The Role of Elements Partition and Austenite Grain Size in the Ferrite-Bainite Banding Formation during Hot Rolling
Abstract
:1. Introduction
2. Materials and Methods
3. Results
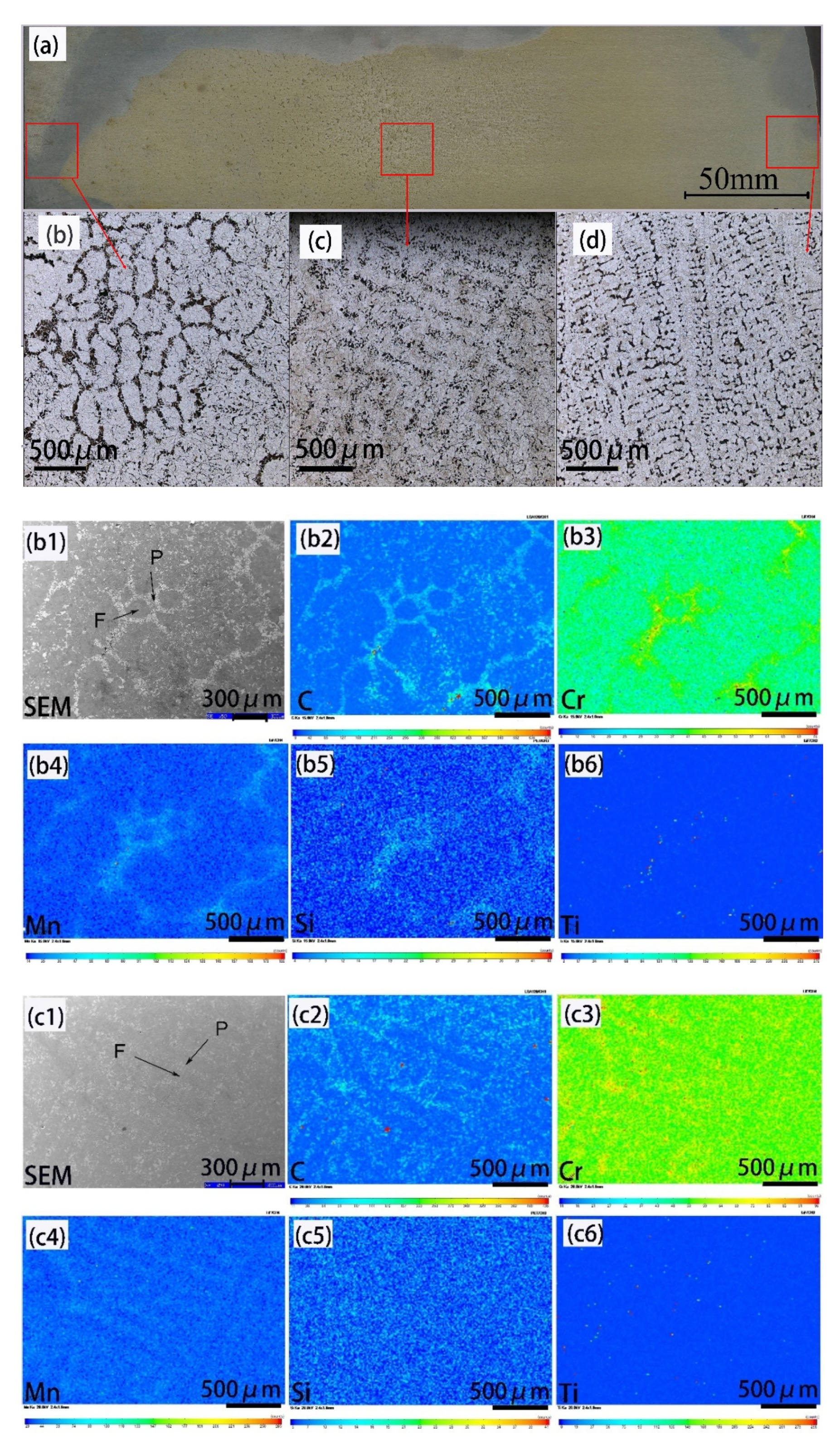
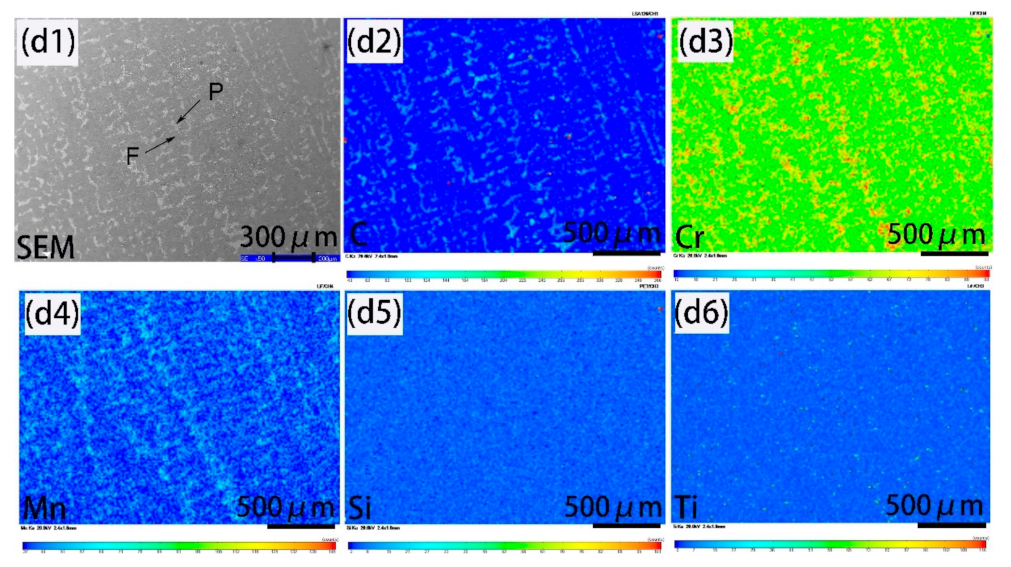
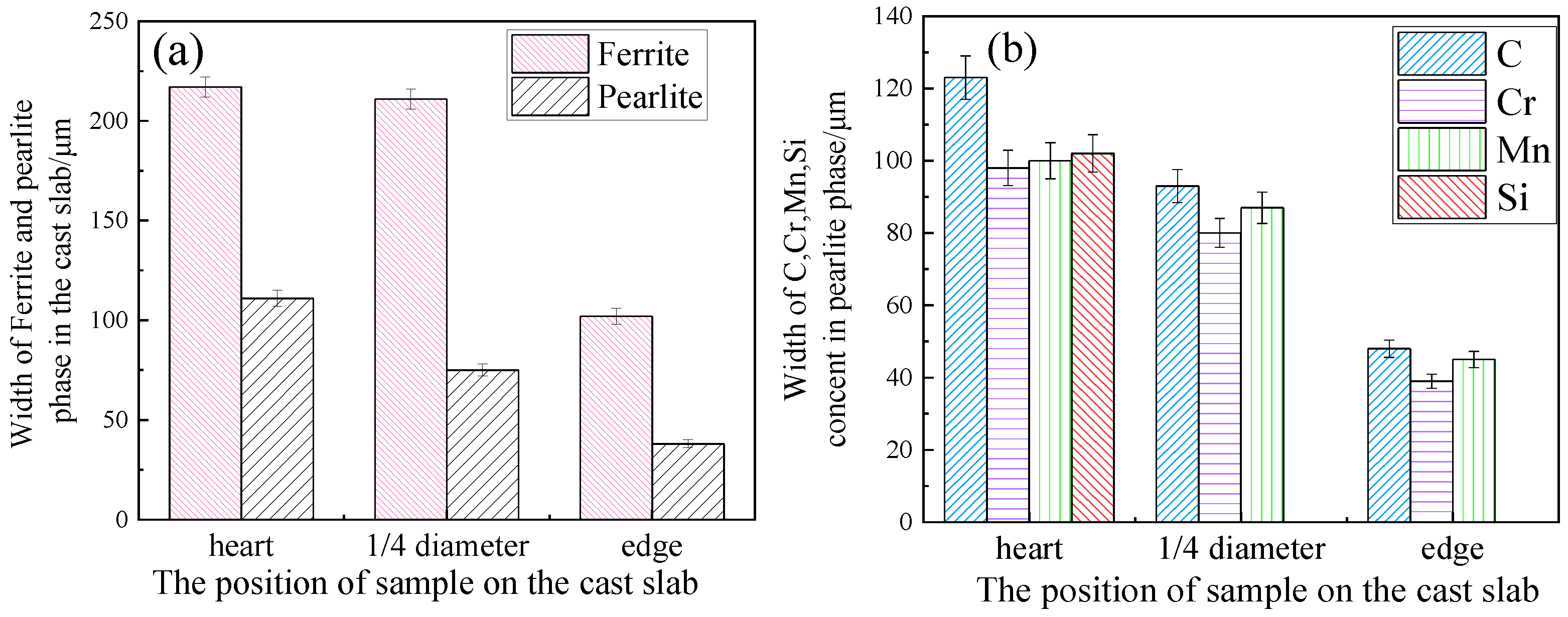
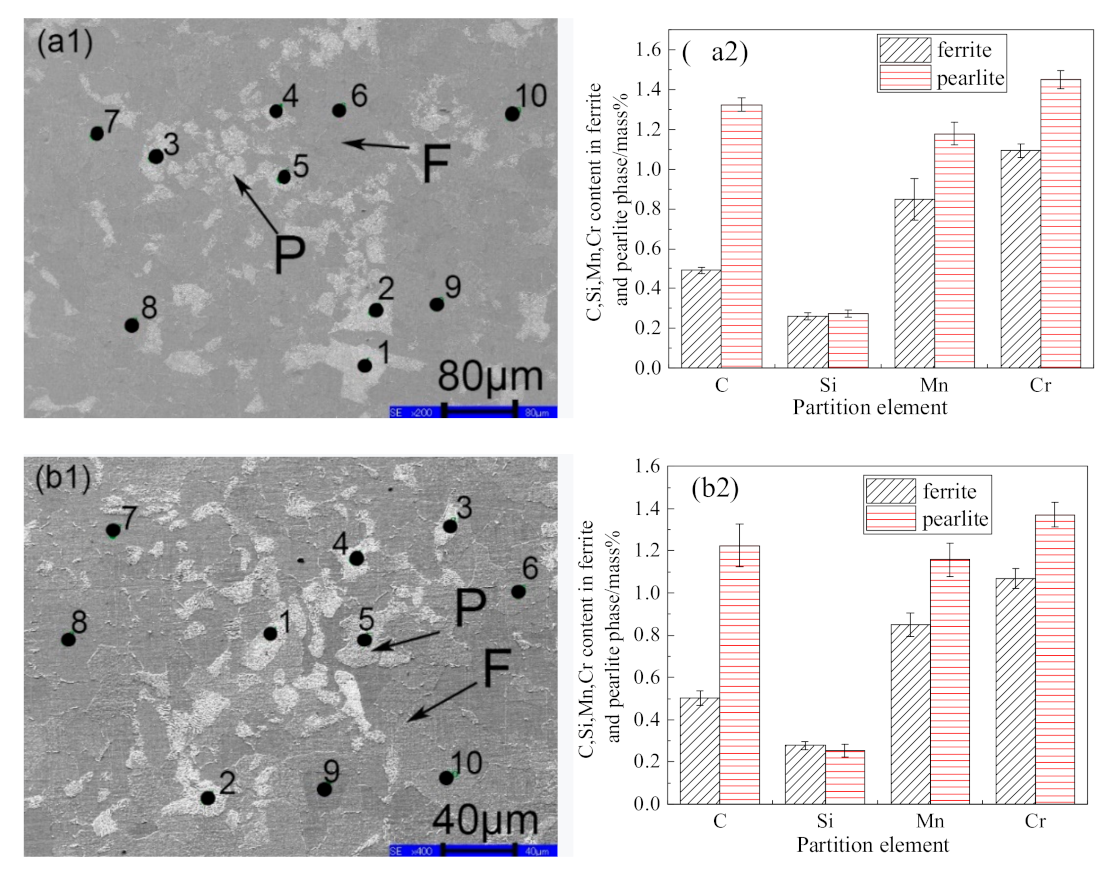
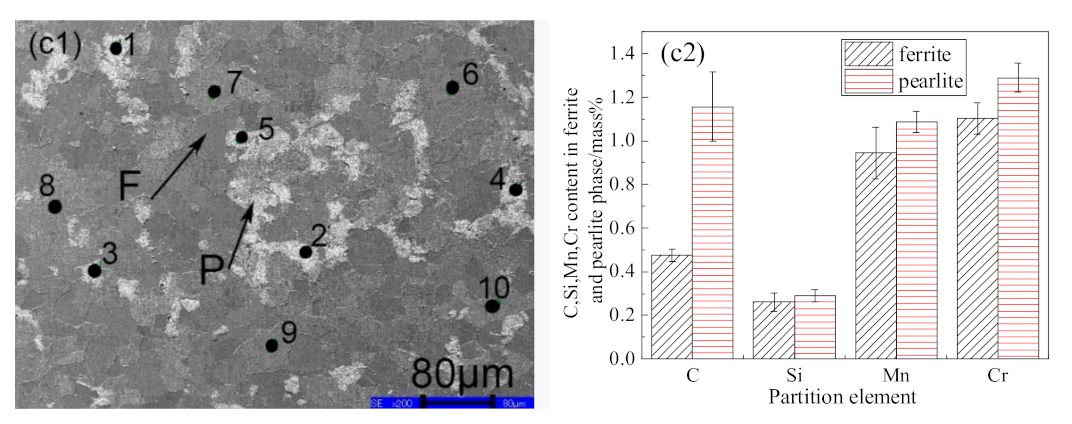
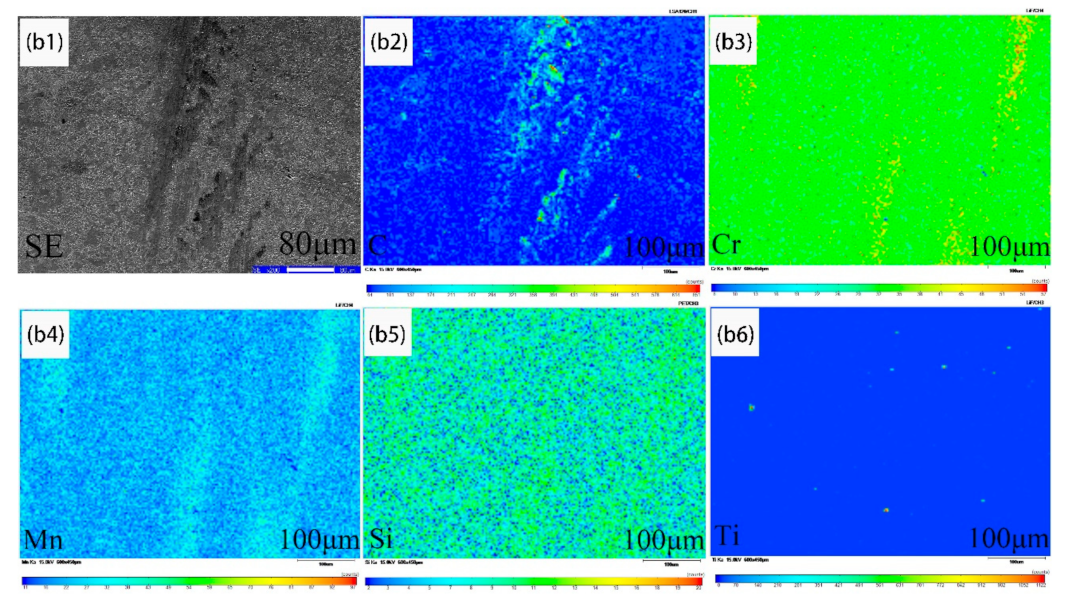
3.1. Ferrite/Pearlite Distribution and Partitioning of Solute Elements in the Cast Slab
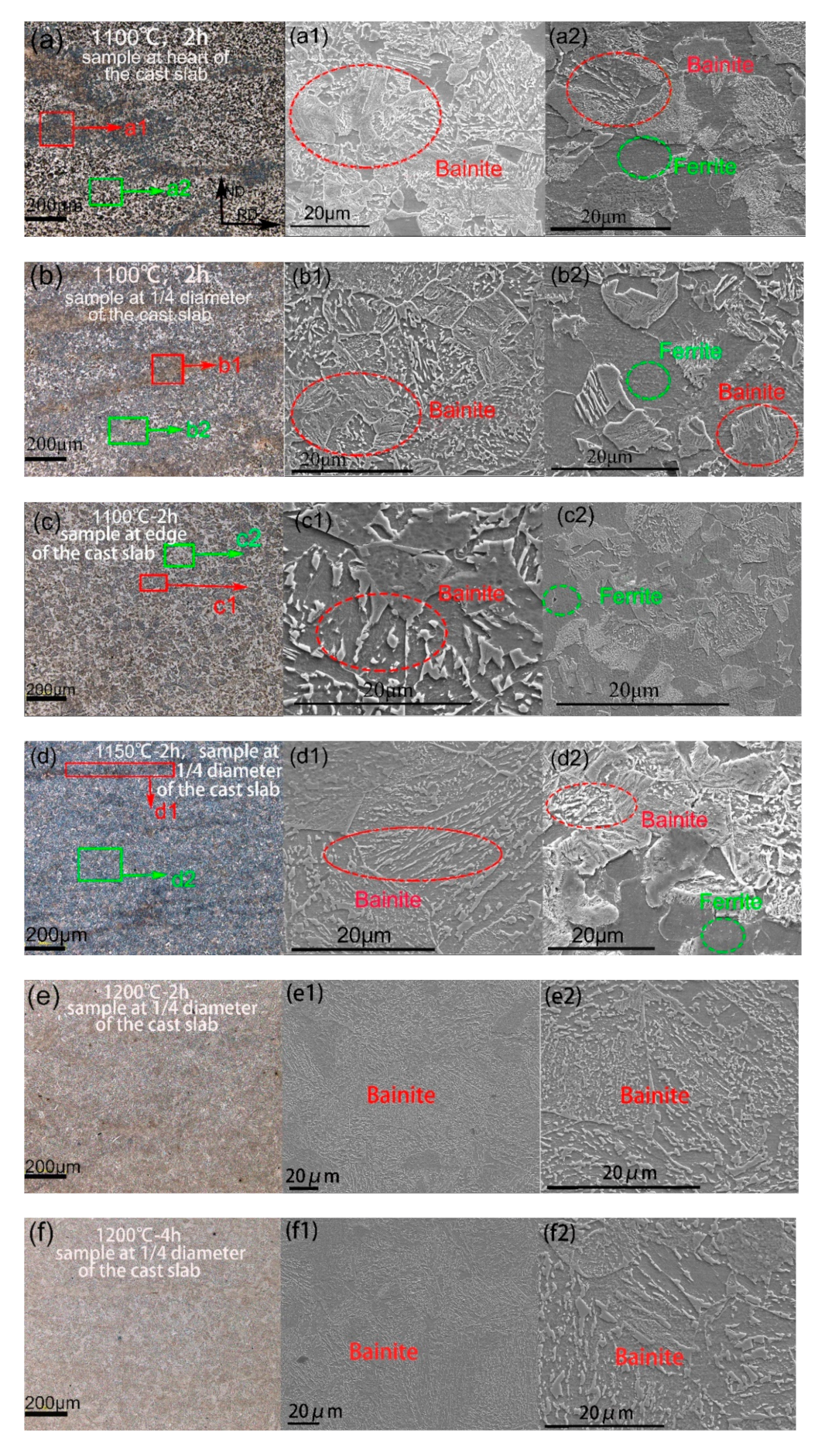
3.2. Ferrite-Bainite Banding Formation after Hot Rolling
4. Discussion
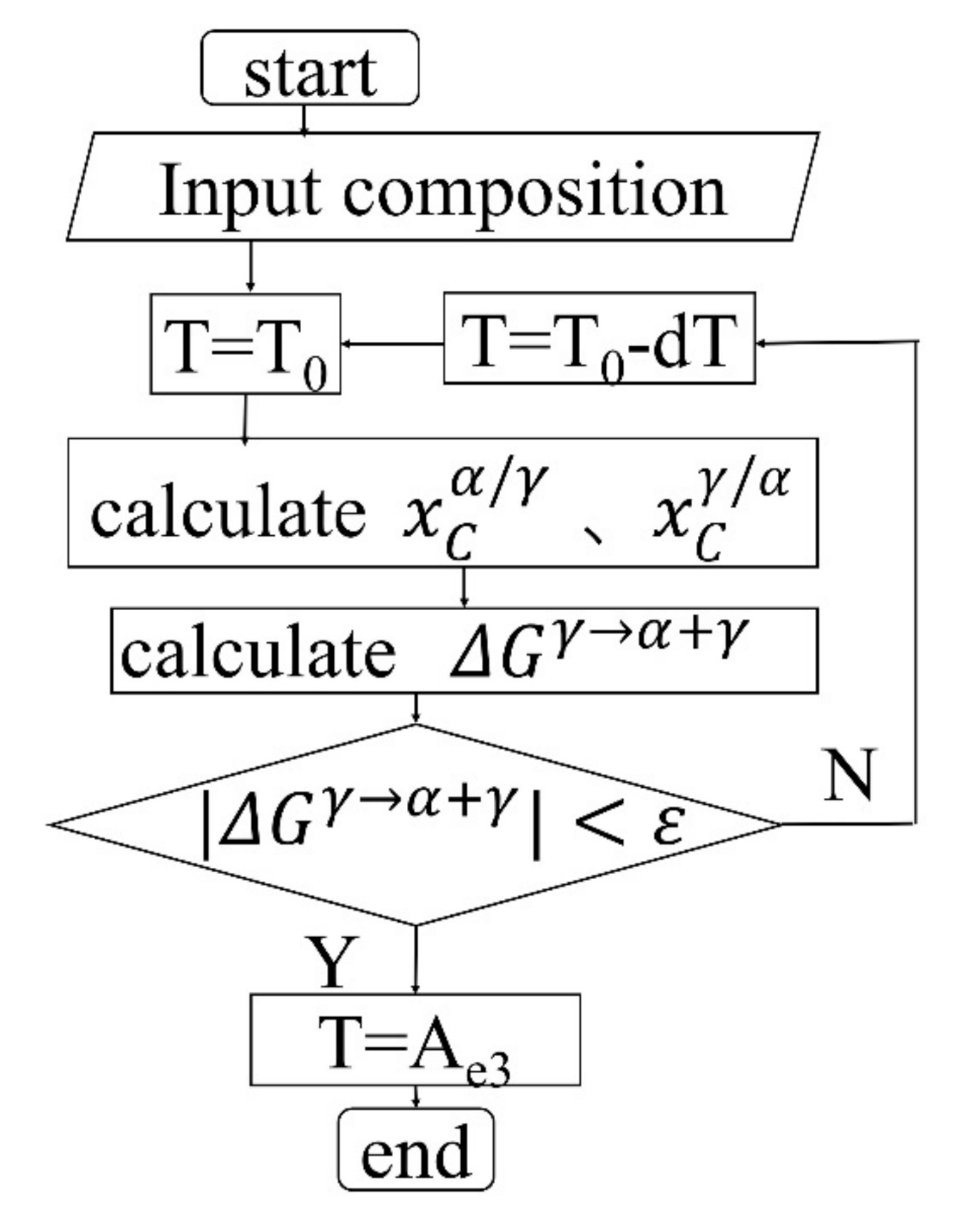
4.1. The Influence of Element Segregation and Diffusion on the γ→α Transformation Temperature
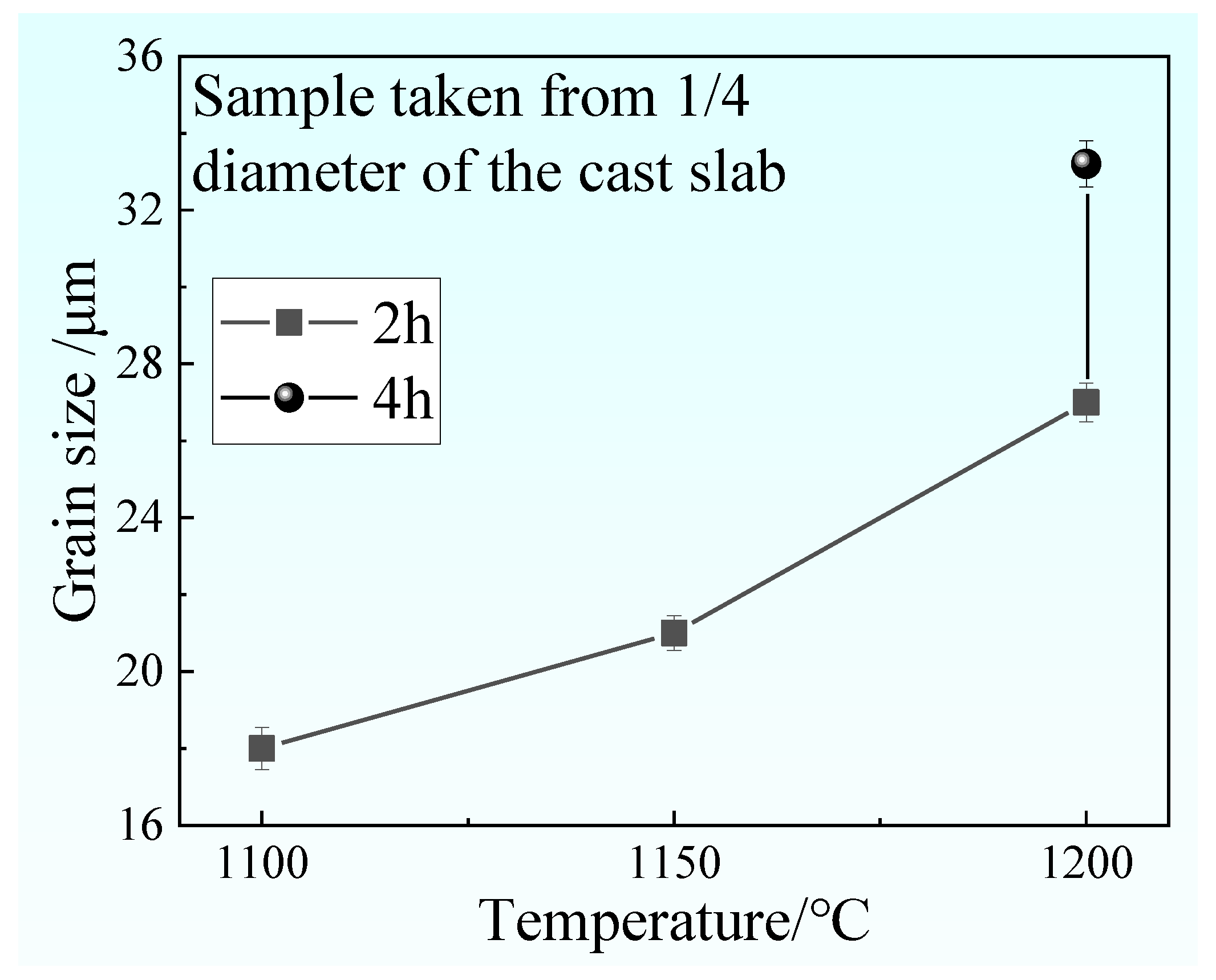
4.2. The Role of Austenite Grain Size in the Ferrite-Bainite Banding Formation during Hot Rolling
5. Conclusions
- (1)
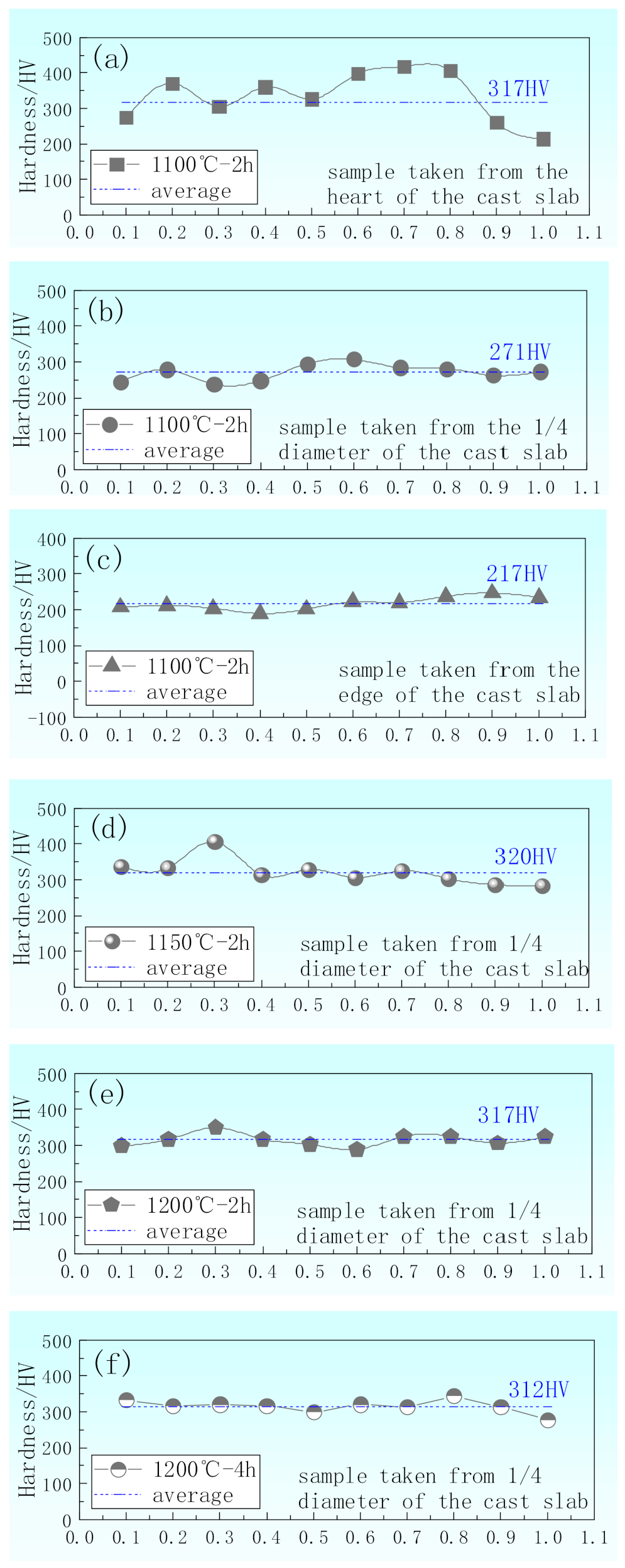
- Samples without banding have the most uniform hardness distribution, which are taken from edge of the cast slab and 1/4 diameter position of the cast slab, heating at 1100 °C for 2 h and above 1200 °C for 2–4 h during the hot rolling, respectively.
- (2)
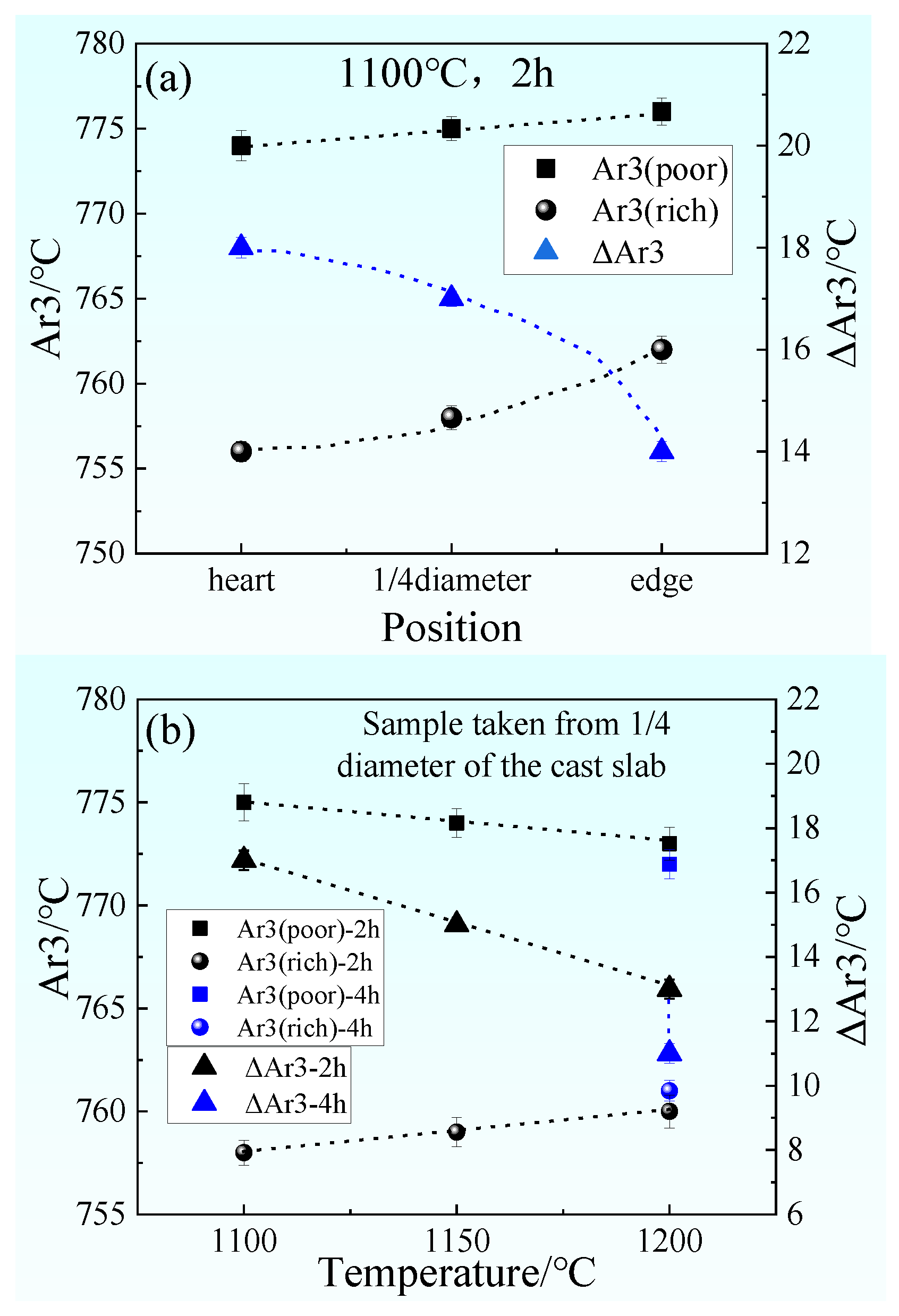
- In the cast slab, solute elements are segregated and distribute in ferrite and pearlite. The segregation width and segregation coefficient K of elements for samples taken from the heart of the cast slab are larger than those that taken from the 1/4 diameter position and edge of the cast slab. Cr, Mn, and Si diffuse and inhomogeneously concentrate in austenite during hot rolling, while C homogeneously concentrates in austenite. After the same hot rolling process, ΔAe3 increases and ferrite-bainite banding intensifies with increasing initial segregation width and segregation coefficient K of solute elements. Under the same initial segregation of solute elements, ΔAe3 drops and ferrite-bainite banding reduces with increasing heating temperature and extension heating time. To sum up, when ΔAe3 drops below 14 °C, ferrite-bainite banding even disappears.
- (3)
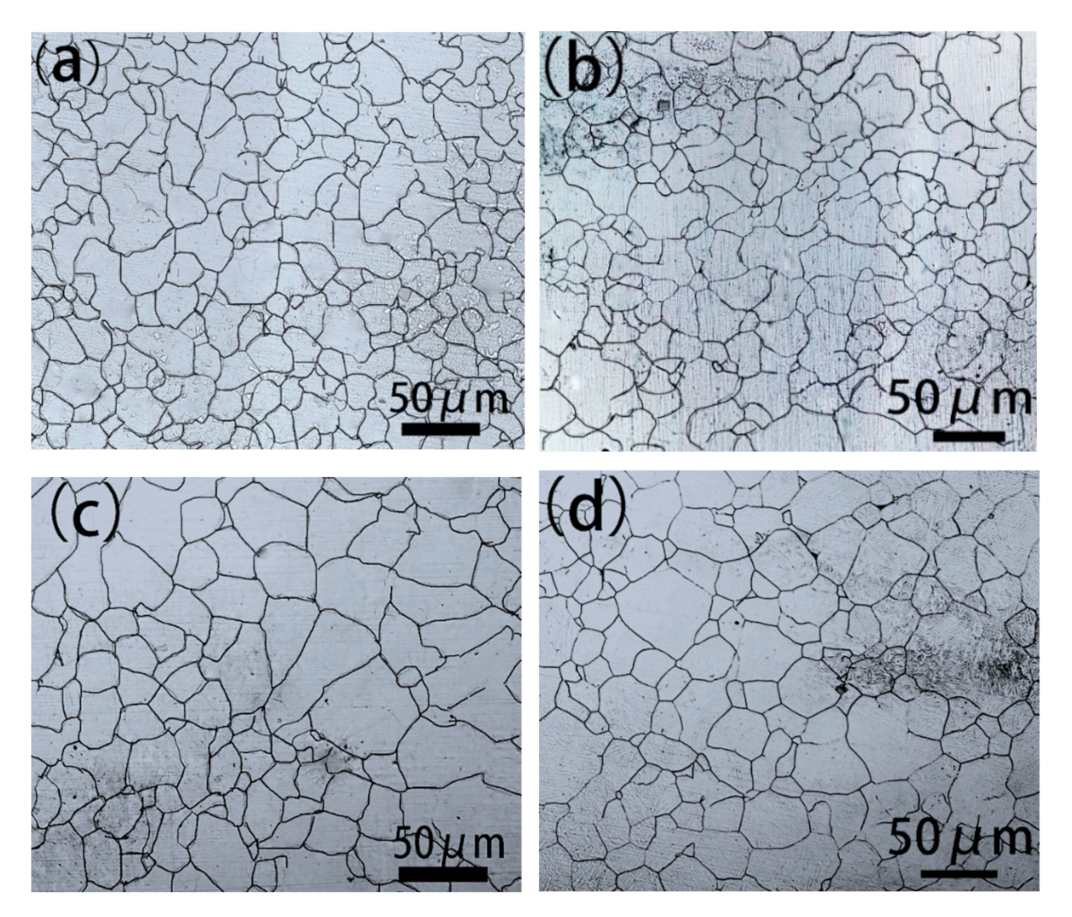
- The austenite grain size increase with increasing heating temperature and extension heating time. It can be concluded that when the austenite grain size is above 21 μm, the sample will not appear to have a banded structure after hot rolling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Ma, S.; Jiang, Z.; Liu, J.; Li, S.; Zha, C.; Ding, W.; Wang, F. Analysis of several common banded structures in high-grade pipeline steel. Steel Roll. 2009, 26, 16–22. (In Chinese) [Google Scholar]
- Ye, Q.; Xie, Q.; Liu, Z.; Wang, G. The inhibitory effect of ultra-rapid cooling process on the abnormal banded structure in the core of the steel plate. J. Northeast. Univ. Nat. Sci. 2017, 38, 1696–1701. (In Chinese) [Google Scholar]
- Yan, M. Talking about the Quality Requirements and Level of Gear Steel for Automobiles in China. Jiangsu Metall. 2007, 1, 7–16. (In Chinese) [Google Scholar]
- Mo, C.L.; Zhang, Y.T.; Li, D.Z.; Li, Y.Y. The Microstructural Banding in the Center of Hot Rolling Strip. Acta Metall. Sin. 2005, 18, 664–668. [Google Scholar]
- Khalid, F.A.; Farooque, M.; Haq, A.U.; Khan, A.Q. Role of ferrite/pearlite banded structure and segregation on mechanical properties of microalloyed hot rolled steel. Mater. Sci. Technol. 2013, 15, 1209–1215. [Google Scholar] [CrossRef]
- Grange, R.A. Effect of microstructural banding in steel. Metall. Trans. 1971, 2, 417–426. [Google Scholar] [CrossRef]
- Verhoeven, J.D. A review of microsegregation induced banding phenomena in steels. J. Mater. Eng. Perform. 2000, 9, 86–96. [Google Scholar] [CrossRef]
- Clover, D.; Kinsella, B.; Pejcic, B.; De Marco, R. The influence of microstructure on the corrosion rate of various carbon steels. J. Appl. Electrochem. 2005, 35, 39–49. [Google Scholar] [CrossRef]
- Bastien, P.G. The mechanism of formation of banded structures. J. Iron Steel Inst. 1957, 187, 281–291. [Google Scholar]
- Kirkaldy, J.S.; von Destinon-Forstmann, J.; Brigham, R. Simulation of banding in steels. Can. Metall. Q. 1962, 1, 59–81. [Google Scholar] [CrossRef]
- Großterlinden, R.; Kawalla, R.; Lotter, U.; Pircher, H. Formation of pearlitic banded structures in ferritic-pearlitic steels. Steel Res. 1992, 63, 331–336. [Google Scholar] [CrossRef]
- Thompson, S.W.; Howell, P. Factors influencing ferrite/pearlite banding and origin of large pearlite nodules in a hypoeutectoid plate steel. Mater. Sci. Technol. 1992, 8, 777–784. [Google Scholar] [CrossRef]
- Offerman, S.E.; Van Dijk, N.H.; Rekveldt, M.T.; Sietsma, J.; Van Der Zwaag, S. Ferrite/pearlite band formation in hot rolled medium carbon steel. Mater. Sci. Technol. 2002, 18, 297–303. [Google Scholar] [CrossRef]
- Maalekian, M.; Azizi-Alizamini, H.; Militzer, M. Phase Field Modeling of Microstructure Banding in Steels. Metall. Mater. Trans. A 2016, 47, 608–622. [Google Scholar] [CrossRef]
- Smith, W.F. Structure and Properties of Engineering Alloys; McGraw-Hill: New York, NY, USA, 1993; pp. 125–128. [Google Scholar]
- Reed-Hill, R.E.; Abbashian, R. Physical Metallurgy Principles; PWS-Kent: Boston, MA, USA, 1992; pp. 459–461. [Google Scholar]
- Fisher, R.M.; Speich, G.R.; Cudy, L.J.; Hu, H. Physical Chemistry in Metallurgy; Proceedings of the Darken conference, United States Steel: Monroeville, PA, USA, 1976; pp. 466–473. [Google Scholar]
- Georges, S.; Reinhart, G.; Nguyen-Thi, H. In situ experimental observation of the time evolution of a dendritic mushy zone in a fixed temperature gradient. Comptes Rendus Mécanique 2013, 341, 421–428. [Google Scholar]
- Grange, R.A. The rapid heat treatment of steel. Metall. Trans. 1971, 2, 17–26. [Google Scholar] [CrossRef]
- Leslie, W.C. The Physical Metallurgy of Steels; Tech Books: New York, NY, USA, 1981; pp. 172–173. [Google Scholar]
- Liu, Y. A method for displaying dendrites of gear steel continuous casting billet [A]. The Chinese Society for Metals, Baosteel Group Coporation. In Proceedings of the 10th China Iron and Steel Annual Conference and the first Proceedings of the 6th Baosteel Academic Annual Conference, Shanghai, China, 21–23 October 2015; Volume 5. [Google Scholar]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]
- Morais, L.; Magnabosco, R. Experimental investigations and DICTRA simulation of sigma phase formation in a duplex stainless steel. Calphad 2017, 58, 214–218. [Google Scholar] [CrossRef]
- Borgenstam, A.; Engstrom, A.; Hoglund, L.; Agren, J. DICTRA, a tool for simulation of diffusional transformations in alloys. Phase Equilib. 2000, 21, 269–280. [Google Scholar] [CrossRef]
- Yao, Y.; Ye, J.; Dong, J.; Yao, Z.; Zhang, M.; Guo, W. Elements diffusion law of DD407/FGH95 diffusion bonding under hot isostatic pressure Ⅰ. Establishment of diffusion bonding model. Acta Metall. Sin. 2013, 49, 1041–1050. [Google Scholar] [CrossRef]
- Hu, Z.; Li, P.; Lu, Z.; Ma, B.; Ding, J.; Zhao, Y.; Tang, P.; Huang, Q. DICTRA® Simulation of Mg and Mn Micro-segregations in Al-Mg5.0-Mn0.5 Alloy during Homogenization Annealing. Rare Metal Mater. Eng. 2021, 50, 23–28. [Google Scholar]
- Peng, N.Q.; Tang, G.B.; Liu, Z.D. Modification of Zener’s two-parameter in the superelement model for Fe—∑Xi—C alloy systems. Acta Metall. Sin. 2009, 45, 331. [Google Scholar]
- Li, Z.; Cao, Y.; Hao, Q.; Qi, L.; Jing, F.; Lin, X. Calculation of super component model modified for X100 pipeline steel. J. Iron Steel Res. 2018, 30, 576–580. [Google Scholar]
- Wang, P.; Meng, L.Y.; You, K. Calculation of phase equilibrium temperature of Ae3 and Ae1 for TRIP steel. Phys. Exam. Test. 2011, 29, 12–15. [Google Scholar]
- Wang, P.; Zhou, X.; Wu, D. Simulation of ferrite transformation temperature during continuous cooling. J. Mater. Metall. 2006, 5, 57–60. [Google Scholar]
- Zhao, Y.; Chen, Y.; Yu, W.; Wang, Y.; Fan, W. Effect of annealing on dendrite segregation and element diffusion of gear steel. Iron Steel 2019, 54, 60–66. [Google Scholar]
- Wang, X. Iron and Steel Metallurgy-Steelmaking; Higher Education Press: Beijing, China, 2007; pp. 305–306. [Google Scholar]
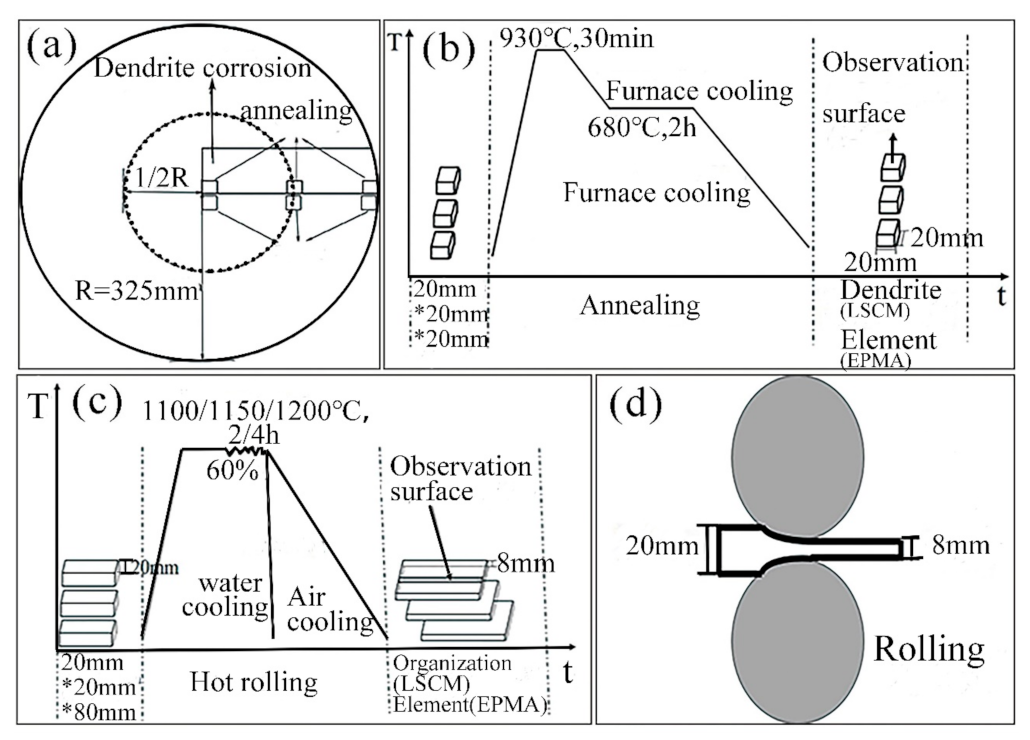






| Element | C | Mn | Si | Cr | Ti | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| mass% | 0.21 | 0.995 | 0.264 | 1.15 | 0.0821 | 0.0132 | 0.0021 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Chen, Y.; Wei, H.; Sun, J.; Yu, W. The Role of Elements Partition and Austenite Grain Size in the Ferrite-Bainite Banding Formation during Hot Rolling. Materials 2021, 14, 2356. https://doi.org/10.3390/ma14092356
Zhao Y, Chen Y, Wei H, Sun J, Yu W. The Role of Elements Partition and Austenite Grain Size in the Ferrite-Bainite Banding Formation during Hot Rolling. Materials. 2021; 14(9):2356. https://doi.org/10.3390/ma14092356
Chicago/Turabian StyleZhao, Yina, Yinli Chen, He Wei, Jiquan Sun, and Wei Yu. 2021. "The Role of Elements Partition and Austenite Grain Size in the Ferrite-Bainite Banding Formation during Hot Rolling" Materials 14, no. 9: 2356. https://doi.org/10.3390/ma14092356
APA StyleZhao, Y., Chen, Y., Wei, H., Sun, J., & Yu, W. (2021). The Role of Elements Partition and Austenite Grain Size in the Ferrite-Bainite Banding Formation during Hot Rolling. Materials, 14(9), 2356. https://doi.org/10.3390/ma14092356






