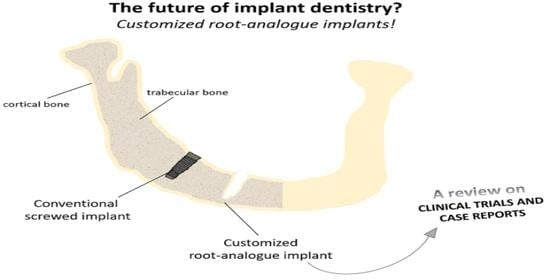
Customized Root-Analogue Implants: A Review on Outcomes from Clinical Trials and Case Reports
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Collection and Extraction
3. Results
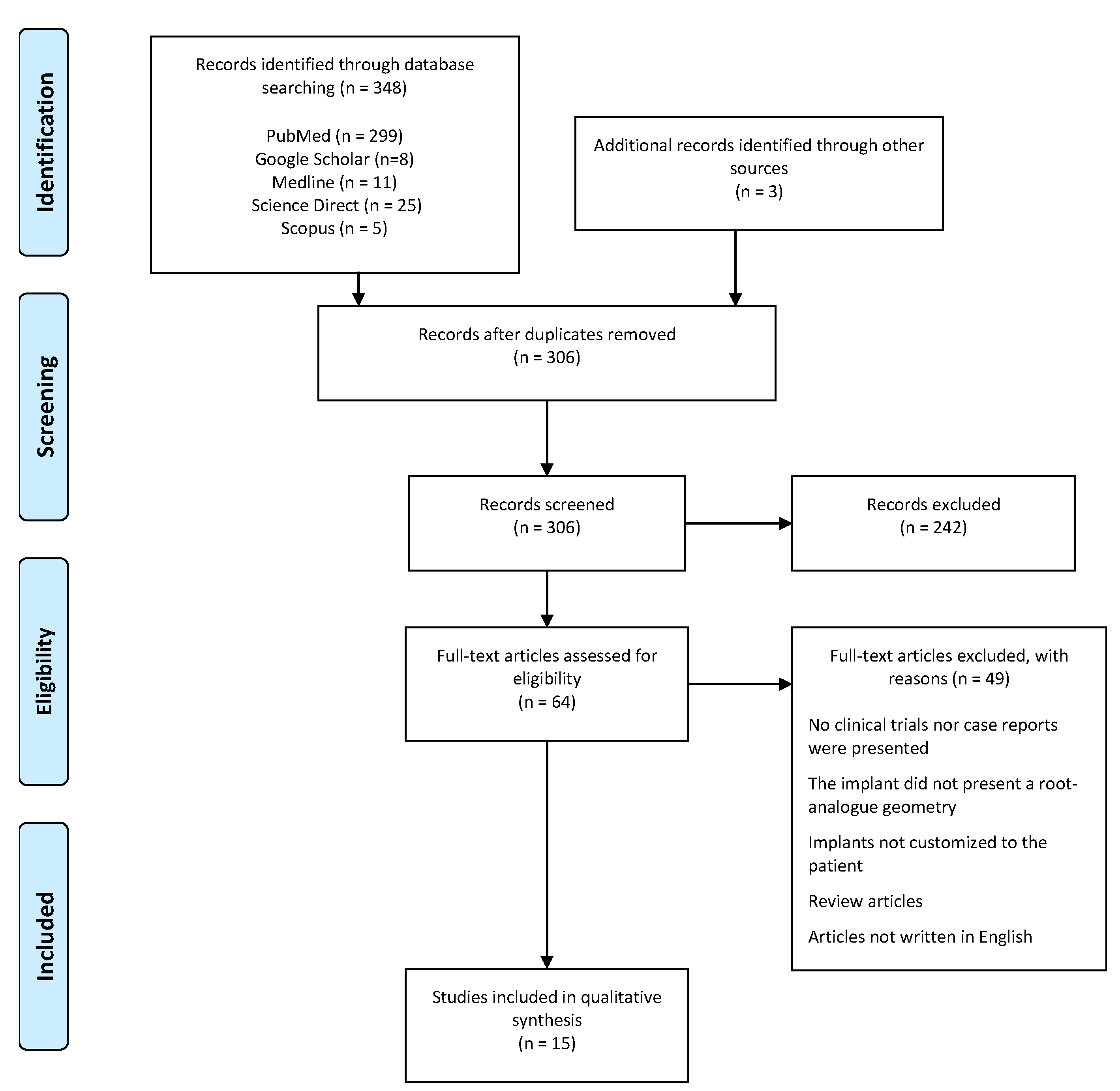
3.1. Study Selection
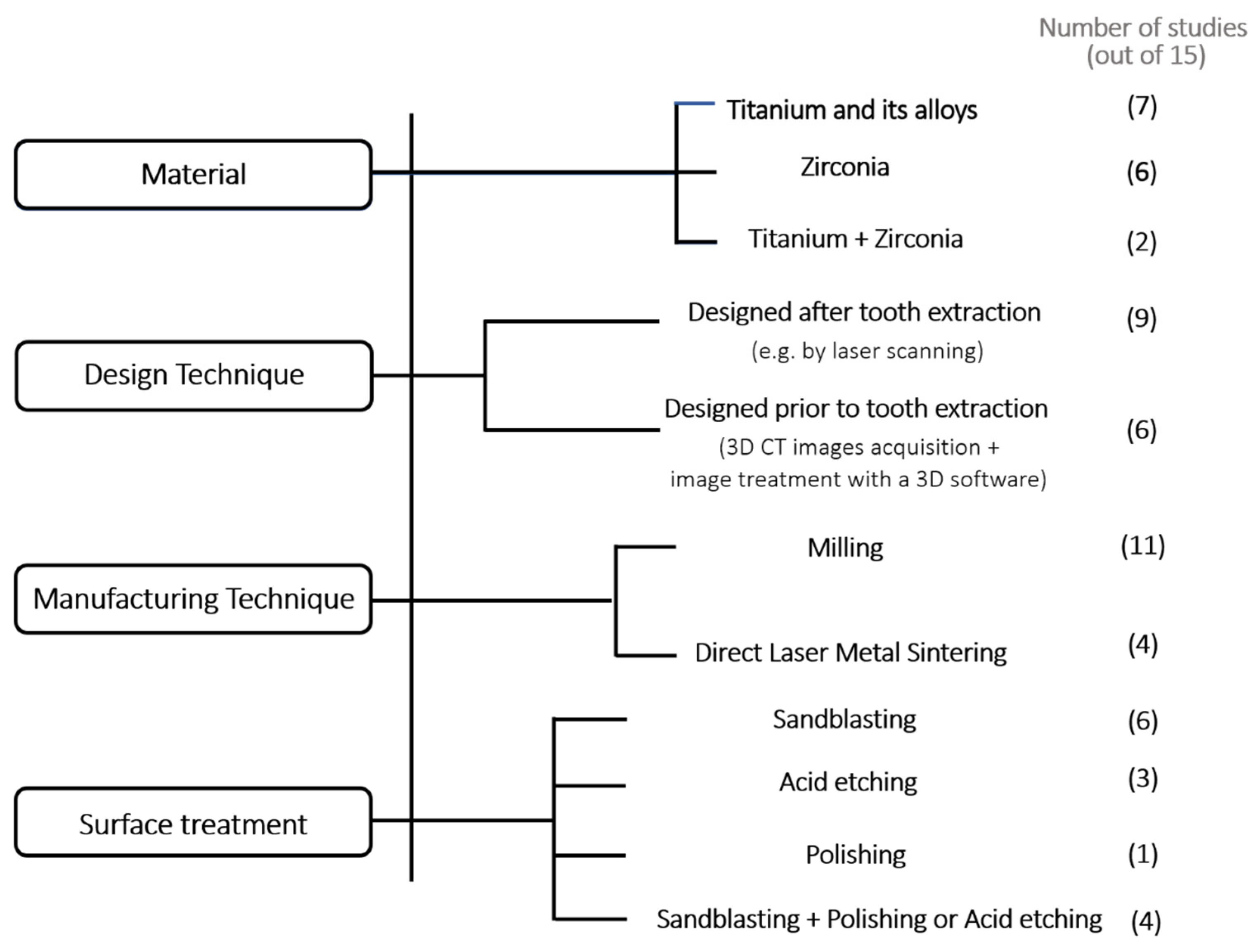
3.2. Design and Manufacturing Techniques, Implant Material and Surface Treatments
3.3. Clinical Trials/Case Reports Summary
4. Discussion
- Titanium and zirconia are the selected materials for the manufacturing of RAI.
- CAD/CAM technology followed by surface treatments such as sandblasting has been the preferred manufacturing technique for such applications.
- The clinical outcomes of the analysed studies suggest that further investigations should be performed aiming to evaluate whether RAI may be considered a promising solution for the replacement of missing teeth or not.
5. Conclusions
- Clinically tested RAI are made of titanium and/or zirconia; no other materials were reported for the analysed period.
- CAD/CAM is an effective technology to design and manufacture RAI and it is being implemented in the dental practice;
- Sandblasting and/or acid etching on the implant surface seems to be effective in promoting the implant osseointegration;
- The addition of macro-retentions on the implant surface induces a positive effect on the stress distribution in the bone surrounding the implant. However, this strategy alone may not be enough to promote the implant mechanical stability, due to its conical geometry;
- Immediate implant placement may be considered successful in RAI, unless there are no clinical contraindications;
- The performance of some RAI with specific features on its surface, namely the incorporation of macro-retentions, was proved to be successful, with no signs of infection, periodontitis nor bleeding during the follow-up periods.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- Baldi, D.; Lombardi, T.; Colombo, J.; Cervino, G.; Perinetti, G.; Di Lenarda, R.; Stacchi, C. Correlation between insertion torque and implant stability quotient in tapered implants with knife-edge thread design. Biomed. Res. Int. Hindawi 2018, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Malet, J.; Mora, F.; Bouchard, F. Implant Dentistry at a Glance; John Wiley & Sons: London UK, 2012. [Google Scholar]
- Lindhe, J.; Karring, T.; Lang, N.P. (Eds.) Clinical Periodontology and Implant Dentistry, 4th ed.; Blackwell Munksgaard: Copenhagen, Denmark, 2003. [Google Scholar]
- Chen, X.; Xie, L.; Chen, J.; Du, R.; Deng, F. Design and fabrication of custom-made dental implants. J. Mech. Sci. Technol. 2012, 26, 1993–1998. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Antonelli, A.; Barone, S.; Wagner, F.; Fortunato, L.; Traxler, H. Influence of clinician’s skill on primary implant stability with conventional and piezoelectric preparation techniques: An ex-vivo study. J. Biol. Regul. Homeost. Agents. 2020, 34, 739–746. [Google Scholar] [PubMed]
- Chen, J.; Zhang, Z.; Chen, X.; Zhang, C.; Zhang, G.; Xu, Z. Design and manufacture of customized dental implants by using reverse engineering and selective laser melting technology. J. Prosthet. Dent. 2014, 112, 1088–1095.e1. [Google Scholar] [CrossRef] [PubMed]
- Regish, K.M.; Sharma, D.; Prithviraj, D.R. An overview of immediate root analogue zirconia implants. J. Oral. Implantol. 2013, 39, 225–233. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Milone, D.; Risitano, G. FEM Investigation of the Stress Distribution over. Mandibular Bone Due to Screwed Overdenture Positioned on Dental Implants. Materials 2018, 11, 1512. [Google Scholar] [CrossRef]
- Dantas, T.A.; Carneiro Neto, J.P.; Alves, J.L.; Vaz, P.C.S.; Silva, F.S. In silico evaluation of the stress fields on the cortical bone surrounding dental implants: Comparing root-analogue and screwed implants. J. Mech. Behav. Biomed. Mater. 2020, 104, 103667. [Google Scholar] [CrossRef]
- Hodosh, M.; Shklar, G.; Povar, M. The porous vitreous carbon/polymethacrylate tooth implant: Preliminary studies. J. Prosthet. Dent. Mosby 1974, 32, 326–334. [Google Scholar] [CrossRef]
- Lundgren, D.; Rylander, H.; Andersson, M.; Johansson, C.; Albrektsson, T. Healing of root analogue titanium implants placed in extraction sockets. An experimental study in the beagle dog. Clin. Oral Implant. Res. 1992, 3, 136–144. [Google Scholar] [CrossRef]
- Pessanha-Andrade, M.; Sordi, M.B.; Henriques, B.; Silva, F.S.; Teughels, W.; Souza, J.C.M. Custom-made root-analogue zirconia implants: A scoping review on mechanical and biological benefits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2888–2900. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef]
- Zühlke, A.; Gasik, M.; Shahramian, K.; Närhi, T.; Bilotsky, Y.; Kangasniemi, I. Enhancement of gingival tissue adherence of zirconia implant posts: In vitro study. Materials 2021, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P. (Ed.) Handbook of Oral Biomaterials; Pan Stanford Publ. Pte. Ltd.: Singapore, 2014. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. Public Libr. Sci. 2009, 6, e1000097. [Google Scholar]
- Kohal, R.; Hürzeler, M.; Mota, L.; Klaus, G.; Caffesse, R.; Strub, J. Custom-made root analogue titanium implants placed into extraction sockets: An experimental study in monkeys. Clin. Oral Implant. Res. 1997, 8, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Heydecke, G.; Kohal, R.; Gläser, R. Optimal esthetics in single-tooth replacement with the Re-Implant system: A case report. Int. J. Prosthodont. 1999, 12, 184–189. [Google Scholar] [PubMed]
- Pirker, W.; Kocher, A. Immediate, non-submerged, root-analogue zirconia implant in single tooth replacement. Int. J. Oral Maxillofac. Surg. 2008, 37, 293–295. [Google Scholar] [CrossRef]
- Pirker, W.; Kocher, A. Immediate, non-submerged, root-analogue zirconia implants placed into single-rooted extraction sockets: 2-year follow-up of a clinical study. Int. J. Oral Maxillofac. Surg. 2009, 38, 1127–1132. [Google Scholar] [CrossRef]
- Pirker, W.; Kocher, A. True Anatomic Immediate Dental Implant Method. Implants 2009, 10, 10–14. [Google Scholar]
- Pirker, W.; Wiedemann, D.; Lidauer, A.; Kocher, A. Immediate, single stage, truly anatomic zirconia implant in lower molar replacement: A case report with 2.5 years follow-up. Int. J. Oral Maxillofac. Surg. 2011, 40, 212–216. [Google Scholar] [CrossRef]
- Mangano, F.; Cirotti, B.; Sammons, R.; Mangano, C. Custom-made, root-analogue direct laser metal forming implant: A case report. Lasers Med. Sci. 2012, 27, 1241–1245. [Google Scholar] [CrossRef]
- Figliuzzi, M.; Mangano, F. A novel root analogue dental implant using CT scan and CAD/CAM: Selective laser melting technology. Int. J. Oral Maxillofac. Surg. 2012, 41, 858–862. [Google Scholar] [CrossRef]
- Mangano, F.; De Franco, M.; Caprioglio, A.; MacChi, A.; Piattelli, A.; Mangano, C. Immediate, non-submerged, root-analogue direct laser metal sintering (DLMS) implants: A 1-year prospective study on 15 patients. Lasers Med. Sci. 2014, 29, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Pirker, W.; Kocher, A. Root analog zirconia implants: True anatomical design for molar replacement—A case report. Int. J. Periodontics Restor. Dent. 2015, 31, 663. [Google Scholar]
- Figliuzzi, M.; Giudice, A.; Rengo, C.; Fortunato, L. A direct metal laser sintering (DMLS) root analogue implant placed in the anterior maxilla. Case report. Ann. Ital. Chir. 2016, 8, 1–5. [Google Scholar] [CrossRef][Green Version]
- Patankar, A.; Kshirsagar, R.; Patankar, S.; Pawar, S. Immediate, Non Submerged Root Analog Zirconia Implant in Single Rooted Tooth Replacement: Case Report with 2 years Follow Up. J. Maxillofac. Oral. Surg. 2016, 15, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Pour, R.; Randelzhofer, P.; Edelhoff, D.; Prandtner, O.; Rafael, C.; Liebermann, A. Innovative Single-Tooth Replacement with an Individual Root-Analog Hybrid Implant in the Esthetic Zone: Case Report. Int. J. Oral Maxillofac. Implant. 2017, 32, e153–e160. [Google Scholar] [CrossRef] [PubMed]
- Moin, D.; Hassan, B.; Wismeijer, D. Immediate Nonsubmerged Custom Root Analog Implants: A Prospective Pilot Clinical Study. Int. J. Oral Maxillofac. Implant. 2018, 33, e37–e44. [Google Scholar] [CrossRef]
- Andersson, M.; Bergman, B.; Bessing, C.; Ericson, G.; Lundquist, P.; Nilson, H. Clinical results with titanium crowns fabricated with machine duplication and spark erosion. Acta Odontol. Scand. 1989, 47, 279–286. [Google Scholar] [CrossRef]
- Strub, J.R.; Kohal, R.J.; Klaus, G.; Ferraresso, F. The Re implant® system for immediate implant placement. J. Esthet. Restor. Dent. 1997, 9, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, L.; Gasik, M. Bacterial Attachment and Biofilm Formation on Biomaterials: The case of dental and orthopaedic implants. In Biomater Immune Response Complicat Mech Immunomodulation; Vrana, E.N., Ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 249. [Google Scholar]
- Gasik, M.; Mellaert, L.; van Pierron, D.; Braem, A.; Hofmans, D.; Waelheyns, E.D.; Vleugels, J. Reduction of biofilm infection risks and promotion of osteointegration for optimized surfaces of titanium implants. Adv. Health Mater. 2012, 1, 117–127. [Google Scholar] [CrossRef]
- Shahramian, K.; Gasik, M.; Kangasniemi, I.; Walboomers, X.F.; Willberg, J.; Abdulmajeed, A.; Närhi, T. Zirconia implants with improved attachment to the gingival tissue. J. Periodontol. 2020, 91, 1213–1224. [Google Scholar] [CrossRef]
- Niinomi, M. Biologically and Mechanically Biocompatible Titanium Alloys. Mater. Trans. Japan Inst. Met. Mater. 2008, 49, 2170–2178. [Google Scholar] [CrossRef]
- Reveron, H.; Fornabaio, M.; Palmero, P.; Fürderer, T.; Adolfsson, E.; Lughi, V.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants: Transformation induced plasticity and its consequence on ceramic reliability. Acta Biomater. 2017, 48, 423–432. [Google Scholar] [CrossRef]
- Bona ADella Pecho, O.E.; Alessandretti, R. Zirconia as a dental biomaterial. Materials 2015, 8, 4978–4991. [Google Scholar] [CrossRef]
- Quirynen, M.; Abarca, M.; Van Assche, N.; Nevins, M.; van Steenberghe, D. Impact of supportive periodontal therapy and implant surface roughness on implant outcome in patients with a history of periodontitis. J. Clin. Periodontol. 2007, 34, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Krishna Alla, R.; Ginjupalli, K.; Upadhya, N.; Shammas, M.; Krishna Ravi, R.; Sekhar, R. Surface Roughness of Implants: A Review. Trends Biomater. Artif. Organs 2011, 25, 112–118. [Google Scholar]
- Saghiri, M.A.; Asatourian, A.; Garcia-Godoy, F.; Sheibani, N. The role of angiogenesis in implant dentistry part I: Review of titanium alloys, surface characteristics and treatments. Med. Oral Patol. Oral 2016, 21, e514–e525. [Google Scholar] [CrossRef] [PubMed]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.-P.; Kiattavorncharoen, S.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant Dent. Relat. Res. 2005, 7, S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Bonino, L.; Dalmasso, P.; Aglietta, M. Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin. Oral Implant. Res. 2014, 25, 1105–1112. [Google Scholar] [CrossRef]
- Greenstein, G.; Cavallaro, J.; Romanos, G.; Tarnow, D. Clinical Recommendations for Avoiding and Managing Surgical Complications Associated With Implant Dentistry: A Review. J. Periodontol. 2008, 79, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.P.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. Clin. Oral Implant. Res. 2006, 17, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Davidowitz, G.; Kotick, P.G. The Use of CAD/CAM in Dentistry. Dent. Clin. 2011, 55, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Susic, I.; Travar, M.; Susic, M. The application of CAD / CAM technology in Dentistry. IOP Conf. Ser. Mater. Sci. Eng. 2017, 200, 012020. [Google Scholar] [CrossRef]
- Goto, T. Osseointegration and dental implants. Clin. Calcium. 2014, 24, 265–271. [Google Scholar] [PubMed]
- Dantas, T.A.; Abreu, C.S.; Costa, M.M.; Miranda, G.; Silva, F.S.; Dourado, N.; Gomes, J.R. Bioactive materials driven primary stability on titanium biocomposites. Mater. Sci. Eng. C. 2017, 77, 1104–1110. [Google Scholar] [CrossRef]
- Moin, D.A.; Hassan, B.; Wismeijer, D. A Patient Specific Biomechanical Analysis of Custom Root Analogue Implant Designs on Alveolar Bone Stress: A Finite Element Study. Int. J. Dent. 2016, 2016, 8. [Google Scholar]
- Lin, D.; Li, Q.; Li, W.; Swain, M. Dental implant induced bone remodeling and associated algorithms. J. Mech. Behav. Biomed. Mater. J. Mech. Behav. Biomed. Mater. 2009, 2, 410–432. [Google Scholar] [CrossRef]
- Katz, J.L.; Misra, A.; Marangos, O.; Ye, Q.S.P. Mechanics of hard tissue. In Biomechanics: Principles & Practices; Peterson, D.R., Bronzino, J.D., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Chen, J.; Ahmad, R.; Li, W.; Swain, M.; Li, Q. Biomechanics of oral mucosa. J. R. Soc. Interface. 2015, 12, 20150325. [Google Scholar] [CrossRef]
- Marques, A.; Miranda, G.; Faria, D.; Pinto, P.; Silva, F.; Carvalho, Ó. Novel design of low modulus high strength zirconia scaffolds for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 97, 375–384. [Google Scholar] [CrossRef]
- Dantas, T.A.; Pinto, P.; Vaz, P.C.S.; Silva, F.S. Design and optimization of zirconia functional surfaces for dental implants applications. Ceram Int. 2020, 46, 16328–16336. [Google Scholar] [CrossRef]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors affecting the survival rate of dental implants: A retrospective study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef] [PubMed]
| Author/Year | Title |
|---|---|
| (Lundgren, et al., 1992) [11] | Healing-in of root analogue titanium implants placed in extraction sockets. An experimental study in the beagle dog |
| (Kohal et al., 1997) [17] | Custom-made root analogue titanium implants placed into extraction sockets. An experimental study in monkeys |
| (Heydecke et al., 1999) [18] | Optimal aesthetics in single-tooth replacement with the Re-Implant system: a case report |
| (Pirker and Kocher 2008) [19] | Immediate, non-submerged, root-analogue zirconia implant in single tooth replacement |
| (Pirker and Kocher 2009a) [20] | Immediate, non-submerged, root-analogue zirconia implants placed into single-rooted extraction sockets: 2-year follow-up of a clinical study |
| (Pirker and Kocher 2009b) [21] | True anatomic immediate dental implant method a clinical case |
| (Pirker et al., 2011) [22] | Immediate, single stage, truly anatomic zirconia implant in lower molar replacement: A case report with 2.5 years follow-up |
| (Mangano et al., 2012) [23] | Custom-made, root-analogue direct laser metal forming implant: a case report |
| (Figliuzzi and Mangano 2012) [24] | A novel root analogue dental implant using CT scan and CAD/CAM: selective laser melting technology |
| (Mangano et al., 2014) [25] | Immediate, non-submerged, root-analogue direct laser metal sintering (DLMS) implants: a 1-year prospective study on 15 patients |
| (Pirker and Kocher 2015) [26] | Root analogue zirconia implants: true anatomical design for molar replacement—a case report |
| (Figliuzzi et al., 2016) [27] | A Direct Metal Laser Sintering (DMLS) root analogue implant placed in the anterior maxilla: case report |
| (Patankar et al., 2016) [28] | Immediate, non-submerged root analogue zirconia implant in single rooted tooth replacement: case report with 2 years follow-up |
| (Pour et al., 2017) [29] | Innovative single-tooth replacement with an individual root-analogue hybrid implant in the aesthetic zone: case report |
| (Moin et al., 2018) [30] | Immediate non-submerged custom root analogue implants: a prospective pilot clinical study |
| Author, Year [Reference] | Material | Design Technique | Manufacturing Technique | Surface Treatment |
|---|---|---|---|---|
| Lundgren, et al., 1992 [11] | Commercial purity (CP) titanium | Copying the original tooth, with the help of a detection needle [31] | Milling | Polishing |
| Kohal et al., 1997 [17] | Grade II titanium | Laser scanning of the original tooth | Milling | Sandblasting with Al2O3 (500 µm)—intraosseous Polishing-extraosseous |
| Heydecke et al., 1999 [18] | Grade II titanium | A root model was created using a silicon putty material, based on the extraction socket. Its geometry was modified to perfectly fit in the alveolus and posteriorly laser scanned. The surface of the root analogue implant has a honey-comb pattern [32,33] | Milling | Sandblasting with Al2O3 (500 µm)—intraosseous Polishing-extraosseous |
| Pirker and Kocher 2008 [19] | Zirconia | Laser scanning of the original tooth root. Macro-retentions were designed on the implant surface. A crown stump was designed for later connection to the crown | Milling | Sandblasting |
| Pirker and Kocher 2009a [20] | Laser scanning of the original tooth root (Group A) and laser scanning of the original tooth root plus macro-retentions on the surface (Group B) | |||
| Pirker and Kocher 2009b [21] | Laser scanning of the original tooth root. Macro-retentions were designed on the implant surface. A crown stump was designed for later connection to the crown | |||
| Pirker et al., 2011 [22] | ||||
| Mangano et al., 2012 [23] | Ti6Al4V | DICOM datasets sent to a 3D reconstruction software, following by segmentation and a 3D reconstruction of the non-restorable root. A “virtual extraction” was performed, isolating the root as an STL file sent to a reverse-engineering software where the root was processed, and the prosthetic abutment was added. The diameter of the implant neck was reduced in the area in contact with the thin buccal bone | Direct Laser Metal Forming (DLMF) | Acid etching (50% oxalic acid and 50% maleic acid) |
| Figliuzzi and Mangano 2012 [24] | ||||
| Mangano et al., 2014 [25] | ||||
| Pirker and Kocher 2015 [26] | Zirconia | Laser scanning of the original tooth root. Macro-retentions were designed on the implant surface. A crown stump was designed for later connection to the crown | Milling | Sandblasting |
| Figliuzzi et al., 2016 [27] | Ti6Al4V | DICOM datasets were sent to a 3D reconstruction software, following by segmentation and a 3D reconstruction of the non-restorable root. A “virtual extraction” was performed, isolating the root as an STL file sent to a reverse-engineering software where the root was processed, and the prosthetic abutment was added. The diameter of the implant neck was reduced in the area in contact with the thin buccal bone | Direct Metal Laser Sintering (DMLS) | No information |
| Patankar et al., 2016 [28] | Zirconia | After the extraction, the tooth was modified with light cured composite material to receive the crown afterward. Macro-retentions were designed on the root surface only on the mesial and distal surface with light cured flowable composite material. The modified root was laser scanned | Milling | Sandblasting |
| Pour et al., 2017 [29] | Titanium (root) Zirconia (abutment) | Impressions of the maxilla and a digital volume tomography (DVT) were taken. The DVT data with the impression used to determine the exact dimensions of the implant, were sent to Natural Dental Implants (NDI). Utilizing the 3D data derived from the DVT and the digitized casts, NDI designed and fabricated a patient-specific root-analogue immediate implant with a predesigned abutment. Additionally, microretentions were designed on the implant surface | Milling Materials bonded with a glass solder for sealing the interface between the implant and abutment | Radiation Acid etching Sandblasting (on the titanium part only) |
| Moin et al., 2018 [30] | Grade IV titanium (root) Zirconia (abutment) | After patient DICOM files acquisition and STL files of stone casts and bite registrations, a 3D envelope was created for the selected tooth representing the extension of the root, alveolar bone, marginal bone level, gingival margins, adjacent and antagonist dental structures, and anatomical structures. Within this 3D envelope, CAD designs of the root analogue implant were made consisting of root/implant portion and an abutment portion | Milling The two parts were bonded with a glass solder | Sandblasting Acid-etching (on the titanium part only) |
| Author/Year | Type of Study | Subjects * | Implant Per Patient | Tooth | Time between Extraction and Implantation | Implant Placement Details | Time between Placement and Reconstruction | Follow-Up | Technical and/or Biological Complications; Overall Performance |
|---|---|---|---|---|---|---|---|---|---|
| (Lundgren, et al., 1992) [11] | Pre-clinical trial (beagle dogs) | 4 (2–3-years-old) | 8 | Variable | Dog 1–3 (2 weeks) Dog 4 (0 day) | Intra-alveolar soft tissue was removed, and the bone walls were not scraped. The implants were immediately placed. The mucoperiosteal flaps were repositioned and sutured | 2 months | 3 years | 2 implants were lost in the first week. 30 implants were retained in the socket (of these, 2 were denuded because of late mucosal perforation but remained clinically stable). The remaining 28 implants fulfilled the clinical criteria for osseointegration |
| (Kohal et al., 1997) [17] | Pre-clinical trial (Macaca fascicularis) | 3 | 4 | Upper central and lateral incisors | 0 day | The implants were tapped into their respective socket The mucoperiosteal flaps were repositioned and sutured | - | 6 months | Buccal bone fracture at the time of implantation. Some of the implants could not be inserted to the intended depth. Four implant exposures (6 days, 8 days, 9 days and 2.5 months). None of the implants was surrounded by soft connective tissue. None of the implants were lost and all were clinically stable at the end of the experiment |
| (Heydecke et al., 1999) [18] | Case report (human) | 1 (45, gender not specified) | 1 | Max. Sn. lateral incisor | 1 day | The implant was placed into the socket using the attached insertion bar under finger pressure and subsequent tapping with a hammer and a mallet. Primary stability was checked with the handles of 2 dental mirrors. The insertion bar was removed and a custom-made healing cap was placed | 6 months | No data | Bony resorption and buccal soft tissue recession The final result was compromised by metal shining through the thin gingiva |
| (Pirker and Kocher 2008) [19] | 1 (63, gender not specified) | 1 | 1st max. Dx premolar | 4 days | The implant was placed into the socket under finger pressure and subsequent gentle tapping with a hammer and a mallet. Primary stability was achieved as checked by palpation and percussion | 4 months | 2 years | Stable implant. No changes on the peri-implant marginal bone level. No bleeding No signs of periodontitis nor bone resorption | |
| (Pirker and Kocher 2009a) [20] | Clinical trial (human) | 18 Group A (4 F, 2 M, 27–60-years-old) Group B (4 F, 8 M, 27–65-years-old) | 1 | Variable | Group A 1–4 days Group B 1–8 days | Group A No restoration Group B 3–13 months | 2 years | Primary implant stability was achieved in all patients and no complications, such as swelling, inflammation, bleeding and pain (Group A) 5 implants were lost within 26–128 days (Group A). Implants were lost suddenly without prior pain or infection (Group A). Implant lost after 624 days. However, the lack of osseointegration was already observed on day 18 (Group B) All 11 remaining implants healed uneventfully with no complications (Group B). Soft tissue retraction ranged from 0–1.5 mm within the first year and remained stable thereafter. Many implants (58%) had no observed soft tissue retraction and maintained an aesthetic gingival architecture (Group B). There was no wound infection, no signs of periodontitis, and no implant mobility/dislocation (Group B) | |
| (Pirker and Kocher 2009b) [21] | Case report | 1 (27 M) | 1 | Dx. lateral maxillary incisor | 7 days | 3 months | 15 months | Stable implant, unchanged peri-implant marginal bone level. No bleeding. Excellent aesthetic result. No signs of periodontitis nor bone or soft tissue recession | |
| (Pirker et al., 2011) [22] | Case report | 1 (50 F) | 1 | 1st mand. Sn. molar | 4 months | 2.5 years | Stable implant, unchanged peri-implant marginal bone level. Complete apical peri-implant ossification with no signs of peri-implantitis | ||
| (Mangano et al., 2012) [23] | Case report | 1 (55 M) | 1 | 1st max. Dx. premolar | 0 day | 3 months | 1 year | Primary stability was achieved, due to the perfect correspondence between the implant and the post-extraction socket The implant was still in function after a one-year follow-up The implant was stable, with no signs of infection, unchanged peri-implant marginal bone level and no peri-implant radiolucency The radiographic profile of the implant–crown complex was very similar to that of a natural tooth No prosthetic complications. The prosthetic restoration showed optimal functional and aesthetic integration | |
| (Figliuzzi and Mangano 2012) [24] | Case report | 1 (50 F) | 1 | 2nd max. Dx premolar | 0 day | The implant was placed into the socket under finger pressure and subsequent gentle tapping with a hammer and a mallet. Primary stability was achieved as checked by palpation and percussion | 3 months | 1 year | Implant in function after one year. The implant was stable with no signs of infection. Good conditions of the peri-implant tissues. Unchanged peri-implant marginal bone level and no peri-implant radiolucency. No prosthetic complications |
| (Mangano et al., 2014) [25] | Clinical trial | 15 (8 M, 7 F, 39–55-years-old) | 1 | Premolars (8 max; 7 mand) | 0 day | No implants were lost, leading to a survival rate of 100%. All implants were stable with no signs of infection. Unchanged peri-implant marginal bone level and no peri-implant radiolucency. The radiographic profile of the implant–crown complex was very similar to that of natural teeth. No prosthetic complications | |||
| (Pirker and Kocher 2015) [26] | Case report | 1 (41 F) | 1 | Maxillary 2nd Sn. molar | 6 day | 7 months | 3 years | The implant completely filled the extraction socket, ensuring perfect osseointegration. Unchanged peri-implant marginal bone levels. No signs of periodontitis, bone resorption nor bleeding. Excellent aesthetic result | |
| (Figliuzzi et al., 2016) [27] | Case report | 1 (45 M) | 1 | Lateral Dx. max. incisor | 0 days | The implant was gently inserted in the socket using a little percussion hammer. Primary stability was achieved, as a consequence of the congruence between the implant and the socket. Then, sutures were positioned | 3 months | 1 year | After one year, the implant was still in function. No biological complications were reported. The peri-implant tissues were mature and stable. Little or no peri-implant bone loss, and no soft tissue recession |
| (Patankar et al., 2016) [28] | Case report | 1 (22 F) | 1 | Dx. mand. 1st premolar | 3 days | The implant was placed into the socket under finger pressure and subsequent gentle tapping with a hammer and a mallet. Primary stability was achieved as checked by palpation and percussion | 4 months | 18 months | Stable implant. Unchanged peri-implant marginal bone level and complete apical peri-implant ossification. No signs of peri-implantitis and no bleeding |
| (Pour et al., 2017) [29] | Case report | 1 (35 F) | 1 | Max. Sn. central incisor | 0 day | The implant was inserted and seated with cautious tapping into the socket and buccal augmentation was achieved with Bio-Oss for stabilizing the tissue architecture. The relief cut was sewn up with three single button sutures | 6 months | 16 months | Satisfactory aesthetics and stability of the surrounding tissues. Stability of the bone and implant functionality observed |
| (Moin et al., 2018) [30] | Clinical trial | 5 | 1 | Premolars | 0 day | The implant was placed into the socket under finger pressure and subsequent gentle tapping with a hammer and a mallet. Primary stability was achieved as checked by palpation and percussion | 3 months | 1 year | In one patient, the implant showed mobility and symptoms of peri-implant infection after 4 weeks. The implant was removed at the 12 months evaluation, all remaining implants were successful. Two patients showed an absence of buccal bone around the implant. Healthy mucosal appearance in all remaining implants |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantas, T.; Madeira, S.; Gasik, M.; Vaz, P.; Silva, F. Customized Root-Analogue Implants: A Review on Outcomes from Clinical Trials and Case Reports. Materials 2021, 14, 2296. https://doi.org/10.3390/ma14092296
Dantas T, Madeira S, Gasik M, Vaz P, Silva F. Customized Root-Analogue Implants: A Review on Outcomes from Clinical Trials and Case Reports. Materials. 2021; 14(9):2296. https://doi.org/10.3390/ma14092296
Chicago/Turabian StyleDantas, Telma, Sara Madeira, Michael Gasik, Paula Vaz, and Filipe Silva. 2021. "Customized Root-Analogue Implants: A Review on Outcomes from Clinical Trials and Case Reports" Materials 14, no. 9: 2296. https://doi.org/10.3390/ma14092296
APA StyleDantas, T., Madeira, S., Gasik, M., Vaz, P., & Silva, F. (2021). Customized Root-Analogue Implants: A Review on Outcomes from Clinical Trials and Case Reports. Materials, 14(9), 2296. https://doi.org/10.3390/ma14092296