Enhanced Thermo–Mechanical Reliability of Ultralow-K Dielectrics with Self-Organized Molecular Pores
Abstract
1. Introduction
2. Experimental Procedure
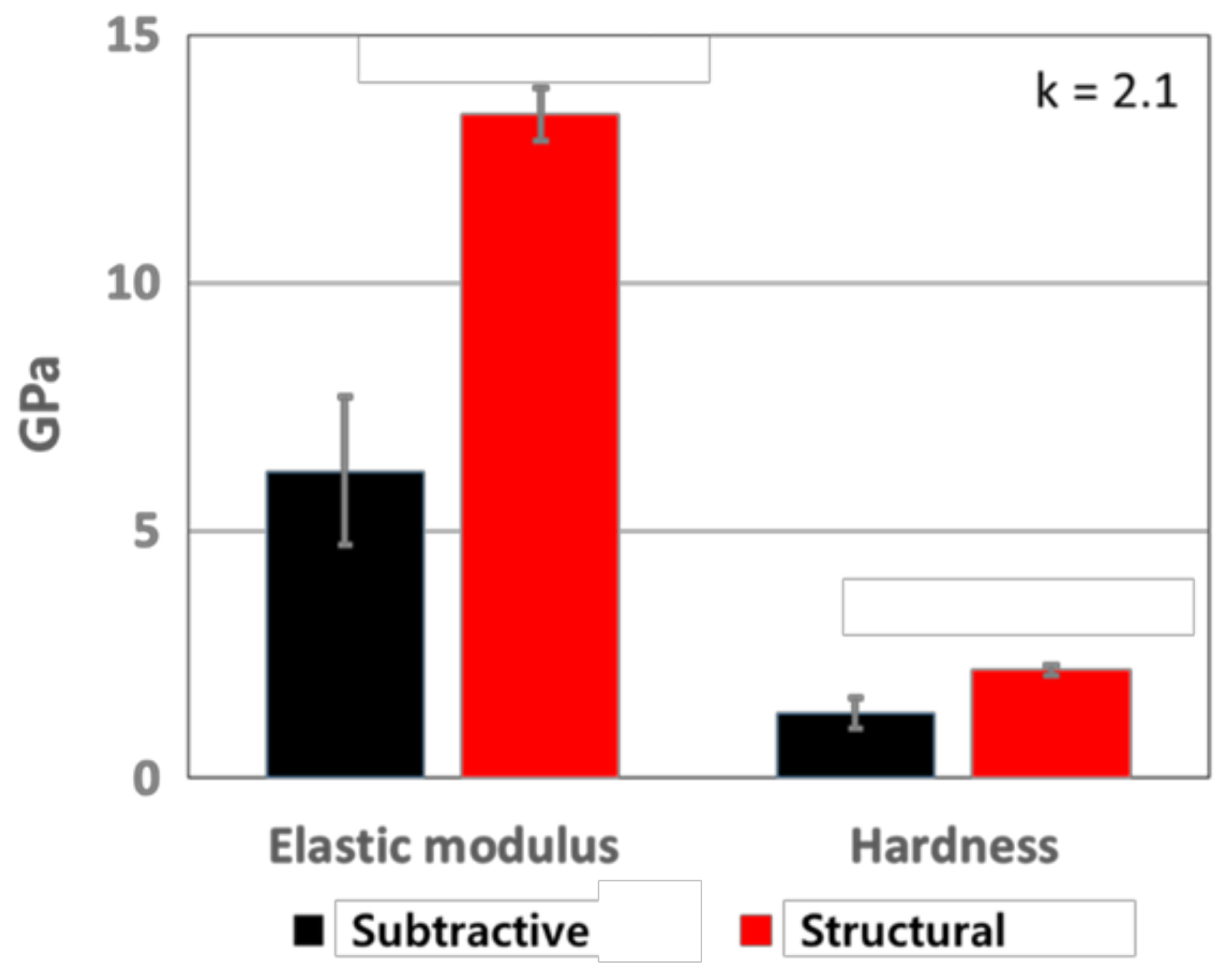
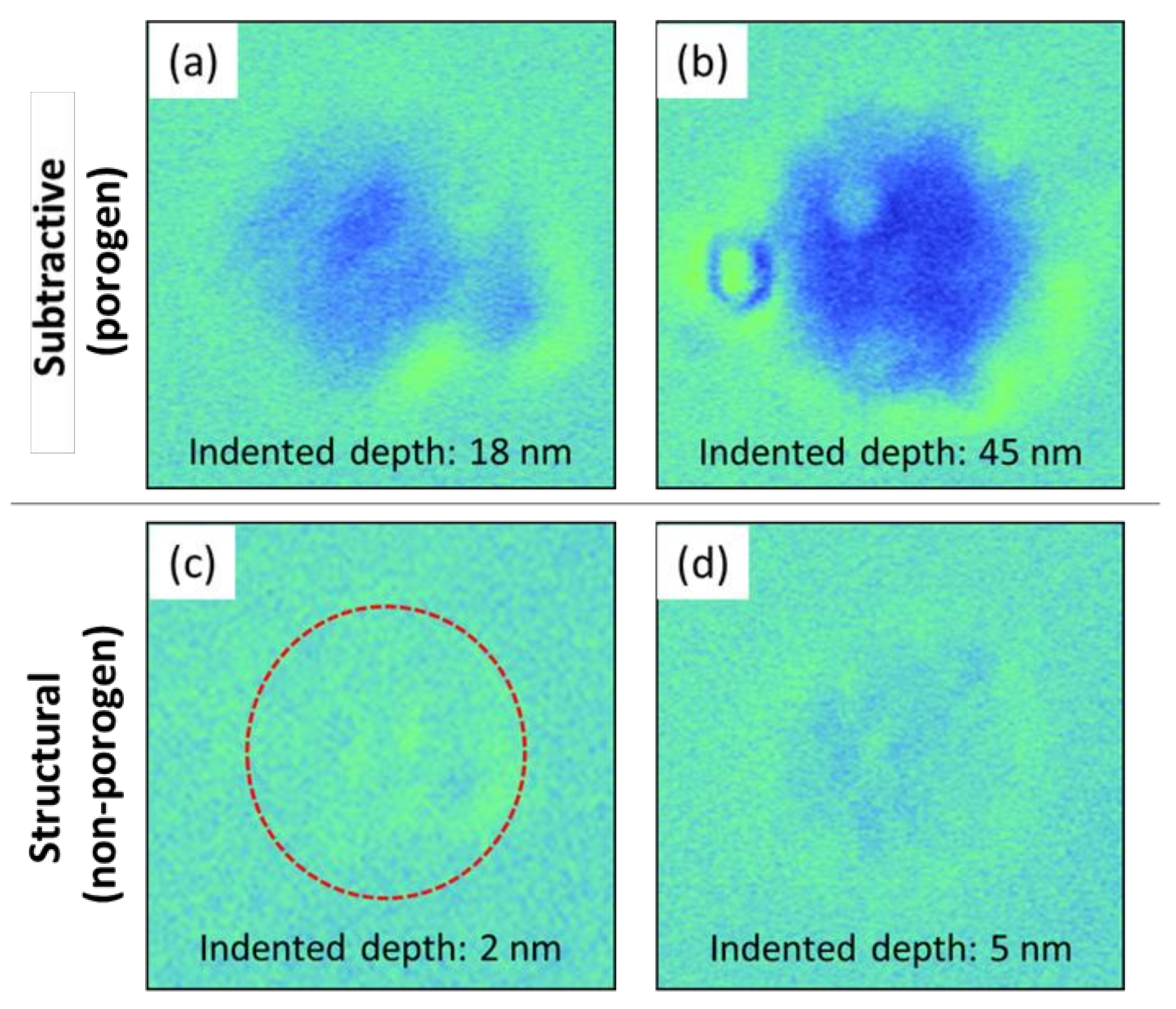
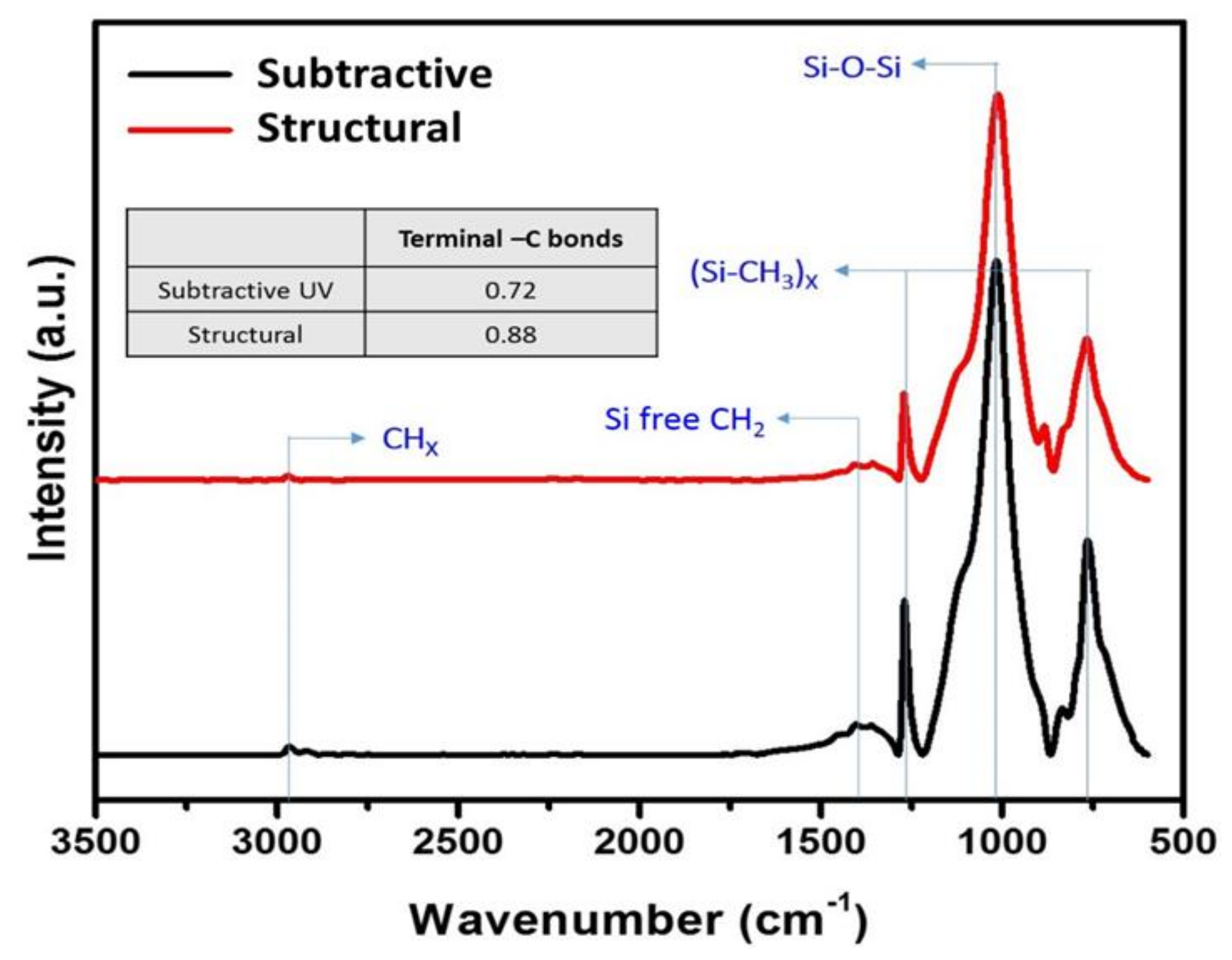
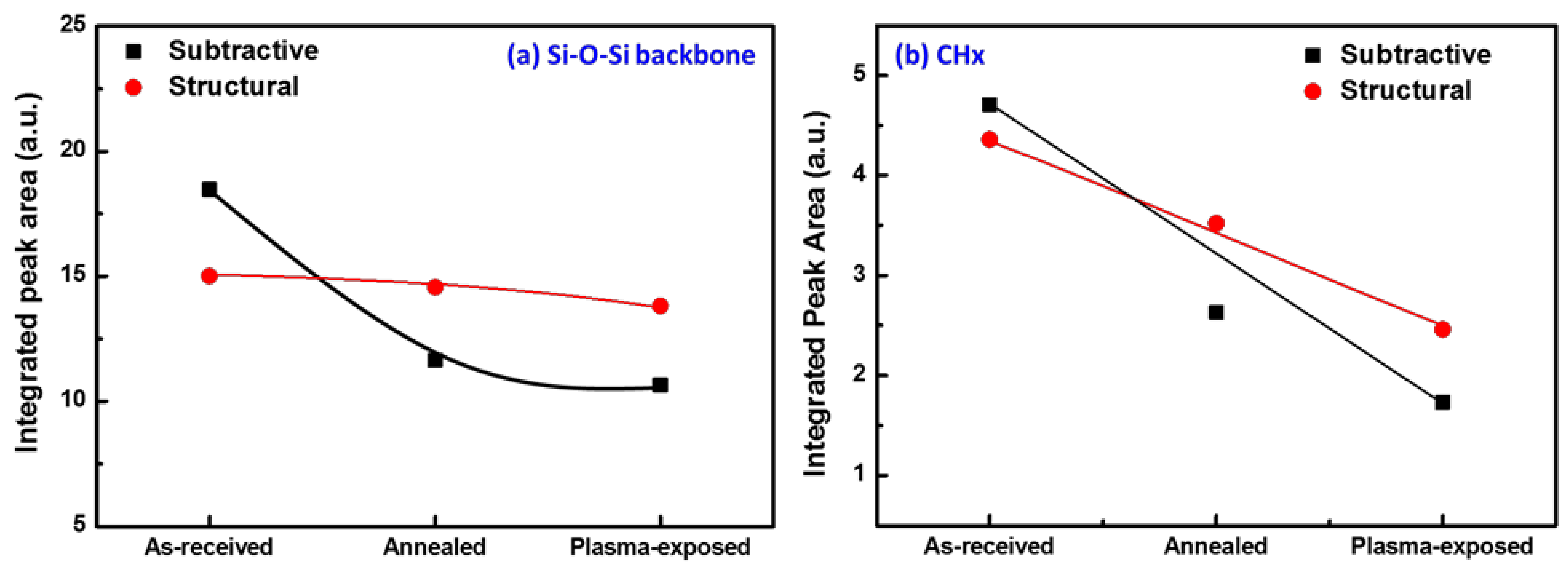
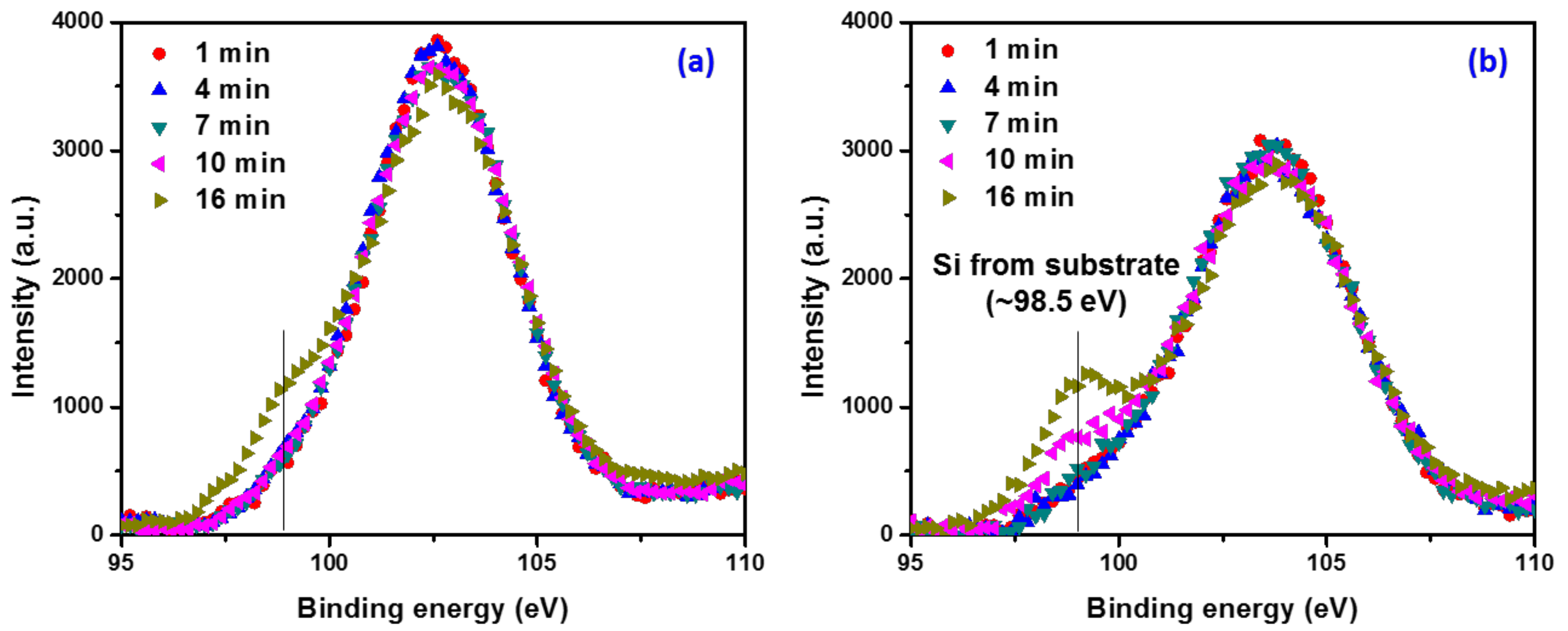
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatton, B.D.; Landskron, K.; Hunks, W.J.; Bennett, M.R.; Shukaris, D.; Perovic, D.D.; Ozin, G.A. Material chemistry for low K-materials. Mater. Today 2006, 9, 22–28. [Google Scholar] [CrossRef]
- Grill, A. PECVD low and ultralow dielectric constant materials: From invention and research to products. J. Vac. Sci. Technol. B 2016, 34, 020801. [Google Scholar] [CrossRef]
- Treichel, H. Low dielectric constant materials. J. Electron. Mater. 2001, 30, 290–298. [Google Scholar] [CrossRef]
- Moors, K.; Soree, B.; Tokei, Z.; Magnus, W. Resistivity scaling and electron relaxation times in metallic nanowires. J. Appl. Phys. 2014, 116, 063714. [Google Scholar] [CrossRef]
- Maex, K.; Baklanov, M.; Shamiryan, D.; Iacopi, F.; Brongersma, S.; Yanovitskaya, Z. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- King, S.W.; Simka, H.; Herr, D.; Akinaga, H.; Garner, M. Research Updates: The three M’s (materials, metrology, and modeling) together pave the path to future nanoelectronic technologies. APL Mater. 2013, 1, 040701. [Google Scholar] [CrossRef]
- Volksen, W.; Miller, R.; Dubois, G. Low Dielectric Constant Materials. Chem. Rev. 2010, 110, 56–110. [Google Scholar] [CrossRef]
- Grill, A.; Gates, S.; Ryan, T.; Nguyen, S.; Priyadarshini, D. Progress in the development and understanding of advanced low k and ultralow k dielectrics for very large-scale integrated interconnects State of the art. Appl. Phys. Rev. 2014, 1, 011306. [Google Scholar] [CrossRef]
- Jousseaume, V.; el Sabahy, J.; Yeromonahos, C.; Castellan, G.; Bouamrani, A.; Ricoul, F. SiOCH thin films deposited by chemical vapor deposition: From low-κ to chemical and biochemical sensors. Microelectron. Eng. 2017, 167, 69–79. [Google Scholar] [CrossRef]
- King, S.W. Dielectric Barrier, Etch Stop, and Metal Capping Materials for State of the Art and beyond Metal Interconnects. ECS J. Solid State Sci. Technol. 2014, 4, N3029. [Google Scholar] [CrossRef]
- Andideh, E.; Lerner, M.; Palmrose, G.; El-Mansy, S.; Scherban, T.; Xu, G.; Blaine, J. Compositional effects on electrical and mechanical properties in carbon-doped oxide dielectric films: Application of Fourier-transform infrared spectroscopy. J. Vac. Sci. Technol. B 2004, 22, 196–201. [Google Scholar] [CrossRef]
- Michalak, D.J.; Blackwell, J.M.; Torres, J.M.; Sengupta, A.; Kreno, L.F.; Clarke, J.S.; Pantuso, D. Porosity scaling strategies for low-k films. J. Mater. Res. 2015, 30, 3363–3385. [Google Scholar] [CrossRef]
- Kemeling, N.; Matsushita, K.; Tsuji, N.; Kagami, K.; Kato, M.; Kaneko, S.; Sprey, H.; de Roest, D.; Kobayashi, N. A robust k∼2.3 SiCOH low-k film formed by porogen removal with UV-cure. Microelectron. Eng. 2007, 84, 2575–2581. [Google Scholar] [CrossRef]
- Jousseaume, V.; Zenasni, A.; Favennec, L.; Gerbaud, G.; Bardet, M.; Simon, J.P.; Humbert, A. Comparison Between E-beam and Ultraviolet Curing to Perform Porous a-SiOC: H. J. Electrochem. Soc. 2007, 154, G103. [Google Scholar] [CrossRef]
- Urbanowicz, A.; Vanstreels, K.; Verdonck, P.; van Besien, E.; Christos, T.; Shamiryan, D.; de Gendt, S.; Baklanov, M. Effect of UV wavelength on the hardening process of porogen-containing and porogen-free ultralow-k plasma-enhanced chemical vapor deposition dielectrics. J. Vac. Sci. Technol. B 2011, 29, 032201. [Google Scholar] [CrossRef]
- Baklanov, M.; de Marneffe, J.-F.; Shamiryan, D.; Urbanowicz, A.M.; Rakhimova, H.S.T.V.; Huang, H.; Ho, P. Plasma processing of low-k dielectrics. J. Appl. Phys. 2013, 113, 041101. [Google Scholar] [CrossRef]
- Hoofman, R.J.O.M.; Verheijden, G.J.A.M.; Michelon, J.; Iacopi, F.; Travaly, Y.; Baklanov, M.R.; Tokei, Z.; Beyer, G.P. Challenges in the implementation of low-k dielectrics in the back-end of line. Microelectron. Eng. 2005, 80, 337–344. [Google Scholar] [CrossRef]
- Hu, Q.; Kjoller, K.; Myers, A.; Singh, K.J.; King, S.W. Nanoscale chemical structure variations in nano-patterned and nano-porous low-k dielectrics: A comparative photothermal induced resonance and infrared spectroscopy investigation. Vib. Spect. 2016, 86, 223–232. [Google Scholar] [CrossRef]
- Rimsza, J.M.; Du, J. Surface reactions and structural evolution of organosilicate glass under Ar plasma bombardment. Comp. Mater. Sci. 2015, 110, 287–294. [Google Scholar] [CrossRef]
- Antonelli, G.A.; Jiang, G.; Shaviv, R.; Mountsier, T.; Dixit, G.; Park, K.J.; Karim, I.; Wu, W.; Shobha, H.; Spooner, T.; et al. Synergistic combinations of dielectrics and metallization process technology to achieve 22 nm interconnect performance targets. Microelectron. Eng. 2012, 92, 9–14. [Google Scholar] [CrossRef]
- Shamuilia, S.; Afanas’ev, V.V.; Somers, P.; Stesmans, A.; Li, Y.-L.; Tokei, Z.; Groeseneken, G.; Maex, K. Internal photoemission of electrons at interfaces of metals with low-κ insulators. Appl. Phys. Lett. 2006, 89, 202909. [Google Scholar] [CrossRef]
- Tanbara, K.; Kamigaki, Y. Paramagnetic Defect Generation and Microstructure Change in Porous Low-k SiOCH Films with Vacuum Baking. J. Electrochem. Soc. 2010, 157, G95. [Google Scholar] [CrossRef]
- Lauer, J.L.; Sinha, H.; Nichols, M.T.; Antonelli, G.A.; Nishi, Y.; Shohet, J.L. Charge Trapping within UV and Vacuum UV Irradiated Low-k Porous Organosilicate Dielectrics. J. Electrochem. Soc. 2010, 157, G177. [Google Scholar] [CrossRef]
- Bittel, B.C.; Lenahan, P.M.; King, S.W. Ultraviolet radiation effects on paramagnetic defects in low-κ dielectrics for ultralarge scale integrated circuit interconnects. Appl. Phys. Lett. 2010, 97, 063506. [Google Scholar] [CrossRef]
- Baklanov, M.; Zhao, L.; van Besien, E.; Pantouvaki, M. Effect of porogen residue on electrical characteristics of ultra low-k materials. Microelectron. Eng. 2011, 88, 990–993. [Google Scholar] [CrossRef]
- Pomorski, T.A.; Bittel, B.C.; Lenahan, P.M.; Mays, E.; Ege, C.; Bielefeld, J.; Michalak, D.; King, S.W. Defect structure and electronic properties of SiOC:H films used for back end of line dielectrics. J. Appl. Phys. 2014, 115, 234508. [Google Scholar] [CrossRef]
- Hussein, M.A.; He, J. Materials’ impact on interconnect process technology and reliability. IEEE Trans. Semi. Manf. 2011, 18, 69–85. [Google Scholar] [CrossRef]
- Volinsky, A.A.; Vella, J.B.; Gerberich, W.W. Fracture toughness, adhesion and mechanical properties of low-K dielectric thin films measured by nanoindentation. Thin Solid Films 2003, 429, 201–210. [Google Scholar] [CrossRef]
- Lin, Y.; Tsui, T.Y.; Vlassak, J.J. Adhesion Degradation and Water Diffusion in Nanoporous Organosilicate Glass Thin Film Stacks. J. Electrochem. Soc. 2009, 157, G53. [Google Scholar] [CrossRef]
- Tambat, A.; Lin, H.-Y.; Subbarayan, G.; Jung, D.Y.; Sammakia, B. Simulations of Damage, Crack Initiation, and Propagation in Interlayer Dielectric Structures: Understanding Assembly-Induced Fracture in Dies. IEEE Trans. Dev. Mater. Rel. 2012, 12, 241–254. [Google Scholar] [CrossRef]
- Zin, E.H.; Bang, W.H.; Ryan, E.T.; King, S.; Kim, C.-U. Study of viscoplastic deformation in porous organosilicate thin films for ultra low-k applications. Appl. Phys. Lett. 2013, 102, 221909. [Google Scholar] [CrossRef]
- Li, Y.; Ciofi, I.; Carbonell, L.; Heylen, N.; van Aelst, J.; Baklanov, M.R.; Groeseneken, G.; Maex, K.; Tokei, Z. Influence of absorbed water components on SiOCH low-k reliability. J. Appl. Phys. 2008, 104, 034113. [Google Scholar] [CrossRef]
- Darnon, M.; Chevolleau, T.; Licitra, C.; Rochat, N.; Zocco, J. Analysis of water adsorption in plasma-damaged porous low-k dielectric by controlled-atmosphere infrared spectroscopy. J. Vac. Sci. Technol. B 2013, 31, 061206. [Google Scholar] [CrossRef]
- Chang, T.C.; Chen, C.W.; Liu, P.T.; Mor, Y.S.; Tsai, H.M.; Tsai, T.M.; Yan, S.T.; Tu, C.H.; Tseng, T.Y.; Sze, S.M. Moisture-Induced Material Instability of Porous Organosilicate Glass. Electrochem. Sol. Stat. Lett. 2003, 6, F13. [Google Scholar] [CrossRef]
- Imada, T.; Nakata, Y.; Ozaki, S.; Kobayashi, Y.; Nakamura, T. Systematic investigation of silylation materials for recovery use of low-k material plasma damage. Jpn. J. Appl. Phys. 2015, 54, 071502. [Google Scholar] [CrossRef]
- Bohm, O.; Leitsmann, R.; Planitz, P.; Radehaus, C.; Schreiber, M.; Schaller, M. k-Restoring Processes at Carbon Depleted Ultralow-k Surfaces. J. Phys. Chem. A 2011, 115, 8282–8287. [Google Scholar] [CrossRef]
- Forster, A.; Wagner, C.; Gemming, S.; Schuster, J. Theoretical investigation of an in situ k-restore process for damaged ultra-low-k materials based on plasma enhanced fragmentation. J. Vac. Sci. Technol. B 2015, 33, 052203. [Google Scholar] [CrossRef]
- Fischer, T.; Ahner, N.; Zimmermann, S.; Schaller, M.; Schul, S.E. Influence of thermal cycles on the silylation process for recovering k-value and chemical structure of plasma damaged ultra-low-k materials. Microelectron. Eng. April. 2012, 92, 53–58. [Google Scholar] [CrossRef]
- Chaabouni, H.; Chapelon, L.L.; Aimadeddine, M.; Vitiello, J.; Farcy, A.; Delsol, R.; Brun, P.; Fossati, D.; Arnal, V.; Chevolleau, T.; et al. Sidewall restoration of porous ultra low-k dielectrics for sub-45 nm technology nodes. Microelectron. Eng. 2007, 84, 2595–2599. [Google Scholar] [CrossRef]
- Oszinda, T.; Schaller, M.; Schulz, S.E. Chemical Repair of Plasma Damaged Porous Ultra Low-κ SiOCH Film Using a Vapor Phase Process. J. Electrochem. Soc. 2010, 157, H1140. [Google Scholar] [CrossRef]
- Jung, J.M.; Kwon, H.S.; Li, W.-K.; Choi, B.-C.; Kim, H.G.; Lim, K.T. Repair of plasma-damaged p-SiOCH dielectric films in supercritical CO2. Microelectron. Eng. 2010, 87, 1680–1684. [Google Scholar] [CrossRef]
- Vyhmeister, E.; Reyes-Bozo, L.; Valdes-Gonzalez, H.; Salazar, J.-L.; Muscat, A.; Estevez, L.A.; Suleiman, D. In situ FTIR experimental results in the silylation of low-k films with hexamethyldisilazane dissolved in supercritical carbon dioxide. J. Supercrit. Fluid. 2014, 90, 134–143. [Google Scholar] [CrossRef]
- Li, H.; Knaup, J.M.; Kaxiras, E.; Vlassak, J.J. Stiffening of organosilicate glasses by organic cross-linking. Acta Mater. 2011, 59, 44–52. [Google Scholar] [CrossRef]
- Krishtab, M.; de Marneffe, J.-F.; de Gendt, S.; Baklanov, M.R. Plasma induced damage mitigation in spin-on self-assembly based ultra low-k dielectrics using template residues. Appl. Phys. Lett. 2017, 110, 013105. [Google Scholar] [CrossRef]
- Urbanowicz, A.M.; Vanstreels, K.; Verdonck, P.; Shamiryan, D.; de Gendt, S.; Baklanov, M.R. Improving mechanical robustness of ultralow-k SiOCH plasma enhanced chemical vapor deposition glasses by controlled porogen decomposition prior to UV-hardening. J. Appl. Phys. 2010, 107, 104122. [Google Scholar] [CrossRef]
- Iacopi, F.; Travaly, Y.; Eyckens, B.; Waldfried, C.; Abell, T.; Guyer, E.P.; Gage, D.M.; Dauskardt, R.H.; Sajavaara, T.; Houthoofd, K.; et al. Short-ranged structural rearrangement and enhancement of mechanical properties of organosilicate glasses induced by ultraviolet radiation. J. Appl. Phys. 2006, 99, 053511. [Google Scholar] [CrossRef]
- Zenasni, A.; Jousseaume, V.; Holliger, P.; Favennec, L.; Gourhant, O.; Maury, P.; Berbaud, G. The role of ultraviolet radiation during ultralow k films curing: Strengthening mechanisms and sacrificial porogen removal. J. Appl. Phys. 2007, 102, 094107. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Wada, A.; Kurotori, T.; Sakamoto, M.; Nozawa, T.; Samukawa, S. Non-porous ultra-low-k SiOCH (k = 2.3) for damage-free integration and Cu diffusion barrier. J. Phys. D Appl. Phys. 2013, 46, 395203. [Google Scholar] [CrossRef]
- Burkey, D.D.; Gleason, K.K. Organosilicon Thin Films Deposited from Cyclic and Acyclic Precursors Using Water as an Oxidant. J. Electrochem. Soc. 2004, 151, 105. [Google Scholar] [CrossRef]
- Rathore, J.S.; Interrante, L.V.; Dubois, G. Ultra Low-k Films Derived from Hyperbranched Polycarbosilanes (HBPCS). Adv. Funct. Mater. 2008, 18, 4022. [Google Scholar] [CrossRef]
- Kubasch, C.; Klaus, C.; Ruelke, H.; Mayer, U.; Bartha, J.W. Investigation of Moisture Uptake in Low-κ Dielectric Materials. IEEE Trans. Electron Dev. 2010, 57, 1865–1872. [Google Scholar] [CrossRef]
- Kubasch, C.; Olawumi, T.; Ruelke, H.; Mayer, U.; Bartha, J.W. Erratum: Investigation of Argon Plasma Damage on Ultra Low-κ Dielectrics. ECS J. Solid State Sci. Technol. 2014, 4, N3023. [Google Scholar] [CrossRef]
- French, B.L.; King, S.W. Detection of surface electronic defect states in low and high-k dielectrics using reflection electron energy loss spectroscopy. J. Mater. Res. 2013, 28, 2771. [Google Scholar] [CrossRef]
- Stan, G.; Gates, R.S.; Kavuri, P.; Torres, J.; Michalak, D.; Ege, C.; Bielefeld, J.; King, S.W. Mechanical property changes in porous low-k dielectric thin films during processing. Appl. Phys. Lett. 2014, 105, 152906. [Google Scholar] [CrossRef]
- Grill, A.; Neumayer, D.A. Structure of low dielectric constant to extreme low dielectric constant SiCOH films: Fourier transform infrared spectroscopy characterization. J. Appl. Phys. 2003, 94, 6697. [Google Scholar] [CrossRef]
- Grill, A.; Patel, V. Ultralow-k dielectrics prepared by plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2001, 79, 803. [Google Scholar] [CrossRef]
- King, S.W.; French, M.; Bielefeld, J.; Lanford, W.A. Fourier transform infrared spectroscopy investigation of chemical bonding in low-k a-SiC:H thin films. J. Non-Cryst. Sol. 2011, 357, 2970–2983. [Google Scholar] [CrossRef]
- Oh, K.S.; Choi, C.K. Nano Pore Structure of Low-k SiOC(-H) Films Measured by Small Angle Neutron Scattering. J. Korean Phys. Soc. 2004, 45, S855–S860. [Google Scholar]
- Wang, W.; Grozea, D.; Kim, A.; Perovic, D.D.; Ozin, G.A. Vacuum-Assisted Aerosol Deposition of a Low-Dielectric-Constant Periodic Mesoporous Organosilica Film. Adv. Mater. 2009, 21, 99–102. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sa, Y.K.; Bang, J.; Son, J.; Yu, D.-Y.; Kim, Y.-C. Enhanced Thermo–Mechanical Reliability of Ultralow-K Dielectrics with Self-Organized Molecular Pores. Materials 2021, 14, 2284. https://doi.org/10.3390/ma14092284
Sa YK, Bang J, Son J, Yu D-Y, Kim Y-C. Enhanced Thermo–Mechanical Reliability of Ultralow-K Dielectrics with Self-Organized Molecular Pores. Materials. 2021; 14(9):2284. https://doi.org/10.3390/ma14092284
Chicago/Turabian StyleSa, Y. K., Junghwan Bang, Junhyuk Son, Dong-Yurl Yu, and Yun-Chan Kim. 2021. "Enhanced Thermo–Mechanical Reliability of Ultralow-K Dielectrics with Self-Organized Molecular Pores" Materials 14, no. 9: 2284. https://doi.org/10.3390/ma14092284
APA StyleSa, Y. K., Bang, J., Son, J., Yu, D.-Y., & Kim, Y.-C. (2021). Enhanced Thermo–Mechanical Reliability of Ultralow-K Dielectrics with Self-Organized Molecular Pores. Materials, 14(9), 2284. https://doi.org/10.3390/ma14092284





