Influence of Sintering Temperature of Kaolin, Slag, and Fly Ash Geopolymers on the Microstructure, Phase Analysis, and Electrical Conductivity
Abstract
1. Introduction
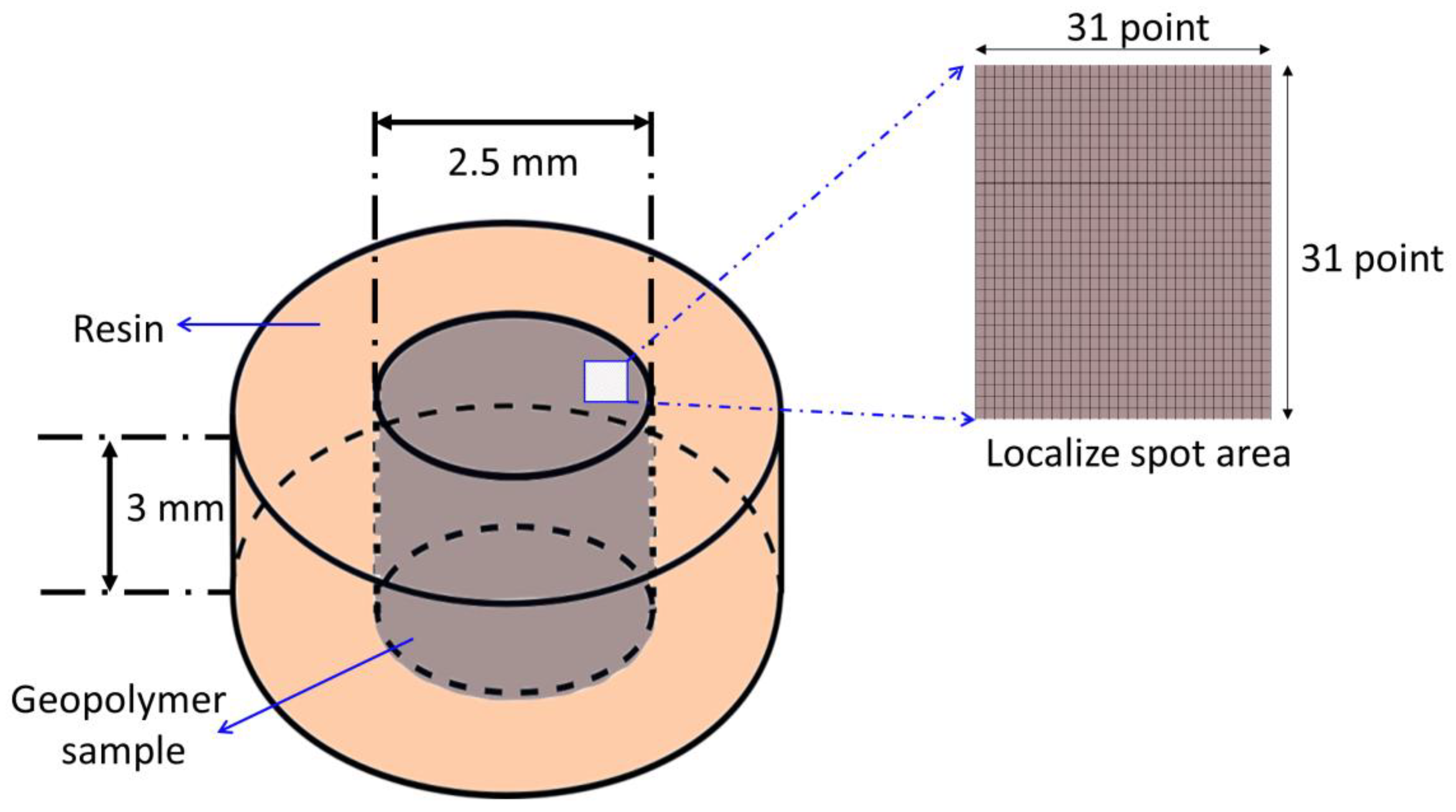
2. Experimental Details
Materials and Sample Preparation
3. Characterisation and Analysis
4. Result and Discussion
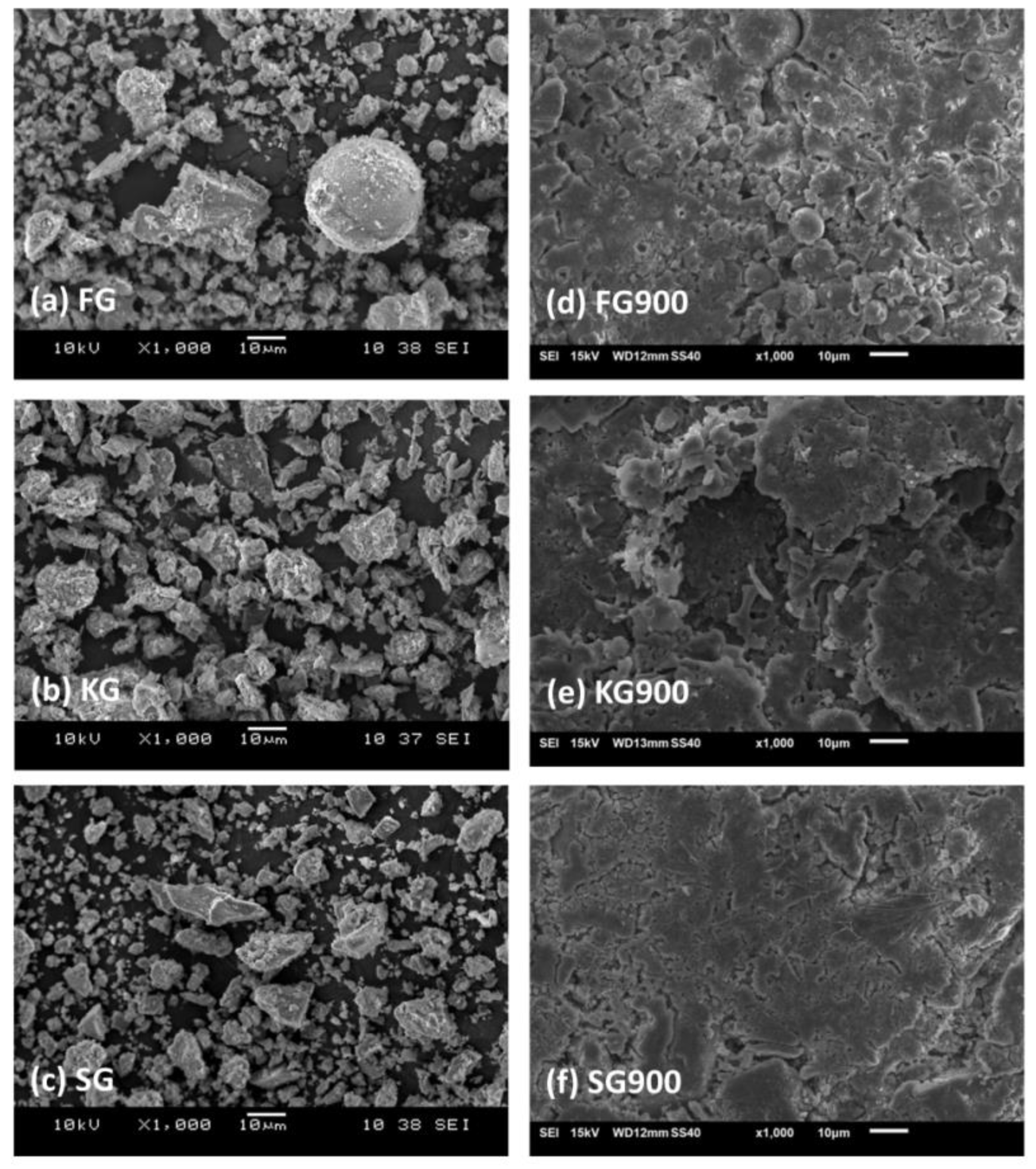
4.1. Microstructure Analysis
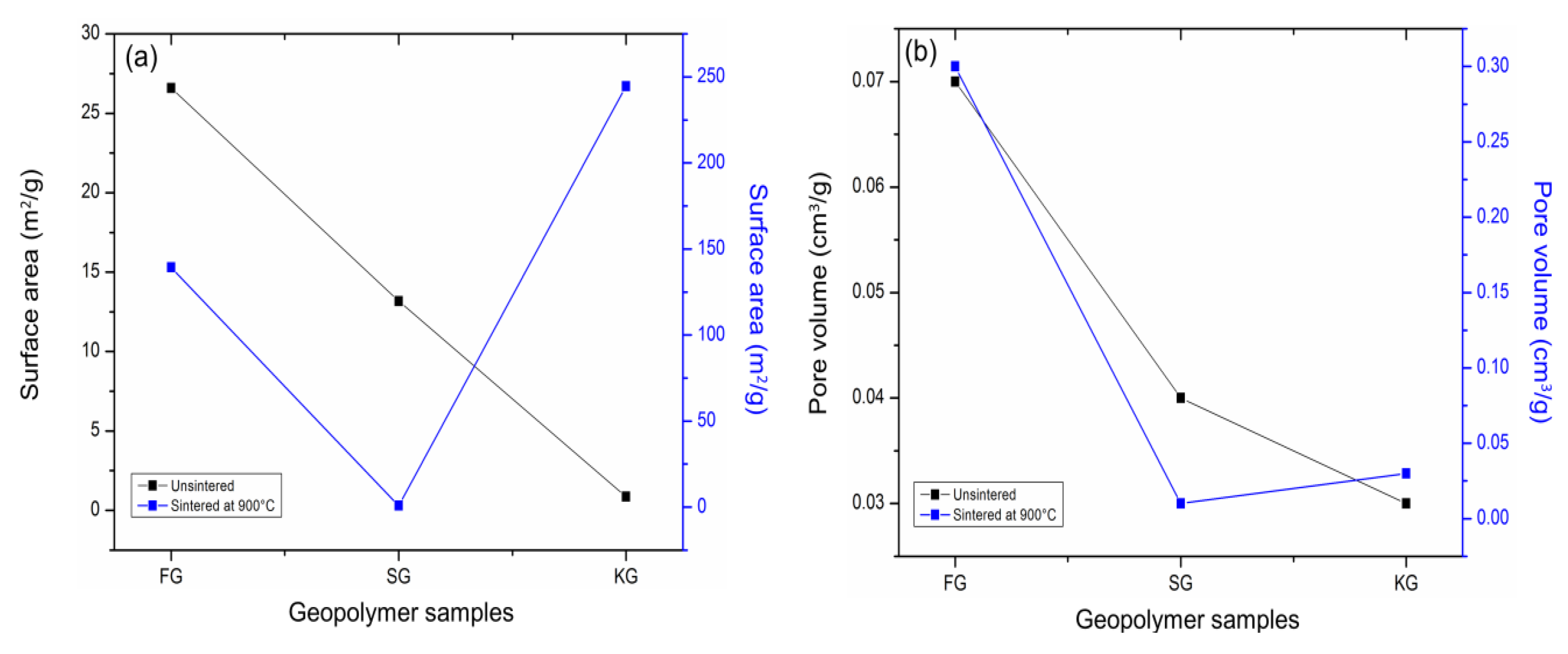
4.2. Pore Structure Analysis
4.3. Structural Spectra Analysis
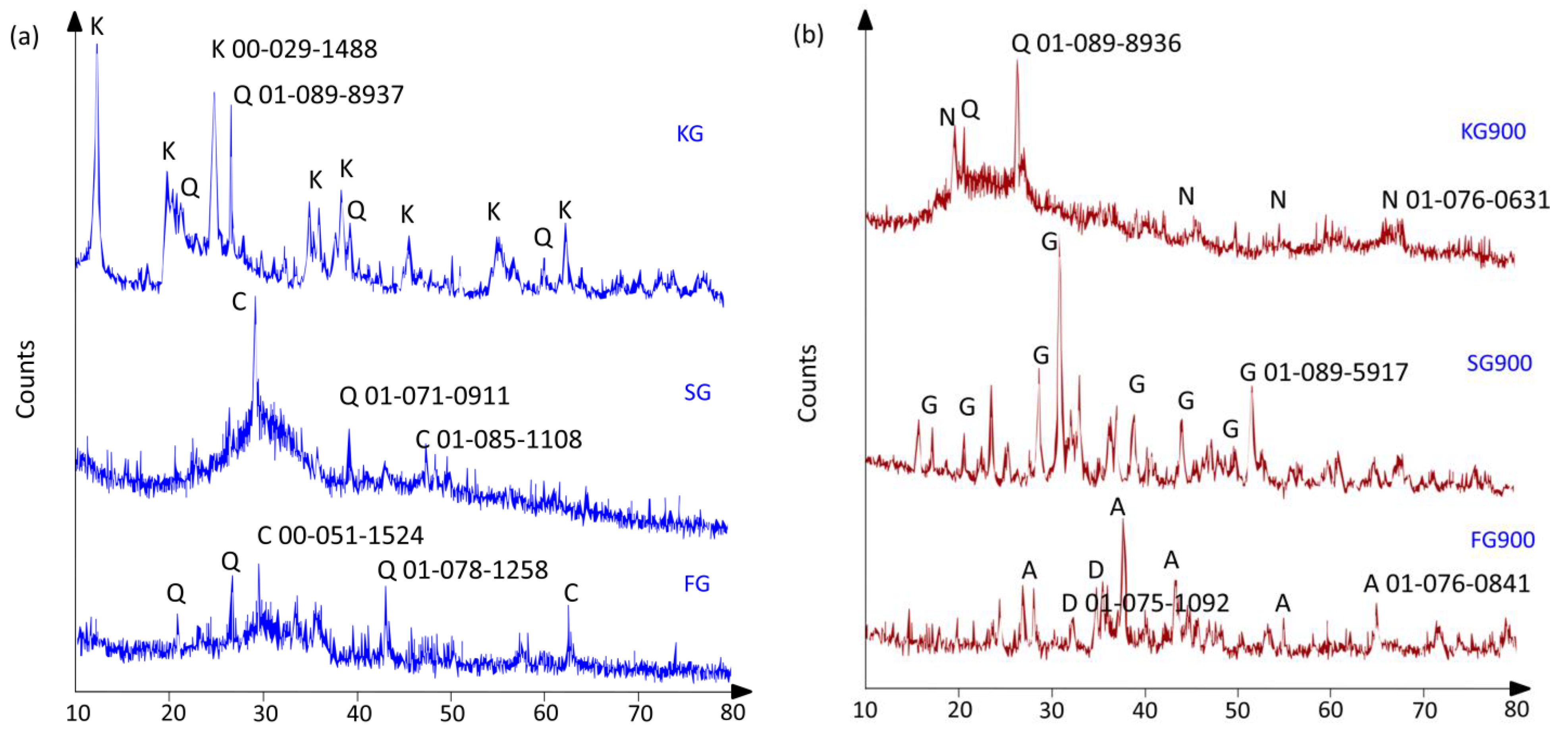
4.4. Phase Transformation
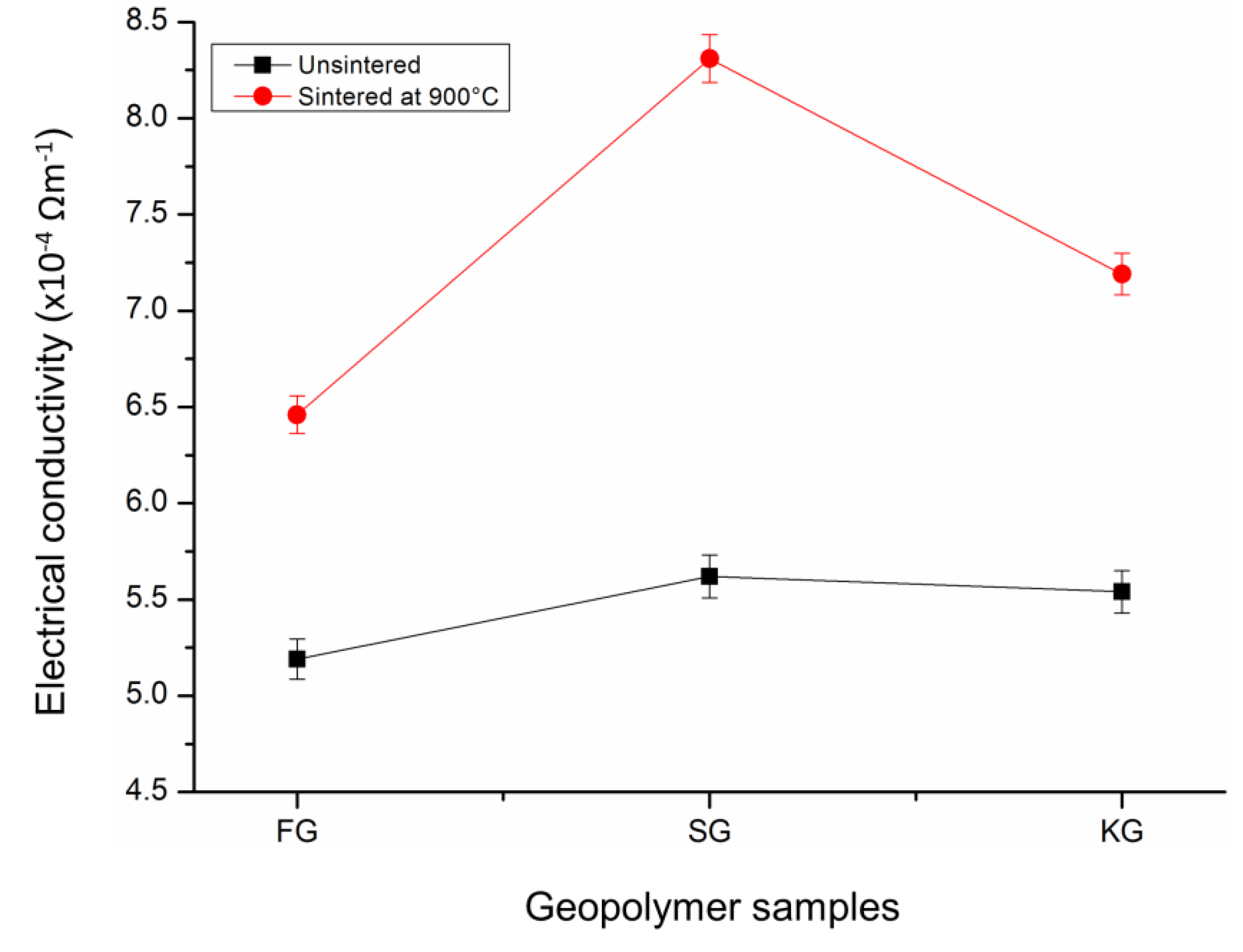
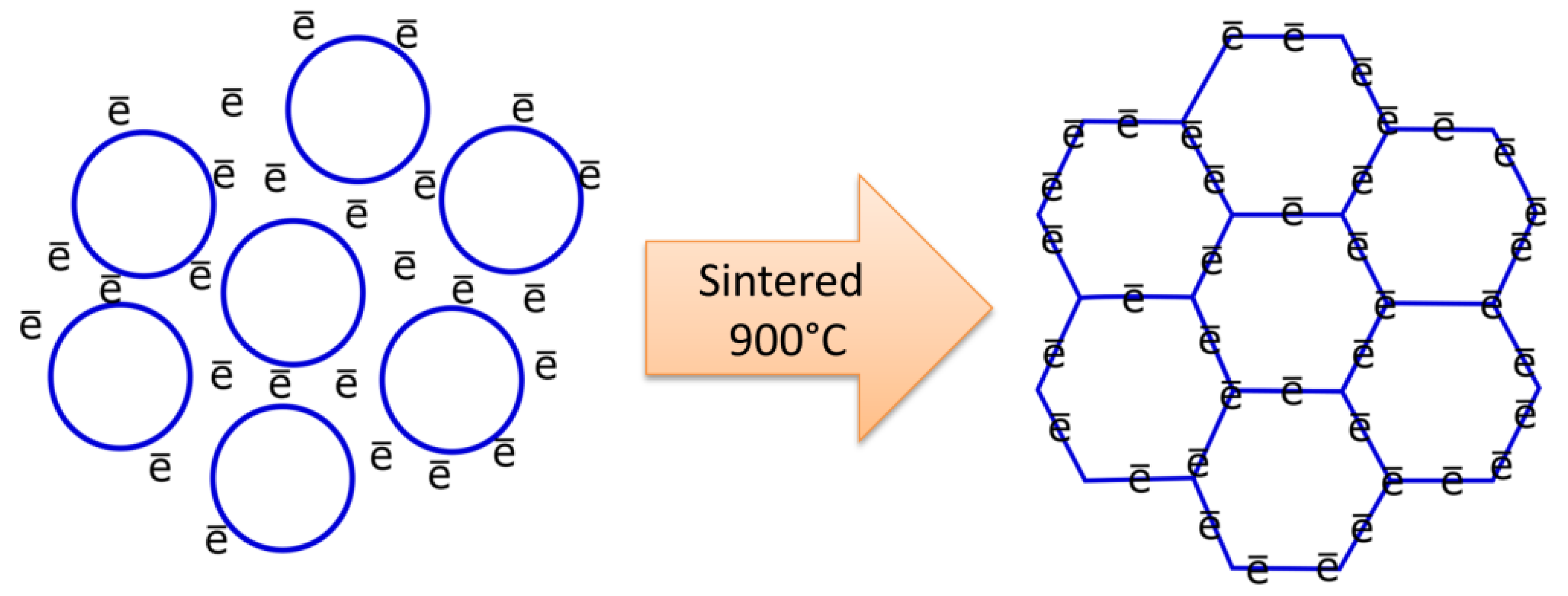
4.5. Electrical Conductivity
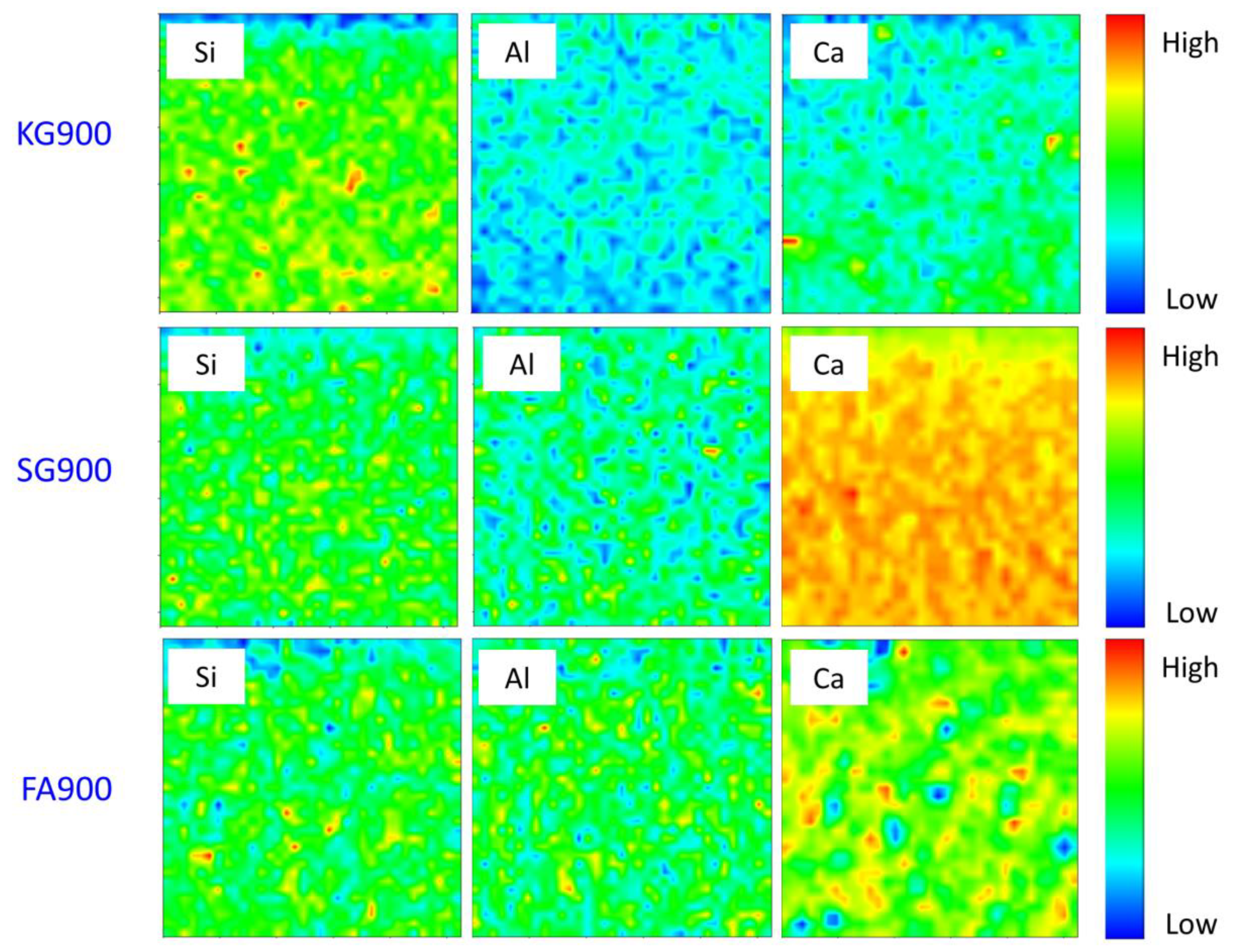
4.6. Elemental Distribution Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, D.L.; Sanjayan, J.G. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Taye, E.; Roether, J.; Schubert, D.; Redda, D.; Boccaccini, A. Hemp Fiber Reinforced Red Mud/Fly Ash Geopolymer Composite Materials: Effect of Fiber Content on Mechanical Strength. Materials 2021, 14, 511. [Google Scholar] [CrossRef]
- Shahedan, N.; Abdullah, M.; Mahmed, N.; Kusbiantoro, A.; Tammas-Williams, S.; Li, L.-Y.; Aziz, I.; Vizureanu, P.; Wysłocki, J.; Błoch, K.; et al. Properties of a New Insulation Material Glass Bubble in Geo-Polymer Concrete. Materials 2021, 14, 809. [Google Scholar] [CrossRef]
- Zailani, W.W.A.; Abdullah, M.M.A.B.; Arshad, M.F.; Razak, R.A.; Tahir, M.F.M.; Zainol, R.R.M.A.; Nabialek, M.; Sandu, A.V.; Wysłocki, J.J.; Błoch, K. Characterisation at the Bonding Zone between Fly Ash Based Geopolymer Repair Materials (GRM) and Ordinary Portland Cement Concrete (OPCC). Materials 2020, 14, 56. [Google Scholar] [CrossRef]
- Faris, M.; Abdullah, M.; Muniandy, R.; Abu Hashim, M.; Błoch, K.; Jeż, B.; Garus, S.; Palutkiewicz, P.; Mortar, N.M.; Ghazali, M. Comparison of Hook and Straight Steel Fibers Addition on Malaysian Fly Ash-Based Geopolymer Concrete on the Slump, Density, Water Absorption and Mechanical Properties. Materials 2021, 14, 1310. [Google Scholar] [CrossRef]
- Li, O.H.; Yun-Ming, L.; Cheng-Yong, H.; Bayuaji, R.; Abdullah, M.M.A.B.; Loong, F.K.; Jin, T.S.; Teng, N.H.; Nabiałek, M.; Jeż, B.; et al. Evaluation of the Effect of Silica Fume on Amorphous Fly Ash Geopolymers Exposed to Elevated Temperature. Magnetochemistry 2021, 7, 9. [Google Scholar] [CrossRef]
- Lancellotti, I.; Piccolo, F.; Traven, K.; Češnovar, M.; Ducman, V.; Leonelli, C. Alkali Activation of Metallurgical Slags: Reactivity, Chemical Behavior, and Environmental Assessment. Materials 2021, 14, 639. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.; Abdullah, M.; Pa, F.C.; Hasmaliza, M.; Ibrahim, W.W.; Aziz, I.A.; Jeż, B.; Nabiałek, M. Phase Transformation of Kaolin-Ground Granulated Blast Furnace Slag from Geopolymerization to Sintering Process. Magnetochemistry 2021, 7, 32. [Google Scholar] [CrossRef]
- Elimbi, A.; Tchakoute, H.; Njopwouo, D. Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr. Build. Mater. 2011, 25, 2805–2812. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.; Postawa, P.; AbdRahim, S.Z.; Zainol, M.R.R.M.A.; Jaya, R.; Śliwa, A.; Omar, M.; Wysłocki, J.; Błoch, K.; et al. Effect of Aluminium Powder on Kaolin-Based Geopolymer Characteristic and Removal of Cu2+. Materials 2021, 14, 814. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Cement. A Review; Technical Paper 21; Geopolymer Institute: Saint-Quentin, France, 2013; pp. 1–11. [Google Scholar]
- Abdulkareem, O.A.; Al Bakri, A.M.; Kamarudin, H.; Nizar, I.K.; Saif, A.A. Effects of elevated temperatures on the thermal behavior and mechanical performance of fly ash geopolymer paste, mortar and lightweight concrete. Constr. Build. Mater. 2014, 50, 377–387. [Google Scholar] [CrossRef]
- Wang, M.; Jia, D.; He, P.; Zhou, Y. Influence of calcination temperature of kaolin on the structure and properties of final geopolymer. Mater. Lett. 2010, 64, 2551–2554. [Google Scholar] [CrossRef]
- Rovnaník, P.; Bayer, P.; Rovnaníková, P. Characterization of alkali activated slag paste after exposure to high temperatures. Constr. Build. Mater. 2013, 47, 1479–1487. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Yliniemi, J.; Ismailov, A.; Levanen, E.; Tanskanen, P.; Kinnunen, P.; Roning, J.; Illikainen, M. Recycling lithium mine tailings in the production of low temperature (700–900 °C) ceramics: Effect of ladle slag and sodium compounds on the processing and final properties. Constr. Build. Mater. 2019, 221, 332–344. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Yong, H.C.; Ming, L.Y.; Hussin, K.; Surleva, A.; Azimi, E.A. Manufacturing parameters influencing fire resistance of geopolymers: A review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2016, 233, 721–733. [Google Scholar] [CrossRef]
- Cui, X.-M.; Zheng, G.-J.; Han, Y.-C.; Su, F.; Zhou, J. A study on electrical conductivity of chemosynthetic Al2O3–2SiO2 geoploymer materials. J. Power Sources 2008, 184, 652–656. [Google Scholar] [CrossRef]
- Vladimirov, I.; Kühn, M.; Geßner, T.; May, F.; Weitz, R.T. Energy barriers at grain boundaries dominate charge carrier transport in an electron-conductive organic semiconductor. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Heah, C.-Y.; Liew, Y.-M. Behaviour changes of ground granulated blast furnace slag geopolymers at high temperature. Adv. Cem. Res. 2020, 32, 465–475. [Google Scholar] [CrossRef]
- Liew, Y.; Kamarudin, H.; Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.; Heah, C. Optimization of solids-to-liquid and alkali activator ratios of calcined kaolin geopolymeric powder. Constr. Build. Mater. 2012, 37, 440–451. [Google Scholar] [CrossRef]
- Abdullah, A.; Hussin, K.; Abdullah, M.; Yahya, Z.; Sochacki, W.; Razak, R.; Błoch, K.; Fansuri, H. The Effects of Various Concentrations of NaOH on the Inter-Particle Gelation of a Fly Ash Geopolymer Aggregate. Materials 2021, 14, 1111. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.M.; Yoriya, S.; Chaiprapa, J.; Rojviriya, C.; Li, L.Y. Microstructure and porosity evolution of alkali activated slag at various heating temperatures. J. Mater. Res. Technol. 2020, 9, 15894–15907. [Google Scholar] [CrossRef]
- Solé, V.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Dudek, M.; Sitarz, M. Analysis of Changes in the Microstructure of Geopolymer Mortar after Exposure to High Temperatures. Materials 2020, 13, 4263. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ishida, T.; Gu, R. A comparison of the specific surface area of fly ash measured by image analysis with conventional methods. Constr. Build. Mater. 2018, 190, 1163–1172. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.M.; Azimi, E.A.; Chaiprapa, J.; Sandu, A.V. Strength development of solely ground granulated blast furnace slag geopolymers. Constr. Build. Mater. 2020, 250, 118720. [Google Scholar] [CrossRef]
- Bai, T.; Song, Z.; Wang, H.; Wu, Y.; Huang, W. Performance evaluation of metakaolin geopolymer modified by different solid wastes. J. Clean. Prod. 2019, 226, 114–121. [Google Scholar] [CrossRef]
- Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Hussin, K. Thermal Resistance Variations of Fly Ash Geopolymers: Foaming Responses. Sci. Rep. 2017, 7, srep45355. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Y.; Wei, Q.; Cui, S.; Hao, L. Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag. Materials 2020, 13, 1462. [Google Scholar] [CrossRef] [PubMed]
- Harech, M.A.; Mesnaoui, M.; Abouliatim, Y.; El Hafiane, Y.; Benhammou, A.; Abourriche, A.; Smith, A.; Nibou, L. Effect of temperature and clay addition on the thermal behavior of phosphate sludge. Bol. Soc. Esp. Cerám. V 2020. [Google Scholar] [CrossRef]
- Ahmad, R.; Abdullah, M.; Ibrahim, W.; Hussin, K.; Zaidi, F.A.; Chaiprapa, J.; Wysłocki, J.; Błoch, K.; Nabiałek, M. Role of Sintering Temperature in Production of Nepheline Ceramics-Based Geopolymer with Addition of Ultra-High Molecular Weight Polyethylene. Materials 2021, 14, 1077. [Google Scholar] [CrossRef]
- Sabbatini, A.; Pettinari, C.; Sobrados, I.; Rossignol, S.; Vidal, L. Control of shaping and thermal resistance of metakaolin-based geopolymers. Mater. Des. 2017, 116, 374–385. [Google Scholar] [CrossRef]
- Sheng, Y.; Hua, Y.; Wang, X.; Zhao, X.; Chen, L.; Zhou, H.; Wang, J.; Berndt, C.C.; Li, W. Application of High-Density Electropulsing to Improve the Performance of Metallic Materials: Mechanisms, Microstructure and Properties. Materials 2018, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Y.; Lv, Y.-Q.; Han, Y.; Fan, J.-L.; Ye, L. Sintering densification behavior and kinetic mechanism of nano-tungsten powder prepared by sol-spray drying. Tungsten 2020, 2, 371–380. [Google Scholar] [CrossRef]
- Sellami, M.; Barre, M.; Toumi, M. Synthesis, thermal properties and electrical conductivity of phosphoric acid-based geopolymer with metakaolin. Appl. Clay Sci. 2019, 180, 105192. [Google Scholar] [CrossRef]

| Element | Mass (wt.%) | ||
|---|---|---|---|
| Fly Ash | Kaolin | Slag | |
| SiO2 | 31.70 | 55.50 | 31.8 |
| Al2O3 | 14.30 | 31.20 | 10.5 |
| CaO | 23.40 | N/A | 50.37 |
| Fe2O3 | 23.92 | 4.40 | 0.53 |
| K2O | 1.54 | 5.41 | N/A |
| TiO2 | 0.94 | 1.37 | 0.98 |
| MgO | 3.60 | N/A | 3.2 |
| ZrO2 | N/A | 0.08 | 0.05 |
| MnO2 | N/A | 0.11 | 0.71 |
| LOI | 1.60 | 1.93 | 1.86 |
| Mix Design | Material | ||
|---|---|---|---|
| Fly Ash | Kaolin | Slag | |
| NaOH (M) | 12 | 8 | 10 |
| Alkaline activator ratio | 1.0 | 0.32 | 2.5 |
| Solid-to-liquid ratio | 2.5 | 1.0 | 3.0 |
| Curing temperature (°C) | ~25 | 60 | ~25 |
| Condition | Classification | ||
| Unsintered | FG | KG | SG |
| Sintered at 900°C | FG900 | KG900 | SG900 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkifli, N.N.I.; Abdullah, M.M.A.B.; Przybył, A.; Pietrusiewicz, P.; Salleh, M.A.A.M.; Aziz, I.H.; Kwiatkowski, D.; Gacek, M.; Gucwa, M.; Chaiprapa, J. Influence of Sintering Temperature of Kaolin, Slag, and Fly Ash Geopolymers on the Microstructure, Phase Analysis, and Electrical Conductivity. Materials 2021, 14, 2213. https://doi.org/10.3390/ma14092213
Zulkifli NNI, Abdullah MMAB, Przybył A, Pietrusiewicz P, Salleh MAAM, Aziz IH, Kwiatkowski D, Gacek M, Gucwa M, Chaiprapa J. Influence of Sintering Temperature of Kaolin, Slag, and Fly Ash Geopolymers on the Microstructure, Phase Analysis, and Electrical Conductivity. Materials. 2021; 14(9):2213. https://doi.org/10.3390/ma14092213
Chicago/Turabian StyleZulkifli, Nur Nadiah Izzati, Mohd Mustafa Al Bakri Abdullah, Anna Przybył, Paweł Pietrusiewicz, Mohd Arif Anuar Mohd Salleh, Ikmal Hakem Aziz, Dariusz Kwiatkowski, Marcin Gacek, Marek Gucwa, and Jitrin Chaiprapa. 2021. "Influence of Sintering Temperature of Kaolin, Slag, and Fly Ash Geopolymers on the Microstructure, Phase Analysis, and Electrical Conductivity" Materials 14, no. 9: 2213. https://doi.org/10.3390/ma14092213
APA StyleZulkifli, N. N. I., Abdullah, M. M. A. B., Przybył, A., Pietrusiewicz, P., Salleh, M. A. A. M., Aziz, I. H., Kwiatkowski, D., Gacek, M., Gucwa, M., & Chaiprapa, J. (2021). Influence of Sintering Temperature of Kaolin, Slag, and Fly Ash Geopolymers on the Microstructure, Phase Analysis, and Electrical Conductivity. Materials, 14(9), 2213. https://doi.org/10.3390/ma14092213







