Effect of Cu Content on Performance of Sn-Zn-Cu Lead-Free Solder Alloys Designed by Cluster-Plus-Glue-Atom Model
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
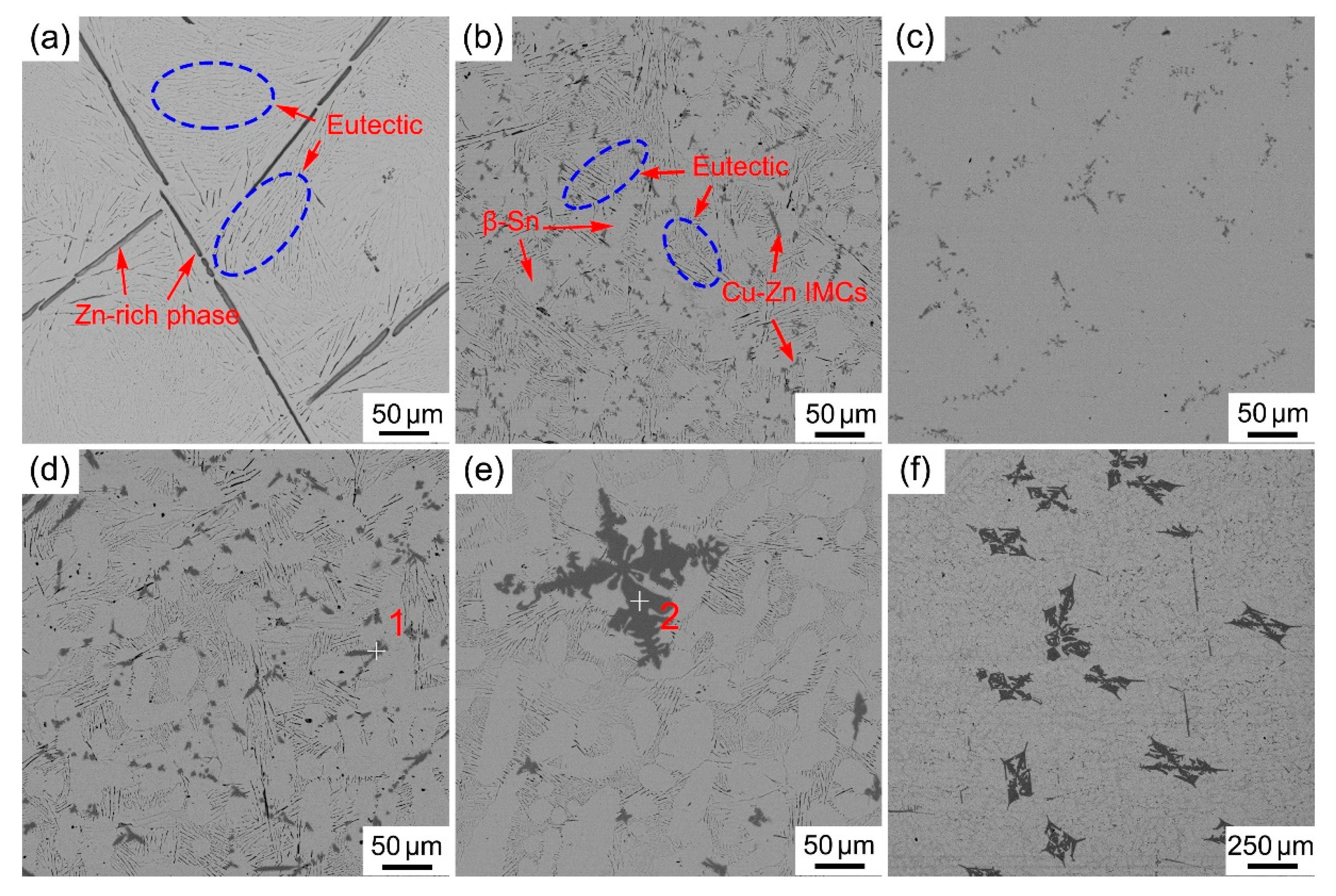
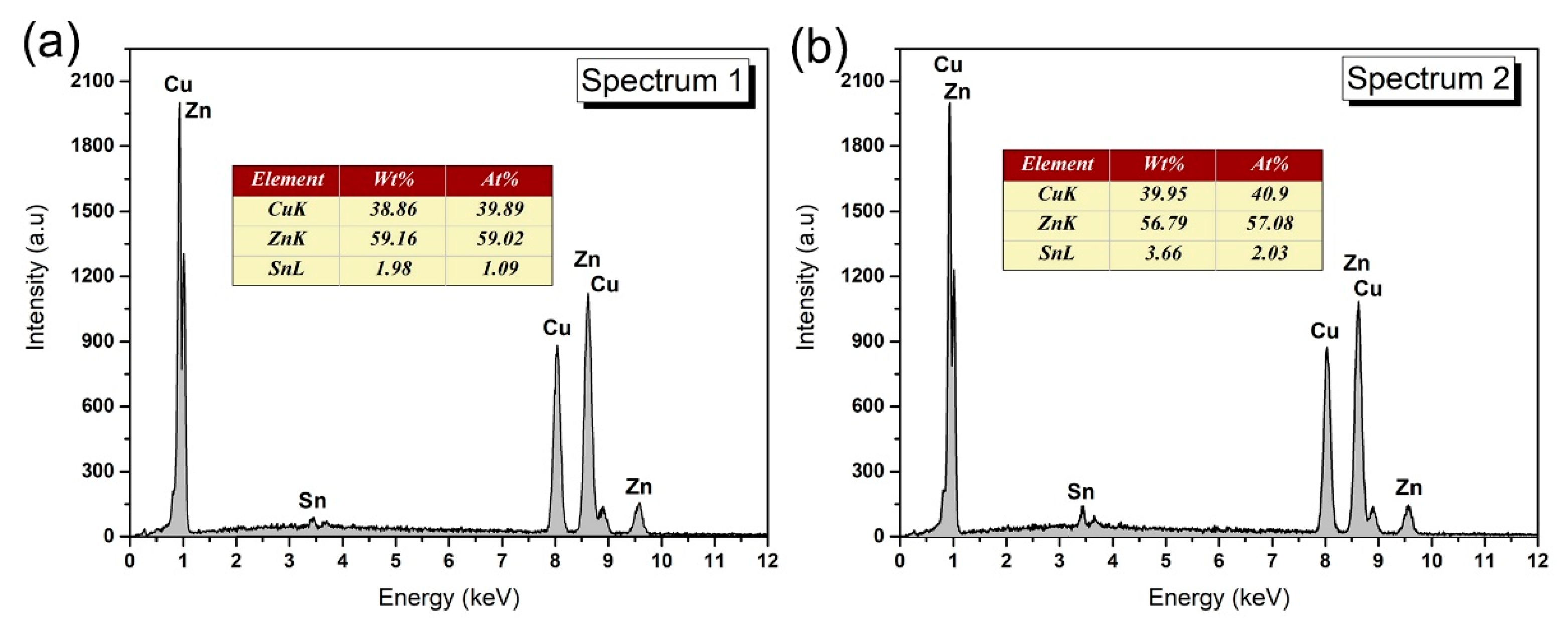
3.1. Microstructure of Solder Alloys
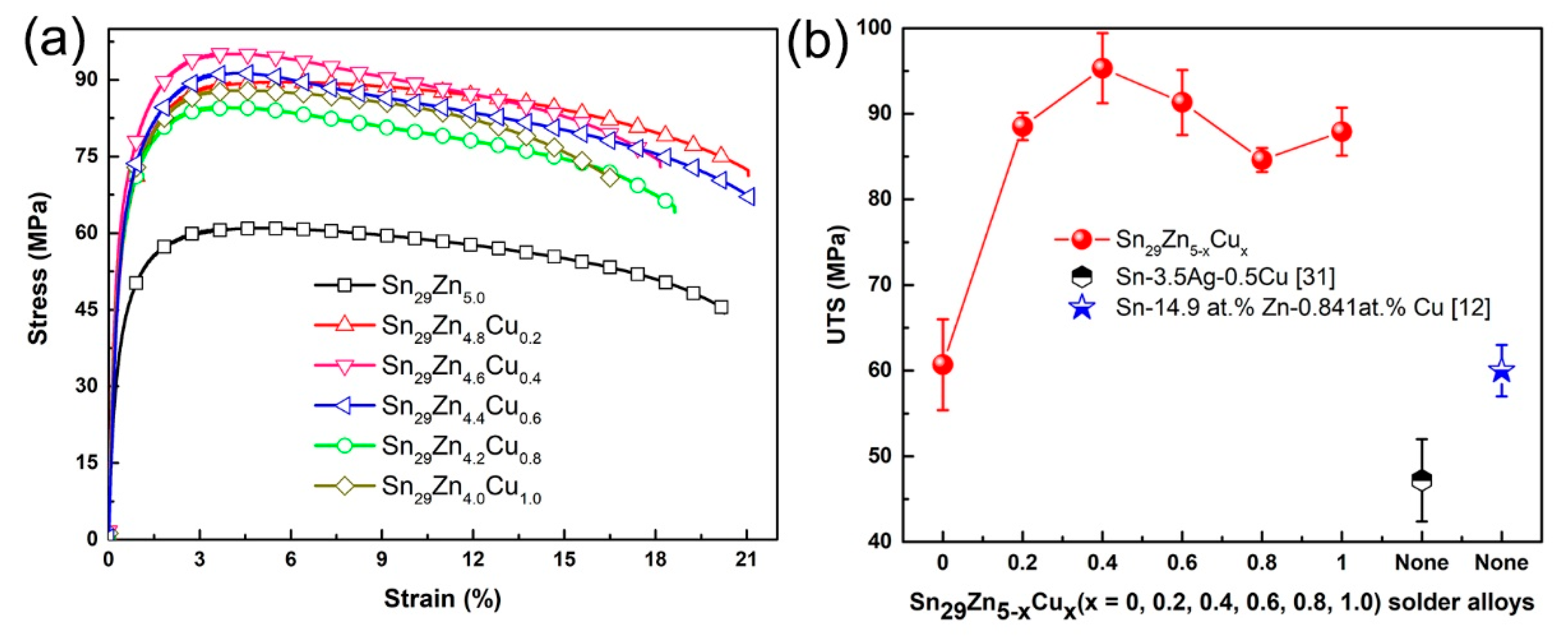
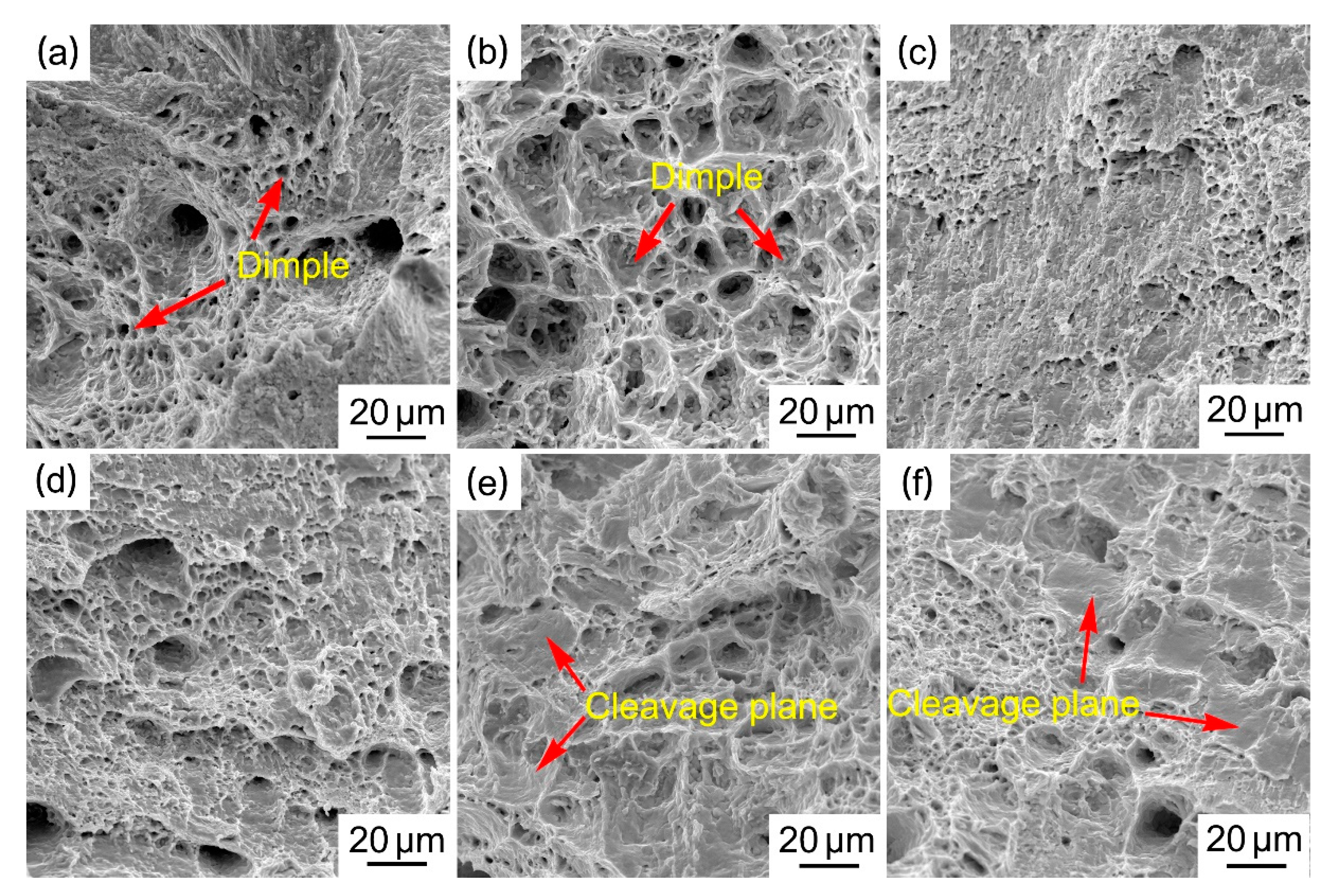
3.2. Mechanical Properties of Solder Alloys
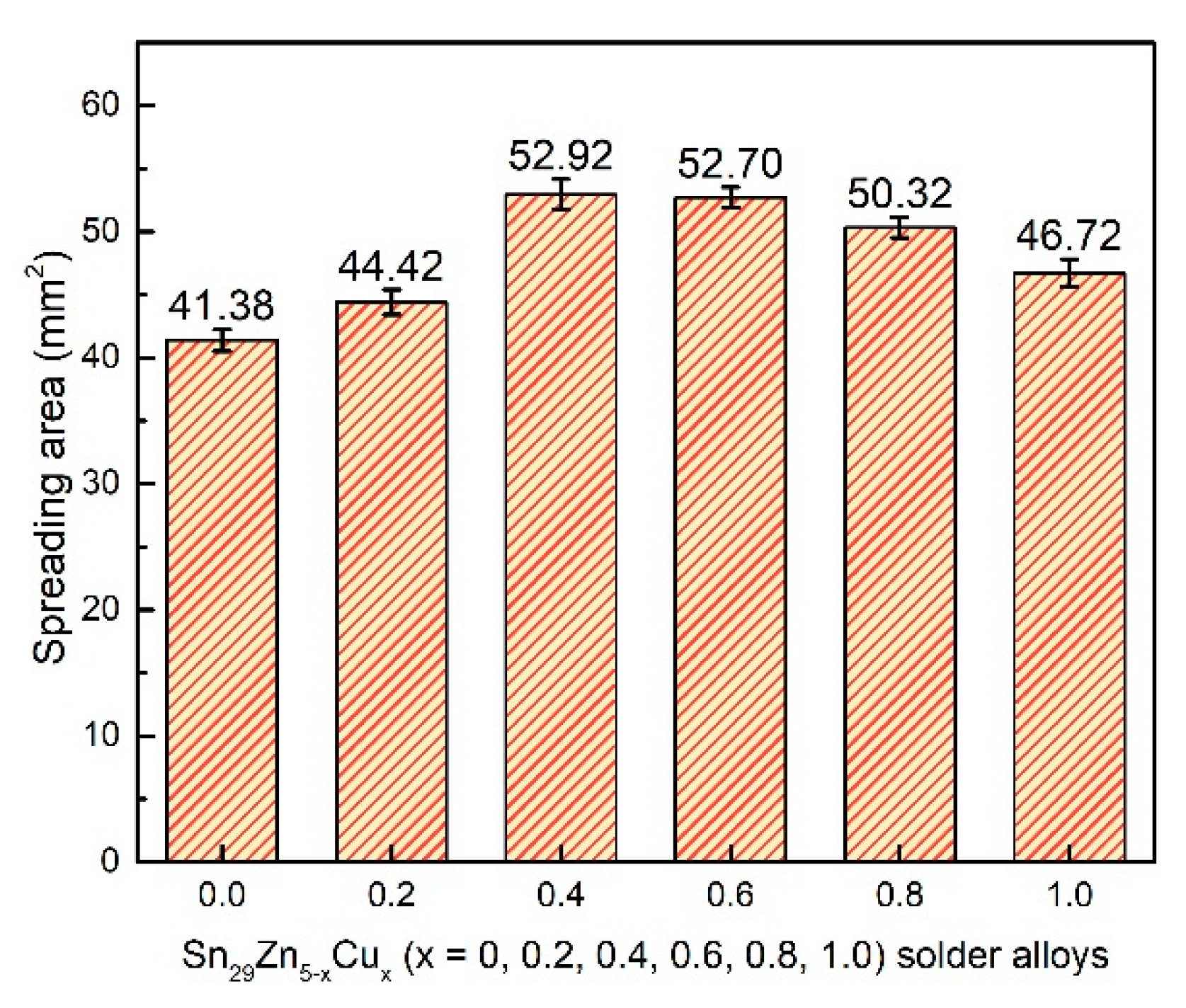
3.3. Wettability of Solder Alloys
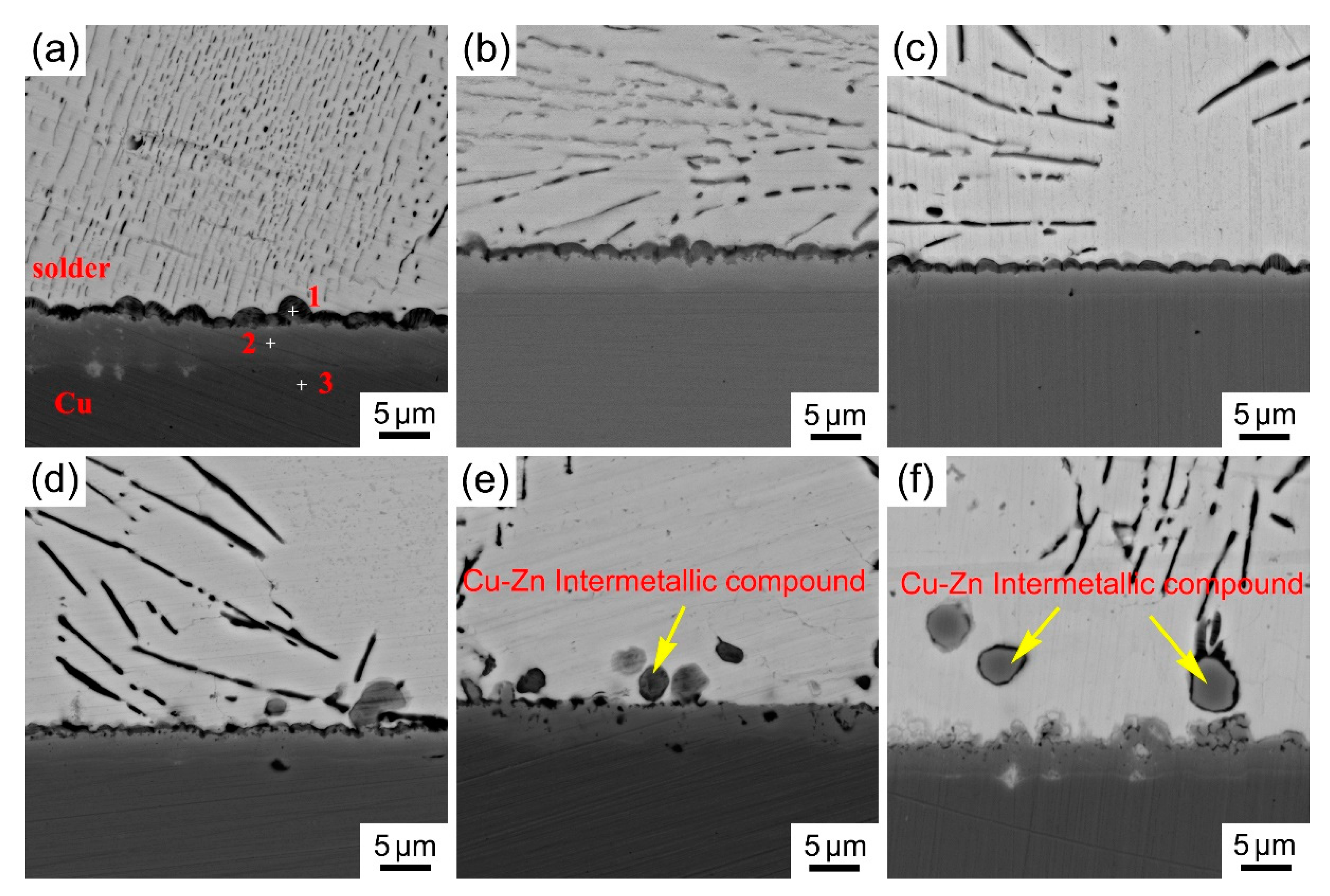
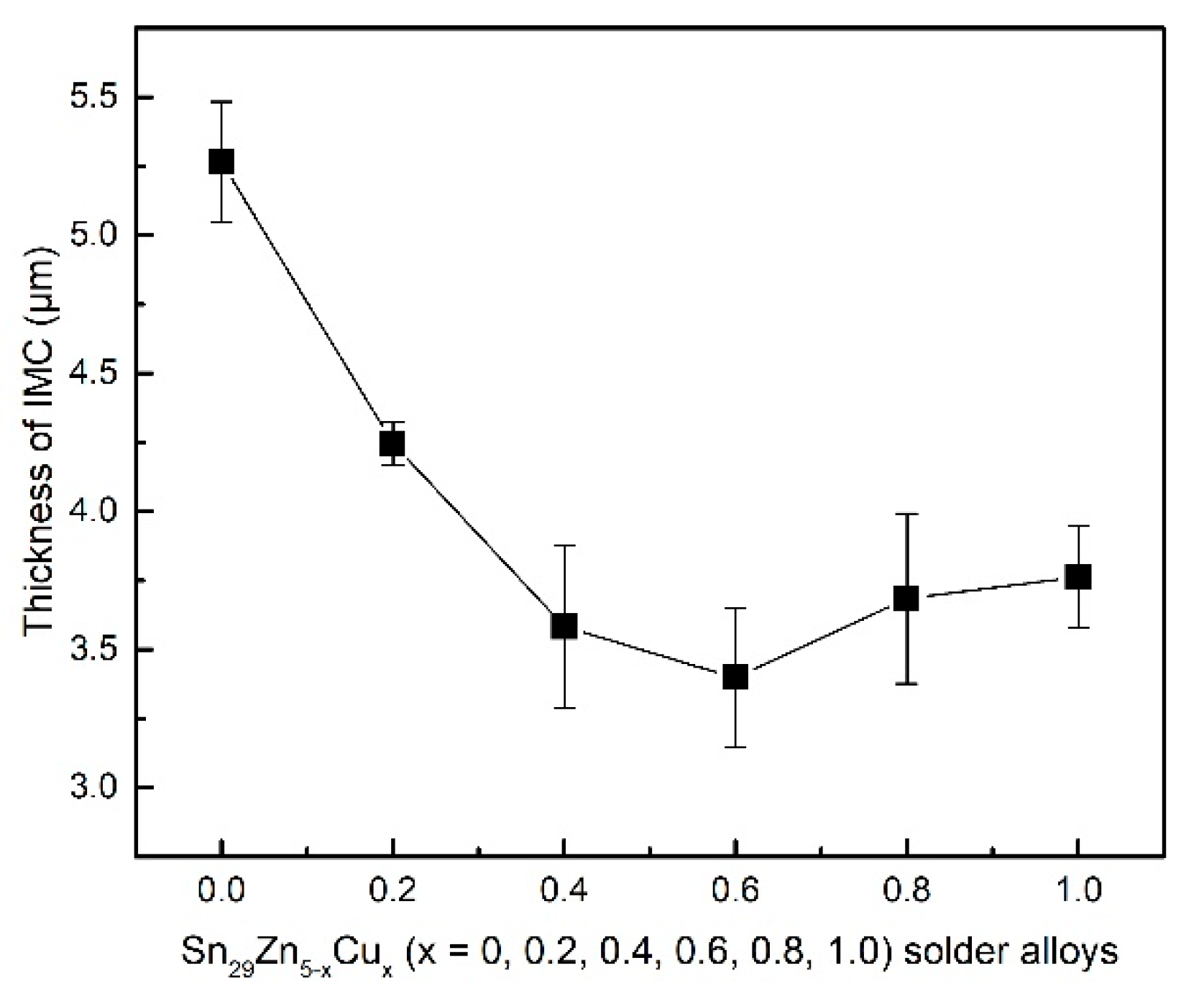
3.4. Interfacial Characterization of Solder Joint
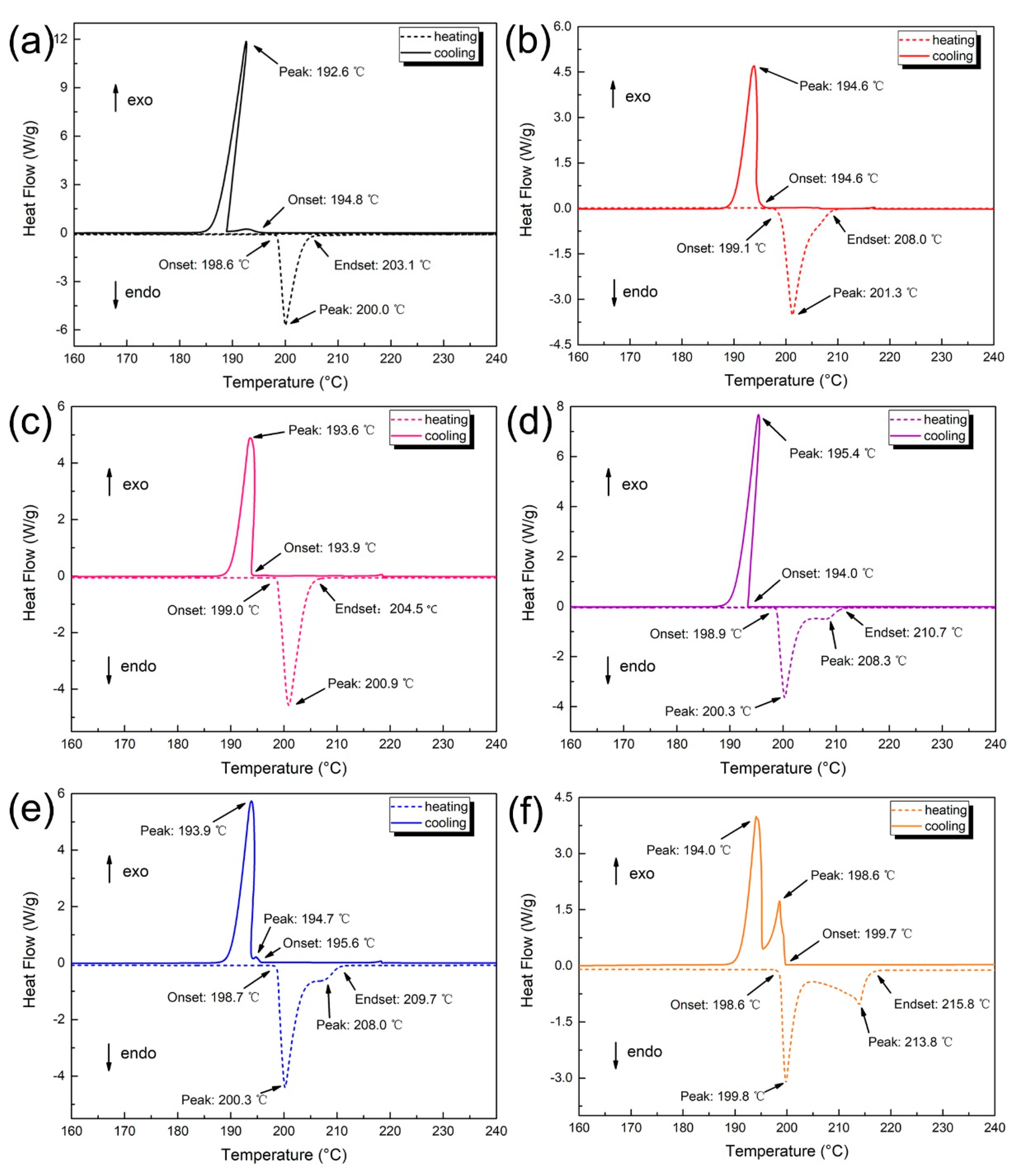
3.5. Thermal Behavior of Solder Alloys
4. Conclusions
- (1)
- The microstructure of the Sn-Zn-Cu solder alloy consists of the α-Zn, β-Sn and Cu5Zn8 phases. The microstructure of the Sn-Zn solder alloy was refined with the addition of Cu, but excessive addition of Cu will result in the appearance of larger size intermetallic compounds.
- (2)
- The tensile test results show that the UTS value increases with the increase in Cu content and the UTS value of the new Sn29Zn4.6Cu0.4 solder alloy is increased by 57% to 95.3 MPa compared with the original Sn-Zn solder. The improved mechanical properties can be attributed to the fine microstructure and dispersion strengthening of the second phase.
- (3)
- The novel Sn29Zn0.6Cu0.4 solder obtains the largest spreading area among the Sn29Zn5-xCux (x = 0, 0.2, 0.4, 0.6, 0.8, 1.0) solder alloys, which was 27.8% higher than that of the plain Sn-Zn solder alloy. However, with the continuous addition of Cu, the spread area tends to decrease. With the increase in the Cu content, the thickness of the IMC layer decreased owing to Cu slowing the diffusion force of Zn to the interface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffery, H.A.; Sabri, M.F.M.; Said, S.M.; Hasan, S.W.; Sajid, I.H.; Nordin, N.I.M.; Megat Hasnan, M.M.I.; Shnawah, D.A.; Moorthy, C.V. Electrochemical corrosion behavior of Sn-0.7Cu solder alloy with the addition of bismuth and iron. J. Alloys Compd. 2019, 810, 151925. [Google Scholar] [CrossRef]
- Lu, T.; Yi, D.; Wang, H.; Tu, X.; Wang, B. Microstructure, mechanical properties, and interfacial reaction with Cu substrate of Zr-modified SAC305 solder alloy. J. Alloys Compd. 2019, 781, 633–643. [Google Scholar] [CrossRef]
- Ren, G.; Collins, M.N. The effects of antimony additions on microstructures, thermal and mechanical properties of Sn-8Zn-3Bi alloys. Mater. Des. 2017, 119, 133–140. [Google Scholar] [CrossRef]
- Liu, J.C.; Park, S.; Nagao, S.; Nogi, M.; Koga, H.; Ma, J.S.; Zhang, G.; Suganuma, K. The role of Zn precipitates and Cl− anions in pitting corrosion of Sn-Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Huang, N.; Hu, A.; Li, M.; Mao, D. Influence of Cr alloying on the oxidation resistance of Sn–8Zn–3Bi solders. J. Mater. Sci. Mater. Electron. 2013, 24, 2812–2817. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Hammad, A.E. Effects of small addition of Ag and/or Cu on the microstructure and properties of Sn–9Zn lead-free solders. Mater. Sci. Eng. A 2010, 527, 5212–5219. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Hammad, A.E.; Al-Ganainy, G.S.; Ibrahiem, A.A. Design of lead-free candidate alloys for low-temperature soldering applications based on the hypoeutectic Sn–6.5Zn alloy. Mater. Des. 2014, 56, 594–603. [Google Scholar] [CrossRef]
- Chen, W.; Xue, S.; Wang, H.; Hu, Y.; Wang, J. Effects of rare earth Ce on properties of Sn-9Zn lead-free solder. J. Mater. Sci. Mater. Electron. 2010, 21, 719–725. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.C.; Yu, L.M.; Chen, H.; Wang, X. Effects of Al on the failure mechanism of the Sn-Ag-Zn eutectic solder. Microelectron. Reliab. 2010, 50, 1142–1145. [Google Scholar] [CrossRef]
- Luo, T.; Hu, A.; Hu, J.; Li, M.; Mao, D. Microstructure and mechanical properties of Sn-Zn-Bi-Cr lead-free solder. Microelectron. Reliab. 2012, 52, 585–588. [Google Scholar] [CrossRef]
- Mohd Nasir, S.S.; Yahaya, M.Z.; Erer, A.M.; Illés, B.; Mohamad, A.A. Effect of TiO2 nanoparticles on the horizontal hardness properties of Sn-3.0Ag-0.5Cu-1.0TiO2 composite solder. Ceram. Int. 2019, 45, 18563–18571. [Google Scholar] [CrossRef]
- Pandey, P.; Tiwary, C.S.; Chattopadhyay, K. Effects of Cu and In Trace Elements on Microstructure and Thermal and Mechanical Properties of Sn-Zn Eutectic Alloy. J. Electron. Mater. 2019, 48, 2660–2669. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Hashem, H.A.; Radwan, N.; El-Tantawy, F.; Dalloul, T.R.; Mansour, N.A.; Abd-Elmoniem, H.M.; Lotfy, E.H. Robust effects of Bi doping on microstructure development and mechanical properties of hypoeutectic Sn-6.5Zn solder alloy. J. Mater. Sci. Mater. Electron. 2016, 27, 2950–2962. [Google Scholar] [CrossRef]
- Chen, W.X.; Xue, S.B.; Wang, H.; Wang, J.X.; Han, Z.J.; Gao, L.L. Effects of Ag on microstructures, wettabilities of Sn-9Zn-xAg solders as well as mechanical properties of soldered joints. J. Mater. Sci. Mater. Electron. 2010, 21, 461–467. [Google Scholar] [CrossRef]
- Chen, X.; Hu, A.; Li, M.; Mao, D. Study on the properties of Sn–9Zn–xCr lead-free solder. J. Alloys Compd. 2008, 460, 478–484. [Google Scholar] [CrossRef]
- Chen, X.; Hu, A.; Li, M.; Mao, D. Effect of a trace of Cr on intermetallic compound layer for tin–zinc lead-free solder joint during aging. J. Alloys Compd. 2009, 470, 429–433. [Google Scholar] [CrossRef]
- Lai, R.S.; Lin, K.L.; Salam, B. Suppressing Growth of the Cu5Zn8 Intermetallic Layer in Sn-Zn-Ag-Al-Ga/Cu Solder Joints. J. Electron. Mater. 2009, 38, 88–92. [Google Scholar] [CrossRef]
- Liu, L.U.; Zhou, W.; Li, B.L.; Wu, P. Interfacial reactions between Sn-8Zn-3Bi-xNi lead-free solders and Cu substrate during isothermal aging. Mater. Chem. Phys. 2010, 123, 629–633. [Google Scholar] [CrossRef]
- Das, S.K.; Sharif, A.; Chan, Y.C.; Wong, N.B.; Yung, W.K.C. Influence of small amount of Al and Cu on the microstructure, microhardness and tensile properties of Sn-9Zn binary eutectic solder alloy. J. Alloys Compd. 2009, 481, 167–172. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Desoky, W.M.; Saad, A.F.; Mansor, N.A.; Lotfy, E.H.; Abd-Elmoniem, H.M.; Hashem, H. The effect of undercooling on the microstructure and tensile properties of hypoeutectic Sn-6.5Zn-xCu Pb-free solders. Mater. Des. 2015, 80, 152–162. [Google Scholar] [CrossRef]
- Dong, C.; Dong, D.D.; Wang, Q. Chemical Units in Solid Solutions and Alloy Composition Design. Acta Metall. Sin. 2018, 54, 293–300. [Google Scholar]
- Ma, Y.P.; Dong, D.D.; Dong, C.; Luo, L.J.; Wang, Q.; Qiang, J.B.; Wang, Y.M. Composition formulas of binary eutectics. Sci. Rep. 2015, 5, 17880. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, Q.; Qiang, J.B.; Wang, Y.M.; Jiang, N.; Han, G.; Li, Y.H.; Wu, J.; Xia, J.H. From clusters to phase diagrams: Composition rules of quasicrystals and bulk metallic glasses. J. Phys. D Appl. Phys. 2007, 40, R273–R291. [Google Scholar] [CrossRef]
- Dong, H.; Xia, Y.; Xu, X.; Naz, G.J.; Hao, X.; Li, P.; Zhou, J.; Dong, C. Performance of GH4169 brazed joint using a new designed nickel-based filler metal via cluster-plus-glue-atom model. J. Mater. Sci. Technol. 2020, 39, 89–98. [Google Scholar] [CrossRef]
- Huang, M.L.; Yang, Y.C.; Chen, Y.; Dong, C. Microstructure and mechanical properties of Sn-rich Au-Sn solders designed using cluster-plus-glue-atom model. Mater. Sci. Eng. A 2016, 664, 221–226. [Google Scholar] [CrossRef]
- Yu, Q.X.; Li, X.N.; Wei, K.R.; Li, Z.M.; Zheng, Y.H.; Li, N.J.; Cheng, X.T.; Wang, C.Y.; Wang, Q.; Dong, C. Cu-Ni-Sn-Si alloys designed by cluster-plus-glue-atom model. Mater. Des. 2019, 167, 107641. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, Y.; Wen, L.; Dong, C. Highly corrosion-resistant Cu70(Ni, Fe, Mn, Cr)30 cupronickel designed using a cluster model for stable solid solutions. J. Alloys Compd. 2010, 505, 179–182. [Google Scholar] [CrossRef]
- Huang, M.L.; Huang, F.F.; Yang, Y.C. Composition design of Sn-rich Sn-Au-Ag solders using cluster-plus-glue-atom model. J. Mater. Sci. Mater. Electron. 2017, 28, 11192–11201. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.S.; Inoue, M.; Jiang, J.X.; Suganuma, K. Effects of Ag and Cu addition on microstructural properties and oxidation resistance of Sn-Zn eutectic alloy. J. Alloys Compd. 2008, 454, 310–320. [Google Scholar] [CrossRef]
- Chuang, C.L.; Tsao, L.C.; Lin, H.K.; Feng, L.P. Effects of small amount of active Ti element additions on microstructure and property of Sn3.5Ag0.5Cu solder. Mater. Sci. Eng. A 2012, 558, 478–484. [Google Scholar] [CrossRef]
- Yang, T.Q.; Zhao, X.C.; Xiong, Z.S.; Tan, W.; Wei, Y.H.; Tan, C.W.; Yu, X.D.; Wang, Y.C. Improvement of microstructure and tensile properties of Sn-Bi-Ag alloy by heterogeneous nucleation of β-Sn on Ag3Sn. Mater. Sci. Eng. A 2020, 785, 139372. [Google Scholar] [CrossRef]
- Wang, S.L.; Murr, L.E. Effect of prestrain and stacking-fault energy on the application of the Hall-Petch relation in fcc metals and alloys. Metallography 1980, 13, 203–224. [Google Scholar] [CrossRef]
- Zeng, X.W.; Liu, Y.C.; Zhang, J.K.; Liu, Y.; Hu, X.W.; Jiang, X.X. Effect of rare earth Ce on the thermal behavior, microstructure and mechanical properties of Zn-30Sn-2Cu high temperature lead-free solder alloy. J. Mater. Sci. Mater. Electron. 2020, 31, 16437–16447. [Google Scholar] [CrossRef]
- Chou, C.Y.; Chen, S.W. Phase equilibria of the Sn-Zn-Cu ternary system. Acta Mater. 2006, 54, 2393–2400. [Google Scholar] [CrossRef]
- Liu, J.C.; Zhang, G.; Wang, Z.H.; Ma, J.S.; Suganuma, K. Thermal property, wettability and interfacial characterization of novel Sn-Zn-Bi-In alloys as low-temperature lead-free solders. Mater. Des. 2015, 84, 331–339. [Google Scholar] [CrossRef]
- Liu, L.; Wu, P.; Zhou, W. Effects of Cu on the interfacial reactions between Sn-8Zn-3Bi-xCu solders and Cu substrate. Microelectron. Reliab. 2014, 54, 259–264. [Google Scholar] [CrossRef]
- Chou, C.Y.; Chen, S.W.; Chang, Y.S. Interfacial reactions in the Sn-9Zn-(xCu)/Cu and Sn-9Zn-(xCu)/Ni couples. J. Mater. Res. 2006, 21, 1849–1856. [Google Scholar] [CrossRef]
- Chen, J.; Shen, J.; Min, D.; Peng, C. Influence of minor Bi additions on the interfacial morphology between Sn–Zn–xBi solders and a Cu layer. J. Mater. Sci. Mater. Electron. 2009, 20, 1112–1117. [Google Scholar] [CrossRef]
| No. | Cluster Formula | Alloy Composition | |
|---|---|---|---|
| at.% | wt.% | ||
| (a) | [Sn-Sn10]Sn5 + [Sn-Sn10]Zn5Sn2 | Sn85.3Zn14.7 | Sn91.33Zn8.67 |
| (b) | [Sn-Sn10]Sn5 + [Sn-Sn10]Zn4.8Cu0.2Sn2 | Sn85.29Zn14.12Cu0.59 | Sn91.33Zn8.33Cu0.34 |
| (c) | [Sn-Sn10]Sn5 + [Sn-Sn10]Zn4.6Cu0.4Sn2 | Sn85.29Zn13.53Cu1.18 | Sn91.34Zn7.98Cu0.68 |
| (d) | [Sn-Sn10]Sn5 + [Sn-Sn10]Zn4.4Cu0.6Sn2 | Sn85.30Zn12.94Cu1.76 | Sn91.37Zn7.63Cu1.00 |
| (e) | [Sn-Sn10]Sn5 + [Sn-Sn10]Zn4.2Cu0.8Sn2 | Sn85.3Zn12.35Cu2.35 | Sn91.36Zn7.29Cu1.35 |
| (f) | [Sn-Sn10]Sn5 + [Sn-Sn10]Zn4.0Cu1.0Sn2 | Sn85.29Zn11.76Cu2.95 | Sn91.37Zn6.94Cu1.69 |
| Point | Sn (at.%) | Zn (at.%) | Cu (at.%) |
|---|---|---|---|
| 1 | 10.1 | 70.2 | 18.7 |
| 2 | 0.9 | 63.6 | 35.4 |
| 3 | - | - | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Peng, Y.; Gao, P.; Li, C. Effect of Cu Content on Performance of Sn-Zn-Cu Lead-Free Solder Alloys Designed by Cluster-Plus-Glue-Atom Model. Materials 2021, 14, 2335. https://doi.org/10.3390/ma14092335
Qiu J, Peng Y, Gao P, Li C. Effect of Cu Content on Performance of Sn-Zn-Cu Lead-Free Solder Alloys Designed by Cluster-Plus-Glue-Atom Model. Materials. 2021; 14(9):2335. https://doi.org/10.3390/ma14092335
Chicago/Turabian StyleQiu, Jialong, Yanzhi Peng, Peng Gao, and Caiju Li. 2021. "Effect of Cu Content on Performance of Sn-Zn-Cu Lead-Free Solder Alloys Designed by Cluster-Plus-Glue-Atom Model" Materials 14, no. 9: 2335. https://doi.org/10.3390/ma14092335
APA StyleQiu, J., Peng, Y., Gao, P., & Li, C. (2021). Effect of Cu Content on Performance of Sn-Zn-Cu Lead-Free Solder Alloys Designed by Cluster-Plus-Glue-Atom Model. Materials, 14(9), 2335. https://doi.org/10.3390/ma14092335






