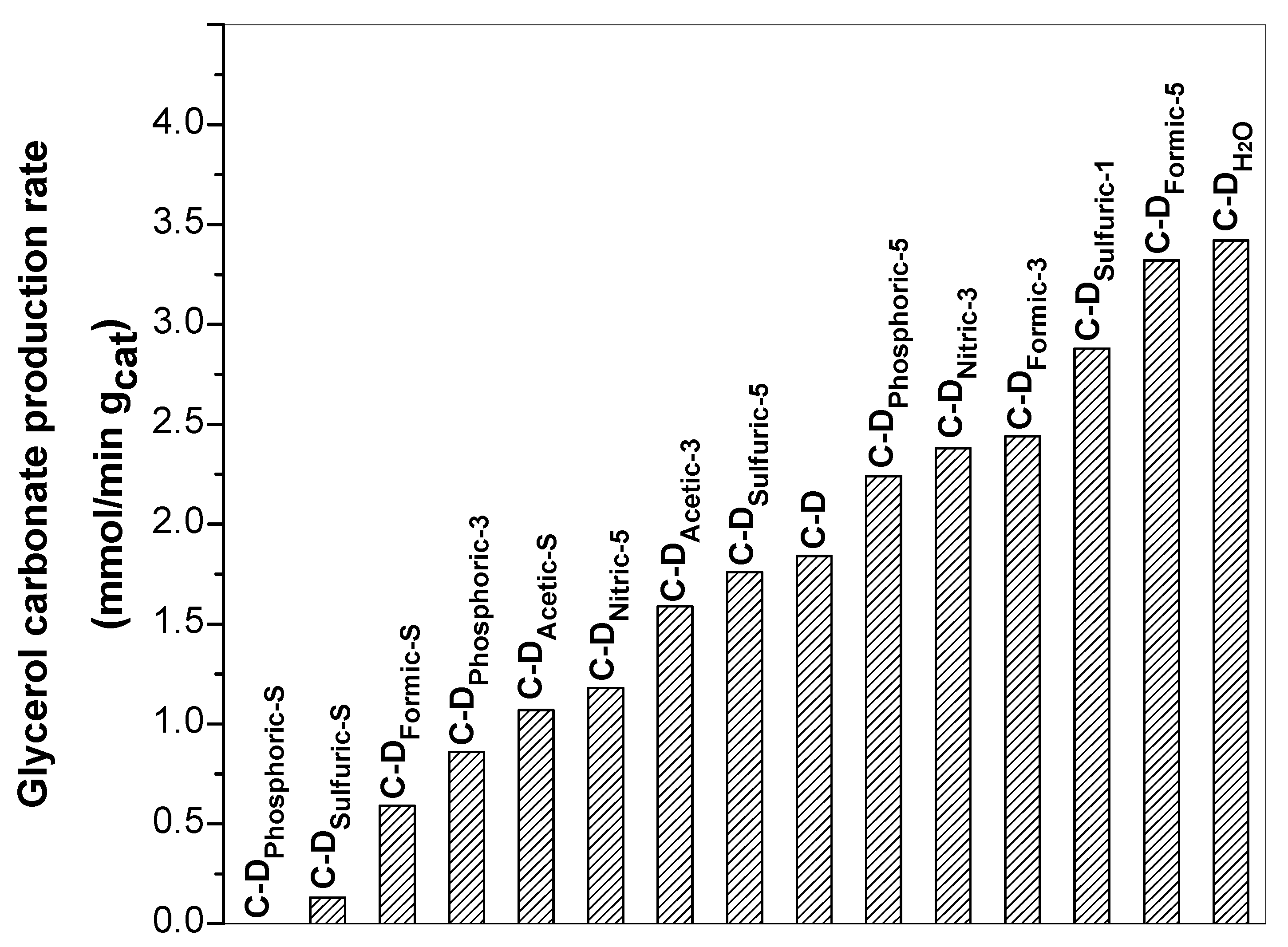
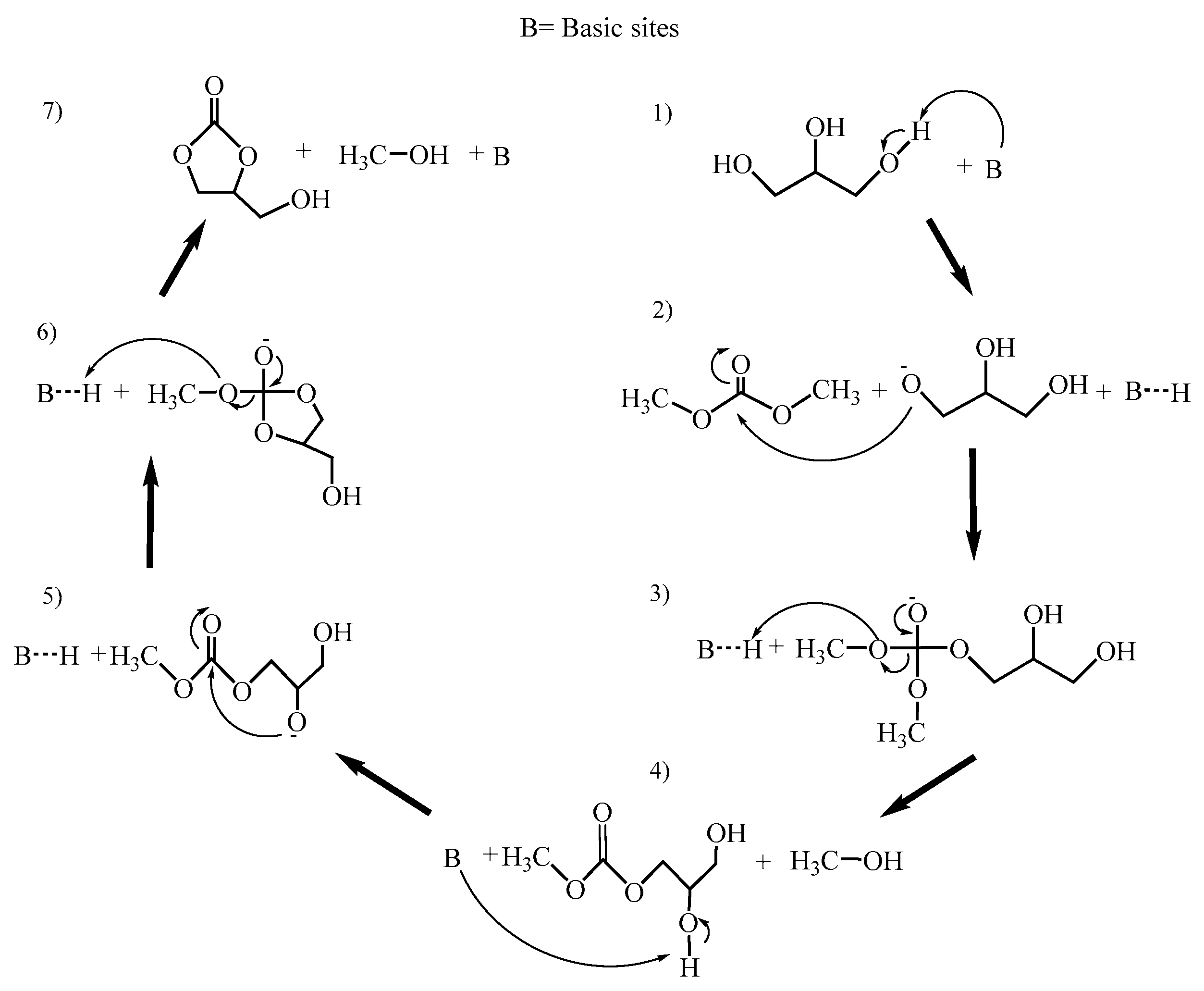
3.7. Catalytic Activity—Synthesis of Glycerol Carbonate
4. Conclusions
- Different treatments led to the reorganization of the pore system of dolomite by diminishing pore diameter from approximately 17 Å to 4~6 Å and enhancing the surface area by 4~5 times. Hydrothermal and formic acid treatments greatly improved the surface area.
- The use of the different treatments and calcination of the dolomite modified the crystalline structure and composition, determined by the XRD analysis and Rietveld refinements. Most of the samples contained crystalline CaO and Ca(OH)2 in different percentages, resulting the hydrothermal treated sample with the higher content of Ca(OH)2. The presence of the hydroxyl species in the structure and surface could be as a result of the fast rehydration of highly active sites of CaO with moisture.
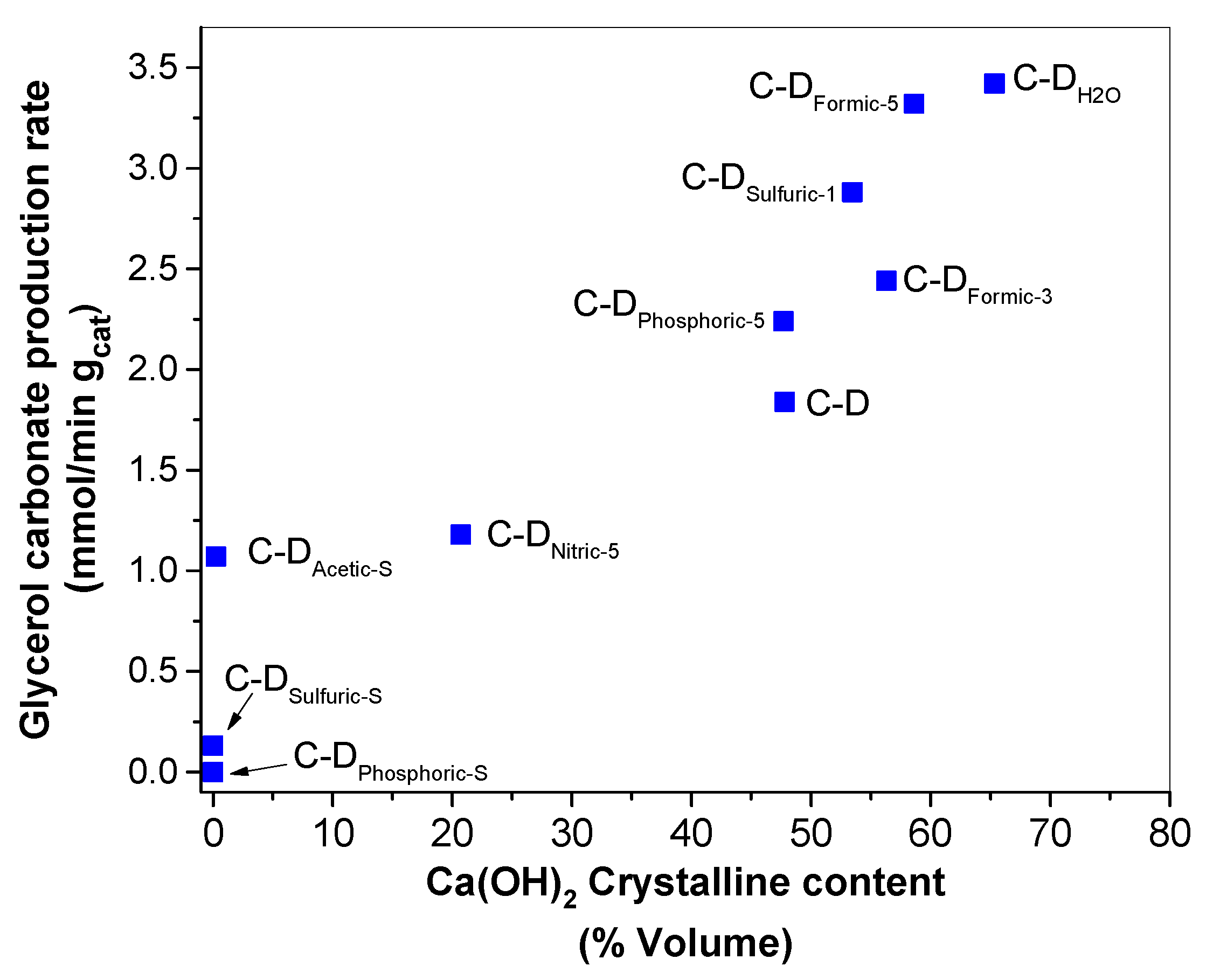
- The presence of Ca(OH)2 in the materials was related to the presence of active sites needed to catalyze the glycerol carbonate synthesis as evidenced by the fact that glycerol carbonate production rate was proportional to the amount of Ca(OH)2. Among the treatments the hydrothermal led to the highest concentration of active sites with weak and moderate strength basicity. However, the sulfuric and phosphoric acids at stoichiometric molar ratio produced inactive crystalline CaSO4, Ca2P2O7, and Mg2P2O7 phases for the glycerol carbonate synthesis, evidenced by the Rietveld refinement and FTIR results.
- The best catalytic performance for the synthesis of glycerol carbonate was achieved on the C-DH2O and C-DFormic-5 catalysts with apparent carbonate production rate of 3.42 and 3.32 mmol/min·gcat, respectively. Even though most of the materials increased their superficial area, which could be related with the catalytic activity, the obtained results suggest that the key parameter required for good catalytic activity was the formation of more basic sites with weak–moderate strength in the catalysts.
- The hydrothermal treatment has been proven to be an economic and environment friendly method for obtaining dolomite with potential use in processes that require a weak-medium basic catalyst for the transesterification reaction of glycerol and dimethyl carbonate. These may lead to new applications of natural dolomite minerals.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Zhang, D.; Guo, G.; Jiang, Y.; Wang, M.; Zhang, P.; Li, J. Dolomite application for the removal of nutrients from synthetic swine wastewater by a novel combined electrochemical process. Chem. Eng. J. 2018, 335, 665–675. [Google Scholar] [CrossRef]
- Alvarado, E.; Torres-Martinez, L.M.; Fuentes, A.F.; Quintana, P. Preparation and characterization of MgO powders obtained from different magnesium salts and the mineral dolomite. Polyhedron 2000, 19, 2345–2351. [Google Scholar] [CrossRef]
- Anbalagan, G.; Gunasekaran, S. Thermal decomposition of natural dolomite. Bull. Mater. Sci. 2007, 30, 339–344. [Google Scholar]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Sun, J.; Li, M.; Yang, X.; Zhang, Y.; Liu, X.; Xu, M. Structurally improved CaO-based sorbent by organic acids for high temperature CO2 capture. Fuel 2016, 167, 17–24. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Wu, S.; Liu, C.; Liu, H.; Lu, C. Enhancement of CO2 capture capacity by modifying limestone with propionic acid. Powder Technol. 2013, 233, 8–14. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Duan, L.; Liang, C.; Li, Q.; Zhou, W.; Chen, H. Cyclic calcination/carbonation looping of dolomite modified with acetic acid for CO2 capture. Fuel Process. Technol. 2008, 89, 1461–1469. [Google Scholar] [CrossRef]
- Clark Westcott, C. PH Measurements; Elsevier: Amsterdam, The Netherlands, 1978; Volume 184, ISBN 9780127451503. [Google Scholar]
- Das, B.; Mohanty, K. A green and facile production of catalysts from waste red mud for the one-pot synthesis of glycerol carbonate from glycerol. J. Environ. Chem. Eng. 2019, 7, 102888. [Google Scholar] [CrossRef]
- Okoye, P.U.; Wang, S.; Xu, L.; Li, S.; Wang, J.; Zhang, L. Promotional effect of calcination temperature on structural evolution, basicity, and activity of oil palm empty fruit bunch derived catalyst for glycerol carbonate synthesis. Energy Convers. Manag. 2019, 179, 192–200. [Google Scholar] [CrossRef]
- Okoye, P.U.; Hameed, B.H. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Kumar, P.; With, P.; Srivastava, V.C.; Gläser, R.; Mishra, I.M. Glycerol Carbonate Synthesis by Hierarchically Structured Catalysts: Catalytic Activity and Characterization. Ind. Eng. Chem. Res. 2015, 54, 12543–12552. [Google Scholar] [CrossRef]
- Chilingar, G.V. Notes on classification of carbonate rocks on basis of chemical composition. J. Sediment. Res. 2003, 30, 157–158. [Google Scholar] [CrossRef]
- Lutterotti, L. MAUD. Available online: http://maud.radiographema.eu/ (accessed on 11 January 2021).
- Young, R.A.; Sakthivel, A.; Moss, T.S.; Paiva-Santos, C.O. DBWS-9411—An upgrade of the DBWS *.* programs for Rietveld refinement with PC and mainframe computers. J. Appl. Crystallogr. 1995, 28, 366–367. [Google Scholar] [CrossRef]
- Gu, Q.; Han, D.; Shi, L.; Sun, Q. Styrene epoxidation with hydrogen peroxide over calcium oxide catalysts prepared from various precursors. J. Nat. Gas Chem. 2012, 21, 452–458. [Google Scholar] [CrossRef]
- Santos, R.C.R.; Vieira, R.B.; Valentini, A. Optimization Study in Biodiesel Production via Response Surface Methodology Using Dolomite as a Heterogeneous Catalyst. J. Catal. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared Spectroscopy in the Analysis of Building and Construction Materials. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Guan, W.; Ji, F.; Cheng, Y.; Fang, Z.; Fang, D.; Yan, P.; Chen, Q. A Novel Synthesis Method of Porous Calcium Silicate Hydrate Based on the Calcium Oxide/Polyethylene Glycol Composites. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Laskina, O.; Young, M.A.; Kleiber, P.D.; Grassian, V.H. Infrared extinction spectroscopy and micro-Raman spectroscopy of select components of mineral dust mixed with organic compounds. J. Geophys. Res. Atmos. 2013, 118, 6593–6606. [Google Scholar] [CrossRef]
- Green, D.W. Perry’s Chemical Engineer’s Handbook 8/E Section 2 Physical & Chem Data, 8th ed.; Professional, M.H., Ed.; McGraw-Hill Education: New York, NY, USA, 2007; ISBN 9780071542098. [Google Scholar]
- Rodriguez-Navarro, C.; Kudlacz, K.; Ruiz-Agudo, E. The mechanism of thermal decomposition of dolomite: New insights from 2D-XRD and TEM analyses. Am. Miner. 2012, 97, 38–51. [Google Scholar] [CrossRef]
- Salvadori, B.; Capitani, G.; Mellini, M.; Dei, L. A novel method to prepare inorganic water-soluble nanocrystals. J. Colloid Interface Sci. 2006, 298, 487–490. [Google Scholar] [CrossRef]
- Salimi, E.; Javadpour, J. Synthesis and Characterization of Nanoporous Monetite Which Can Be Applicable for Drug Carrier. J. Nanomater. 2012, 2012, 931492. [Google Scholar] [CrossRef]
- Wang, K.; Hu, X.; Zhao, P.; Yin, Z. Natural dolomite modified with carbon coating for cyclic high-temperature CO2 capture. Appl. Energy 2016, 165, 14–21. [Google Scholar] [CrossRef]
- Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. Preparation and characterization of CaO nanoparticles from Ca(OH)2 by direct thermal decomposition method. J. Ind. Eng. Chem. 2014, 20, 113–117. [Google Scholar] [CrossRef]
- Brockner, W.; Ehrhardt, C.; Gjikaj, M. Thermal decomposition of nickel nitrate hexahydrate, Ni(NO3)2·6H2O, in comparison to Co(NO3)2·6H2O and Ca(NO3)2·4H2O. Thermochim. Acta 2007, 456, 64–68. [Google Scholar] [CrossRef]
- Ettarh, C.; Galwey, A.K. A kinetic and mechanistic study of the thermal decomposition of calcium nitrate. Thermochim. Acta 1996, 288, 203–219. [Google Scholar] [CrossRef]
- Heimann, R.B. Classic and Advanced Ceramics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; ISBN 9783527630172. [Google Scholar]
- Gupta, H.; Fan, L.-S. Carbonation−Calcination Cycle Using High Reactivity Calcium Oxide for Carbon Dioxide Separation from Flue Gas. Ind. Eng. Chem. Res. 2002, 41, 4035–4042. [Google Scholar] [CrossRef]
- Asmi, D.; Low, I.M. Manufacture of graded ceramic matrix composites using infiltration techniques. In Advances in Ceramic Matrix Composites; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 109–140. ISBN 9780857091208. [Google Scholar]
- Tang, Y.; Xu, J.; Zhang, J.; Lu, Y. Biodiesel production from vegetable oil by using modified CaO as solid basic catalysts. J. Clean. Prod. 2013, 42, 198–203. [Google Scholar] [CrossRef]
- Zadick, T.W.; Zavaleta, R.; McCandless, F.P. Catalytic Reduction of Calcium Sulfate to Calcium Sulfide with Carbon Monoxide. Ind. Eng. Chem. Process Des. Dev. 1972, 11, 283–287. [Google Scholar] [CrossRef]
- Shajaratun Nur, Z.A.; Taufiq-Yap, Y.H.; Rabiah Nizah, M.F.; Teo, S.H.; Syazwani, O.N.; Islam, A. Production of biodiesel from palm oil using modified Malaysian natural dolomites. Energy Convers. Manag. 2014, 78, 738–744. [Google Scholar] [CrossRef]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B. Hydration–dehydration technique for property and activity improvement of calcined natural dolomite in heterogeneous biodiesel production: Structural transformation aspect. Appl. Catal. A Gen. 2011, 395, 87–94. [Google Scholar] [CrossRef]
- Parameswaram, G.; Rao, P.S.N.; Srivani, A.; Rao, G.N.; Lingaiah, N. Magnesia-ceria mixed oxide catalysts for the selective transesterification of glycerol to glycerol carbonate. Mol. Catal. 2018, 451, 135–142. [Google Scholar] [CrossRef]
- Tang, Y.; Xue, Y.; Li, Z.; Yan, T.; Zhou, R.; Zhang, Z. Heterogeneous synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by LiCl/CaO. J. Saudi Chem. Soc. 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Calatayud, M.; Ruppert, A.M.; Weckhuysen, B.M. Theoretical Study on the Role of Surface Basicity and Lewis Acidity on the Etherification of Glycerol over Alkaline Earth Metal Oxides. Chem. A Eur. J. 2009, 15, 10864–10870. [Google Scholar] [CrossRef] [PubMed]

| Sample-Treatment Acid | Label |
|---|---|
| Dolomite | U or C-D |
| Dolomite-H2O | U or C-DH2O |
| Dolomite-Formic Acid | U or C-DFormic-# |
| Dolomite-Acetic Acid | U or C-DAcetic-# |
| Dolomite-Nitric Acid | U or C-DNitric-# |
| Dolomite-Sulfuric Acid | U or C-DSulfuric-# |
| Dolomite-Phosphoric acid | U or C-DPhosphoric-# |
| Sample | Crystalline Phases | Symmetry | Space Group | Lattice Parameters (Å) | Crystallite Size (Å) | % Vol | %RWP | ||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | |||||||
| C-D | Ca(OH)2 | Trigonal | P3m1 | 3.5926 | 3.5926 | 4.9189 | 242.1 | 47.80 | 8.29 |
| MgO | Cubic | Fm-3m | 4.2132 | 4.2132 | 4.2132 | 3908.2 | 38.02 | ||
| CaCO3 | Trigonal | R-3c:H | 4.9950 | 4.9950 | 17.0743 | 692.3 | 7.28 | ||
| Orthorhombic | Pbnm | 4.0604 | 7.1536 | 8.3997 | 266.3 | 4.26 | |||
| Orthorhombic | Pmcn | 4.9535 | 8.0206 | 5.7447 | 207.8 | 1.53 | |||
| CaO | Cubic | Fm-3m | 4.7990 | 4.7990 | 4.7990 | 30.8 | 1.11 | ||
| C-DH2O | Ca(OH)2 | Trigonal | P3m1 | 3.5926 | 3.5926 | 4.9226 | 239.9 | 65.35 | 9.12 |
| MgO | Cubic | Fm-3m | 4.2152 | 4.2152 | 4.2152 | 377.7 | 24.61 | ||
| CaCO3 | Trigonal | R-3c:H | 4.9956 | 4.9956 | 17.0659 | 607.1 | 9.91 | ||
| Orthorhombic | Pbnm | 4.1291 | 7.1581 | 8.4764 | 25.3 | 0.13 | |||
| C-DFormic-5 | Ca(OH)2 | Trigonal | P3m1 | 3.5935 | 3.5935 | 4.9130 | 249.0 | 58.63 | 10.14 |
| MgO | Cubic | Fm-3m | 4.2154 | 4.2154 | 4.2154 | 349.7 | 32.47 | ||
| CaCO3 | Trigonal | R-3c:H | 4.9970 | 4.9970 | 17.0600 | 590.5 | 7.10 | ||
| Orthorhombic | Pbnm | 4.0768 | 7.1546 | 8.4337 | 127.4 | 1.80 | |||
| C-DFormic-3 | Ca(OH)2 | Trigonal | P3m1 | 3.5914 | 3.5914 | 4.9137 | 234.0 | 56.29 | 9.65 |
| MgO | Cubic | Fm-3m | 4.2159 | 4.2159 | 4.2159 | 349.6 | 31.92 | ||
| CaCO3 | Trigonal | R-3c:H | 4.9950 | 4.9950 | 17.0707 | 530.0 | 9.65 | ||
| Orthorhombic | Pbnm | 4.1291 | 7.1581 | 8.4764 | 19.8 | 2.14 | |||
| C-DAcetic-S | Ca(OH)2 | Trigonal | P3m1 | 3.5931 | 3.5931 | 4.9164 | 258.8 | 0.25 | 10.03 |
| MgO | Cubic | Fm-3m | 4.2131 | 4.2131 | 4.2131 | 583.4 | 32.98 | ||
| CaCO3 | Trigonal | R-3c:H | 4.9978 | 4.9978 | 17.0689 | 617.5 | 9.48 | ||
| Orthorhombic | Pbnm | 4.0847 | 7.1554 | 8.4764 | 34.2 | 0.49 | |||
| CaO | Cubic | Fm-3m | 4.7391 | 4.7391 | 4.7391 | 66.6 | 56.80 | ||
| C-DNitric-5 | Ca(OH)2 | Trigonal | P3m1 | 3.5937 | 3.5937 | 4.9110 | 248.8 | 20.71 | 11.31 |
| MgO | Cubic | Fm-3m | 4.2144 | 4.2144 | 4.2144 | 422.1 | 74.29 | ||
| CaCO3 | Trigonal | R-3c:H | 4.9932 | 4.9932 | 17.0576 | 292.9 | 4.88 | ||
| Orthorhombic | Pbnm | 4.0929 | 7.1364 | 8.2680 | 182.4 | 0.12 | |||
| C-DSulfuric-1 | Ca(OH)2 | Trigonal | P3m1 | 3.5923 | 3.5923 | 4.9114 | 198.3 | 53.45 | 12.99 |
| CaO | Cubic | Fm-3m | 4.8107 | 4.8107 | 4.8107 | 992.5 | 11.52 | ||
| MgO | Cubic | Fm-3m | 4.2122 | 4.2122 | 4.2122 | 628.1 | 31.18 | ||
| CaSO4 | Orthorhombic | Amma | 6.9929 | 6.9907 | 6.2481 | 802.8 | 3.85 | ||
| C-DSulfuric-S | CaSO4 | Orthorhombic | Amma | 6.9978 | 6.9899 | 6.2405 | 2955.7 | 100.00 | 17.51 |
| C-DPhosphoric-5 | Ca(OH)2 | Trigonal | P3m1 | 3.5931 | 3.5931 | 4.9164 | 254.0 | 47.56 | 11.77 |
| MgO | Cubic | Fm-3m | 4.2131 | 4.2131 | 4.2131 | 435.8 | 39.85 | ||
| CaCO3 | Trigonal | R-3c:H | 4.9978 | 4.9978 | 17.0689 | 260.4 | 7.10 | ||
| Orthorhombic | Pbnm | 4.0847 | 7.1554 | 8.4764 | 185.8 | 5.50 | |||
| C-DPhosphoric-S | Ca2P2O7 | Tetragonal | P41 | 6.6807 | 6.6807 | 24.1191 | 1334.6 | 67.37 | 14.16 |
| Mg2P2O7 | Monoclinic | P21/c:b1 | 6.9438 | 8.2855 | 9.0582 | 539.7 | 32.63 | ||
| * Beta = 114° | |||||||||
| Reaction Number | Reaction | ΔH°r (kJ/mol) | ΔG°r (kJ/mol) |
|---|---|---|---|
| 1 | CaO + H2O → Ca(OH)2 | −65.16 | −65.15 |
| 2 | CaO + 2CHOOH → Ca(CHOO)2 + H2O | 2585.8 | 2560.6 |
| 3 | Ca(OH)2 + 2CHOOH → Ca(CHOO)2 + 2H2O | 2650.4 | 2664.8 |
| 4 | CaO + 2CH3–CHOOH → Ca(CH3–CHOO)2 + H2O | −196.0 | −227.1 |
| 5 | Ca(OH)2 + 2CH3–CHOOH → Ca(CH3–CHOO)2 + 2H2O | −131.3 | −123.0 |
| 6 | CaO + 2HNO3 → Ca(NO3)2 + H2O | −240.0 | −239.9 |
| 7 | Ca(OH)2 + 2HNO3 → Ca(NO3)2 + 2H2O | −75.4 | −109.3 |
| 8 | CaO + H2SO4 → CaSO4 + H2O | −262.6 | −256.6 |
| 9 | Ca(OH)2 + H2SO4 → CaSO4 + 2H2O | −198.0 | −199.7 |
| 10 | 3CaO + 2H3PO4 → Ca3(PO4)2 + 3H2O | −528.1 | −598.1 |
| 11 | 3Ca(OH)2 + 2H3PO4 → Ca3(PO4)2 + 6H2O | −334.1 | −364.7 |
| Sample | Brunauer-Emmett-Teller (BET) Surface Area (m2/g) | Barrett-Joyner-Halenda (BJH) Pore Volume (cm3/g) | Barrett–Joyner–Halenda (BJH) Pore Diameter (Å) |
|---|---|---|---|
| U-D | 8.61 | 0.017 | 16.95 |
| C-D | 18.51 | 0.038 | 18.82 |
| C-DH2O | 31.88 | 0.059 | 3.56 |
| C-DFormic-S | 42.64 | 0.099 | 3.58 |
| C-DFormic-1 | N. D. | N. D. | N. D. |
| C-DFormic-3 | 46.08 | 0.085 | 3.57 |
| C-DFormic-5 | 38.30 | 0.077 | 3.58 |
| C-DAcetic-S | 42.01 | 0.137 | 5.57 |
| C-DAcetic-1 | 31.39 | 0.070 | 3.59 |
| C-DAcetic-3 | 33.46 | 0.072 | 3.58 |
| C-DAcetic-5 | 45.79 | 0.086 | 3.60 |
| C-DNitric-S | 25.40 | 0.063 | 3.98 |
| C-DNitric-1 | 22.00 | 0.050 | 3.58 |
| C-DNitric-3 | 33.26 | 0.075 | 3.58 |
| C-DNitric-5 | 44.58 | 0.080 | 4.42 |
| C-DSulfuric-S | 6.93 | 0.017 | 3.97 |
| C-DSulfuric-1 | 20.32 | 0.036 | 3.59 |
| C-DSulfuric-3 | 21.87 | 0.050 | 3.96 |
| C-DSulfuric-5 | 34.57 | 0.074 | 3.58 |
| C-DPhosphoric-S | 6.32 | 0.007 | 14.4 |
| C-DPhosphoric-1 | 19.22 | 0.048 | 3.25 |
| C-DPhosphoric-3 | 30.99 | 0.056 | 3.97 |
| C-DPhosphoric-5 | 40.09 | 0.078 | 3.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-García, J.; Chen, L.; Campuzano-Calderon, O.; Núñez-Correa, S.; López-Guajardo, E.A.; Wang, J.A.; Montesinos-Castellanos, A. Modified Natural Dolomite and Its Influence on the Production of Glycerol Carbonate: Effects of Structural and Basicity Properties. Materials 2021, 14, 2358. https://doi.org/10.3390/ma14092358
González-García J, Chen L, Campuzano-Calderon O, Núñez-Correa S, López-Guajardo EA, Wang JA, Montesinos-Castellanos A. Modified Natural Dolomite and Its Influence on the Production of Glycerol Carbonate: Effects of Structural and Basicity Properties. Materials. 2021; 14(9):2358. https://doi.org/10.3390/ma14092358
Chicago/Turabian StyleGonzález-García, Julio, Lifang Chen, Omar Campuzano-Calderon, Sara Núñez-Correa, Enrique A. López-Guajardo, Jin An Wang, and Alejandro Montesinos-Castellanos. 2021. "Modified Natural Dolomite and Its Influence on the Production of Glycerol Carbonate: Effects of Structural and Basicity Properties" Materials 14, no. 9: 2358. https://doi.org/10.3390/ma14092358
APA StyleGonzález-García, J., Chen, L., Campuzano-Calderon, O., Núñez-Correa, S., López-Guajardo, E. A., Wang, J. A., & Montesinos-Castellanos, A. (2021). Modified Natural Dolomite and Its Influence on the Production of Glycerol Carbonate: Effects of Structural and Basicity Properties. Materials, 14(9), 2358. https://doi.org/10.3390/ma14092358









