Visible Light-Cured Antibacterial Collagen Hydrogel Containing Water-Solubilized Triclosan for Improved Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
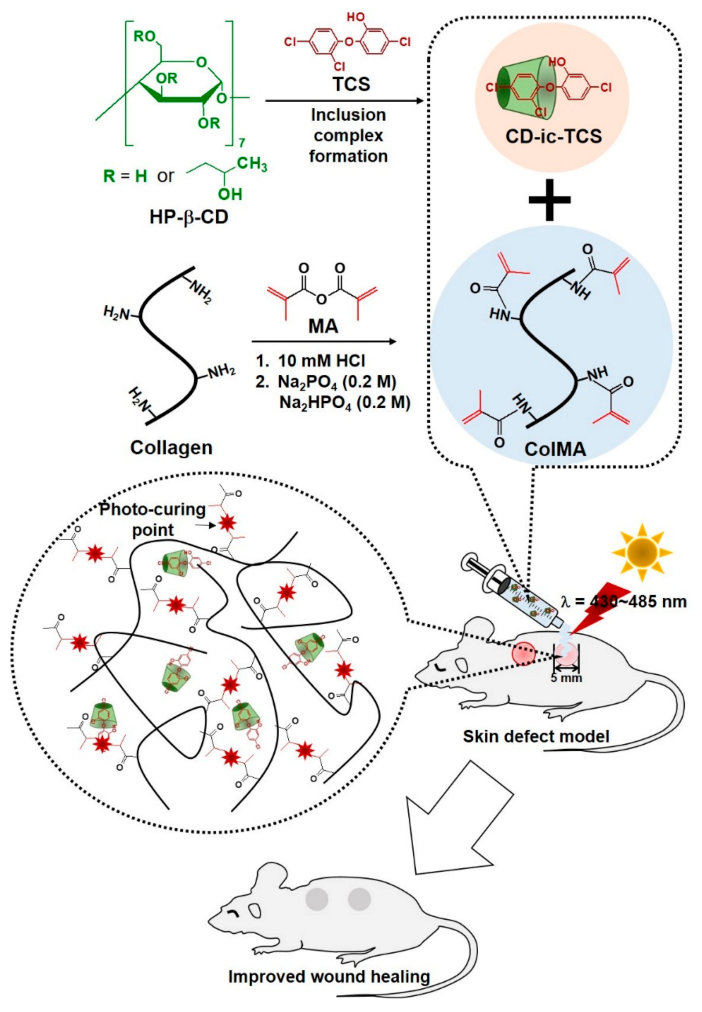
2.2. Preparation of Methacrylated Collagen (ColMA) Derivative
2.3. Inclusion Complex between 2-Hydroxypropyl-Beta-Cyclodextrin and Triclosan (CD-ic-TCS)
2.4. Preparation of CD-ic-TCS-Loaded ColMA Hydrogel (ColMA/CD-ic-TCS)
2.5. In Vitro Release Test
2.6. In Vitro Biocompatibility
2.7. Antibacterial Assay
2.8. In Vivo Animal Testing
2.9. Histological Evaluation
2.10. Statistical Analysis
3. Results
3.1. 1H NMR Spectra of CD-ic-TCS and ColMA
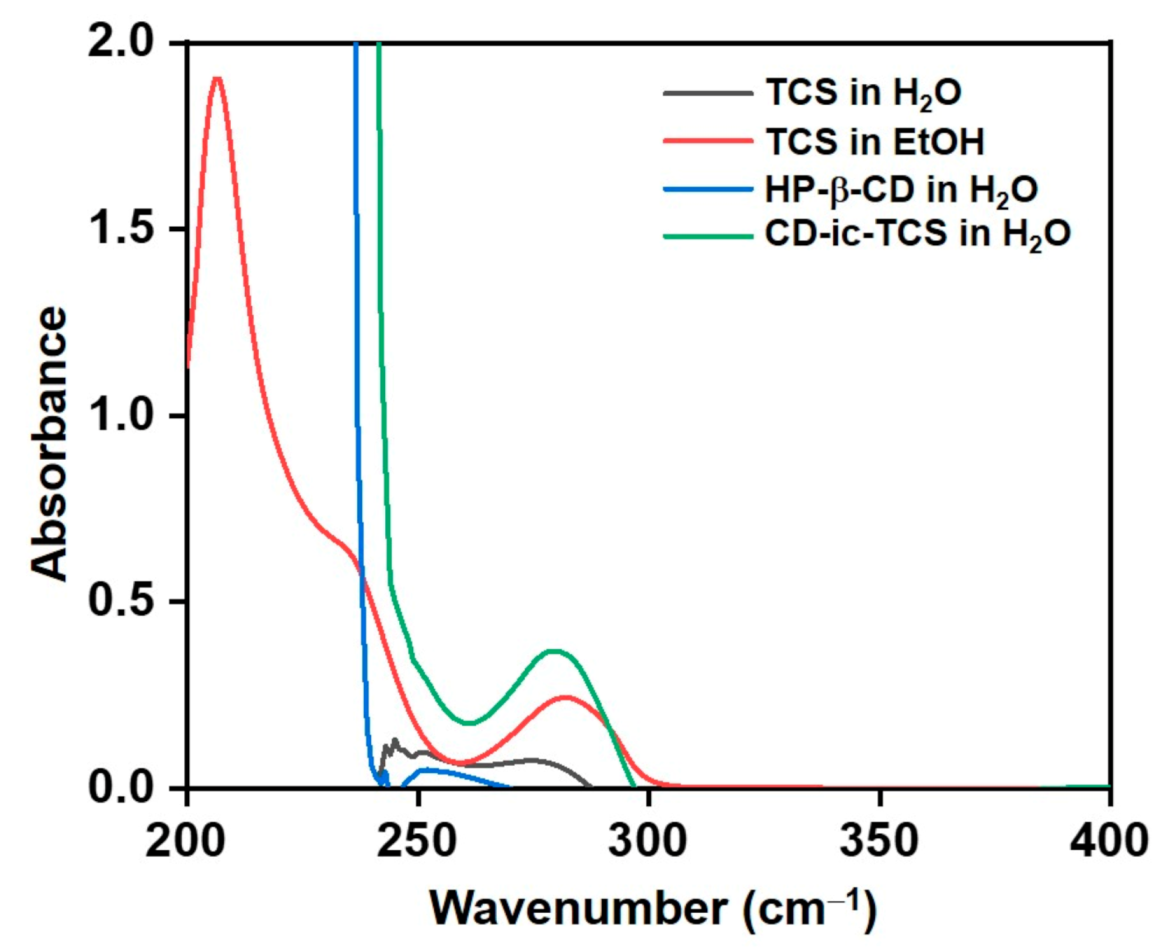
3.2. UV Spectrum of CD-ic-TCS
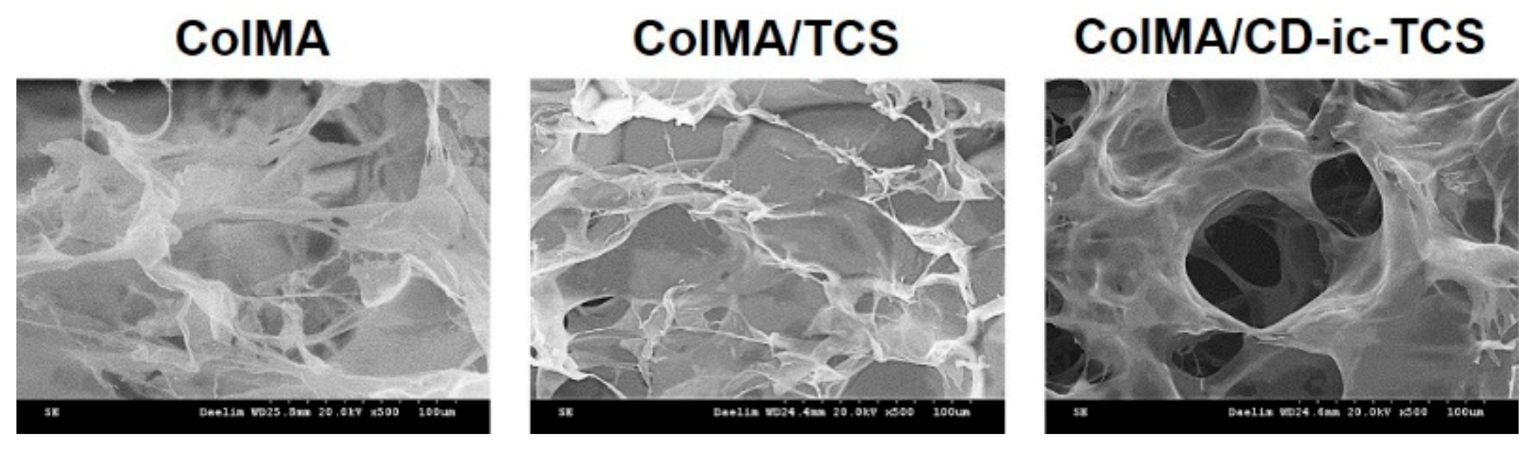
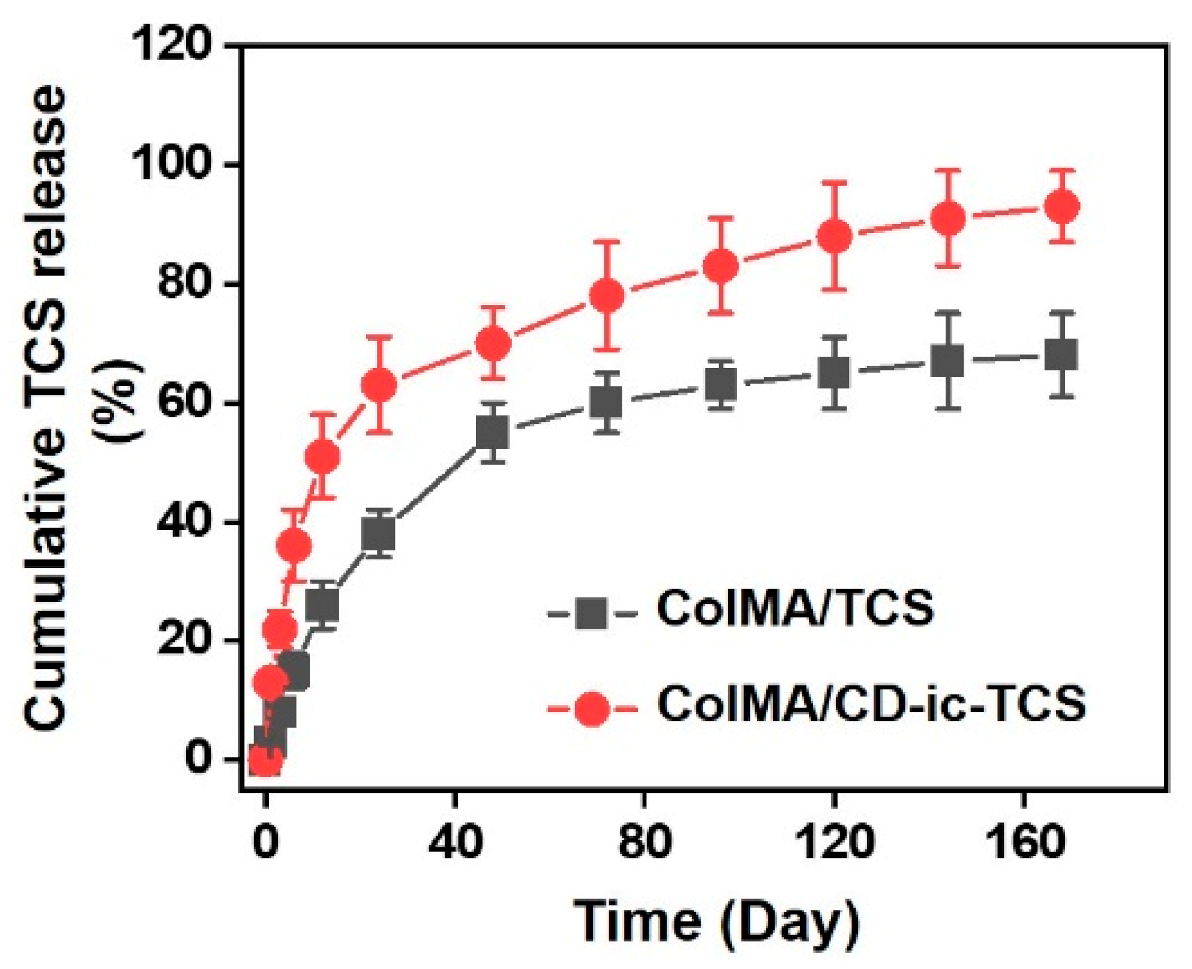
3.3. SEM Images, TCS Release, and Cell Proliferation
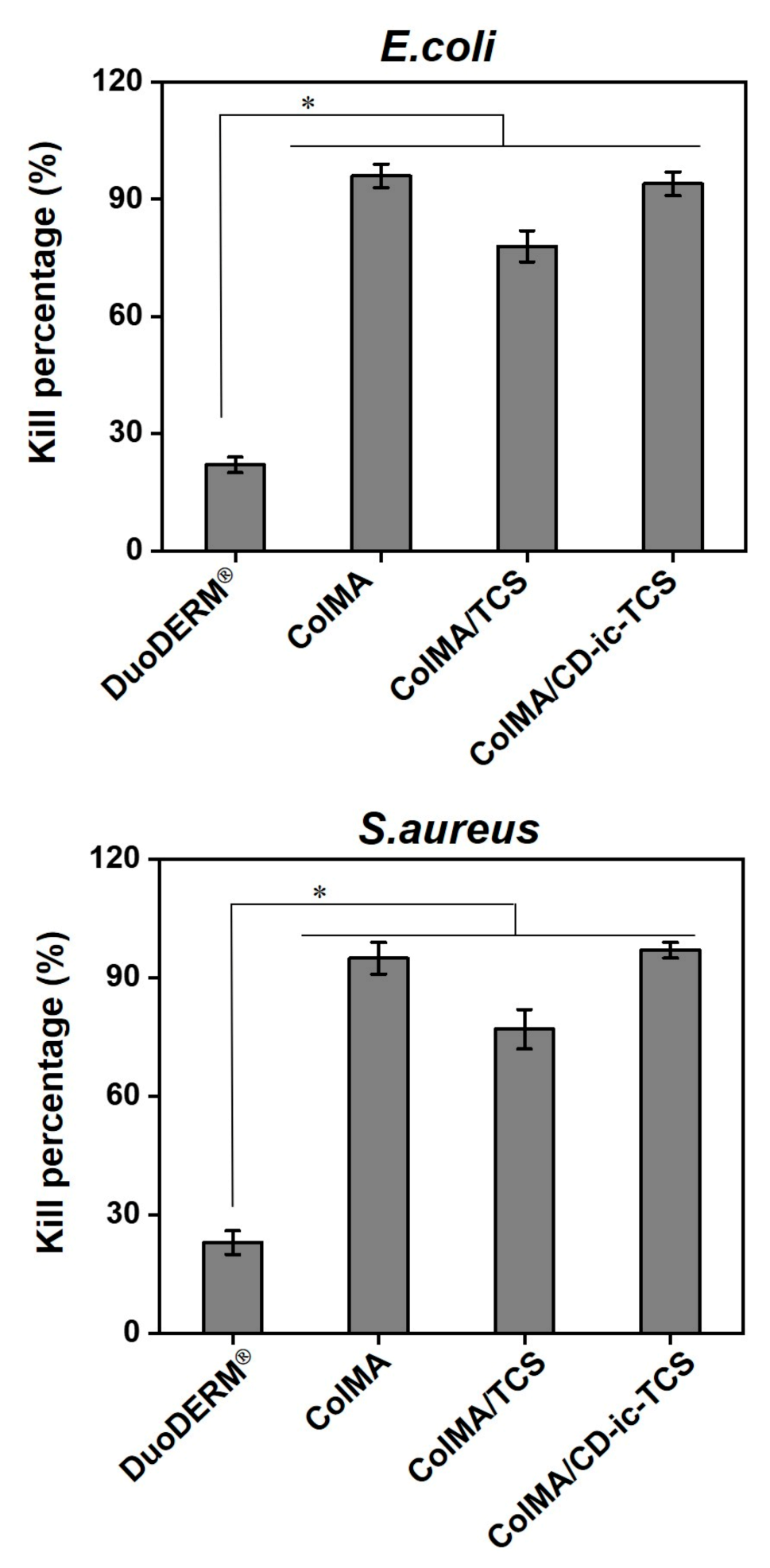
3.4. Antibacterial Activity
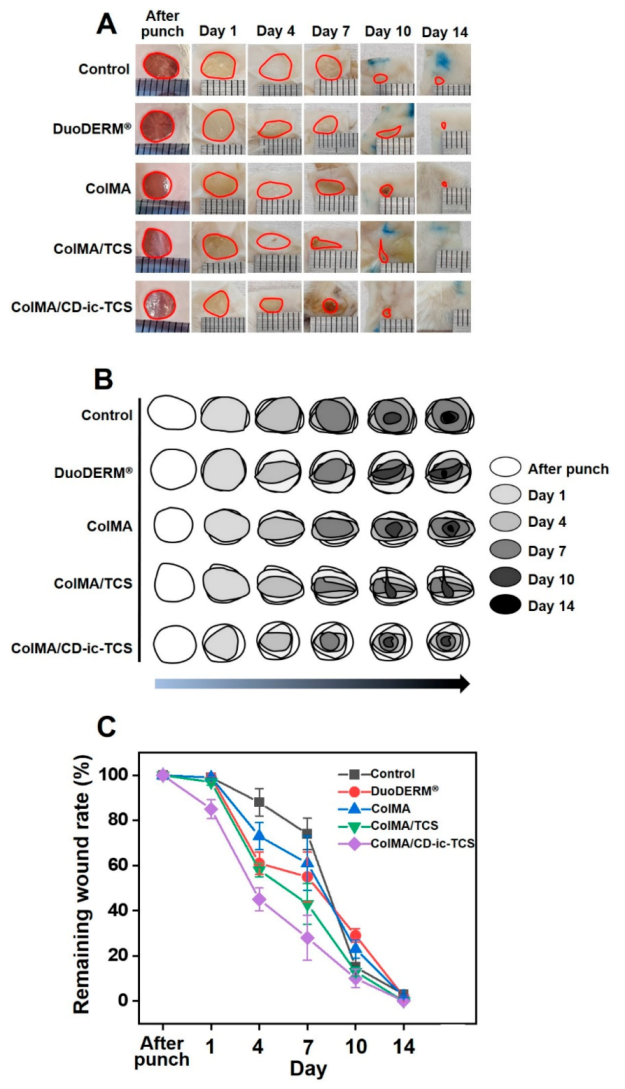
3.5. Gross Appearances and Wound Healing
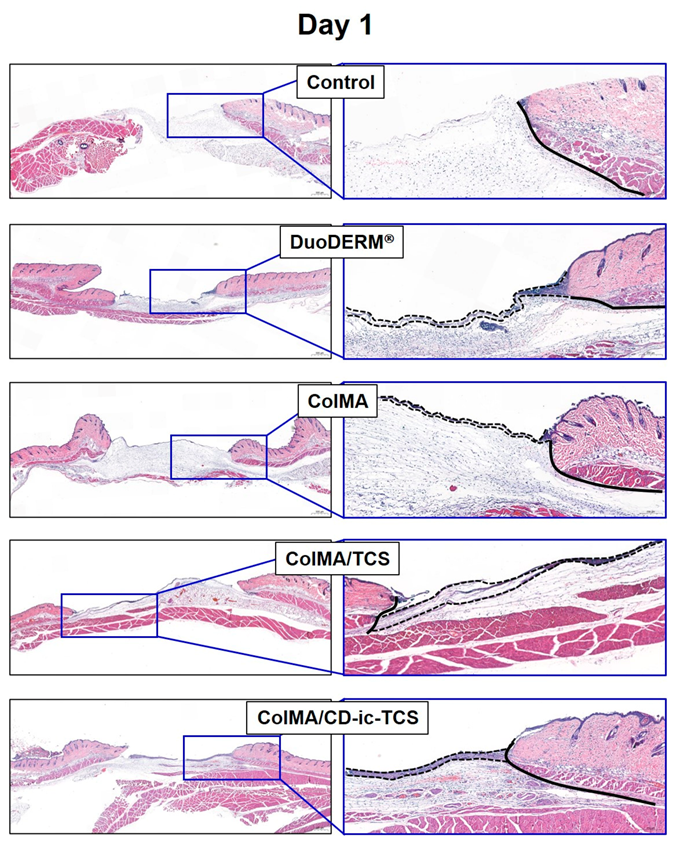
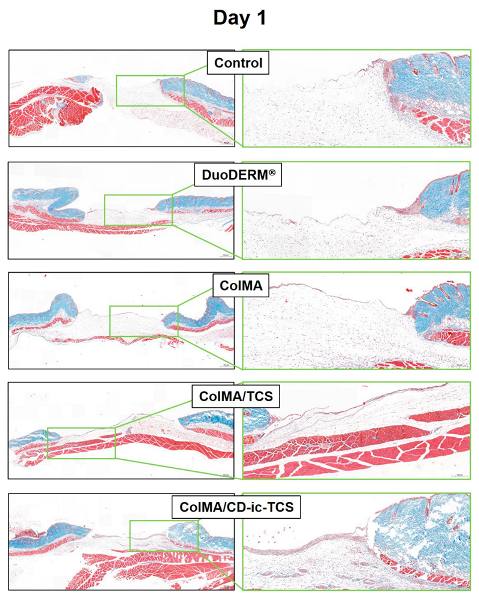
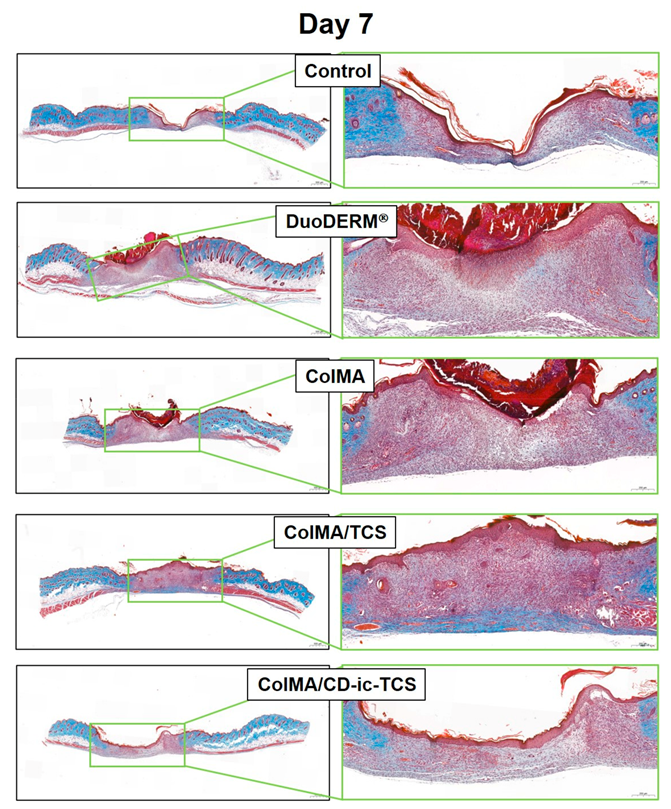
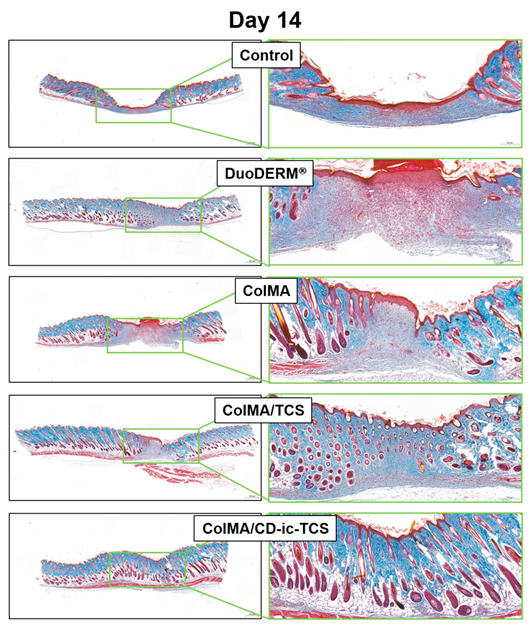
3.6. Histological Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Cutting, K.F. Wound dressings: 21st century performance requirements. J. Wound Care 2010, 19, 4–9. [Google Scholar] [CrossRef]
- Yang, D.H.; Seo, D.I.; Lee, D.-W.; Bhang, S.H.; Park, K.; Jang, G.; Kim, C.H.; Chun, H.J. Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J. Eng. Ind. Chem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Basha, S.I.; Ghosh, S.; Vinothkumar, K.; Ramehs, B.; Hema Praksh Kumari, P.; Murali Mohan, K.V.; Sukumar, E. Fumaric acid incorporated Ag/agar-agar hybrid hyudrogel: A multifunctional avenue to tackle wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110743. [Google Scholar] [CrossRef]
- Mele, A.; Castiglione, F.; Malpezzi, L.; Ganazzoli, F.; Raffaini, G.; Trotta, F.; Rossi, B.; Fontana, A.; Giunchi, G. HR MAS NMR, powder XRD and Raman spectroscopy study of inclusion phenomena in bCD nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 403–409. [Google Scholar] [CrossRef]
- Crupi, V.; Majolino, D.; Mele, A.; Rossi, B.; Trotta, F.; Venuti, V. Modelling the interplay between covalent and physical interactions in cyclodextrin-based hydrogel: Effect of water confinement. Soft Matter 2013, 9, 6457. [Google Scholar] [CrossRef]
- Singh, G.; Lohani, A.; Bhattacharya, S.S. Hydrogel as a novel drug delivery system: A review. J. Fundam. Pharm. Res. 2014, 2, 35–48. [Google Scholar]
- Brandl, F.; Sommer, F.; Goepferich, A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials 2007, 28, 134–146. [Google Scholar] [CrossRef]
- Doillon, C.J.; Sliver, F.H. Collagen-based wound dressing: Effects of hyaluronic acid and firponectin on wound healing. Biomaterials 1986, 7, 3–8. [Google Scholar] [CrossRef]
- Wiegand, C.; Buhren, B.A.; Bünemann, E.; Schrumpf, H.; Homey, B.; Frykberge, R.G.; Lurie, F.; Gerber, P.A. A novel native collagen dressing with advantageous properties to promote physiological wound healing. J. Wound Care 2016, 25, 713–720. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Wei, M. Synthesis and characterization of a novel injectable alginate-collagen-hydroxyapatite hydrogel for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Xu, H.; Mu, B.; Xie, K.; Yang, Y. Non-toxic and clean crosslinking system for protein materials: Effect of extenders on crosslinking performance. J. Clean. Prod. 2017, 150, 214–223. [Google Scholar] [CrossRef]
- Choi, J.R.; Young, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.J.G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-cured glycol chitosan hydrogel for ovarian cancer drug delivery. Mar. Drug 2019, 17, 41–52. [Google Scholar] [CrossRef]
- Yoon, S.J.; Moon, Y.J.; Chun, H.J.; Yang, D.H. Doxorubicin⋅hydrochloride/cisplatin-loaded hydrogel/nanosized (2-hydroxypropyl)-beta-cyclodextrin local drug-delivery system for osteosarcoma treatment in vivo. Nanomaterials 2019, 9, 1652–1663. [Google Scholar] [CrossRef]
- Yoo, Y.; Yoon, S.J.; Kim, S.Y.; Lee, D.W.; Um, S.; Hyun, H.; Hong, S.O.; Yang, D.H. A local drug delivery system based on visible light-cured glycol chitosan and doxorubicin⋅hydrochloride for thyroid cancer treatment in vitro and in vivo. Drug Deliv. 2018, 25, 1664–1671. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Lim, W.; Kim, S.Y.; Jo, D.; Jung, J.S.; Jo, G.; Um, S.; Lee, D.W.; Yang, D.H. Injectable visible light-cured glycol chitosan hydrogels with controlled release of anticancer drugs for local cancer therapy in vivo: A feasible study. Artif. Cells Nanomed. Biotech. 2018, 46, S874–S882. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mat. Today 2011, 14, 88–95. [Google Scholar]
- Yoo, Y.; Hyun, H.; Yoon, S.-J.; Kim, S.Y.; Lee, D.-W.; Um, S.; Hong, S.O.; Yang, D.H. Visible light-cured glycol chitosan hydrogel dressing containing endothelial growth factor and basic fibroblast growth factor accelerates wound healing in vivo. J. Ind. Eng. Chem. 2018, 67, 365–372. [Google Scholar] [CrossRef]
- Jones, R.D.; Jampani, H.B.; Newman, J.L.; Lee, A.S. Triclosan: A review of effectiveness and safety in health care settings. Am. J. Infect. Control 2000, 28, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, W.T.; Nagapudi, K.; Thomas, B.S.; Chaikof, E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules 2003, 3, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Fagui, A.E.; Dubot, P.; Loftsson, T.; Amiel, C. Triclosan-loaded with high encapsulation efficiency into PLA nanoparticles coatged with β-cyclodextrin polymer. J. Incl. Phenom. Macro. Chem. 2013, 75, 277–283. [Google Scholar] [CrossRef]
- Moon, Y.J.; Yoon, S.J.; Koo, J.H.; Yoo, Y.; Byun, H.J.; Kim, H.S.; Khang, G.; Chun, H.J.; Yang, D.H. β-Cyclodextrin/triclosan complex-grafted methacrylated glycol chitosan hydrogel by photocrosslinking via visible light irradiation for a tissue bio-adhesive. Int. J. Mol. Sci. 2021, 22, 700. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Maniyazagan, M.; Chakraborty, S.; Pérez-Sánchez, H.; Stalin, T. Encapsulation of triclosan within 2-hydroxypropyl-β-cyclodextrin cavity and its application in the chemisorption of rhodamine B dye. J. Mol. Liq. 2019, 282, 235–243. [Google Scholar] [CrossRef]
- Kacsó, I.; Borodi, G.; Farcas, S.I.; Hernanz, A.; Bratu, I. Host-guest system of vitamin B10 in β-cyclodextrin: Characterization of the interaction in soluytion and in solid state. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 175–182. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Rains, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Lei, J.; Chen, P.; Li, Y.; Wang, X.; Tang, S. Collagen hydrogel dressing for wound healing and angiogenesis in diabetic rat models. Int. J. Clin. Exp. Med. 2017, 10, 16319–16327. [Google Scholar]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Hyun, H.; Lee, D.-W.; Yang, D.H. Visible light-cured glycol chitosan hydrogel containing a beta-cyclodextrin-curcumin inclusion complex improves wound healing in vivo. Molecules 2017, 22, 1513. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mat. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Bai, Z.; Dan, W.; Yu, G.; Wang, Y.; Chen, Y.; Huang, Y.; Yang, C.; Dan, N. Tough and tissue-adhesive polyacrylamide/collagen hydrogel with dopamine-grafted oxidized sodium alginate as crosslinker for cutaneous wound healing. RSC Adv. 2018, 8, 42123–42132. [Google Scholar] [CrossRef]
- Nguyen, T.U.; Watkins, K.E.; Kishore, V. Photochemically crosslinked cell-laden methacrylated collagen hydrogels with high cell viability and functionality. J. Biomed. Mater. Res. A 2019, 107, 1541–1550. [Google Scholar] [CrossRef]
- Peila, R.; Vineis, C.; Varesano, A.; Ferri, A. Different methods for β-cyclodextrin/triclosan complexation as antibacterial treatment of cellulose substrates. Cellulose 2013, 20, 2115–2123. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Park, K.; Yoon, Y.; Kim, H.S.; Kim, H.J.; Choi, J.W.; Lee, D.Y.; Chun, H.J.; Yang, D.H. Visible Light-Cured Antibacterial Collagen Hydrogel Containing Water-Solubilized Triclosan for Improved Wound Healing. Materials 2021, 14, 2270. https://doi.org/10.3390/ma14092270
Jin L, Park K, Yoon Y, Kim HS, Kim HJ, Choi JW, Lee DY, Chun HJ, Yang DH. Visible Light-Cured Antibacterial Collagen Hydrogel Containing Water-Solubilized Triclosan for Improved Wound Healing. Materials. 2021; 14(9):2270. https://doi.org/10.3390/ma14092270
Chicago/Turabian StyleJin, Longhao, Kyeongsoon Park, Yihyun Yoon, Hyeon Soo Kim, Hyeon Ji Kim, Jae Won Choi, Deuk Yong Lee, Heung Jae Chun, and Dae Hyeok Yang. 2021. "Visible Light-Cured Antibacterial Collagen Hydrogel Containing Water-Solubilized Triclosan for Improved Wound Healing" Materials 14, no. 9: 2270. https://doi.org/10.3390/ma14092270
APA StyleJin, L., Park, K., Yoon, Y., Kim, H. S., Kim, H. J., Choi, J. W., Lee, D. Y., Chun, H. J., & Yang, D. H. (2021). Visible Light-Cured Antibacterial Collagen Hydrogel Containing Water-Solubilized Triclosan for Improved Wound Healing. Materials, 14(9), 2270. https://doi.org/10.3390/ma14092270








