Hydrothermal Crystallization of Bismuth Oxychlorides (BiOCl) Using Different Shape Control Reagents
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization Methods
2.2.1. Diffuse Reflectance Spectroscopy (DRS)
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Powder X-ray Diffractometry (XRD)
2.2.4. Specific Surface Area
2.2.5. XPS Measurements
2.2.6. Raman Spectroscopy
2.2.7. Surface Tension
2.3. Evaluation of Photocatalytic Efficiency
3. Results and Discussion
3.1. Evaluation of Crystal Phase Composition, Primary Crystallite Size and Specific Surface Area
3.2. Morphological Characterization
- Oriented growth of crystals by blocking a crystallographic direction through surface anchoring.
- Aggregation state of the primary crystallites, the possibility of Ostwald ripening
- Hierarchical particle size and specific surface area tuning, by non-selective surface adsorption
3.3. Assessment of the Band Gap Energy
3.4. Photocatalytic Efficiencies of the Obtained BiOX Materials
3.5. The Reason behind the Photocatalytic Activity of the Samples
3.5.1. The Effect of the Surface Tension
3.5.2. Evaluation of the Raman spectra
3.5.3. XPS Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Liu, L.; Zhou, Z. Towards Better Photocatalysts: First-principles Studies of the Alloying Effects on the Photocatalytic Activities of Bismuth Oxyhalides under Visible Light. Phys. Chem. Chem. Phys. 2012, 14, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Vajda, K.; Kasa, Z.; Dombi, A.; Nemeth, Z.; Kovacs, G.; Danciu, V.; Radu, T.; Ghica, C.; Baia, L.; Hernadi, K.; et al. “Crystallographic” Holes: New Insights for a Beneficial Structural Feature for Photocatalytic Applications. Nanoscale 2015, 7, 5776–5786. [Google Scholar] [CrossRef] [PubMed]
- Sujuan, W.; Jiawei, X.; Jianguo, S.; Zachary, D.H.; Wen, Z.; Zhenzhong, Y.; Lin, G.; Xixiang, Z.; Yang, S. Hydroxyl-dependent Evolution of Oxygen Vacancies Enables the Regeneration of BiOCl Photocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 16620–16626. [Google Scholar]
- Zou, Z.; Xu, H.; Li, D.; Sun, J.; Xia, D. Facile Preparation and Photocatalytic Activity of Oxygen Vacancy Rich BiOCl with {001} Exposed Reactive Facets. App. Sur. Sci. 2019, 463, 1011–1018. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Liu, G.; Zhang, C.; Liu, G.; Xu, S.; Cui, P.; Li, D. Rapid Synthesis of BiOCl Graded Microspheres with Highly Exposed (110) Facets and Oxygen Vacancies at Room Temperature to Enhance Visible Light Photocatalytic Activity. Catal. Commun. 2019, 130, 105769. [Google Scholar] [CrossRef]
- Kedves, E.-Z.; Pap, Z.; Hernádi, K.; Baia, L. Significance of the Surface and Bulk Features of Hierarchical TiO2 in Their Photocatalytic Properties. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Vajda, K.; Saszet, K.; Kedves, E.Z.; Kása, Z.; Danciu, V.; Baia, L.; Magyari, K.; Hernádi, K.; Kovács, G.; Pap, Z. Shape-controlled Agglomeration of TiO2 Nanoparticles. New Insights on Polycrystallinity vs. Single Crystals in Photocatalysis. Ceram. Int. 2016, 42, 3077–3087. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Q.; Ma, L.; Liu, X.; Zhou, C.; Yang, C.; Tian, T.; Tian, X. Hierarchical BiOCl Hollow Microspheres Assembled by Ultrathin Nanosheets with Large Surface Area for the Exceptional Visible Light Photocatalytic Activity. J. Nanosci. Nanotechnol. 2017, 17, 6328–6336. [Google Scholar] [CrossRef]
- Cao, F.; Wang, Y.A.; Wang, J.M.; Lv, X.; Liu, D.Y.; Ren, J.; Zhou, J.; Deng, R.P.; Li, S.; Qin, G.W. Oxygen Vacancy Induced Superior Visible-light-driven Photodegradation Pollutant Performance in BiOCl Microflowers. New J. Chem. 2018, 42, 3614–3618. [Google Scholar] [CrossRef]
- Sun, J.; Cai, Y.; Xu, H.; Zou, Z.; Hu, M.; Jin, X.; Sun, L.; Li, D.; Xia, D. Synthesis of Porous BiOCl Nanocubes with Enhanced Visible Light Photocatalytic Performance. Chem. Phys. Lett. 2018, 711, 207–212. [Google Scholar] [CrossRef]
- Tian, J.; Chen, Z.; Deng, X.; Sun, Q.; Sun, Z.; Li, W. Improving Visible Light Driving Degradation of Norfloxacin over Core-shell Hierarchical BiOCl Microspherical Photocatalyst by Synergistic Effect of Oxygen Vacancy and Nanostructure. App. Sur. Sci. 2018, 453, 373–382. [Google Scholar] [CrossRef]
- Garg, S.; Yadav, M.; Chandra, A.; Gahlawat, S.; Ingole, P.P.; Pap, Z.; Hernadi, K. Plant Leaf Extracts as Photocatalytic Activity Tailoring Agents for BiOCl Towards Environmental Remediation. Ecotoxicol. Environ. Saf. 2018, 165, 357–366. [Google Scholar] [CrossRef]
- Ye, L.; Zan, L.; Tian, L.; Peng, T.; Zhang, J. The {001} Facets-dependent High Photoactivity of BiOCl Nanosheets. Chem. Commun. 2011, 47, 6951–6953. [Google Scholar] [CrossRef]
- Cui, Z.; Mi, L.; Zeng, D. Oriented Attachment Growth of BiOCl Nanosheets with Exposed {110} Facets and Photocatalytic Activity of the Hierarchical Nanostructures. J. Alloys Compd. 2013, 549, 70–76. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Thongtem, S.; Thongtem, T. Characterization of BiOCl Nanoplates Synthesized by PVP-assisted Hydrothermal Method and Their Photocatalytic Activities. Appl. Phys. A 2020, 126. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, D.; Pu, X.; Li, M.; Yu, Y.M.; Shim, J.J.; Cai, P.; Kim, S.I.; Seo, H.J.; Johnson, D. Combustion Synthesis of BiOCl with Tunable Percentage of Exposed {001} Facets and Enhanced Photocatalytic Properties. J. Am. Ceram. Soc. 2015, 98, 1515–1519. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Figlarz, M. Preparing Monodisperse Metal Powders in Micrometer and Submicrometer Sizes by the Polyol Process. MRS Bull. 2013, 14, 29–34. [Google Scholar] [CrossRef]
- Yu, S.-H. A Solvothermal Decomposition Process for Fabrication and Particle Sizes Control of Bi2S3 Nanowires. J. Mater. Res. 1999, 14, 4157. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, X.; Zhang, D.; Chen, S.; Guo, X.; Ding, W.; Chen, Y. Hollow Microscale Organization of Bi2S3nanorods. Nanotechnology 2006, 17, 3806–3811. [Google Scholar] [CrossRef]
- Bárdos, E.; Márta, V.; Baia, L.; Todea, M.; Kovács, G.; Baán, K.; Garg, S.; Pap, Z.; Hernadi, K. Hydrothermal Crystallization of Bismuth Oxybromide (BiOBr) in the Presence of Different Shape Controlling Agents. App. Sur. Sci. 2020, 518. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Jenkins, R.; Snyder, R.L. Introduction to X-Ray Powder Diffractometry; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Zhao, L.; Zhang, X.; Fan, C.; Liang, Z.; Han, P. First-principles Study on the Structural, Electronic and Optical Properties of BiOX (X = Cl, Br, I) Crystals. Phys. B Condens. Matter. 2012, 407, 3364–3370. [Google Scholar] [CrossRef]
- Huang, W.L.; Zhu, Q. DFT Calculations on the Electronic Structures of BiOX (X = F, Cl, Br, I) Photocatalysts with and without Semicore Bi 5d States. J. Comput. Chem. 2009, 30, 183–190. [Google Scholar] [CrossRef]
- Huang, W.L. Electronic Structures and Optical Properties of BiOX (X = F, Cl, Br, I) via DFT Calculations. J. Comput. Chem. 2009, 30, 1882–1891. [Google Scholar] [CrossRef]
- Wu, T.X.; Liu, G.M.; Zhao, J.C.; Hidaka, H.; Serpone, N. Photoassisted Degradation of Dye Pollutants. V. Self-photosensitized Oxidative Transformation of Rhodamine B under Visible Light Irradiation in Aqueous TiO2 Dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Du, D.; Li, W.; Chen, S.; Yan, T.; You, J.; Kong, D. Synergistic Degradation of Rhodamine B on BiOClxBr1−xsheets by Combined Photosensitization and Photocatalysis under Visible Light Irradiation. New J. Chem. 2015, 39, 3129–3136. [Google Scholar] [CrossRef]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, M.; Wojtyła, S.; Macyk, W. On Oxygen Activation at Rutile- and Anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Saqlain, M.A.; Hussain, A.; Siddiq, M.; Leitão, A.A. A DFT+U Study of the Mars Van Krevelen Mechanism of CO Oxidation on Au/TiO2 Catalysts. Appl. Catal. A Gen. 2016, 519, 27–33. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, B.-H.; Yang, K.S. TiO2 Nanoparticles Loaded on Graphene/Carbon Composite Nanofibers by Electrospinning for Increased Photocatalysis. Carbon 2012, 50, 2472–2481. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, Y.; Dong, F.; Wu, F.; Li, B.; Zhang, Q.; Zhou, Y. The Activation of Oxygen through Oxygen Vacancies in BiOCl/PPy to Inhibit Toxic Intermediates and Enhance the Activity of Photocatalytic Nitric Oxide Removal. Nanoscale 2019, 11, 6360–6367. [Google Scholar] [CrossRef]
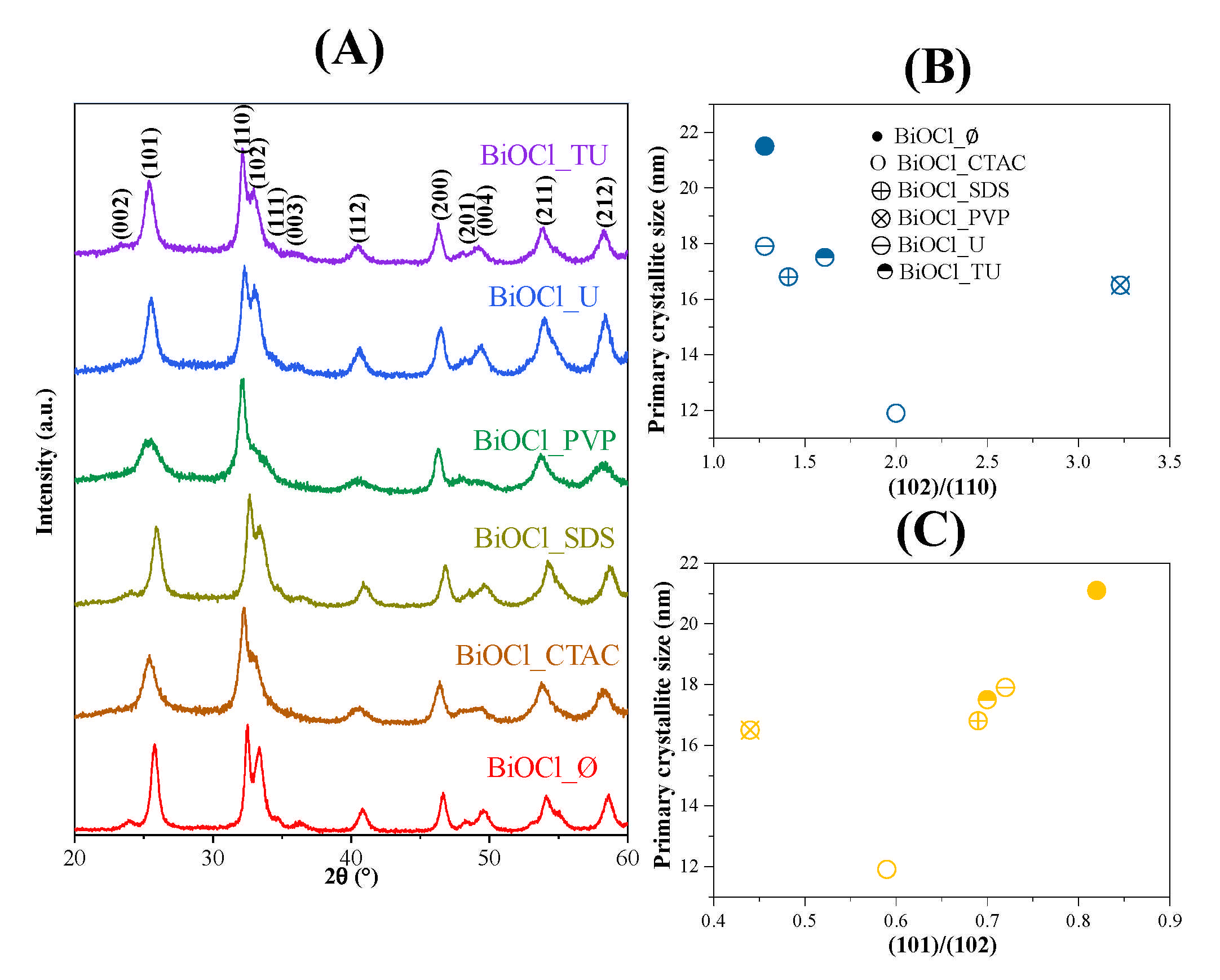
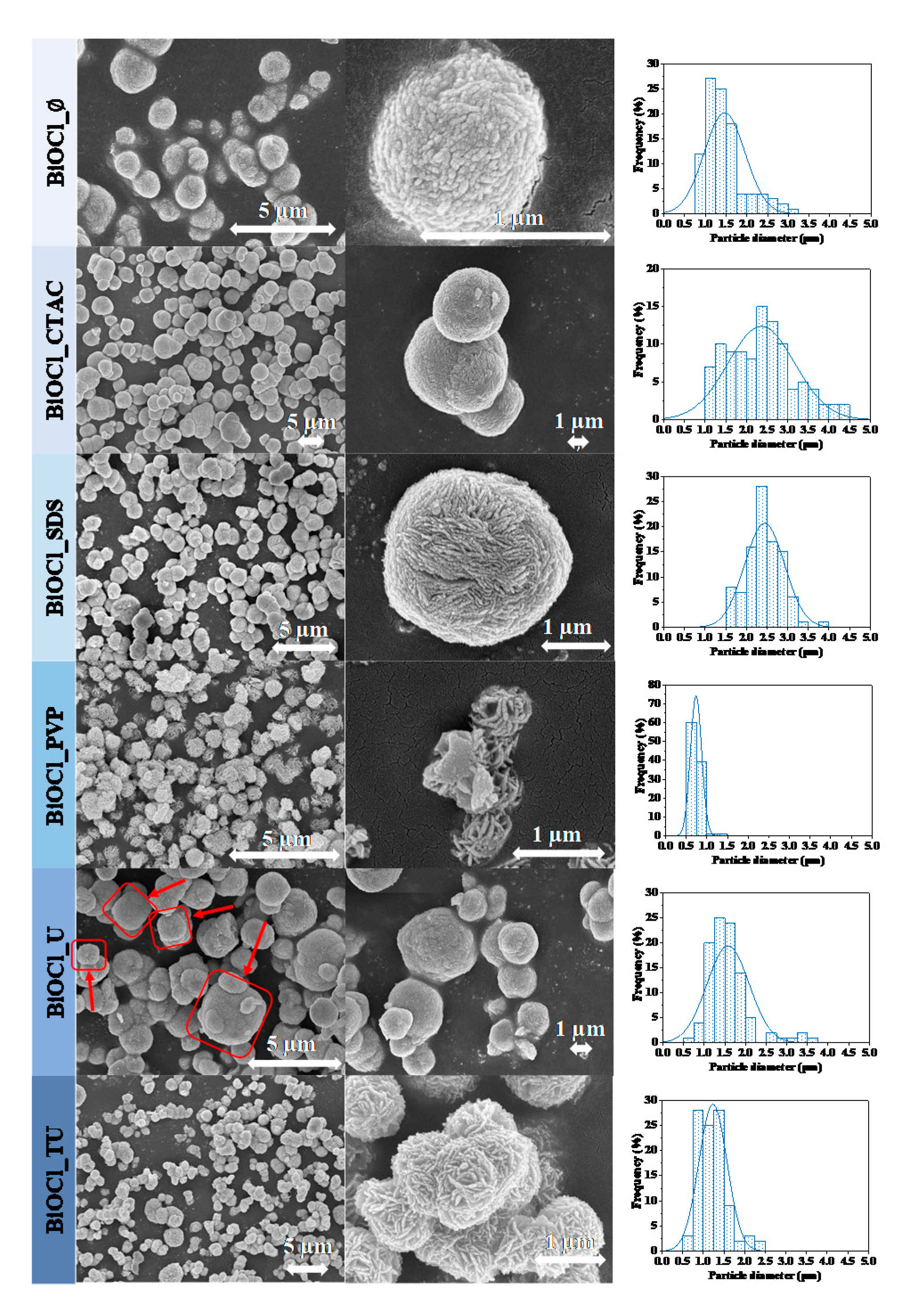
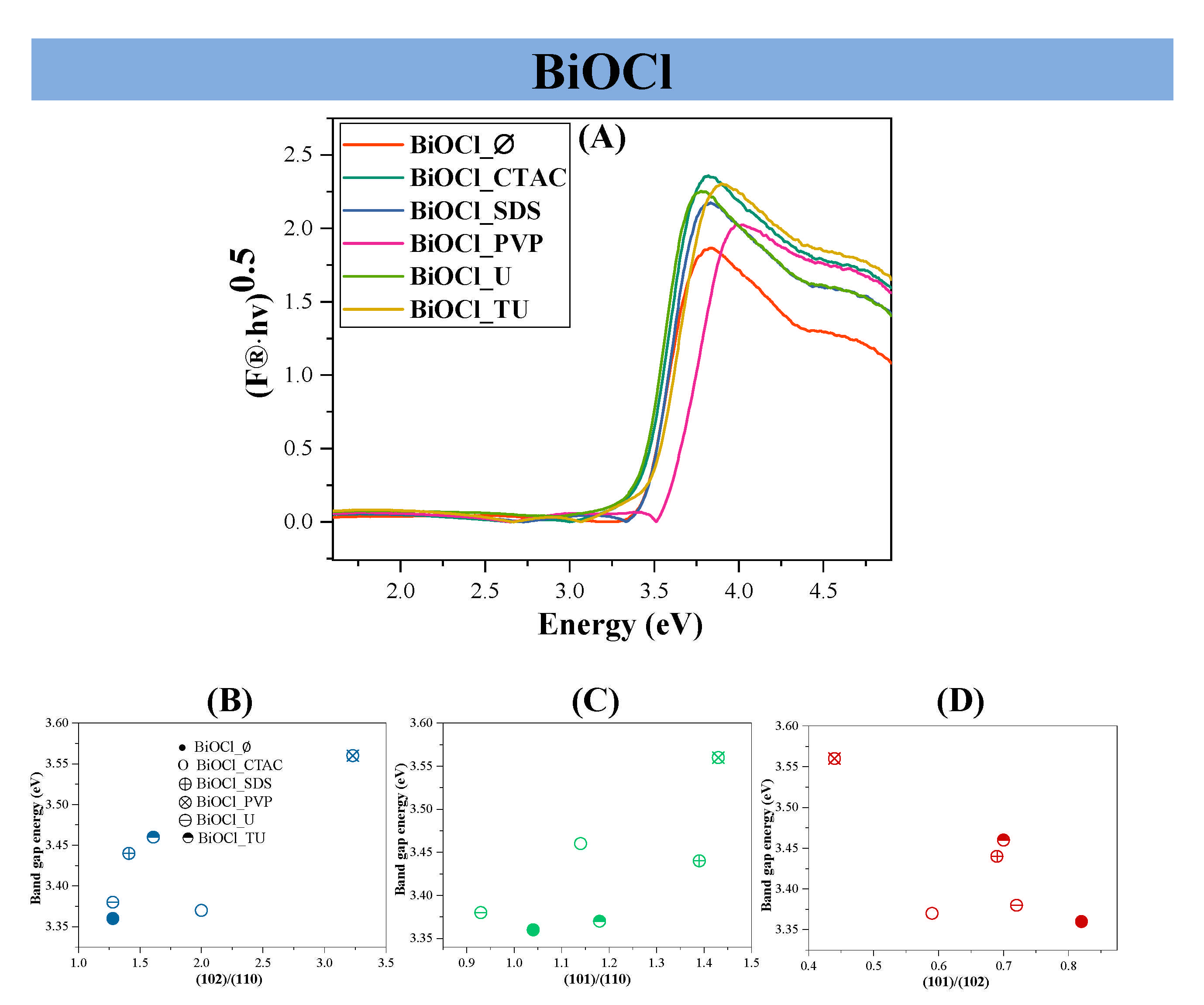
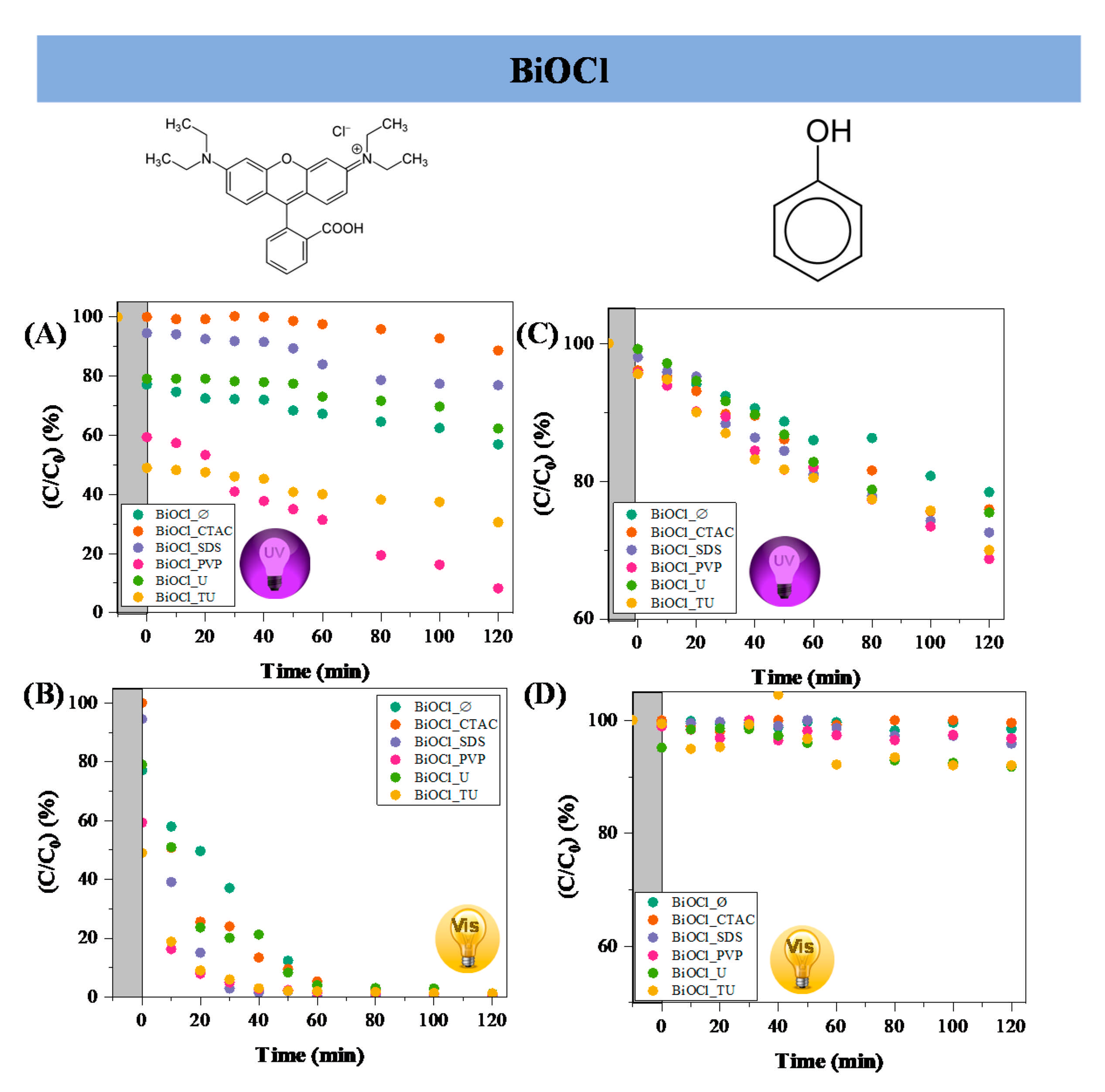
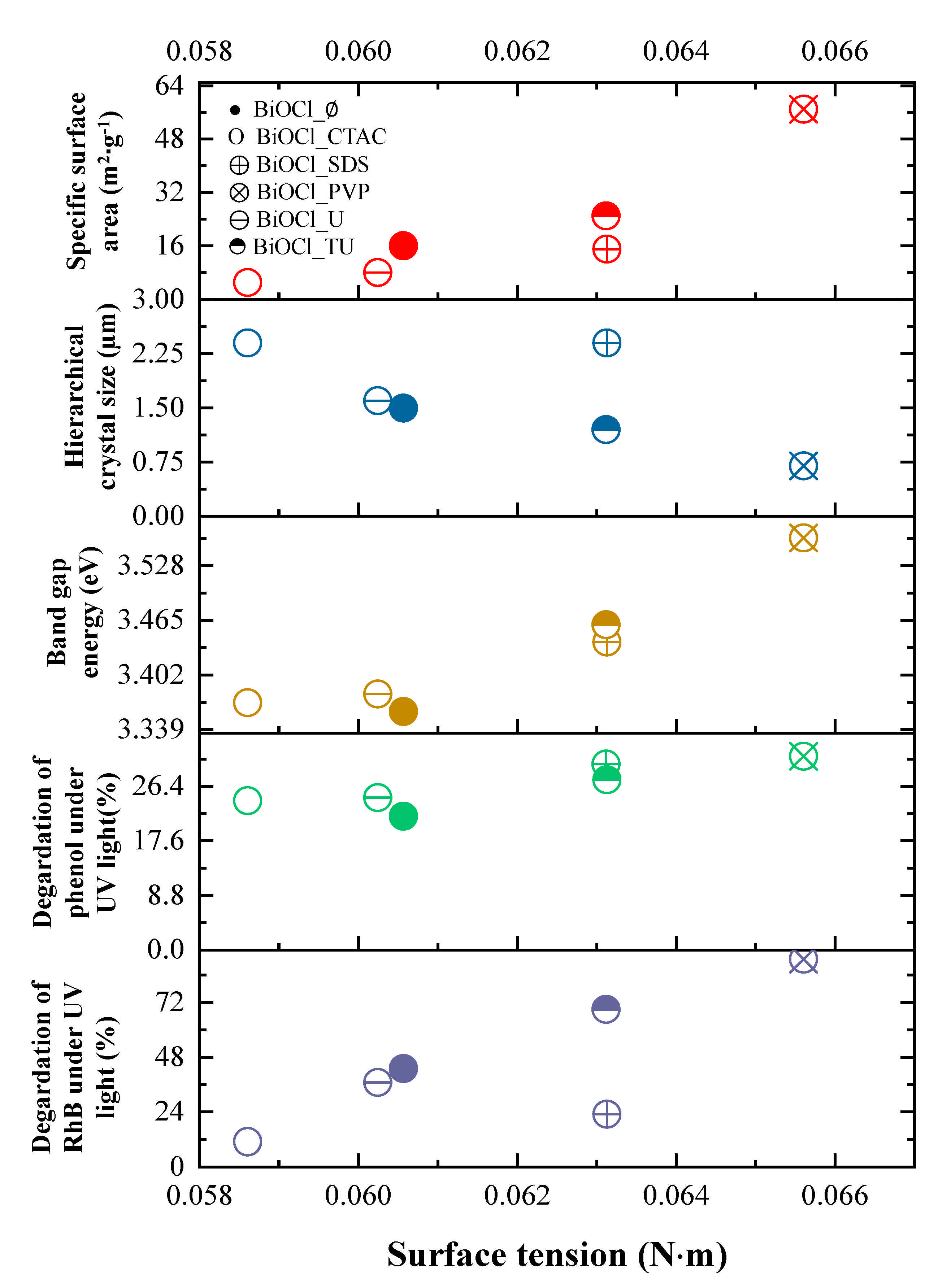
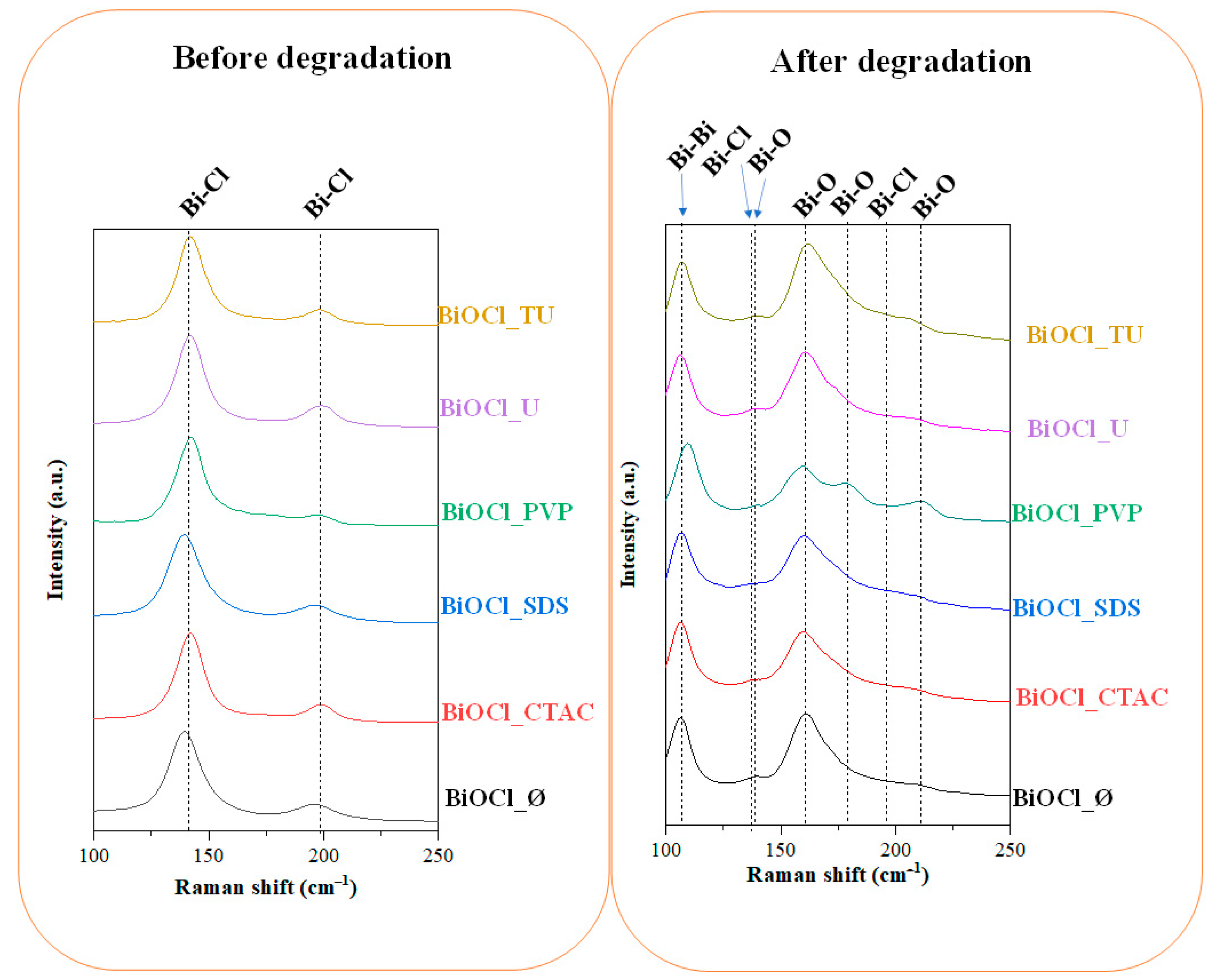
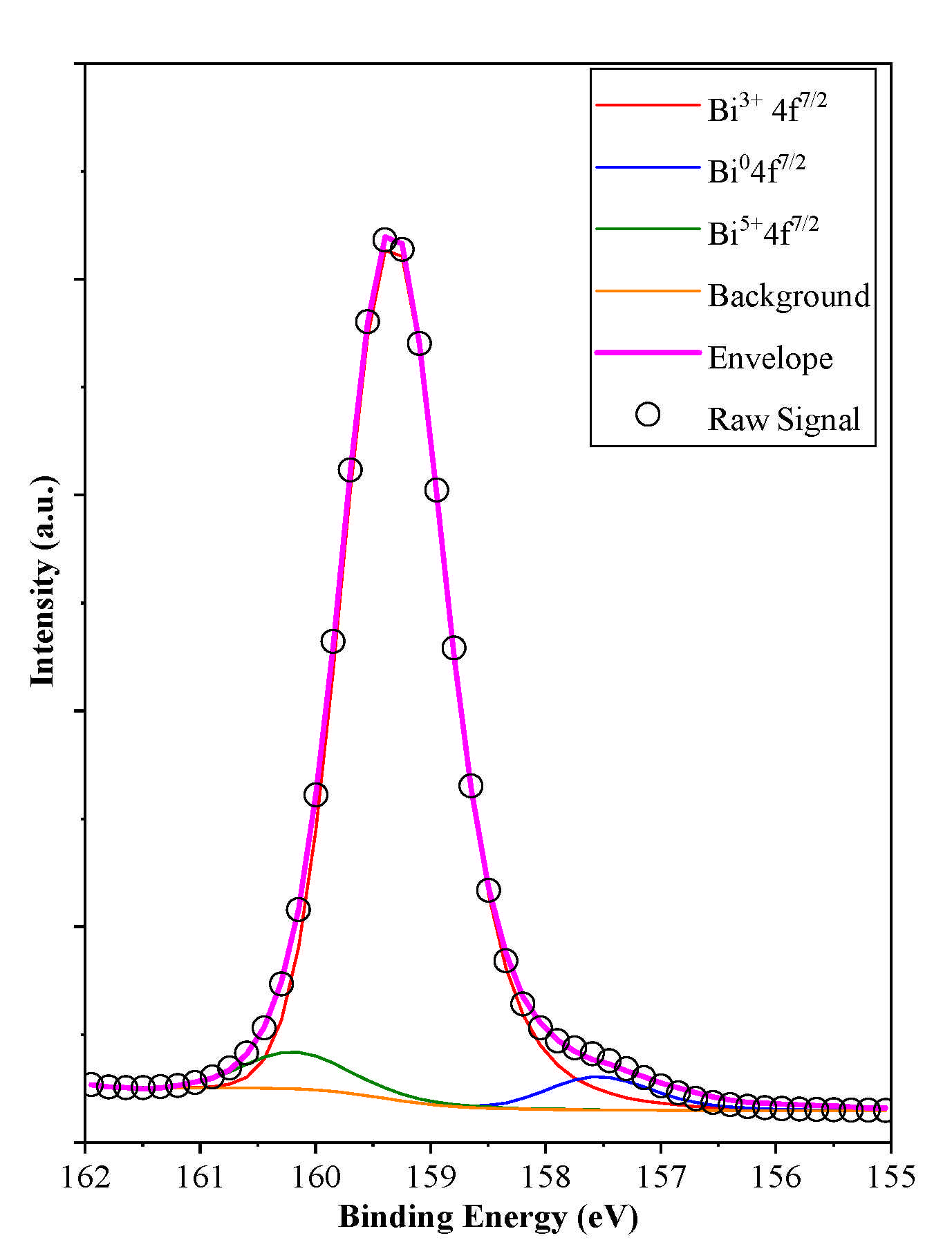
| Sample Name | Mean Primary Crystallite Size (nm) | Median Hierarchical Crystal Size (µm) | Band Gap Energy (eV) | (101), (102) and (110) Intensity Ratio. | Specific Surface Area (m2·g−1) | Conversion of Phenol (%) ** | Conversion of RhB ** (%) | Adsorbed RhB (%) | Normalized Degraded Phenol (mM·m−2) | Normalized Degraded RhB (mM·m−2) | Kinetic Constant (mol L−1 min−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UV | Vis | UV | Vis | ||||||||||
| BiOCl_∅ | 21.1 | 1.5 | 3.36 | 0.82:1.00:0.78 | 16 | 21.6 | 1.5 | 43 | - | 22.7 | 1.35 | 2.69 | 0.1603 |
| BiOCl_CTAC | 11.9 | 2.4 | 3.37 | 0.59:1.00:0.5 | 5 | 24.1 | 0.0 | 11 | 99 | 0.0 | 4.82 | 2.20 | 0.1865 |
| BiOCl_SDS | 16.8 | 2.4 | 3.44 | 0.69:1.00:0.71 | 15 | 27.5 | 4.1 | 23 | 99 | 5.1 | 2.83 | 1.53 | 0.2259 |
| BiOCl_PVP | 16.5 | 0.7 | 3.56 | 0.44:1.00:0.31 | 57 | 31.3 | 3.2 | 91 | 99 | 40.4 | 0.55 | 1.60 | 0.2229 |
| BiOCl_U | 17.9 | 1.6 | 3.38 | 0.72:1.00:0.78 | 8 | 24.6 | 8.2 | 37 | 99 | 21.0 | 3.08 | 4.63 | 0.2178 |
| BiOCl_TU | 17.5 | 1.2 | 3.46 | 0.70:1.00:0.62 | 25 | 30.0 | 8.0 | 69 | 99 | 51.1 | 1.20 | 2.76 | 0.2063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárdos, E.; Márta, V.A.; Fodor, S.; Kedves, E.-Z.; Hernadi, K.; Pap, Z. Hydrothermal Crystallization of Bismuth Oxychlorides (BiOCl) Using Different Shape Control Reagents. Materials 2021, 14, 2261. https://doi.org/10.3390/ma14092261
Bárdos E, Márta VA, Fodor S, Kedves E-Z, Hernadi K, Pap Z. Hydrothermal Crystallization of Bismuth Oxychlorides (BiOCl) Using Different Shape Control Reagents. Materials. 2021; 14(9):2261. https://doi.org/10.3390/ma14092261
Chicago/Turabian StyleBárdos, Enikő, Viktória A. Márta, Szilvia Fodor, Endre-Zsolt Kedves, Klara Hernadi, and Zsolt Pap. 2021. "Hydrothermal Crystallization of Bismuth Oxychlorides (BiOCl) Using Different Shape Control Reagents" Materials 14, no. 9: 2261. https://doi.org/10.3390/ma14092261
APA StyleBárdos, E., Márta, V. A., Fodor, S., Kedves, E.-Z., Hernadi, K., & Pap, Z. (2021). Hydrothermal Crystallization of Bismuth Oxychlorides (BiOCl) Using Different Shape Control Reagents. Materials, 14(9), 2261. https://doi.org/10.3390/ma14092261








