Innovative Method for Coating of Natural Corrosion Inhibitor Based on Artemisia vulgaris
Abstract
1. Introduction
2. Materials and Methods
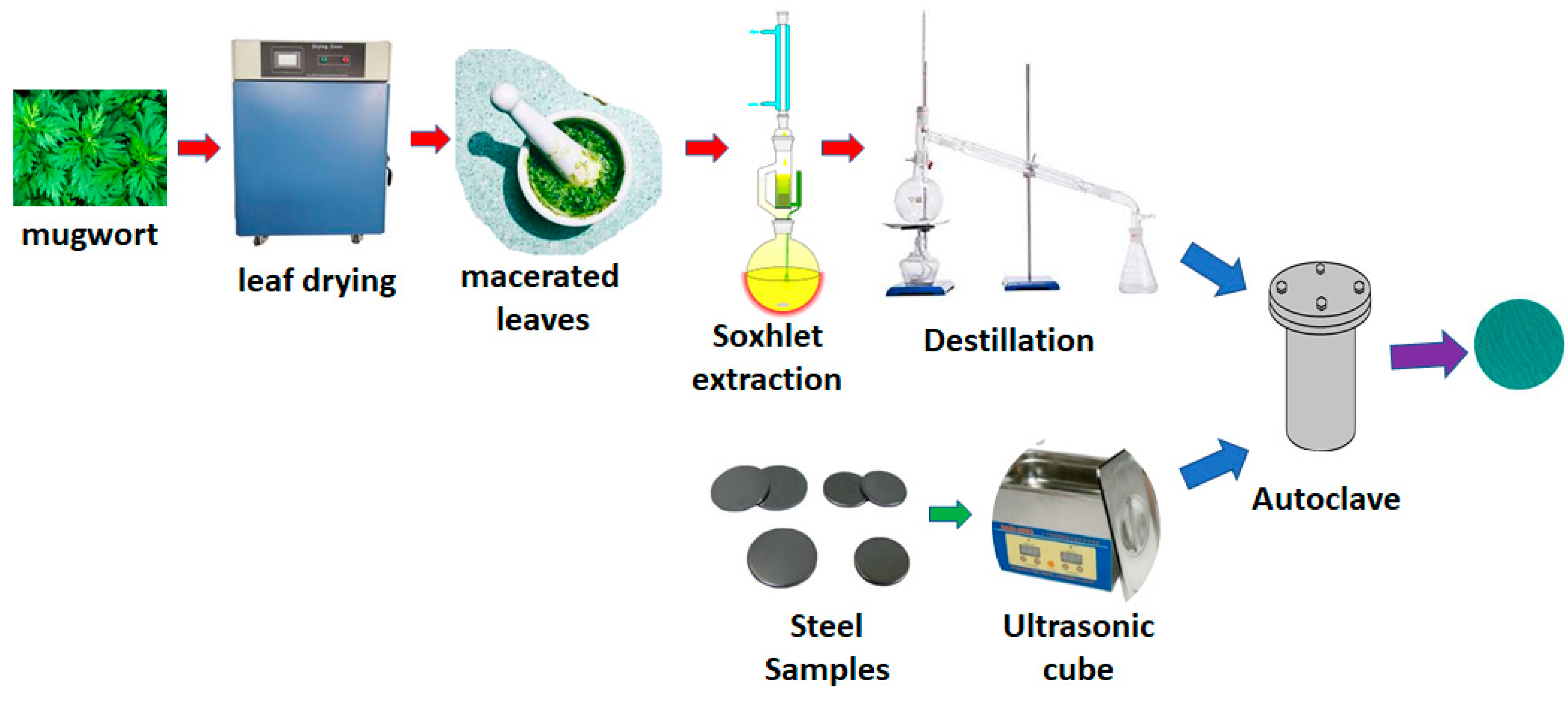
2.1. Extraction and Inhibitor Application
2.2. Materials Characterization
3. Results
3.1. Compositional Characterization
3.1.1. Fourier Transform Infrared
3.1.2. Thermogravimetric Analysis
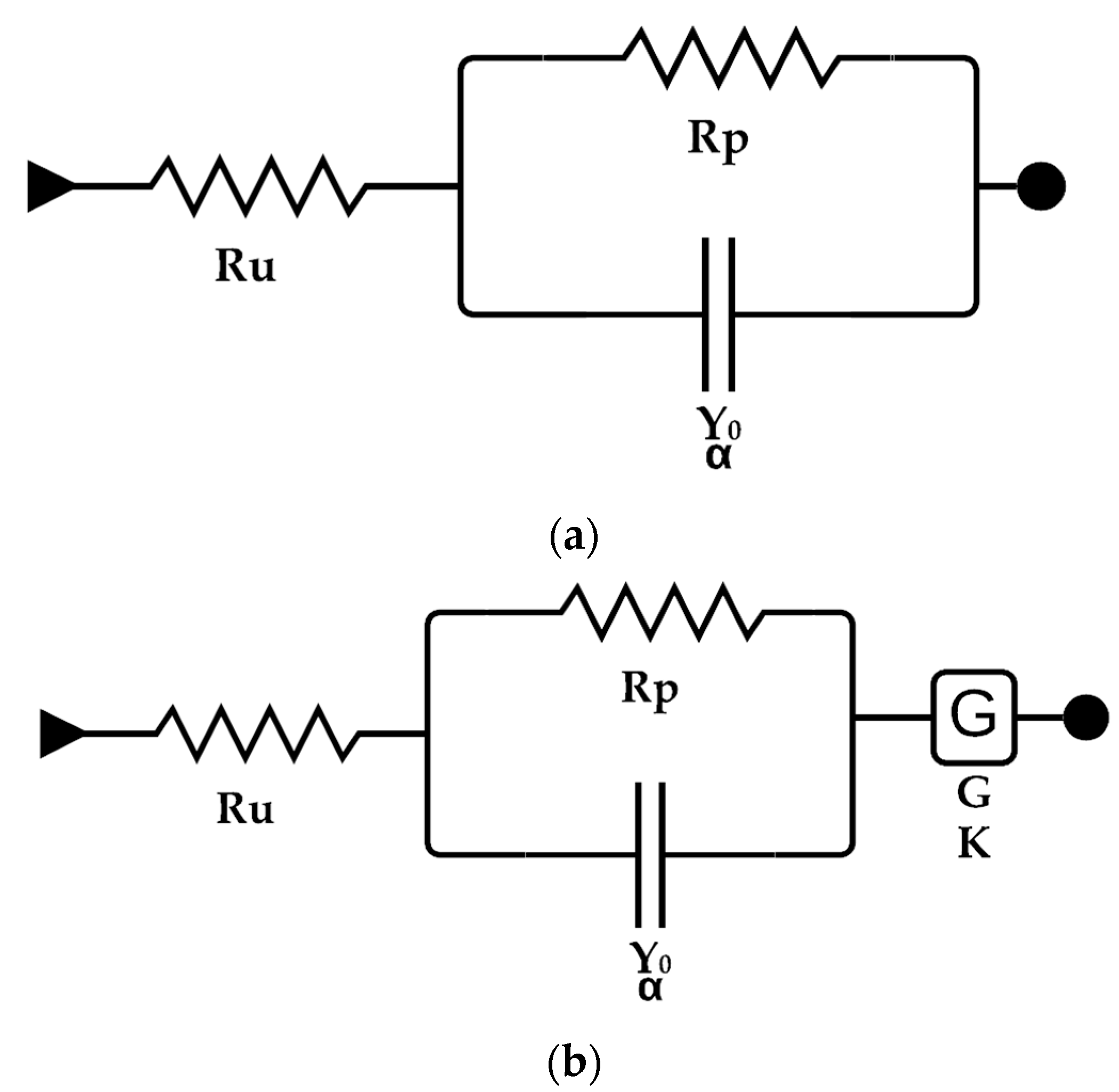
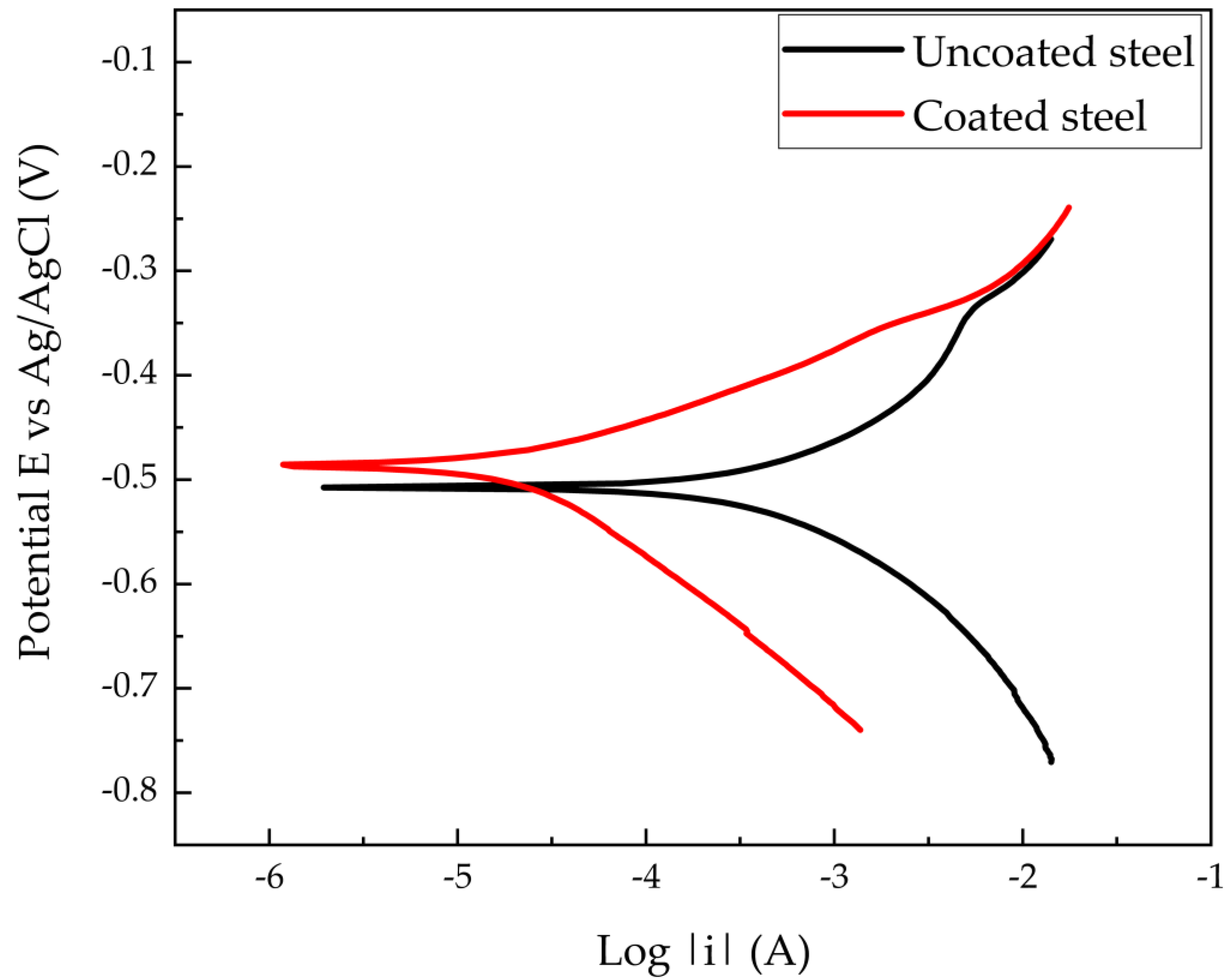
3.2. Electrochemical Measurements
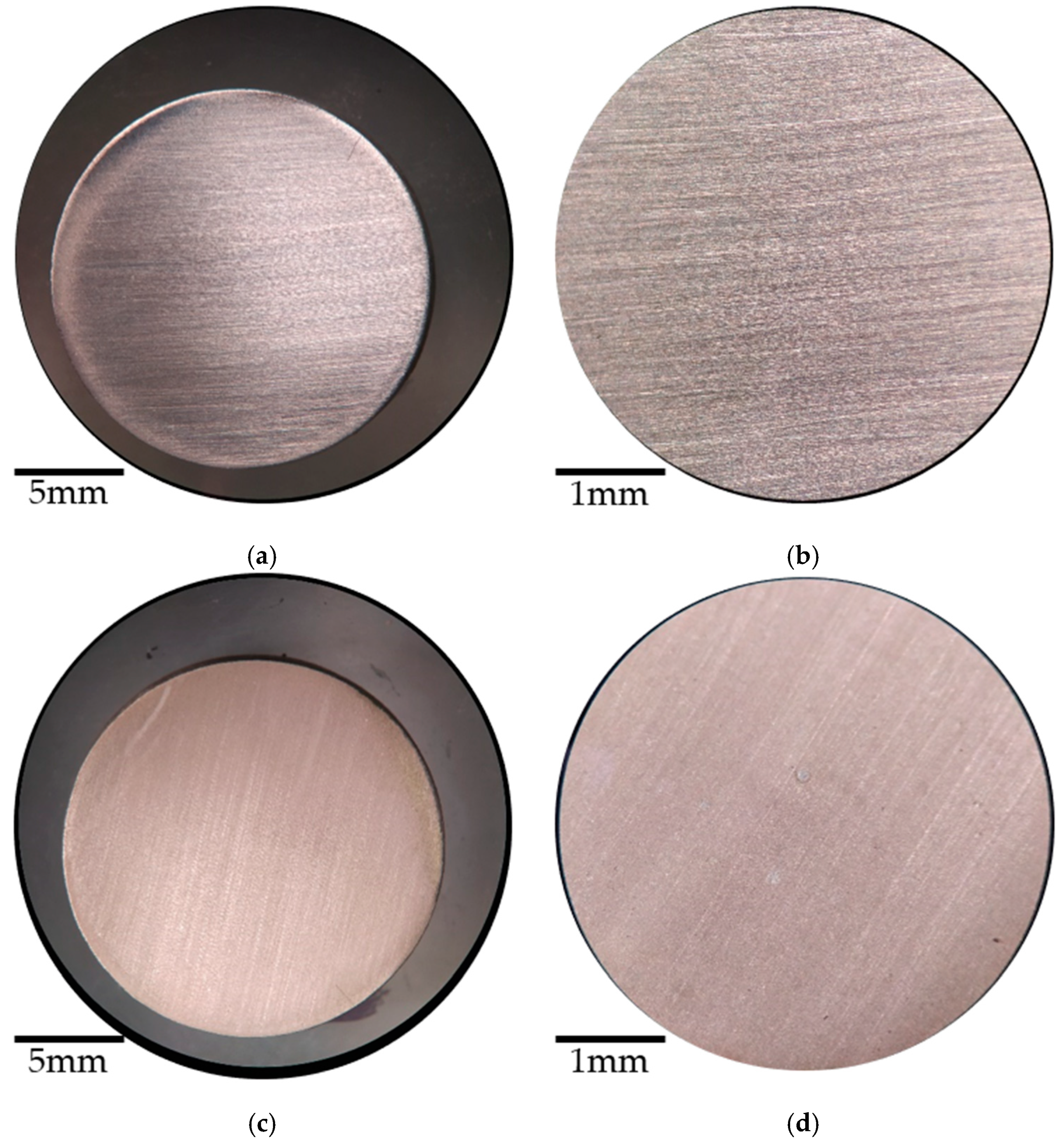
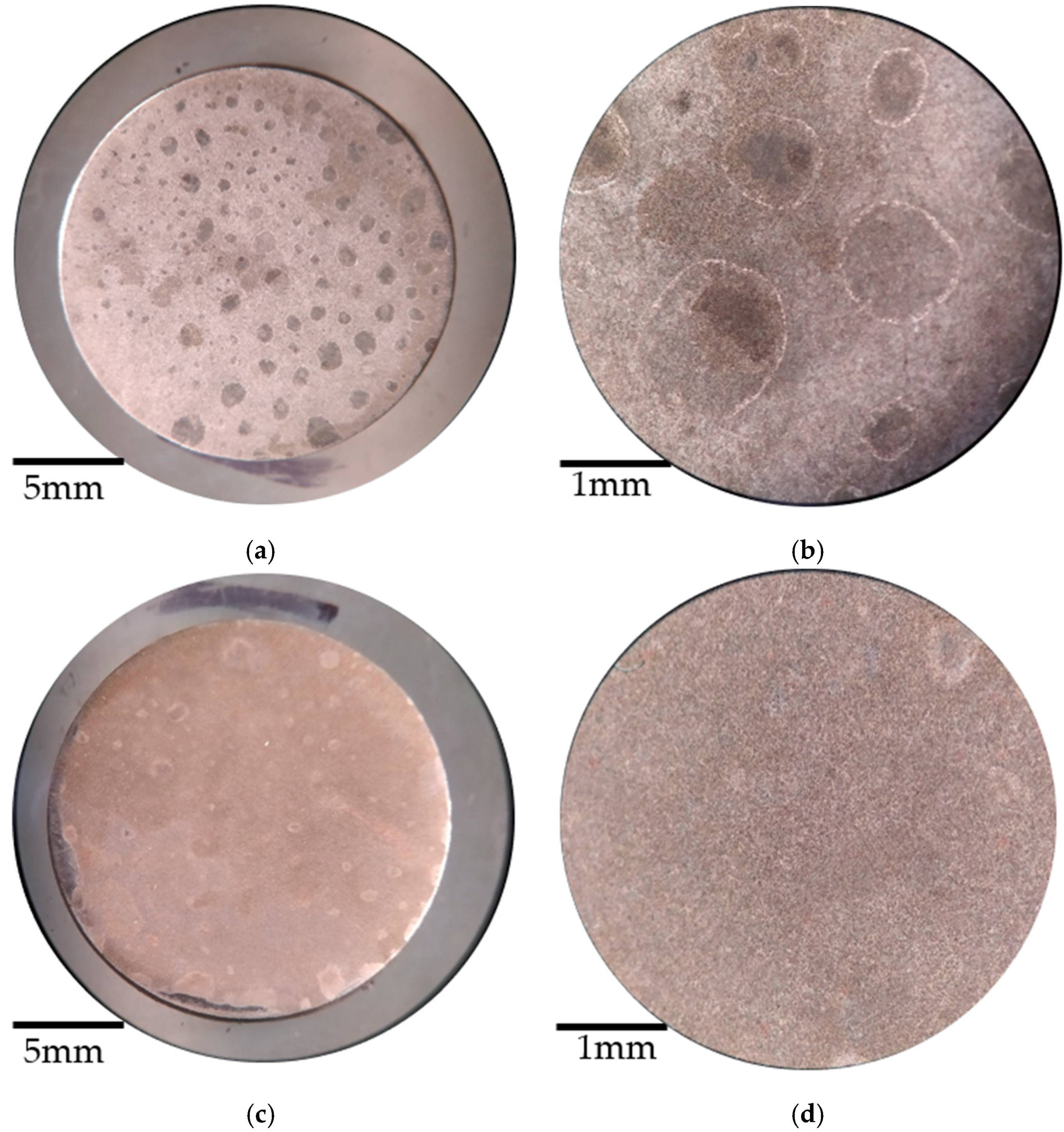
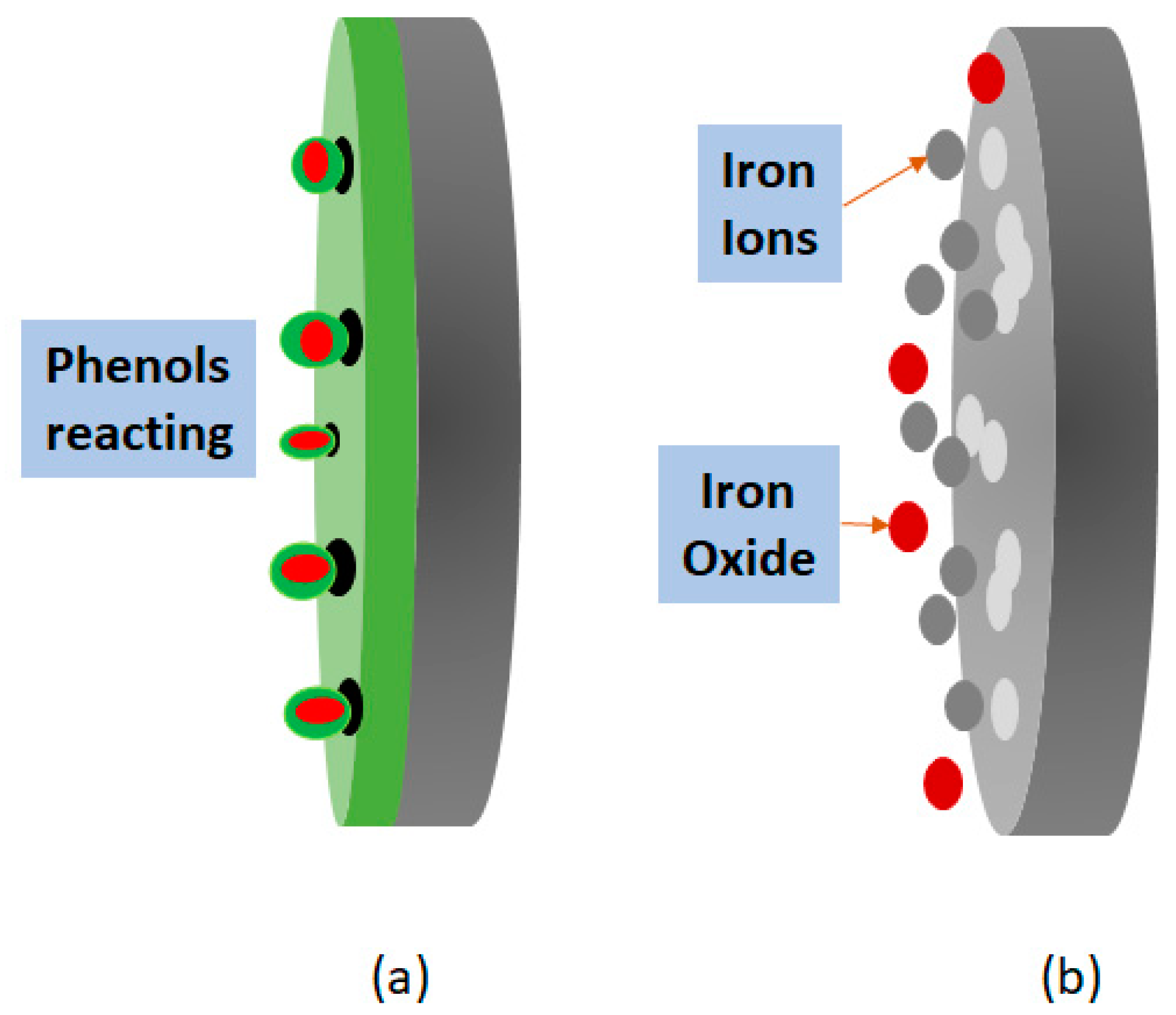
3.3. Visual Inspection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiche, H.A.T.; Derry, T.K.; Williams, T.I. A Short History of Technology from the Earliest Times to A.D. 1900. Class World 2010, 54, 296. [Google Scholar] [CrossRef]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. NACE 2016 Impact Study; NACE International: Huston, TX, USA, 2016. [Google Scholar]
- Jiang, L.; Syed, J.A.; Gao, Y.; Lu, H.; Meng, X. Electrodeposition of Ni(OH)2 reinforced polyaniline coating for corrosion protection of 304 stainless steel. Appl. Surf. Sci. 2018, 440, 1011–1021. [Google Scholar] [CrossRef]
- Singh, A.K.; Alam, S.; Rani, N. Preparation and characterisation of acrylic–polyurethane-based waterborne anticorrosion coating on galvanised steel. Trans. IMF 2017, 95, 165–172. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, Q.; Wang, Q.; Zhou, Z.; Li, L.K.Y. Electrochemical and tribological properties of CrBCN coatings with various B concentrations in artificial seawater. Tribol. Int. 2017, 116, 19–25. [Google Scholar] [CrossRef]
- Garai, S.; Garai, S.; Jaisankar, P.; Singh, J.K.; Elango, A. A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution. Corros. Sci. 2012, 60, 193–204. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Kashani, M.K.; Mosavizadeh, A.; Oguzie, E.E.; Jalali, M.R. Silybum marianum extract as a natural source inhibitor for 304 stainless steel corrosion in 1.0M HCl. J. Ind. Eng. Chem. 2014, 20, 3217–3227. [Google Scholar] [CrossRef]
- Herrera-Hernández, H.; Franco-Tronco, M.I.; Miranda-Hernández, G.J.; Hernández-Sánchez, E.; Espinoza-Vázquez, A.; Fajardo, G. Gel de aloe-vera como potencial inhibidor de la corrosión del acero de refuerzo estructural. Av. Cienc. Ing. 2015, 6, 9–23. [Google Scholar]
- EPA. The Environmental Protection (Controls on Dangerous Substances) Regulations 2003; Legislation: London, UK, 2003; Volume 57. [Google Scholar]
- Patricia, L.; Benítez, T.; Javier, P.; Castellar, M.; David, E.; Percy, A. Uso de extractos de plantas como inhibidores de corrosión. Rev. Inf. Tec. 2014, 78, 155–165. [Google Scholar]
- Nikpour, S.; Naderi, R.; Mahdavian, M. Fabrication of silane coating with improved protection performance using Mentha longifolia extract. J. Taiwan Inst. Chem. Eng. 2018, 88, 261–276. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M.; Solomon, M.M.; Udoh, A.P. Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab. J. Chem. 2016, 9, S209–S224. [Google Scholar] [CrossRef]
- Umoren, S.A.; Obot, I.B.; Israel, A.U.; Asuquo, P.O.; Solomon, M.M.; Eduok, U.M.; Udoh, A.P. Inhibition of mild steel corrosion in acidic medium using coconut coir dust extracted from water and methanol as solvents. J. Ind. Eng. Chem. 2014, 20, 3612–3622. [Google Scholar] [CrossRef]
- Jakovljević, M.R.; Grujičić, D.; Vukajlović, J.T.; Marković, A.; Milutinović, M.; Stanković, M.; Vuković, N.; Vukić, M.; Milošević-Djordjević, O. In vitro study of genotoxic and cytotoxic activities of methanol extracts of Artemisia vulgaris L. and Artemisia alba Turra. S. Afr. J. Bot. 2020, 132, 117–126. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar] [CrossRef]
- Addo-Mensa, A.; Garcia, G.; Maldonado, I.A.; Anaya, E.; Cadena, G.; Lee, L.G. Evaluation of Antibacterial Activity of Artemisia vulgaris Extracts. Res. J. Med. Plant 2015, 9, 234–240. [Google Scholar] [CrossRef]
- ASTM International. ASTM G59—Standard test method for conducting potentiodynamic polarization resistance measurements. ASTM Stand. 2014, 97, 1–4. [Google Scholar] [CrossRef]
- Liang, T.; Wang, L. Preparation and characterization of a novel edible film based on Artemisia sphaerocephala Krasch. gum: Effects of type and concentration of plasticizers. Food Hydrocoll. 2018, 77, 502–508. [Google Scholar] [CrossRef]
- Kouhbanani, M.A.J.; Beheshtkhoo, N.; Amani, A.M.; Taghizadeh, S.; Beigi, V.; Bazmandeh, A.Z.; Khalaf, N. Green synthesis of iron oxide nanoparticles using Artemisia vulgaris leaf extract and their application as a heterogeneous Fenton-like catalyst for the degradation of methyl orange. Mater. Res. Express 2018, 5, 115013. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; de Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Da Silva Moreira Thiré, R.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria (Rio Janeiro) 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Jarial, R.; Shard, A.; Thakur, S.; Sakinah, M.; Zularisam, A.W.; Rezania, S.; Kanwar, S.S.; Singh, L. Characterization of flavonoids from fern Cheilanthes tenuifolia and evaluation of antioxidant, antimicrobial and anticancer activities. J. King Saud Univ. Sci. 2018, 30, 425–432. [Google Scholar] [CrossRef]
- Cobaleda-Velasco, M.; Almaraz-Abarca, N.; Alanis-Bañuelos, R.E.; Uribe-Soto, J.N.; González-Valdez, L.S.; Muñoz-Hernández, G.; Zaca-Morán, O.; Rojas-López, M. Rapid Determination of Phenolics, Flavonoids, and Antioxidant Properties of Physalis ixocarpa Brot. ex Hornem. and Physalis angulata L. by Infrared Spectroscopy and Partial Least Squares. Anal. Lett. 2018, 51, 523–536. [Google Scholar] [CrossRef]
- Alshamsi, H.A.; Ali, S.K.; Altaa, S.H.A. Green Synthesis and Characterization of Reduced Graphene Oxide (RGO) Using Sabdarriffa L Extract and Its Solubility Property. J. Phys. Conf. Ser. 2020, 1664, 012058. [Google Scholar] [CrossRef]
- Afia, L.; Hamed, O.; Larouj, M.; Lgaz, H.; Jodeh, S.; Salghi, R. Novel Natural Based Diazepines as Effective Corrosion Inhibitors for Carbon Steel in HCl Solution: Experimental, Theoretical and Monte Carlo Simulations. Trans. Indian Inst. Met. 2017, 70, 2319–2333. [Google Scholar] [CrossRef]
- Verma, D.; Khan, F.; Bahadur, I.; Salman, M.; Quraishi, M.A.; Verma, C.; Ebenso, E.E. Inhibition performance of Glycine max, Cuscuta reflexa and Spirogyra extracts for mild steel dissolution in acidic medium: Density functional theory and experimental studies. Results Phys. 2018, 10, 665–674. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Kazemi-Darafshani, M. Root and shoot extracts of Ajuga chamaecistus subsp. scoparia as natural inhibitors for 304 stainless steel corrosion in strong acidic medium. Surf. Eng. Appl. Electrochem. 2017, 53, 560–569. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, L.; Liu, J. Corrosion inhibition of levofloxacin and Ce(NO3)3 for AA2024-T4 in 3.5% NaCl. Corros. Eng. Sci. Technol. 2020, 55, 75–82. [Google Scholar] [CrossRef]
- Meland, A.K.; Bedeaux, D.; Kjelstrup, S. A Gerischer phase element in the impedance diagram of the polymer electrolyte membrane fuel cell anode. J. Phys. Chem. B 2005, 109, 21380–21388. [Google Scholar] [CrossRef]
- Kashkovskiy, R.; Strelnikova, K.; Fedotova, A. Application of electrochemical impedance spectroscopy to study hydrogen sulphide corrosion of steel and its inhibition: A review. Corros. Eng. Sci. Technol. 2019, 54, 493–515. [Google Scholar] [CrossRef]
- Hou, B.S.; Zhang, Q.H.; Li, Y.Y.; Zhu, G.Y.; Liu, H.F.; Zhang, G.A. A pyrimidine derivative as a high efficiency inhibitor for the corrosion of carbon steel in oilfield produced water under supercritical CO2 conditions. Corros. Sci. 2020, 164, 108334. [Google Scholar] [CrossRef]
- Parthipan, P.; Narenkumar, J.; Elumalai, P.; Preethi, P.S.; Nanthini, A.U.R.; Agrawal, A.; Rajasekar, A. Neem extract as a green inhibitor for microbiologically influenced corrosion of carbon steel API 5LX in a hypersaline environments. J. Mol. Liq. 2017, 240, 121–127. [Google Scholar] [CrossRef]
- Tezeghdenti, M.; Etteyeb, N.; Dhouibi, L.; Kanoun, O.; Al-Hamri, A. Natural products as a source of environmentally friendly corrosion inhibitors of mild steel in dilute sulphuric acid: Experimental and computational studies. Prot. Met. Phys. Chem. Surfaces 2017, 53, 753–764. [Google Scholar] [CrossRef]
- Sivakumar, P.R.; Srikanth, A.P. Green corrosion inhibitor: A comparative study. Sādhanā 2020, 45, 56. [Google Scholar] [CrossRef]
- Isaac, C.; Jiménez, E.; Yane, E.; Martínez, C.; Fonseca, J.G. Flavonoides y sus acciones antioxidantes. Rev. la Fac. Med. 2009, 52, 73–75. [Google Scholar]

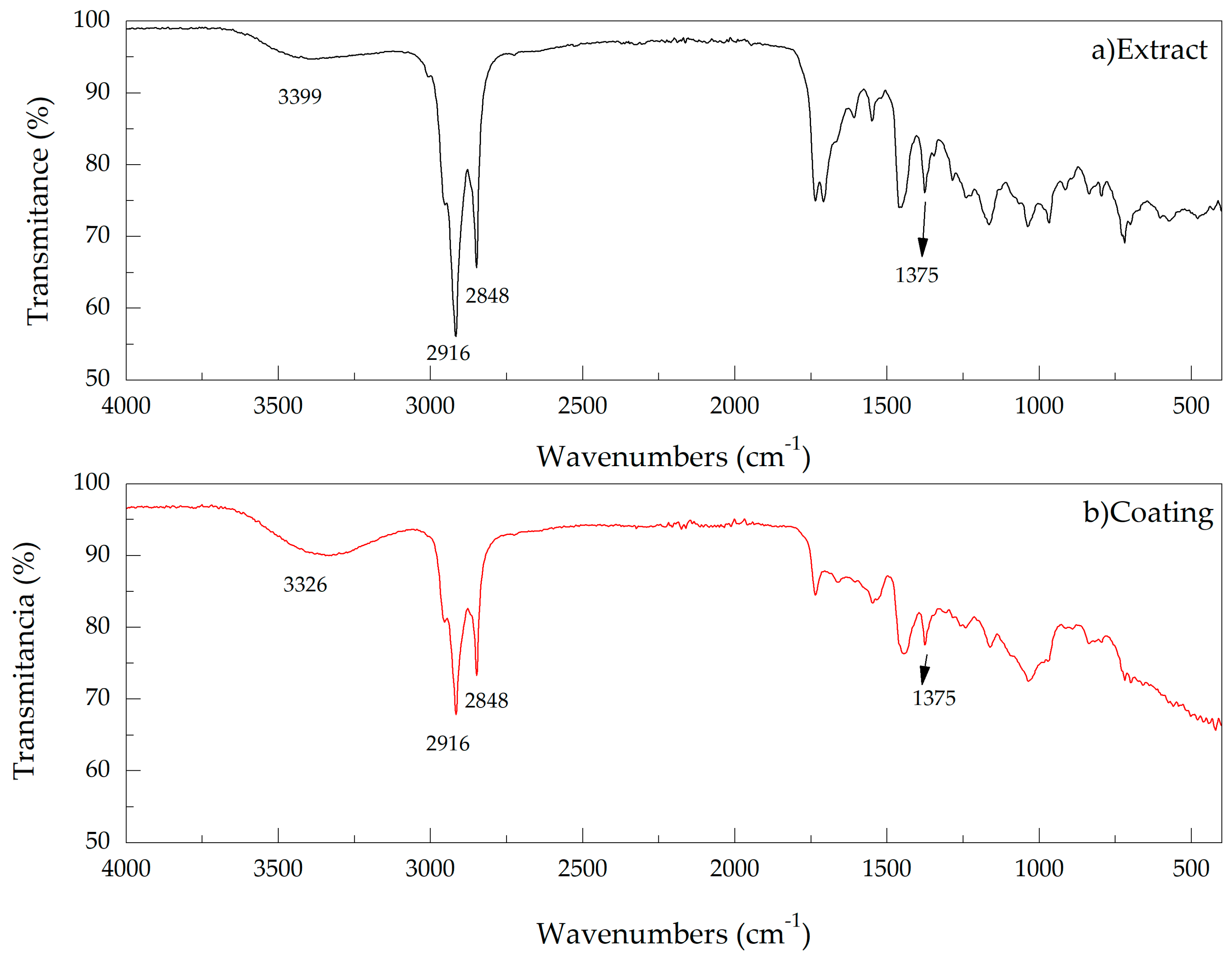


| Wavenumber (cm−1) | Compound | Notes |
|---|---|---|
| 3399.29 | OH | Associated with a polymeric primary alcohol (carrier substance) |
| 3000 | -CH2- | Vs |
| 2917.06 | Vas | |
| 2849.23 | Vs | |
| 1460.79 | Scissor | |
| 719.70 | Rocking | |
| 1376.08 | O-CO-CH3 | |
| 1549.51 | 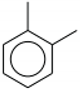 | Confirmed presence of aromatic rings with benzene nucleus. |
| 1608.67 | ||
| 1165.22 | They give information about the branches that the ring presents. | |
| 1037.77 | ||
| 967.94 | ||
| 1735.49 | 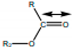 | Esters |
| 1708.87 |  | Carboxylic acids |
| % Weight | Temperature (°C) | |
|---|---|---|
| A | 11.38 | 52.24 |
| B | 20.25 | 288.28 |
| C | 29.22 | 321.64 |
| D | 13.07 | 618.45 |
| Element | Value | ±Error | Units |
|---|---|---|---|
| Ru | 6.607 | 40.05 × 10−5 | Ω |
| Y0 | 454.4 × 10−6 | 19.95 × 10−6 | S × sα |
| α | 841.6 × 10−3 | 7.136 × 10−3 | - |
| Rp | 35.53 | 289.7 × 10−3 | Ω |
| Element | Value | ±Error | Units |
|---|---|---|---|
| Ru | 6.101 | 97.19 × 10−3 | Ω |
| Y0 | 59.29 × 10−6 | 5.415 × 10−6 | S × sα |
| α | 890 × 10−3 | 12.38 × 10−3 | - |
| G | 1232 × 10−3 | 55.50 × 10−6 | S × s(½) |
| K | 181.5 | 77.64 | s−1 |
| Rp | 501.2 | 13.24 | Ω |
| Sample\Charact. | Uncoated Steel | Coated Steel | %Efficiency |
|---|---|---|---|
| Rcorr (Ω) | 35.53 ± 289.7 × 10−3 | 501.2 ± 13.24 | 92.91% |
| Corrosion Rate (mpy) | 52.3 | 6.34 | 88.31% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda Hernández, D.A.; Restrepo Parra, E.; Arango Arango, P.J.; Segura Giraldo, B.; Acosta Medina, C.D. Innovative Method for Coating of Natural Corrosion Inhibitor Based on Artemisia vulgaris. Materials 2021, 14, 2234. https://doi.org/10.3390/ma14092234
Pineda Hernández DA, Restrepo Parra E, Arango Arango PJ, Segura Giraldo B, Acosta Medina CD. Innovative Method for Coating of Natural Corrosion Inhibitor Based on Artemisia vulgaris. Materials. 2021; 14(9):2234. https://doi.org/10.3390/ma14092234
Chicago/Turabian StylePineda Hernández, Daniel Alejandro, Elisabeth Restrepo Parra, Pedro José Arango Arango, Belarmino Segura Giraldo, and Carlos Daniel Acosta Medina. 2021. "Innovative Method for Coating of Natural Corrosion Inhibitor Based on Artemisia vulgaris" Materials 14, no. 9: 2234. https://doi.org/10.3390/ma14092234
APA StylePineda Hernández, D. A., Restrepo Parra, E., Arango Arango, P. J., Segura Giraldo, B., & Acosta Medina, C. D. (2021). Innovative Method for Coating of Natural Corrosion Inhibitor Based on Artemisia vulgaris. Materials, 14(9), 2234. https://doi.org/10.3390/ma14092234







