Research and Development of Novel Refractory of MgO Doped with ZrO2 Nanoparticles for Copper Slag Resistance
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
- (i)
- The cold isostatic pressing method contributes to the application of homogeneous forces on the sample. The mixture of micrometric and nanometric powders helps to achieve maximum packing. Nanoparticles fill cavities between MgO microparticles leading to a more efficient sintering process.
- (ii)
- A suitable sintering temperature: as is usually recognized, the sintering temperature in refractory materials is about 1/2 to 2/3 of the melting point. Particularly, in the present study, a sintering temperature of 1650 °C (0.589 of the magnesia melting point) was used. This temperature promotes higher densification.
- (iii)
- The sintering mechanism: an ionic migration between the Zr, which has a valence of 4+, and Mg, which has a valence of 2+. Cationic magnesium vacancies in the cubic structure of MgO are generated due to the migration of magnesium ions when they replace zirconia ions. Considering that cationic vacancies are formed in the periphery of the grain boundary of magnesium oxide, and these must be replaced with other MgO cations within the crystal volume, the ionic migration takes place towards the grain boundaries at high temperatures. Then, the grain boundaries begin to move, joining each other, generating densification of the sample with larger grains and contraction of the material, causing the closure of the pores.
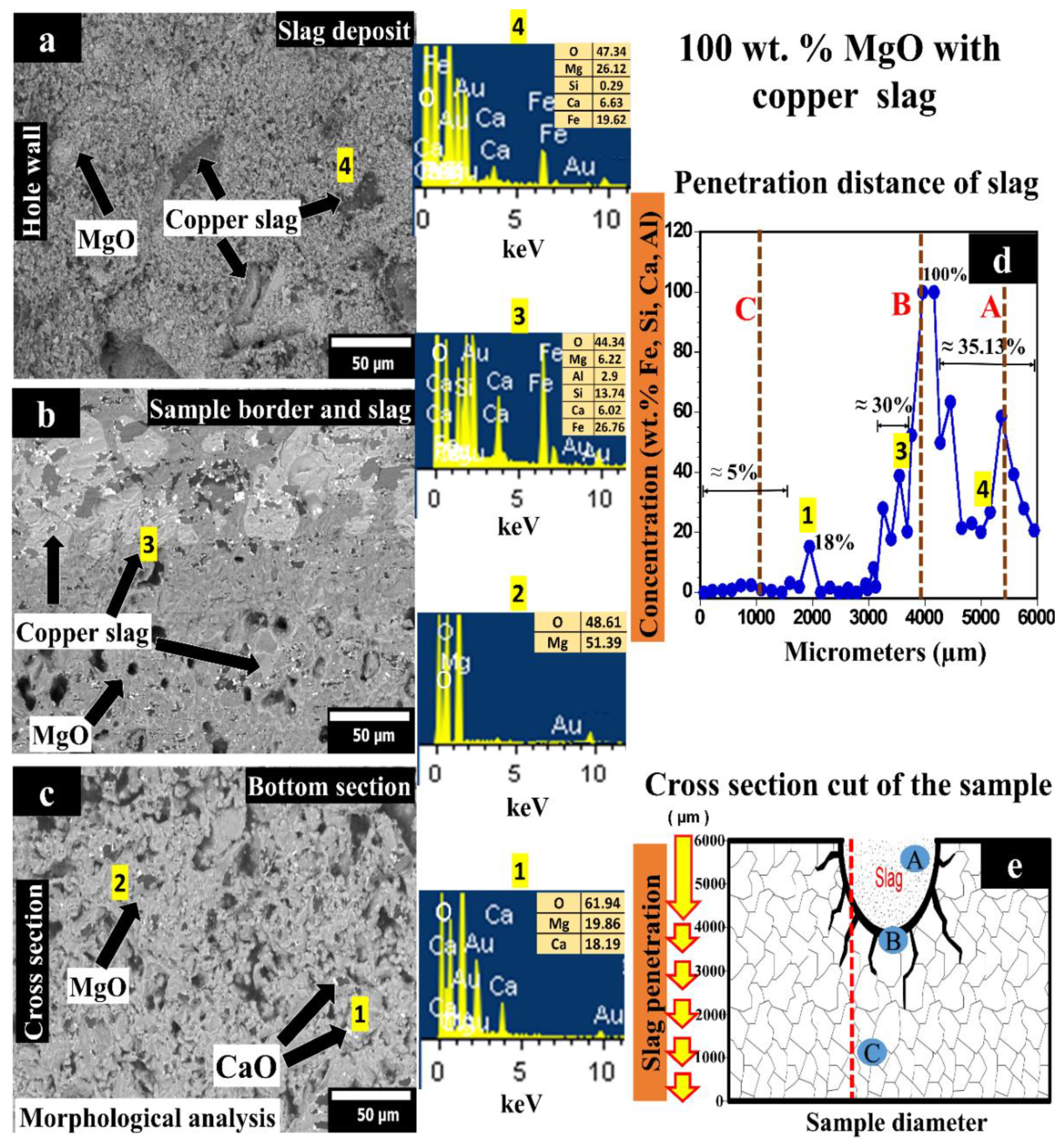
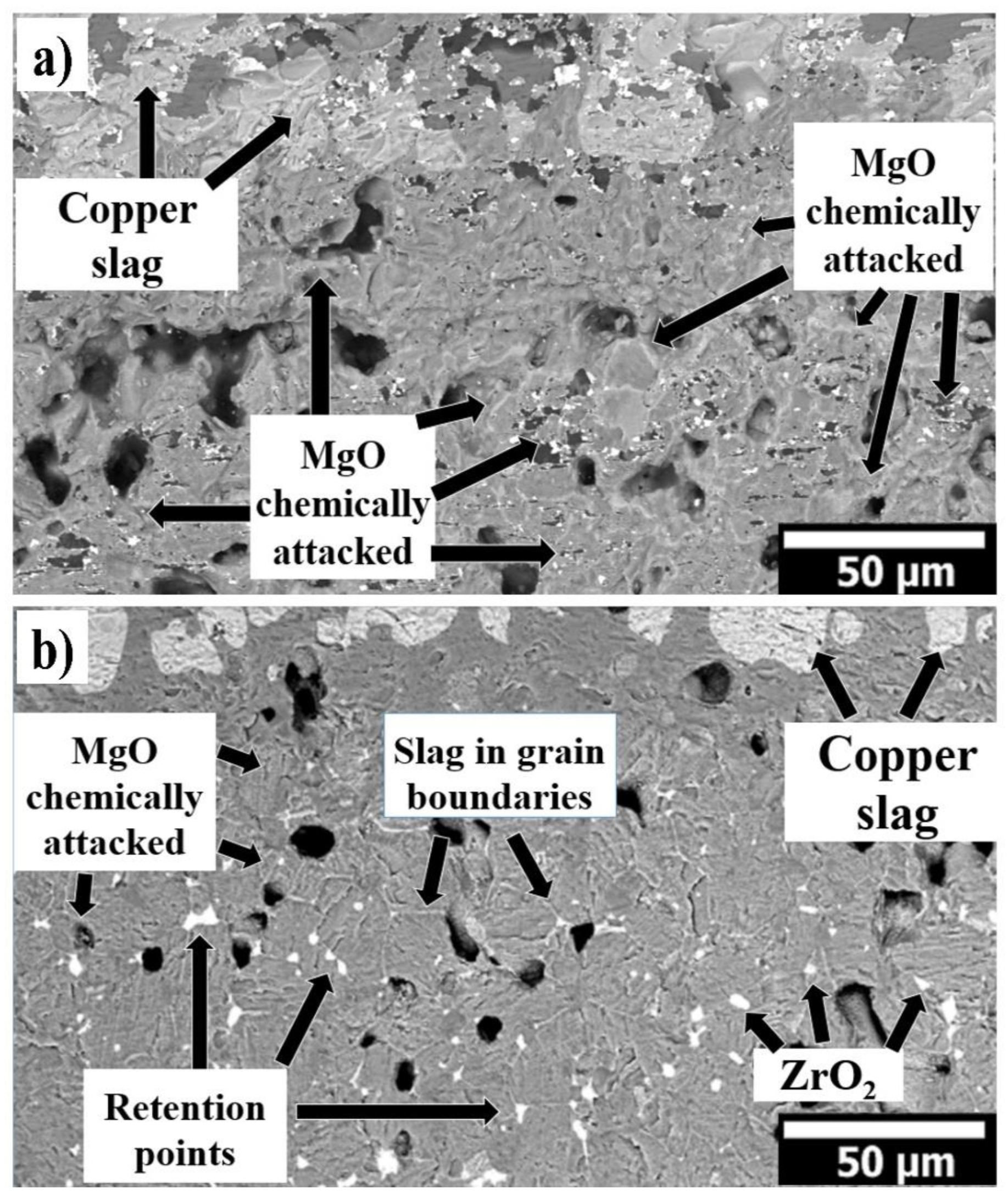
3.1. 100 wt.% MgO Sample Tested with Copper Slag
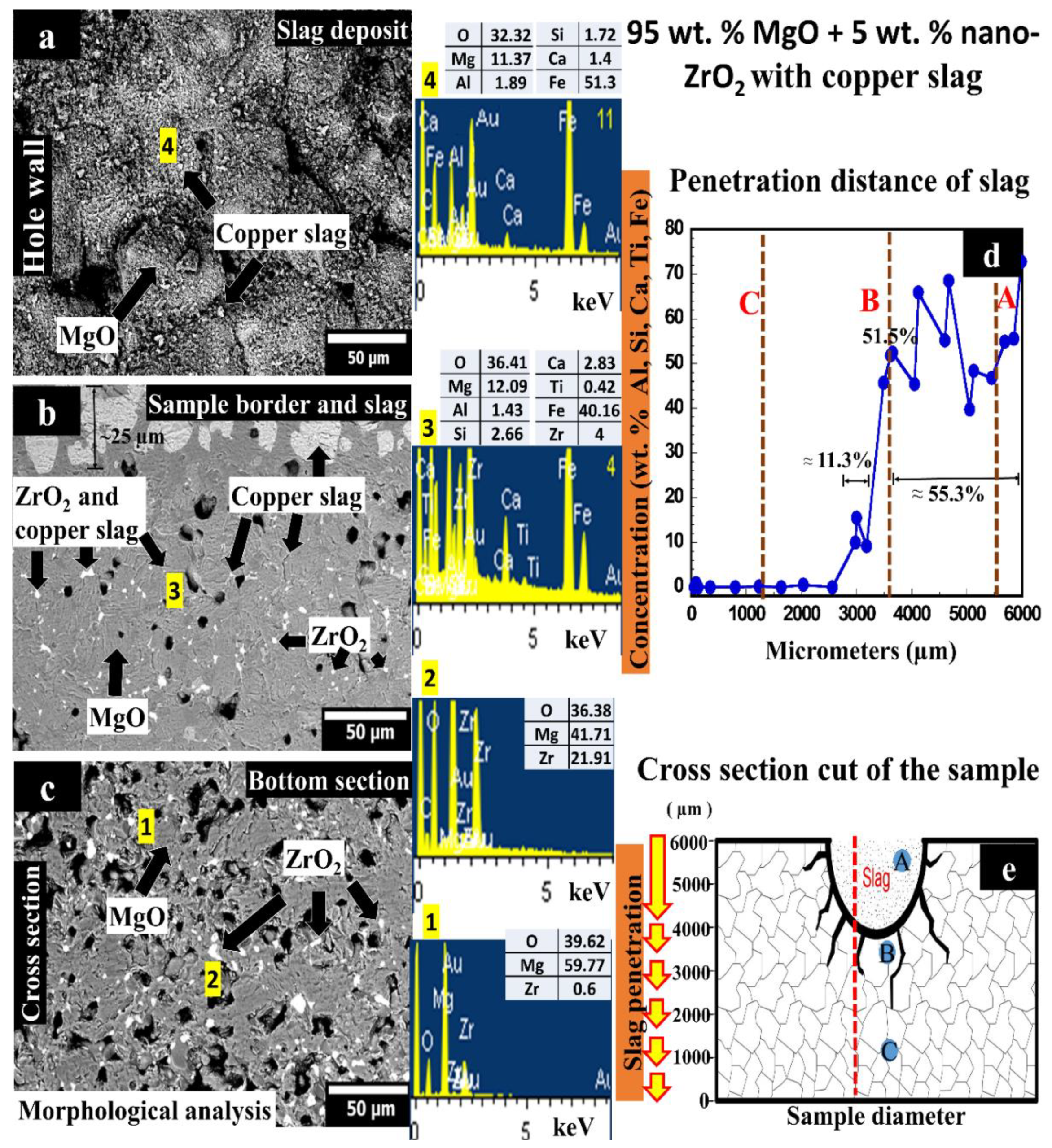
3.2. 95 wt.% MgO with 5 wt.% ZrO2 Sample Tested with Copper Slag
- (i)
- CaZrO3 in situ formation helps to hinder the slag penetration in the refractory by increasing the viscosity of the molten slag, slowing down the intergranular path of the molten slag.
- (ii)
- The slag infiltration was inhibited due to the quasi-spherical ZrO2 nanoparticles since they act as barriers against the penetration of the intergranular liquid. Retention points were observed as non-circular phases due to accumulation in different proportions of slag elements on the periphery of ZrO2 nanoparticles, where the slag was retained around the ZrO2 particles.
- (iii)
- This matrix exhibited a higher density and lower porosity which also help to avoid a considerable slag infiltration.
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, R.E. Refractory usage in non-ferrous metallurgical applications. In 3rd Unified International Conference on Refractories; Krietz, L., Leungs, J.G., Eds.; UNITECR: Sao Paolo, Brazil, 1993; Volume 1, pp. 131–142. [Google Scholar]
- Sancho, J.P.; Verdeja, L.F.; Ballester, A. Metalurgia Extractiva. Volumen II. Procesos de Obtención, 1st ed.; Síntesis: Madrid, Spain, 2000. [Google Scholar]
- Nazer, A. Una revisión de los usos de las escorias de cobre. In Proceedings of the IBEROMET XI. X CONAMET/SAM, Viña del Mar, Chile, 5 November 2010. [Google Scholar]
- Coursol, P.; Cardona, N.; Mackey, P.; Bell, S.; Davis, B. Minimization of Copper Losses in Copper Smelting Slag During Electric Furnace Treatment. JOM 2012, 64, 1305–1313. [Google Scholar] [CrossRef]
- Cardona, N.; Coursol, P.; Mackey, P.J.; Parra, R. Physical chemistry of copper smelting slags and copper losses at the Paipote smelterPart 1—Thermodynamic modelling. Can. Met. Q. 2011, 50, 318–329. [Google Scholar] [CrossRef]
- Cardona, N.; Coursol, P.; Vargas, J.; Parra, R. The Physical Chemistry of Copper Smelting Slags and Copper Losses at the Paipote SmelterPart 2—Characterisation of industrial slags. Can. Met. Q. 2011, 50, 330–340. [Google Scholar] [CrossRef]
- Madheswaran, C.K.; Ambily, P.S.; Dattatreya, J.K.; Rajamane, N.P. Studies on use of Copper Slag as Replacement Material for River Sand in Building Constructions. J. Inst. Eng. Ser. A 2014, 95, 169–177. [Google Scholar] [CrossRef]
- Nazer, A.S.; Pavez, O.; Rojas, F. Use of copper slag in cement mortar. Rem. Rev. Esc. Minas 2012, 65, 87–91. [Google Scholar] [CrossRef][Green Version]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of copper slag in cement and concrete. Resour. Conserv. Recycl. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- Malfliet, A.; Lotfian, S.; Scheunis, L.; Petkov, V.; Pandelaers, L.; Jones, P.T.; Blanpain, B. Degradation mechanisms and use of refractory linings in copper production processes: A critical review. J. Eur. Ceram. 2014, 34, 849–876. [Google Scholar] [CrossRef]
- Schlesinger, M. Refractories for Copper Production. Miner. Process. Extr. Met. Rev. 1996, 16, 125–146. [Google Scholar] [CrossRef]
- Xu, L.; Chen, M.; Wang, N.; Gao, S. Chemical wear mechanism of magnesia-chromite refractory for an oxygen bottom-blown copper-smelting furnace: A post-mortem analysis. Ceram. Int. 2021, 47, 2908–2915. [Google Scholar] [CrossRef]
- Xu, T.; Xu, Y.; Li, Y.; Sang, S.; Wang, Q.; Zhu, T.; Nath, M.; Zhang, B. Corrosion mechanisms of magnesia-chrome refractories in copper slag and concurrent formation of hexavalent chromium. J. Alloys Compd. 2019, 786, 306–313. [Google Scholar] [CrossRef]
- Karakus, M.; Crites, M.E.; Schlesinger, M.E. Cathodoluminescence microscopy characterization of chrome-free refractories for copper smelting and converting furnaces. J. Microsc. 2019, 200, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Malfliet, A.; Vleugels, J.; Blanpain, B.; Guo, M. Degradation mechanisms of alumina-chromia refractories for secondary copper smelter linings. Corros. Sci. 2018, 136, 409–417. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Li, R.; Zhen, Q. Influence of MgAl2O4 spinel addition on properties of alumina-chrome refractory prepared with alumina-chrome slag. J. Ceram. Sci. Tech. 2016, 7, 447–454. [Google Scholar]
- Volker Stein, T.S.; Jansen, H. A New generation of chrome free refractories for copper production. In The Unifed International Technical Conference on Refractories 15th Biennial Worldwide Congress on Refractories; ALAFAR: Santiago de Chile, Chile, 2017; p. 129. [Google Scholar]
- Chen, M.; Chen, J.; Zhao, B. Corrosion Resistances of Cr-Free Refractories to Copper Smelting Slags. In Advances in Molten Slags, Fluxes, and Salts: Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts 2016; Reddy, R.G., Chaubal, P., Pistorius, P.C., Pal, U., Eds.; Springer International Publishing: Berlin, Germany, 2016; pp. 1101–1108. [Google Scholar]
- Chen, M.; Jiang, Y.; Cui, Z.; Wei, C.; Zhao, B. Chemical Degradation Mechanisms of Magnesia–Chromite Refractories in the Copper Smelting Furnace. JOM 2018, 70, 2443–2448. [Google Scholar] [CrossRef]
- Chen, L.; Guo, M.; Shi, H.; Huang, S.; Jones, P.; Blanpain, B.; Malfliet, A. Effect of ZnO Level in Secondary Copper Smelting Slags on Slag/Magnesia-Chromite Refractory Interactions. J. Eur. Ceram. 2016, 36, 1821–1828. [Google Scholar] [CrossRef]
- Rodríguez, E.; Moreno, F.H.; Aguilar-Martínez, J.A.; Montes-Mejía, A.E.; Ruiz-Valdés, J.J.; Puente-Ornelas, R.; Contreras, J.E. Effect of nano-titania (η-TiO2) content on the mechano-physical properties of a magnesia refractory composite. Ceram. Int. 2016, 42, 8445–8452. [Google Scholar] [CrossRef]
- Kahrizsangi, S.G.; Nemati, A.; Shahraki, A.; Farooghi, M. Effect of nano-sized Fe2O3 on microstructure and hydration resistance of MgO-CaO refractories. Int. J. Nanosci. 2016, 12, 19–26. [Google Scholar]
- Gómez Rodríguez, C.; Das Roy, T.K.; Shaji, S.; Castillo Rodríguez, G.A.; García Quiñonez, L.; Rodríguez, E.; González, J.O.; Aguilar-Martínez, J.A. Effect of addition of Al2O3 and Fe2O3 nanoparticle on the microstructural and physico-chemical evolution of dense magnesia composite. Ceram. Int. 2015, 41, 7751–7758. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Gheisari, S.; Boroujerdnia, M. Effect of micro and nano-Al2O3 addition on the microstructure and properties of MgO-C refractory ceramic composite. Matter. Chem. Phys. 2016, 189, 230–236. [Google Scholar] [CrossRef]
- Shahraki, A.; Ghasemi-Kahrizsangi, A.; Nemati, A. Performance improvement of MgO-CaO refractories by the addition of nano-sized Al2O3. Matter. Chem. Phys. 2017, 198, 354–359. [Google Scholar] [CrossRef]
- Dudczig, S.; Veres, D.; Aneziris, C.G.; Skiera, E.; Steinbrech, R.W. Nano- and micrometre additions of SiO2, ZrO2 and TiO2 in fine grained alumina refractory ceramics for improved thermal shock performance. Ceram. Int. 2011, 38, 2011–2019. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Sedeh, B.; Dehsheikh, H.G.; Shahraki, A.; Farooghi, M. Densification and properties of ZrO2 nanoparticles added magnesia-doloma refractories. Ceram. Int. 2016, 42, 15658–15663. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S. Performance improvement of MgO-C refractory bricks by the addition of Nano-ZrSiO4. Matter. Chem. Phys. 2017, 202, 369–376. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Karamian, E.; Dehsheikh, H.G. The impact of ZrSiO4 nanoparticles addition on the microstructure and properties of dolomite based refractories. Ceram. Int. 2017, 43, 13932–13937. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S.; Karamian, E. Addition impact of nano-carbon black on the performance of MgO-CaO compounds. Ceram. Int. 2018, 44, 5524–5527. [Google Scholar] [CrossRef]
- Bag, M.; Adak, S.; Sarkar, R. Study on low carbon containing MgO-C refractory: Use of nano carbon. Ceram. Int. 2012, 38, 2339–2346. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E.; Boroujerdnia, M.; Payandeh, K. Effect of MgAl2O4 nanoparticles addition on the densification and properties of MgO-CaO refractories. Ceram. Int. 2017, 43, 5014–5019. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E. Impact of Titania nanoparticles addition on the microstructure and properties of MgO-C refractories. Ceram. Int. 2017, 43, 15472–15477. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Shahraki, A.; Farooghi, M. Effect of Nano-TiO2 Additions on the Densification and Properties of Magnesite-Dolomite Ceramic Composites. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 567–575. [Google Scholar] [CrossRef]
- Zargar, H.R.; Oprea, C.; Oprea, G.; Troczynski, T. The effect of nano-Cr2O3 on solid-solution assisted sintering of MgO refractories. Ceram. Int. 2012, 38, 6235–6241. [Google Scholar]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Boroujerdnia, M. MgO-CaO-Cr2O3 composition as a novel refractory brick: Use of Cr2O3 nanoparticles. Bol. Soc. Esp. Ceram. V 2017, 56, 83–89. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S. The influence of silica nanoparticles addition on the physical, mechanical, thermo-mechanical as well as microstructure of Mag-Dol refractory composites. Ceram. Int. 2017, 43, 16780–16786. [Google Scholar] [CrossRef]
- Verdeja, L.F.; Sancho, J.P.; Ballester, A.; González, R. Refractory and Ceramic Materials, 1st ed.; Síntesis: Madrid, Spain, 2008. [Google Scholar]
- Gómez-Rodríguez, C.; Fernández-González, D.; García-Quiñonez, L.V.; Castillo-Rodríguez, G.A.; Aguilar-Martínez, J.A.; Verdeja, L.F. MgO Refractory Doped with ZrO2 Nanoparticles: Influence of Cold Isostatic and Uniaxial Pressing and Sintering Temperature in the Physical and Chemical Properties. Metals 2019, 9, 1297. [Google Scholar] [CrossRef]
- Stahleisen, V.; Deutscher, V. Slag Atlas, 2nd ed.; Verlag Stahleisen: Dusseldorf, Germany, 1995. [Google Scholar]
- Rigaud, M.; Palco, S.; Paransky, E. New refractory materials for the copper industry. In Proceedings of the Tehran International Conference on Refractories, Tehran, Iran, 4–6 May 2004; Iran University of Science and Technology: Tehran, Iran, 2004; pp. 277–289. [Google Scholar]
- Paranthaman, M.; David, K.A.; Lindemer, T.B. Phase equilibria of the MgO-Cu2O-CuO system. Mater. Res. Bull. 1997, 32, 165. [Google Scholar] [CrossRef]
- Chen, M.; Lu, C.; Yu, J. Improvement in performance of MgO–CaO refractories by addition of nano-sized ZrO2. J. Eur. Ceram. Soc. 2007, 27, 4633–4638. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Baudín, C.; Pena, P. Relationships between phase constitution and mechanical behaviour in MgO–CaZrO3–calcium silicate materials. J. Eur. Ceram. Soc. 2004, 24, 669–679. [Google Scholar] [CrossRef]
- Rodríguez, E.; Castillo, A.; Contreras, J.; Puente-Ornelas, R.; Aguilar-Martínez, J.A.; García, L.; Gómez, C. Hercynite and magnesium alumi-nate spinels acting as a ceramic bonding in an electrofused MgO–CaZrO3 refractory brick for the cement industry. Ceram. Int. 2012, 38, 6769–6775. [Google Scholar] [CrossRef]
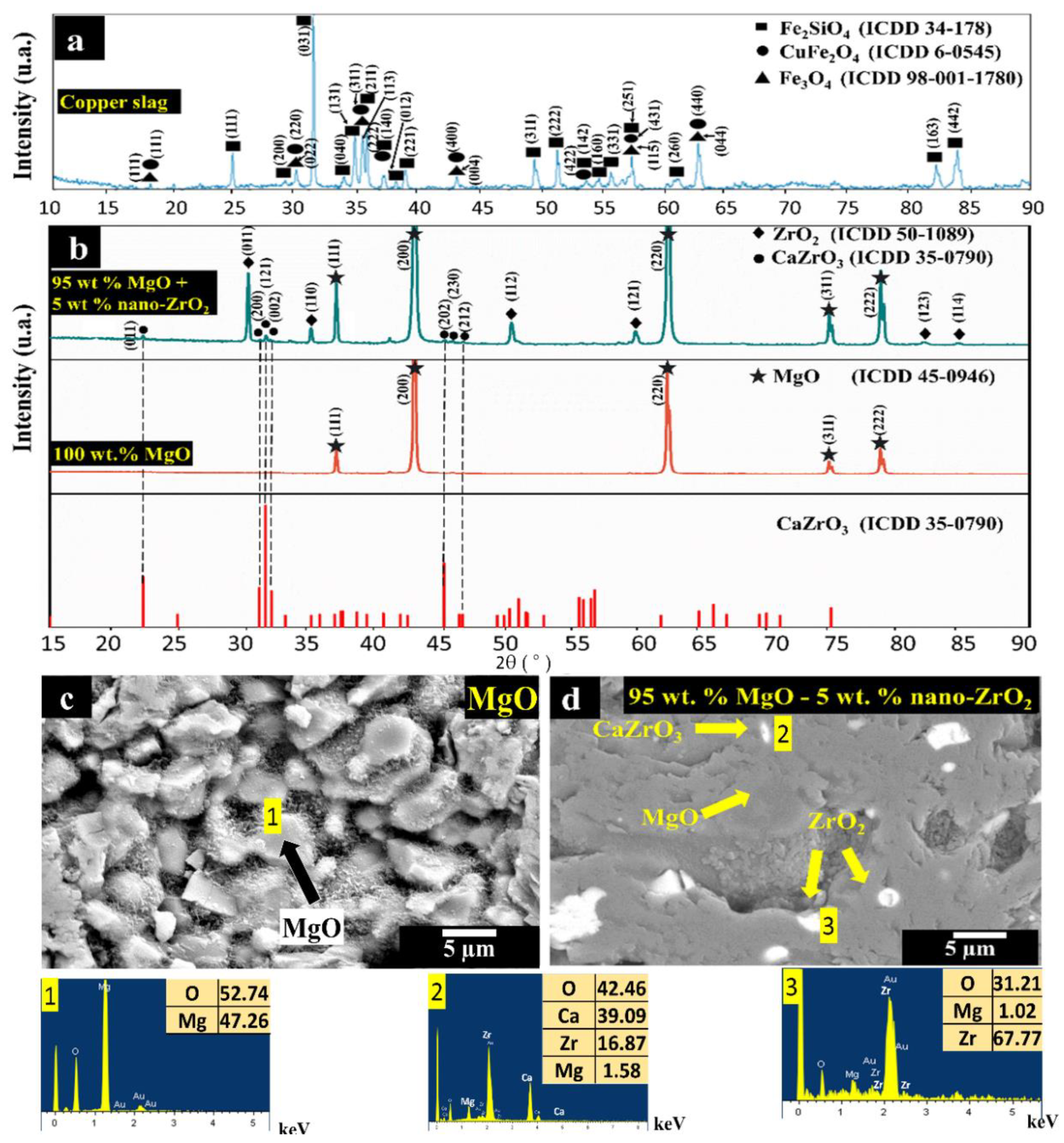
| 1650 °C | |||||
|---|---|---|---|---|---|
| Cold isostatic pressing | Properties | Composition MgO + (x = wt.%) Nano-ZrO2 | * Impurity from MgO, CaO ~ 1.5 | ||
| x = 0 | x = 1 | x = 3 | x = 5 | ||
| Density (g/cm3) [39] | 2.72 | 2.87 | 2.99 | 3.04 | |
| Porosity (%) [39] | 26.24 | 20.56 | 16.43 | 14048 | |
| Cold crushing strenght (MPa) | 119 | 210.07 | 304.72 | 315.78 | |
| Crystallographic phases | MgO X X X | MgO X X X | MgO X X X | MgO X X X | |
| ZrO2 X | ZrO2 X X | ZrO2 X X X | |||
| - | CaZrO3 | CaZrO3 X | CaZrO3 X X | ||
| Copper Slag | |||
|---|---|---|---|
| Element/wt.% | Element/wt.% | Element/wt.% | Element/wt.% |
| Ca/1.756 | Cr/0.02603 | Al/3.239 | Cu/1.84 |
| O/36.15 | Cl/0.02477 | P/0.05097 | Co/0.4598 |
| Fe/42.82 | K/0.6914 | Ti/0.1994 | Mo/0.1796 |
| Si/10.36 | Sr/0.01129 | S/0.2197 | As/0.1535 |
| Mn/0.1327 | Zn/0.2614 | Na/0.993 | Pb/0.1129 |
| Phases (%) | |||
| Fe2SiO4 = 85.80 ± 1.30 | Fe3O4 = 7.90 ± 1.60 | CuFe2O4 = 6.20 ± 1.80 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Rodríguez, C.; Antonio-Zárate, Y.; Revuelta-Acosta, J.; Verdeja, L.F.; Fernández-González, D.; López-Perales, J.F.; Rodríguez-Castellanos, E.A.; García-Quiñonez, L.V.; Castillo-Rodríguez, G.A. Research and Development of Novel Refractory of MgO Doped with ZrO2 Nanoparticles for Copper Slag Resistance. Materials 2021, 14, 2277. https://doi.org/10.3390/ma14092277
Gómez-Rodríguez C, Antonio-Zárate Y, Revuelta-Acosta J, Verdeja LF, Fernández-González D, López-Perales JF, Rodríguez-Castellanos EA, García-Quiñonez LV, Castillo-Rodríguez GA. Research and Development of Novel Refractory of MgO Doped with ZrO2 Nanoparticles for Copper Slag Resistance. Materials. 2021; 14(9):2277. https://doi.org/10.3390/ma14092277
Chicago/Turabian StyleGómez-Rodríguez, Cristian, Yanet Antonio-Zárate, Josept Revuelta-Acosta, Luis Felipe Verdeja, Daniel Fernández-González, Jesús Fernando López-Perales, Edén Amaral Rodríguez-Castellanos, Linda Viviana García-Quiñonez, and Guadalupe Alan Castillo-Rodríguez. 2021. "Research and Development of Novel Refractory of MgO Doped with ZrO2 Nanoparticles for Copper Slag Resistance" Materials 14, no. 9: 2277. https://doi.org/10.3390/ma14092277
APA StyleGómez-Rodríguez, C., Antonio-Zárate, Y., Revuelta-Acosta, J., Verdeja, L. F., Fernández-González, D., López-Perales, J. F., Rodríguez-Castellanos, E. A., García-Quiñonez, L. V., & Castillo-Rodríguez, G. A. (2021). Research and Development of Novel Refractory of MgO Doped with ZrO2 Nanoparticles for Copper Slag Resistance. Materials, 14(9), 2277. https://doi.org/10.3390/ma14092277











