Utilization of Red Mud as a Source for Metal Ions—A Review
Abstract
1. Introduction
2. Sources and Utilization of Red Mud
2.1. Relevant Sources for Literature Review
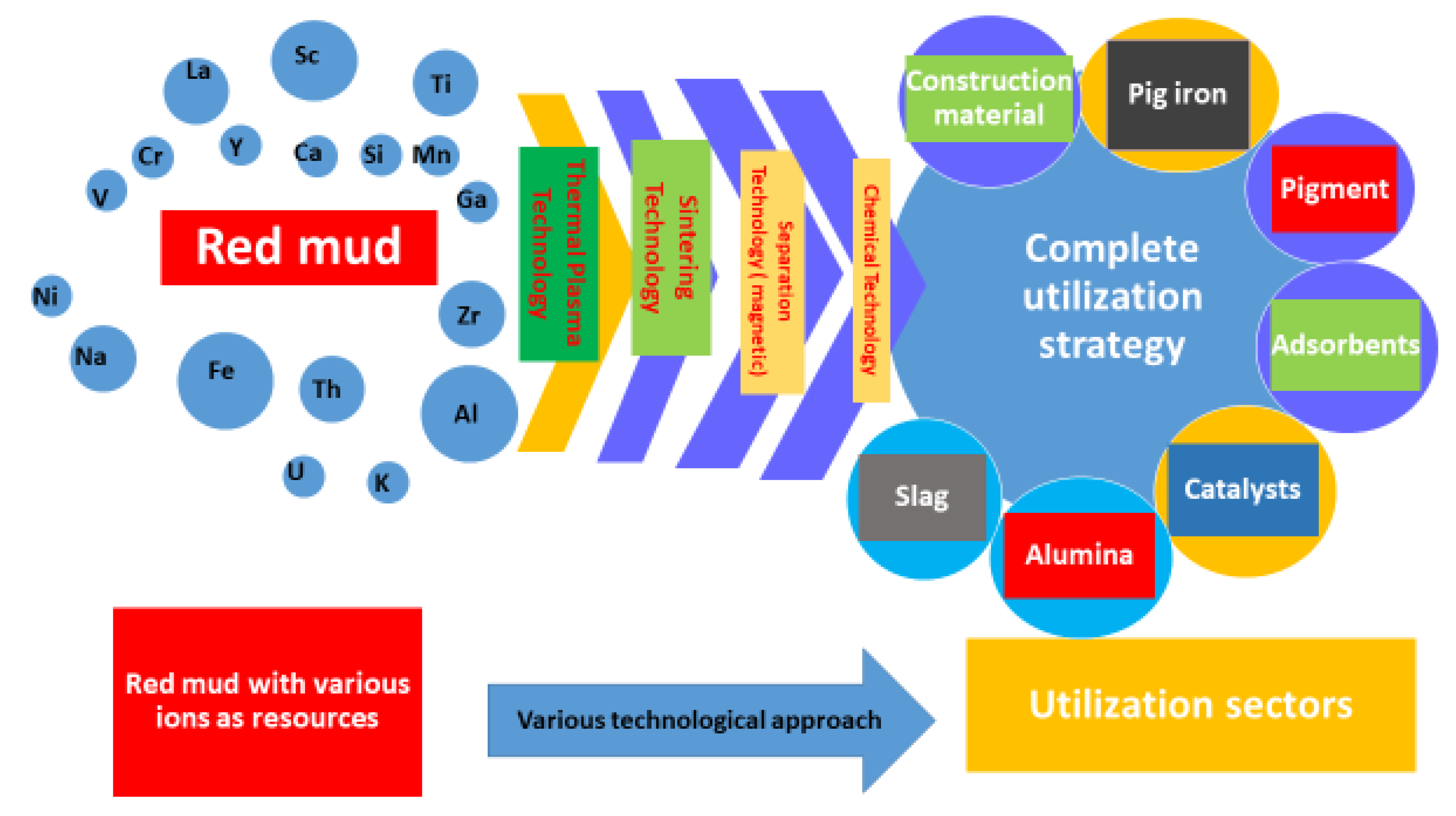
2.2. Utilization of Red Mud as Metal Resource
2.3. Sources of Metal Ions
3. Physical and Chemical Properties of Red Mud
3.1. Particle Size Distribution and pH of Red Mud
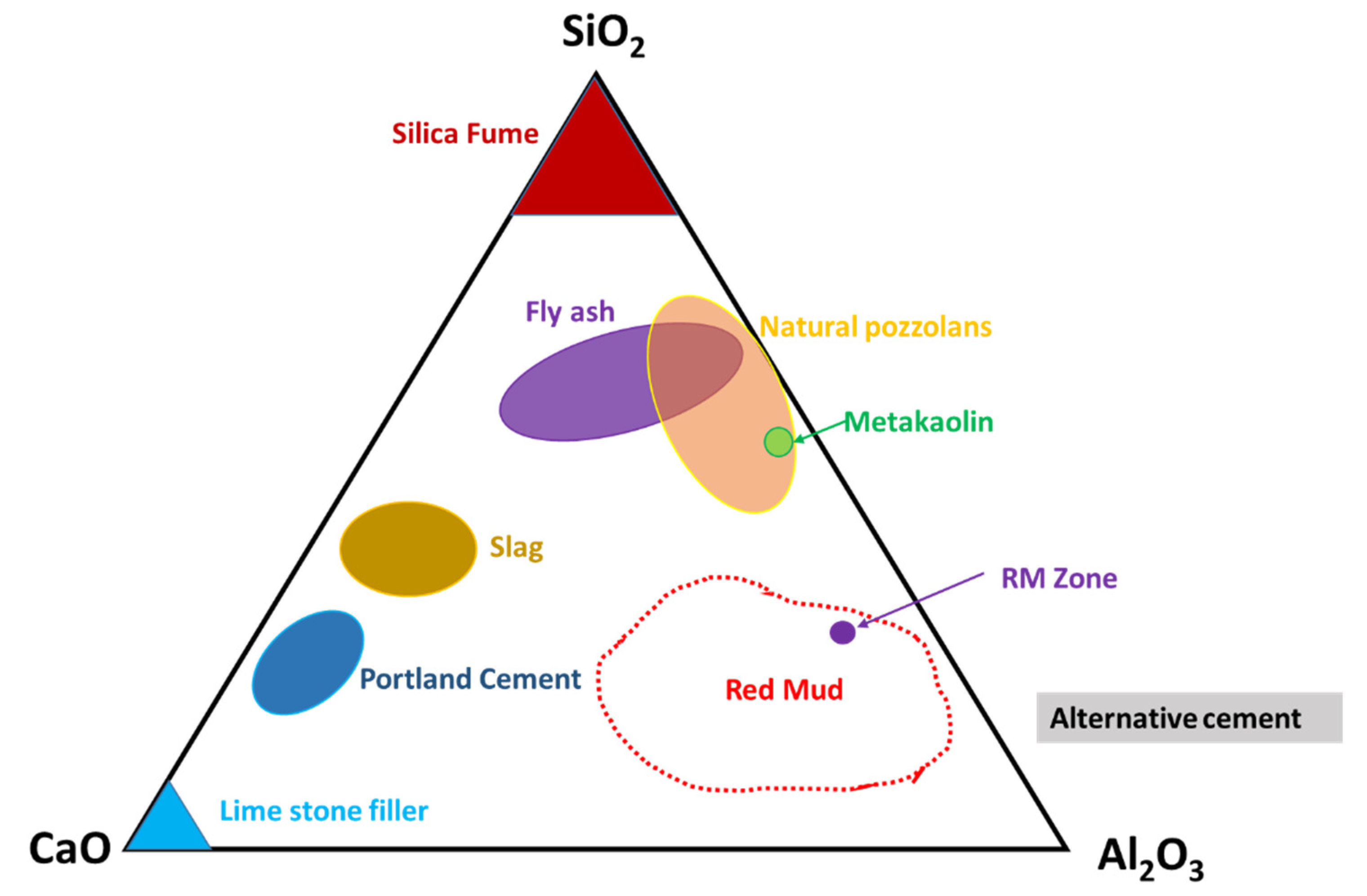
3.2. Ternary Phase Diagram of the CaO–Al2O3–SiO2 System
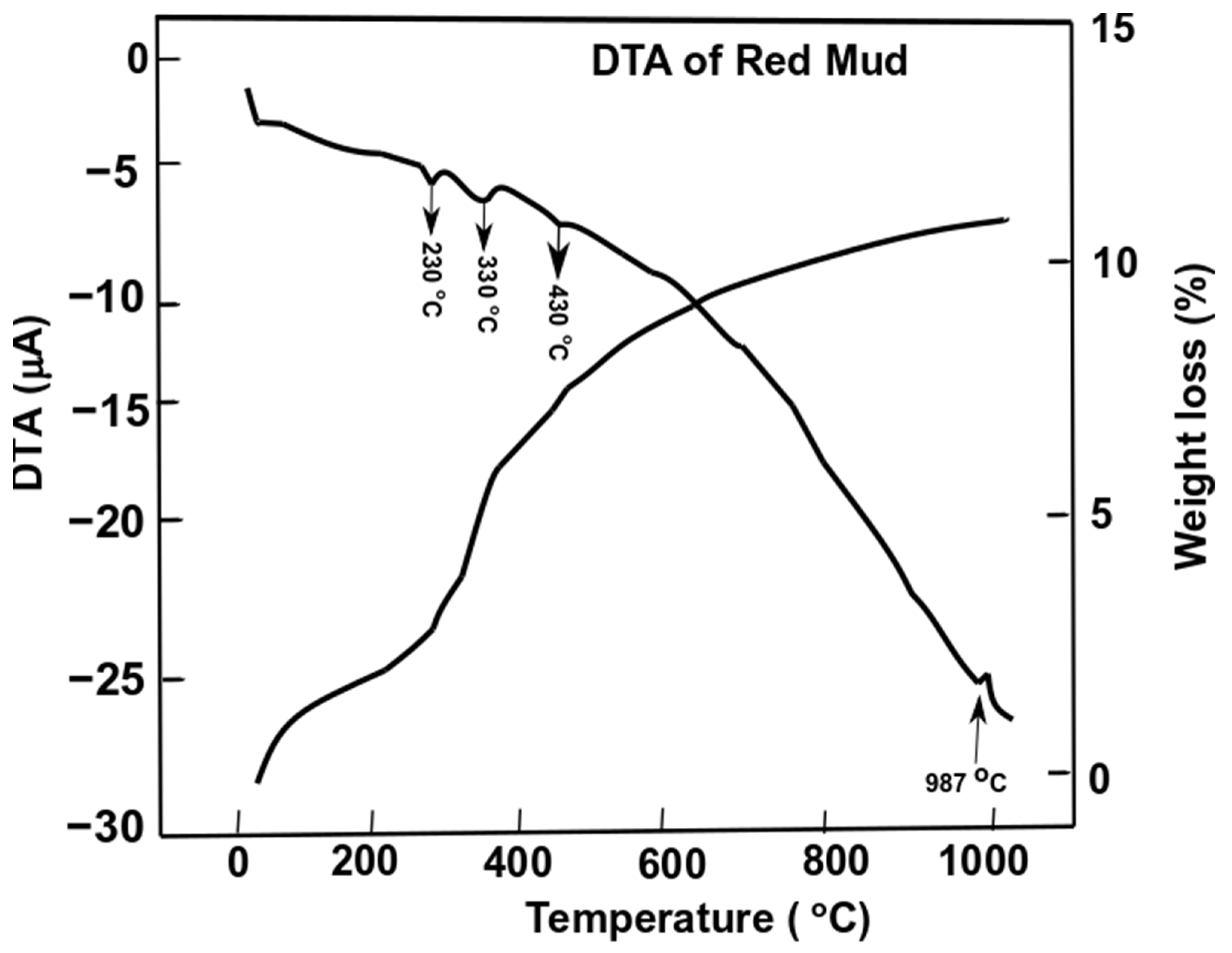
3.3. Phase Transformation during Thermal Decomposition
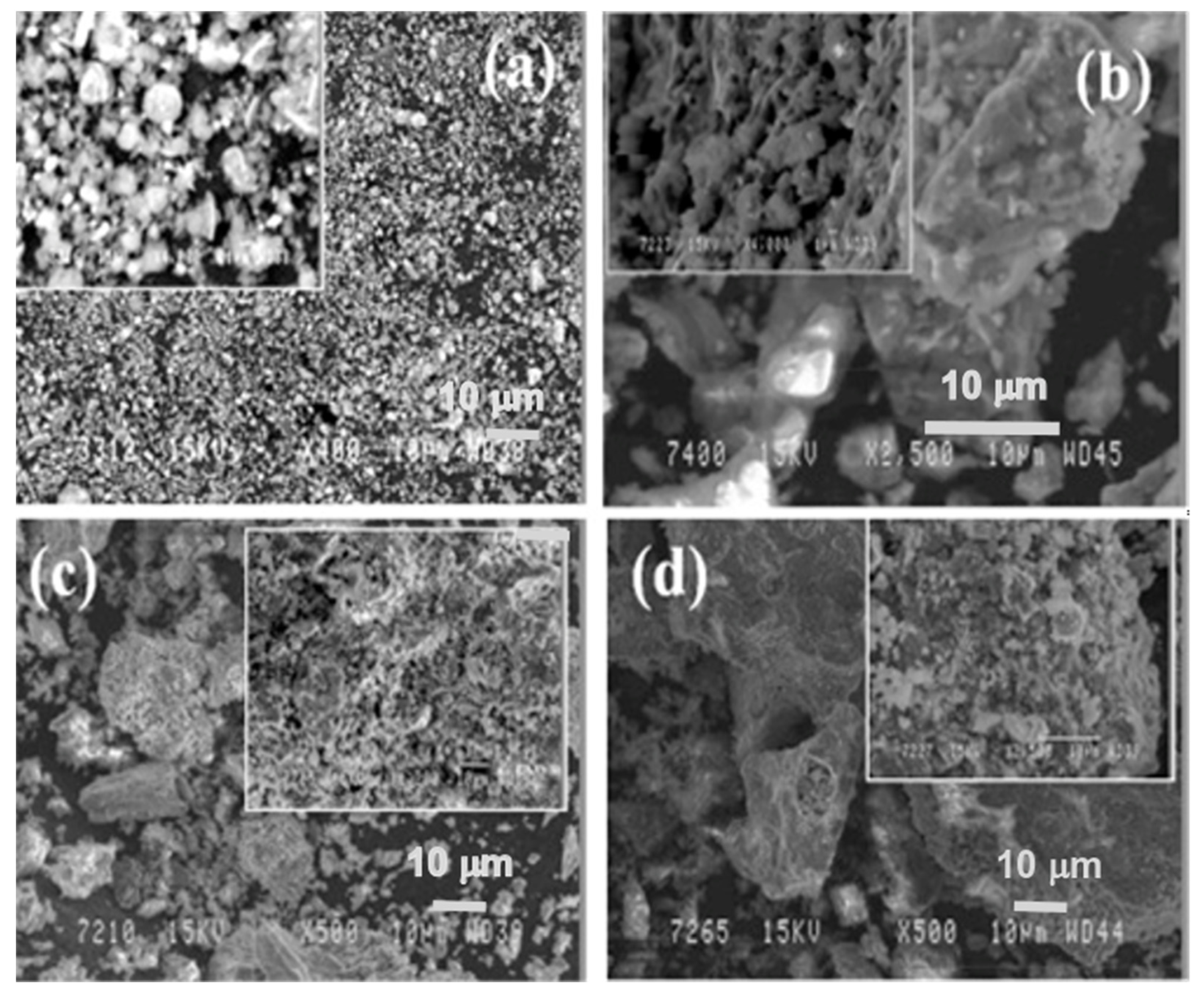
3.4. Microstructure of Sintered Compound at 1100 °C Temperature
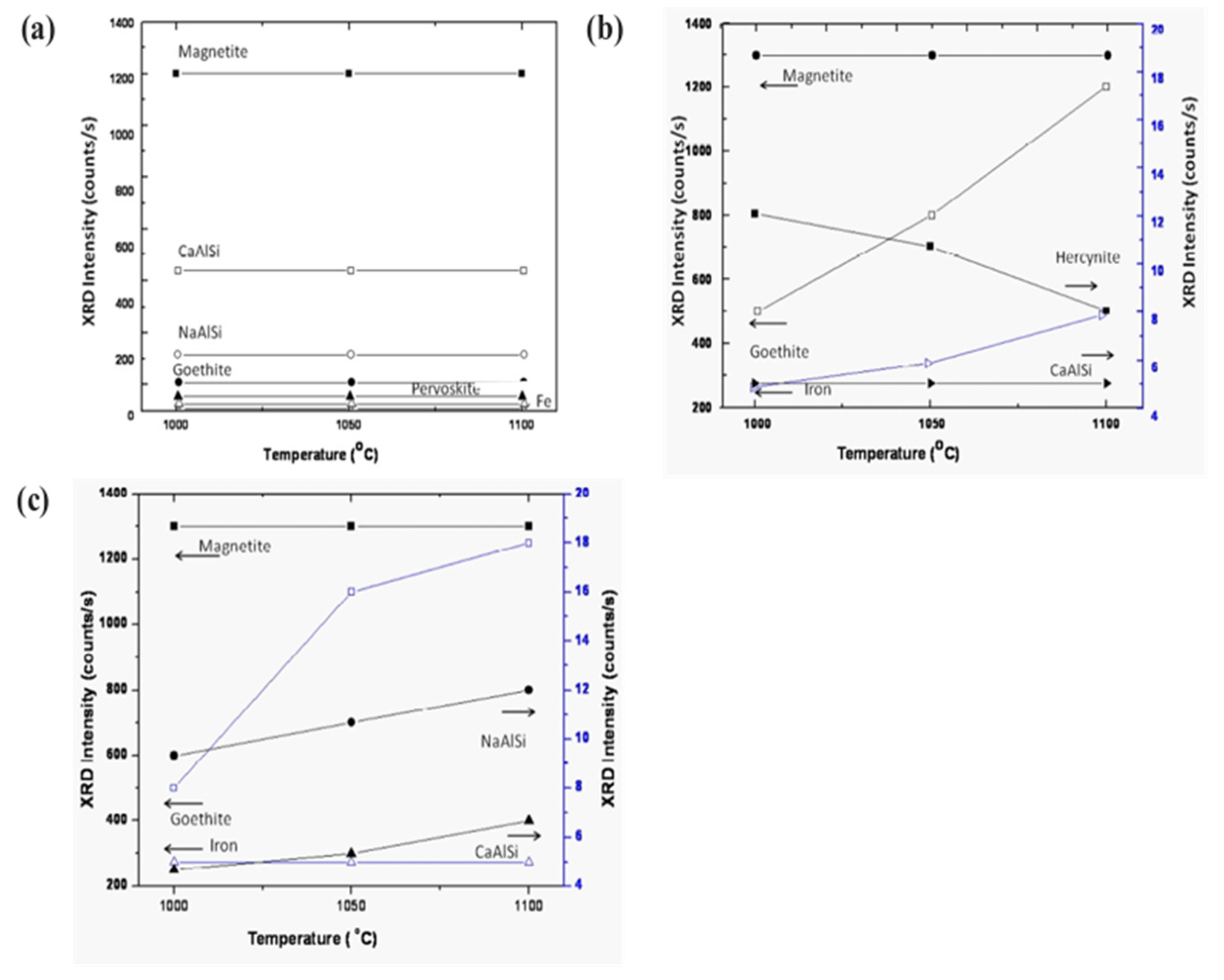
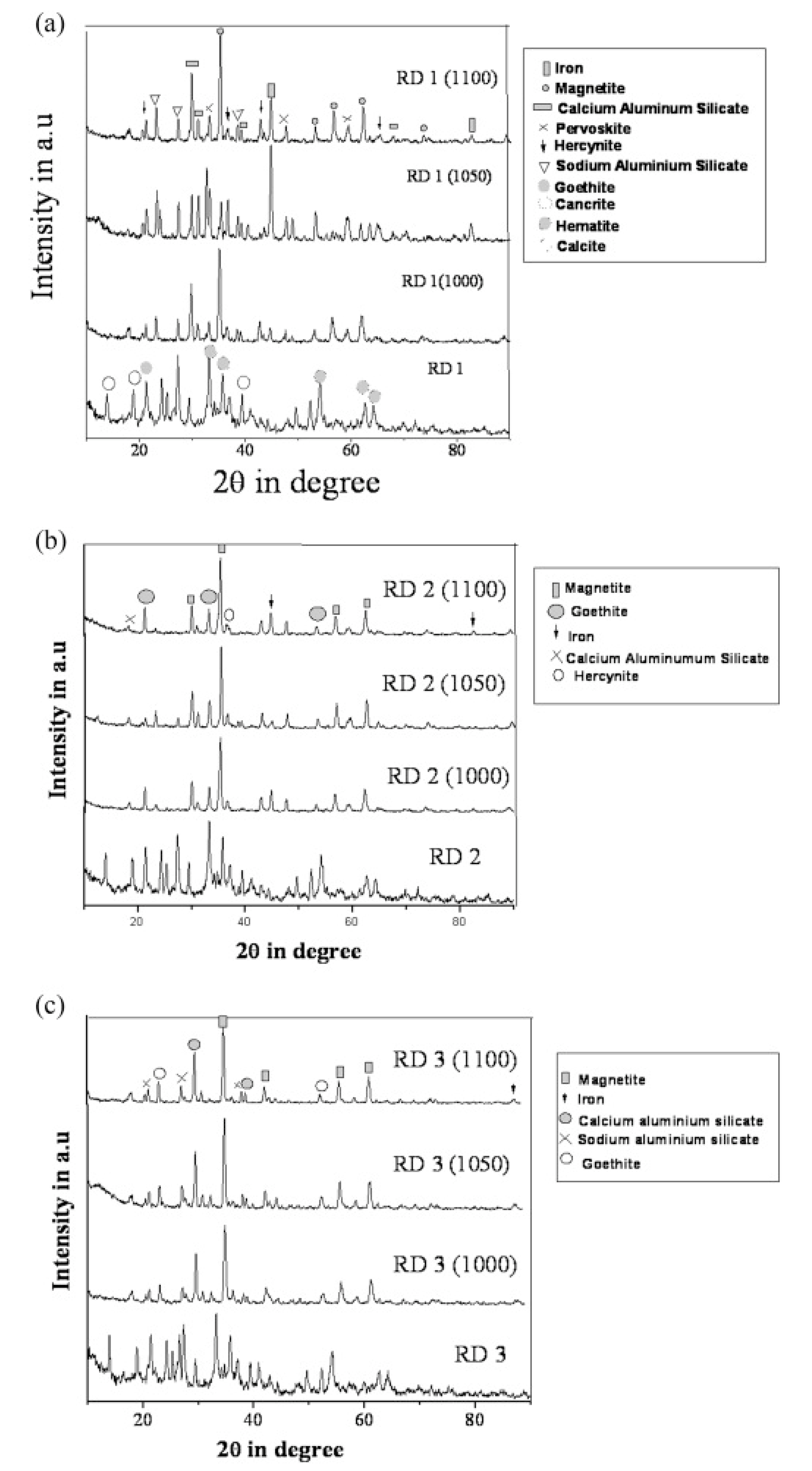
3.5. Phase Evolution of Sintered Sample as a Function of Temperature
3.6. Carbo-Thermal Smelting Technology
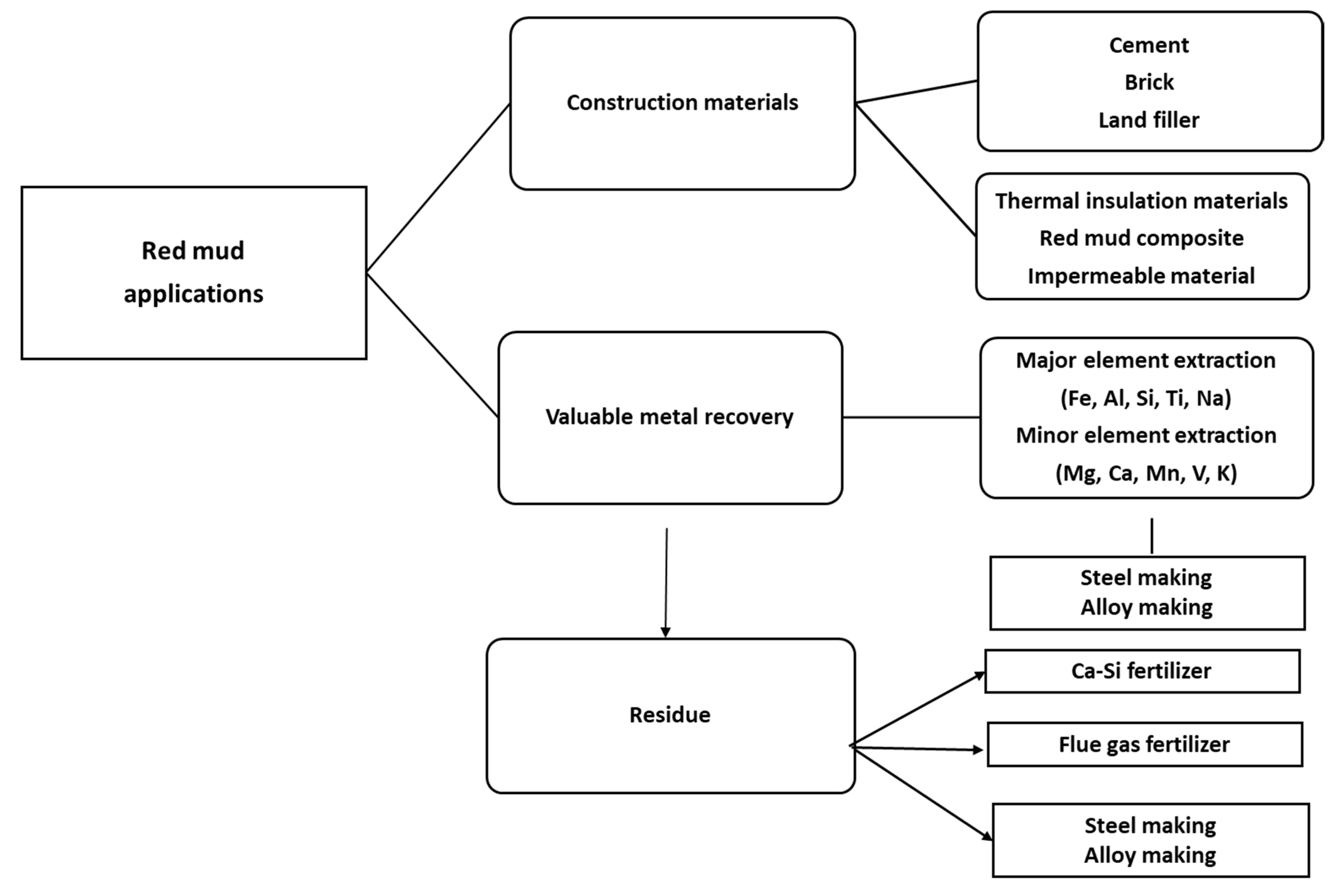
4. Fields of Application of Red Mud
4.1. Thermal Plasma Technology for the Production of By-Products from Red Mud
4.2. Mixing Technology for Use of Red Mud as an Additive for Construction Materials
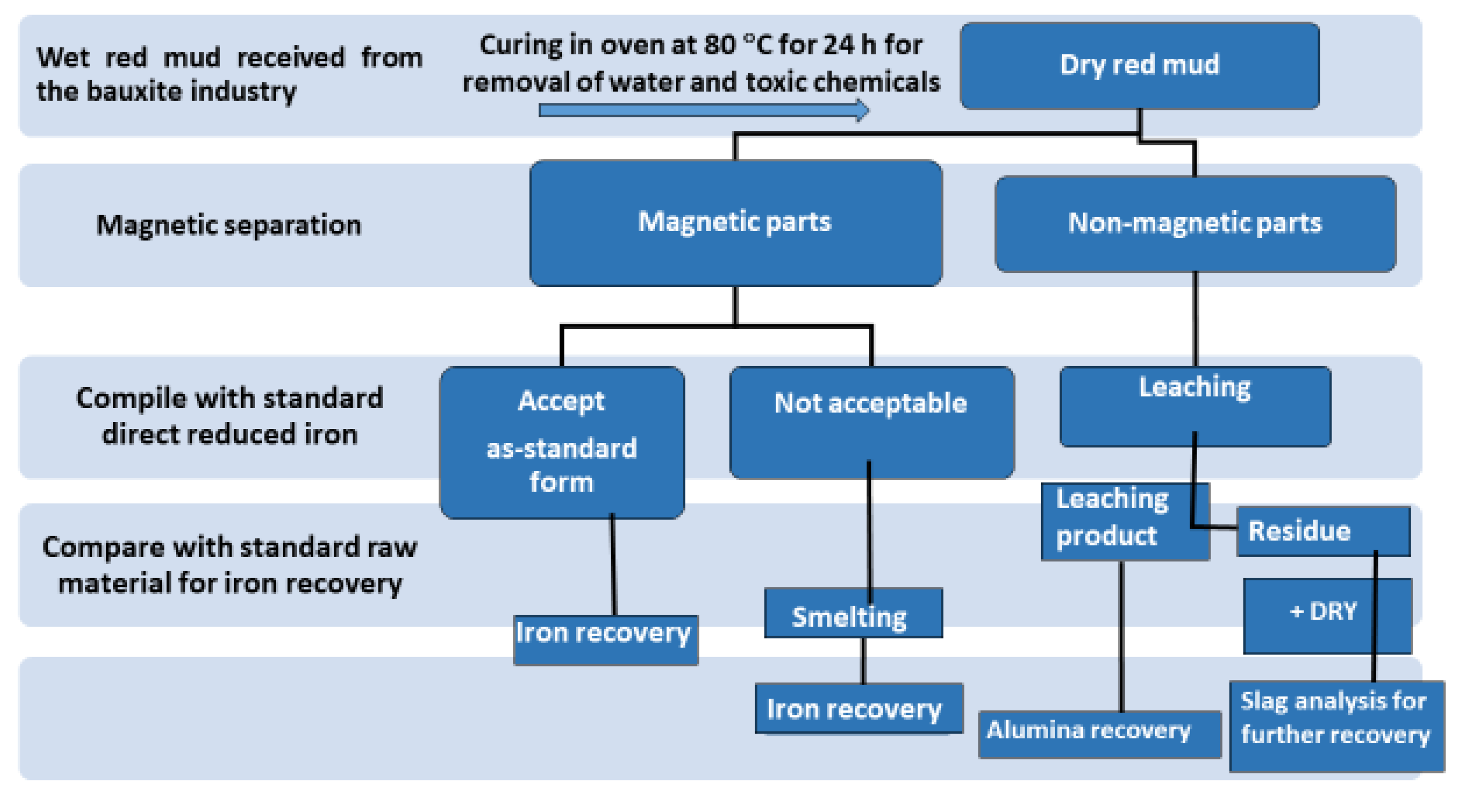
4.3. Separation and Extraction Technology
4.4. Coating Technology
4.5. Economic and Social Impact
4.6. Value Recovery and Strategic Utilization
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, S.; Bahadure, S.; Chaddha, M.J.; Agnihotri, A. Disposal Practices and Utilization of Red Mud (Bauxite Residue): A Review in Indian Context and Abroad. J. Sustain. Metall. 2020, 6, 1. [Google Scholar] [CrossRef]
- Menzies, N. Seawater Neutralization of Alkaline Bauxite residue and Implications for revegetation. J. Environ. Qual. 2004, 33, 1877. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mocadlo, R.; Zhao, M.; Sisson, R.D., Jr.; Tao, M.; Liang, J. Preparation of a geopolymer from red mud slurry and class F fly ash and its behavior at elevated temperatures. Constr. Build. Mat. 2019, 221, 308. [Google Scholar] [CrossRef]
- Samal, S. Effect of high temperature on the microstructural evolution of fiber reinforced geopolymer composite. Heliyon 2019, 5, e01779. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Barrino, F.; Dal Poggetto, G.; Blanco, I.; Cicala, G.; Ognibene, G.; Recca, G. Mechanical and thermal properties of fly ash-filled geopolymers. J. Therm. Anal. Calorim. 2019, 138, 3267. [Google Scholar] [CrossRef]
- Samal, S.; Ray, A.K.; Bandopadhyay, A. Proposal for resources, utilization and processes of red mud in India—A review. Int. J. Miner. Process. 2013, 118, 43. [Google Scholar] [CrossRef]
- Hammond, K.; Mishra, B.; Apelian, D.; Blanpain, B. CR3 communication: Red mud—A resource or a waste? JOM 2013, 65, 340. [Google Scholar] [CrossRef]
- Tulsidas, H.; Gabriel, S.; Kiegiel, K.; Haneklaus, N. Uranium resources in EU phosphate rock imports. Resour. Policy 2019, 61, 151–155. [Google Scholar] [CrossRef]
- Yao, L.; Gao, W.; Ma, X.; Fu, H. Properties Analysis of Asphalt Binders Containing Bayer Red Mud. Materials 2020, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.T.; Gerven, V. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Choe, G.; Kang, S.; Kang, H. Mechanical Properties of Concrete Containing Liquefied Red Mud Subjected to Uniaxial Compression Loads. Materials 2020, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.M.; Cabeza, M.; Tenza-Abril, A.J.; Real-Herraiz, T.; Climent, M.Á.; Sánchez, I. Effects of Red Mud Addition in the Microstructure, Durability and Mechanical Performance of Cement Mortars. Appl. Sci. 2019, 9, 984. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.-Y. Physical and Chemical Properties of Sintering Red Mud and Bayer Red Mud and the Implications for Beneficial Utilization. Materials 2012, 5, 1800–1810. [Google Scholar] [CrossRef]
- Choe, G.; Kang, S.; Kang, H. Characterization of Slag Cement Mortar Containing Nonthermally Treated Dried Red Mud. Appl. Sci. 2019, 9, 2510. [Google Scholar] [CrossRef]
- Rao, P.P. The characteristics and genesis discussion of fracture in dry red mud disposal yard. Ind. Const. 2010, 40, 73–77. [Google Scholar]
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of red mud in geopolymer-based previous concrete with function of adsorption of heavy metal ions. J. Clean. Prod. 2019, 207, 789. [Google Scholar] [CrossRef]
- Ascensao, G.; Seabra, M.P.; Aguiar, B.J. Labrincha. J.A. Red mud-based geopolymers with tailored alkali diffusion properties and pH buffering ability. J. Clean. Prod. 2017, 148, 23. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Karimi, Z.; Allahverdi, A. Recycling of red mud for value-added applications: A comprehensive Review. Encyl. Renew. Sust. Mat. 2020, 2, 561. [Google Scholar]
- Guo, Y.-H.; Gao, J.-J.; Xu, H.-J.; Zhao, K.; Shi, X.-F. Nuggets production by direct reduction of high Iron red mud. J. Iron Steel Res. Int. 2013, 20, 24–27. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, J.; Premchand, P. Utilization of iron values of red mud for metallurgical applications. Environ. Waste Manag. 1998, 108–119. Available online: https://core.ac.uk/download/pdf/297716085.pdf (accessed on 10 April 2021).
- Kumar, S.; Kumar, R.; Bandopadhyay, A. Innovative methodologies for the utilization of wastes from metallurgical and allied industries. Resour. Conserv. Recycl. 2006, 48, 301–314. [Google Scholar] [CrossRef]
- Jayasankar, K.; Ray, P.K.; Chaubey, A.K.; Padhi, A.; Satapathy, B.K.; Mukherjee, P.S. Production of pig iron from red mud waste fines using thermal plasma technology. Int. J. Miner. Metall. Mater. 2012, 19, 679–684. [Google Scholar] [CrossRef]
- Lim, K.; Shon, B. Metal Components (Fe, Al and Ti) recovery from red mud by sulfuric acid leaching assisted with ultrasonic waves. Int. J. Emerg. Technol. Adv. Eng. 2015, 5, 25–32. [Google Scholar]
- Voßenkaul, D.; Birich, A.; Müller, N.; Stoltz, N.; Friedrich, B. Hydrometallurgical processing of eudialyte bearing concentrates to recover rare earth elements via low-temperature dry digestion to prevent the silica gel formation. J. Sustain. Metall. 2017, 3, 79–89. [Google Scholar] [CrossRef]
- Schwarzenbach, G.; Muehlebach, J.; Mueller, K. Peroxo complexes of titanium. Inorg. Chem. 1970, 9, 2381–2390. [Google Scholar] [CrossRef]
- Antonijević, M.; Dimitrijević, M.; Janković, Z. Leaching of pyrite with hydrogen peroxide in sulphuric acid. Hydrometallurgy 1997, 46, 71–83. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Dittrich, C.; Friedrich, B. Precipitation Trends of Scandium in Synthetic Red Mud Solutions with Different Precipitation Agents. J. Sustain. Metall. 2017, 3, 90–98. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.C.; Sahoo, S.K.; Maharana, H. Progress of red mud utilization: An overview. Am. Chem. Sci. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Rickhov, V.; Botalov, M.; Kirilov, E.; Kirilov, S.; Semenishchev, V.; Bunkov, G.; Smyshlyaev, D. The investigation of sulphuric acid sorption recovery of scandium and uranium from the red mud of alumina production. Hydrometallurgy 1997, 45, 249–259. [Google Scholar]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Ning, G.; Zhang, B.; Liu, C.; Li, S.; Ye, Y.; Jiang, M. Large-Scale Consumption and Zero-Waste Recycling Method of Red Mud in Steel Making Process. Minerals 2018, 8, 102. [Google Scholar] [CrossRef]
- Pascual, J.; Corpas, F.; López-Beceiro, J.; Benítez-Guerrero, M.; Artiaga, R. Thermal characterization of a Spanish red mud. J. Therm. Anal. Calorim. 2009, 96, 407–412. [Google Scholar] [CrossRef]
- Mauskar, J. Assessement of Utilization of Industrial Solid Waste in Cement Manufacturing; Central Pollution Control board: Delhi, India, 2006. [Google Scholar]
- Pratt, K.C.; Christoverson, V. Hydrogenatation of a model hydrogen-donor system using activated red mud catalyst. Fuel 1982, 61, 460–462. [Google Scholar] [CrossRef]
- Halász, J.; Hodos, M.; Hannus, I.; Tasi, G.; Kiricsi, I. Catalytic detoxification of C2-chlorohydrocarbons over iron-containing oxide and zeolite catalysts. Colloids Surf. A 2005, 265, 171–177. [Google Scholar] [CrossRef]
- Rivera, R.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Gerven, T. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Koumanova, B.; Drame, M.; Popangelova, M. Phosphate removal from aqueous solutions using red mud wasted in bauxite Bayer’s process. Resour. Conserv. Recy. 1997, 19, 11–20. [Google Scholar] [CrossRef]
- Pera, J.; Boumaza, R.; Ambroise, J. Development of a pozzolanic pigment from red mud. Cem. Concr. Res. 1997, 27, 1513–1522. [Google Scholar] [CrossRef]
- Collazo, A.; Fernández, D.; Izquierdo, M.; Nóvoa, X.R.; Pérez, C. Evaluation of red mud as surface treatment for carbon steel painting. Prog. Org. Coat. 2005, 52, 351–358. [Google Scholar] [CrossRef]
- Park, S.J.; Jun, B.R. Improvement of red mud polymer-matrix nanocomposites by red mud surface treatment. J. Colloid Interface Sci. 2005, 284, 204–209. [Google Scholar] [CrossRef]
- Chunming, G.; Nanr, Y. Effect of phosphate on the hydration of alkali-activated red mud–slag cementitious material. Cem. Concr. Res. 2000, 30, 1013–1016. [Google Scholar]
- Ochsenkuhn, P.M.; Lyberropulu, T.; Ochsenkuhn, K.M.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Buchanan, V.E.; Oliver, G. The potential application of red mud in the production of castings. Mater. Sci. Eng. A 2006, 420, 250–253. [Google Scholar] [CrossRef]
- Li, P.; Miser, D.E.; Rabiei, S.; Yadav, R.T.; Hajaligol, M.R. The removal of carbon monoxide by iron oxide nanoparticles. Appl. Catal. B 2003, 43, 151–162. [Google Scholar] [CrossRef]
- Lopez, E.; Soto, B.; Arias, M.; Nunez, A.; Rubinos, D.; Barral, T. Adsorbent properties of red mud and its use for wastewater treatment. Water Res. 1998, 32, 1314–1322. [Google Scholar] [CrossRef]
- Altundoğan, H.S.; Altundoğan, S.; Tümen, F.; Bildik, M. Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag. 2000, 20, 761–767. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bartzas, G.; Paspaliaris, I. Efficiency of limestone and red mud barriers: Laboratory column studies. Miner. Eng. 2004, 17, 183–194. [Google Scholar] [CrossRef]
- Bertocchi, A.F.; Ghiani, M.; Peretti, R.; Zucca, A. Red mud and fly ash for mine sites contaminated with As, Cd, Cu, Pb and Zn. J. Hazard. Mater. 2006, 134, 112–119. [Google Scholar] [CrossRef]
- Genc, H.; Tjell, J.C.; McConchie, D.; Schuiling, O. Adsorption of arsenate from water using neutralized red mud. Colloid Interface Sci. 2003, 264, 327–334. [Google Scholar] [CrossRef]
- Yalcin, N.; Sevinc, V. Utilization of bauxite waste in ceramic glazes. Ceram. Int. 2000, 26, 485–490. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization. Constr. Build. Mat. 2013, 38, 865. [Google Scholar] [CrossRef]
- Chen, X.; Lu, A.; Qu, G. Preparation and characterization of foam ceramics from red mud and fly ash using sodium silicate as foaming agent. Ceram. Int. 2013, 39, 1923. [Google Scholar] [CrossRef]
- Samal, S.; Ray, A.K.; Bandopadhyay, A. Characterization and microstructure observation of sintered red mud–fly ash mixtures at various elevated temperatures. J. Clean. Prod. 2015, 101, 368. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Samal, S. Study of Porosity on Titania Slag Obtained by Conventional Sintering and Thermal Plasma Process. JOM 2016, 68, 3000. [Google Scholar] [CrossRef]
- Blanco, I.; Cicala, G.; Tosto, C.; Recca, G.; Dal Poggetto, G.; Catauro, M. Kinetic study of the thermal dehydration of fly ash filled Geopolymers. Macromol. Symp. 2020. accepted. [Google Scholar] [CrossRef]
- Samal, S. High temperature oxidation of Metals. InTech Open 2016, 6, 101–121. [Google Scholar] [CrossRef]
- Samal, S. Thermal plasma technology: The prospective future in material processing. J. Clean. Prod. 2017, 142, 3131. [Google Scholar] [CrossRef]
- Samal, S. Thermal Plasma Processing of Materials: High Temperature Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Gomez, E.; Amutha Rani, D.; Cheeseman, C.R.; Deegan, D.; Wise, M.; Boccaccini, A.R. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mat. 2009, 161, 614. [Google Scholar] [CrossRef]
- Xiaoming, L.; Na, Z. Utilization of red mud in cement production: A review. Waste Manag. Res. 2011, 29, 1053. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Campostrini, R.; Maurina, S.; Carturan, G.; Monagheddu, M.; Budroni, G.; Cocco, G. Bauxite “red mud” in the ceramic industry. Part 1: Thermal behavior. J. Eur. Ceram. Soc. 2000, 20, 235. [Google Scholar] [CrossRef]
- Chen, R.; Cai, G.; Dong, X.; Mi, D.; Puppala, A.J.; Duan, W. Mechanical properties and micro mechanism of loess roadbed filling using by product red mud as a partial alternative. Constr. Build. Mater. 2019, 216, 188. [Google Scholar] [CrossRef]
- Alam, S.; Das, S.K.; Rao, B.H. Strength and durability characteristic of alkali activated GGBS stabilized red mud as geo-material. Constr. Build. Mater. 2019, 211, 932. [Google Scholar] [CrossRef]
- Samal, S.; Thanh, N.P.; Marvalova, B.; Petrikova, I. Thermal characterization of metakaolin-based geopolymer. JOM 2017, 69, 2480–2484. [Google Scholar] [CrossRef]
- Jakob, A.; Stucki, S.; Kuhn, P. Evaporation of hevy-metals during the heat treament of municipal solid waste incinerator fly ash. Environ. Sci. Technol. 1995, 29, 2429. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.C.; Wang, Z.; Liu, Y.; Cui, H.Z. Influence of red mud on fresh and hardened properties of self-compacting concrete. Construct. Build. Mater. 2018, 178, 288. [Google Scholar] [CrossRef]
- Patel, S.; Pal, B. Current status of industrial waste: Red mud an overview. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2015, 4, 1–16. Available online: https://www.ijltemas.in/DigitalLibrary/Vol.4Issue8/01-16.pdf (accessed on 10 April 2021).
- Xue, S.G.; Zhu, F.; Kong, X.F.; Wu, C.; Huang, L.; Huang, N.; Hartley, W. A review of the characterization and revegetation of bauxite residues (Red mud). Environ. Sci. Pollut. Res. 2016, 23, 1120. [Google Scholar] [CrossRef]
- Geng, C.; Liu, J.; Wu, S.; Jia, Y.; Du, B.; Yu, S. Novel method for comprehensive utilization of MSWI fly ash through co-reduction with red mud to prepare crude alloy and cleaned slag. J. Hazard. Mater. 2020, 384, 121315. [Google Scholar] [CrossRef]
- Geng, C.; Chen, C.; Shi, X.; Wu, S.; Jia, Y.; Du, B.; Liu, J. Recovery of metals from municipal solid waste incineration fly ash and red mud via a co-reduction process. Resour. Conserv. Recycl. 2020, 154, 104600. [Google Scholar] [CrossRef]
- Okada, T.; Tomikawa, H. Efficiencies of metal separation and recovery in ash-melting of municipal solid waste under non-oxidative atmospheres with different reducing abilities. J. Environ. Manag. 2016, 166, 147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, B.; Tang, Y.; Wan, P.; Chen, Y.; Lv, Z. Recycling of iron from red mud by magnetic separation after co-roasting with pyrite. Thermochim. Acta 2014, 588, 11. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Dimas, D.; Maragkos, I.; Panias, D. Utilization of metallurgical solid by-products for the development of inorganic polymeric construction materials. Glob. NEST J. 2009, 11, 127–136. [Google Scholar]
- Geng, C.; Wang, H.; Hu, W.; Li, L.; Shi, C. Recovery of iron and copper from copper tailings by coal-based direct reduction and magnetic separation. J. Iron Steel Res. Int. 2017, 24, 991. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Zhang, Q.; Zhang, P.; Li, A.; Yao, H.; Naruse, I. Sintering characteristics of CaO-rich municipal solid waste incineration fly ash through the addition of Si/Al-rich ash residues. J. Mater. Cycles. Waste 2016, 18, 340. [Google Scholar] [CrossRef]
- Kang, S.; Kang, H.; Lee, B. Effects of Adding Neutralized Red Mud on the Hydration Properties of Cement Paste. Materials 2020, 13, 4107. [Google Scholar] [CrossRef]
- Cardenia, C.; Balomenos, E.; Panias, D. Optimization of Microwave Reductive Roasting Process of Bauxite Residue. Metals 2020, 10, 1083. [Google Scholar] [CrossRef]
- Keller, V.; Stopić, S.; Xakalashe, B.; Ma, Y.; Ndlovu, S.; Mwewa, B.; Simate, G.S.; Friedrich, B. Effectiveness of Fly Ash and Red Mud as Strategies for Sustainable Acid Mine Drainage Management. Minerals 2020, 10, 707. [Google Scholar] [CrossRef]
- Chaikin, L.; Shoppert, A.; Valeev, D.; Loginova, I.; Napol’skikh, J. Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust. Minerals 2020, 10, 500. [Google Scholar] [CrossRef]
- Nie, Q.; Li, Y.; Wang, G.; Bai, B. Physicochemical and Microstructural Properties of Red Muds under Acidic and Alkaline Conditions. Appl. Sci. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Uthayakumar, M.; Arumugaprabu, V. Potential use of industrial waste-red mud in developing hybrid composites: A waste management approach. J. Clean. Prod. 2020, 276, 124278. [Google Scholar] [CrossRef]
- Singh, S.; Aswath, M.U.; Ranganath, R.V. Performance assessment of bricks and prisms: Red mud based geopolymer composite. J. Build. Eng. 2020, 32, 101462. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Wu, C.-S. Stockpiling and Comprehensive Utilization of Red Mud Research Progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef]
- Laskou, M.; Andreou, G. Rare earth elements distribution and REE-minerals from the Parnassos–Ghiona bauxite deposits, Greece. In Proceedings of the 7th Biennial SGA Meeting on Mineral Exploration and Sustainable Development, Athens, Greece, 24–28 August 2003; pp. 89–92. [Google Scholar]
- Samal, S. Effect of shape and size of filler particle on the aggregation and sedimentation behavior of the polymer composite. Powder Technol. 2020, 366, 43–51. [Google Scholar]
- Samal, S.; Vlach, J.; Kolinova, M.; Kavan, P. Micro-computed tomography characterization of isotropic filler distribution in magnetorheological elastomeric composites. In Advanced Processing and Manufacturing Technologies for Nanostructured and Multifunctional Materials III; The American Ceramic Society: Columbus, OH, USA, 2017. [Google Scholar]
- Samal, S.; Škodová, M.; Blanco, I. Effects of filler distribution on magnetorheological silicon-based composites. Materials 2019, 12, 3017. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, S.; Gronen, L.; Stopic, S.; Frierich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.E.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Rare Earth Element Phases in Bauxite Residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, W.; Huang, B.; Shu, X.; He, Q. Synergistic utilization of red mud for flue-gas desulfurization and fly ash based geopolymer preparation. J. Hazard. Mater. 2019, 369, 503. [Google Scholar] [CrossRef]
- Kaußen, F.M.; Friedrich, B. Phase characterization and thermochemical simulation of (landfilled) bauxite residue (“red mud”) in different alkaline processes optimized for aluminum recovery. Hydrometallurgy 2018, 176, 49–61. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. Options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The rare earth elements: Demand, global resources, and challenges for resourcing future generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Rare earths and the balance problem. J. Sustain. Metall. 2015, 1, 29–38. [Google Scholar] [CrossRef]
- Konkanov, M.; Salem, T.; Jiao, P.; Niyazbekova, R.; Lajnef, N. Environment-Friendly, Self-Sensing Concrete Blended with Byproduct Wastes. Sensors 2020, 20, 1925. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.; Tam, J.; Yang, M.; Azimi, G. Technospheric Mining of Rare Earth Elements from Bauxite Residue (Red Mud): Process Optimization, Kinetic Investigation, and Microwave Pretreatment. Sci. Rep. 2017, 7, 15252. [Google Scholar] [CrossRef] [PubMed]
- Samal, S. Preparation of synthetic rutile from pre-treated ilmenite/Ti-rich slag with phenol and resorcinol leaching solutions. Hydrometallurgy 2013, 137, 8–12. [Google Scholar] [CrossRef]
- Alkan, G.; Schier, C.; Gronen, L.; Stopic, S.; Friedrich, B. A Mineralogical Assessment on Residues after Acidic Leaching of Bauxite Residue (Red Mud) for Titanium Recovery. Metals 2017, 7, 458. [Google Scholar] [CrossRef]
- Sayan, E.; Bayramoglu, M. Statistical modeling of sulfuric acid leaching of TiO2 from red mud. Hydrometallurgy 2004, 71, 397–401. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A Review on Comprehensive Utilization of Red Mud and Prospect Analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]



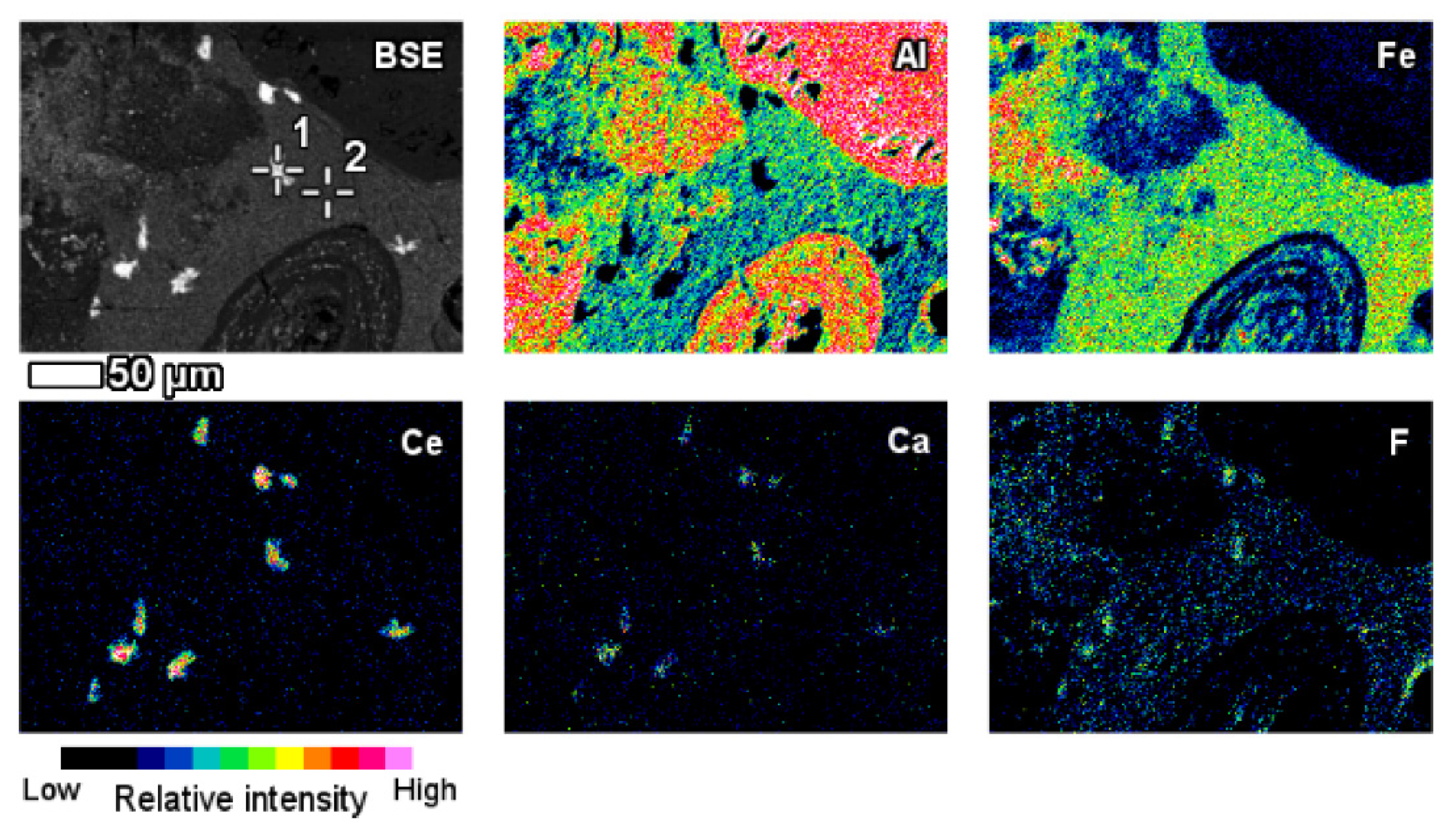
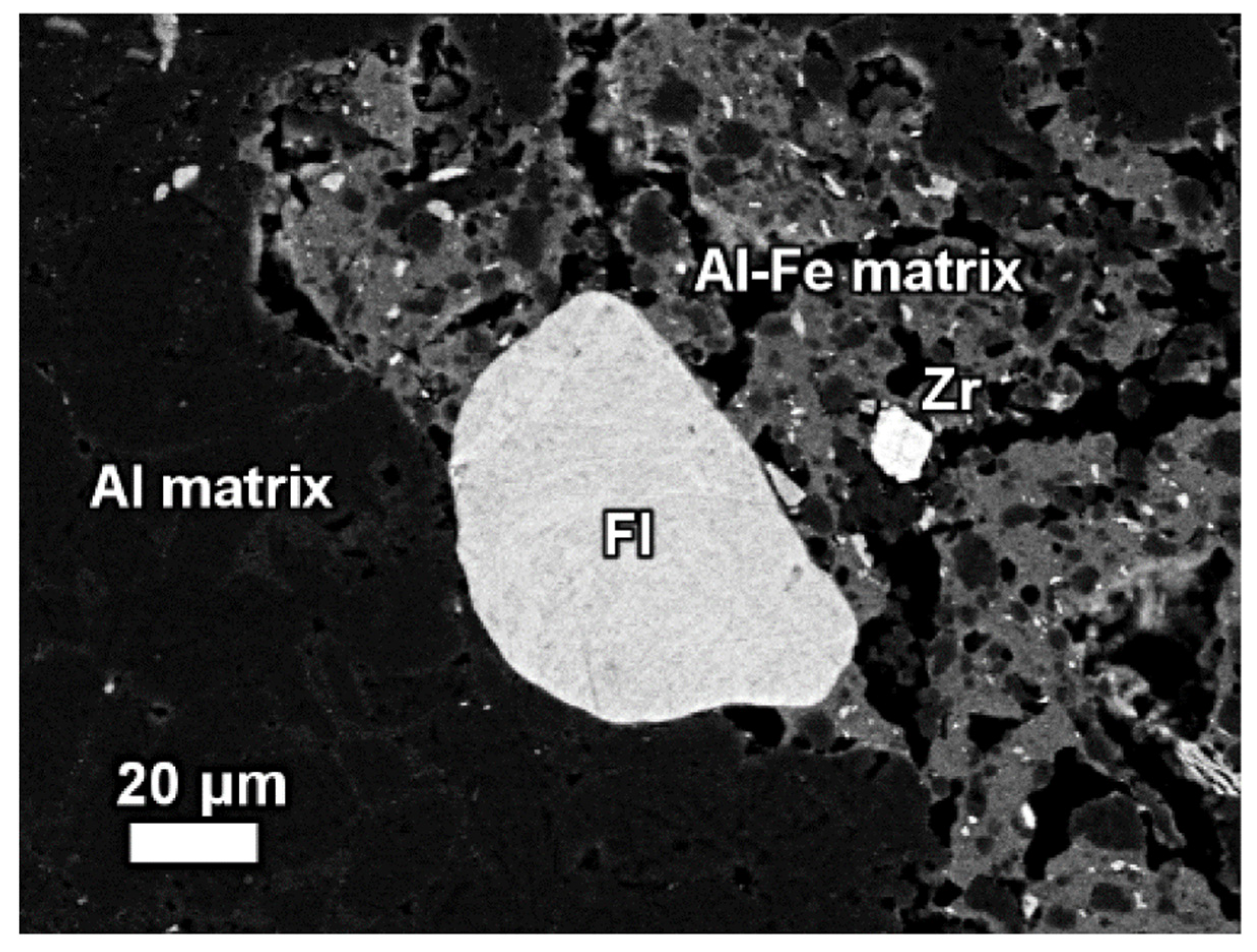
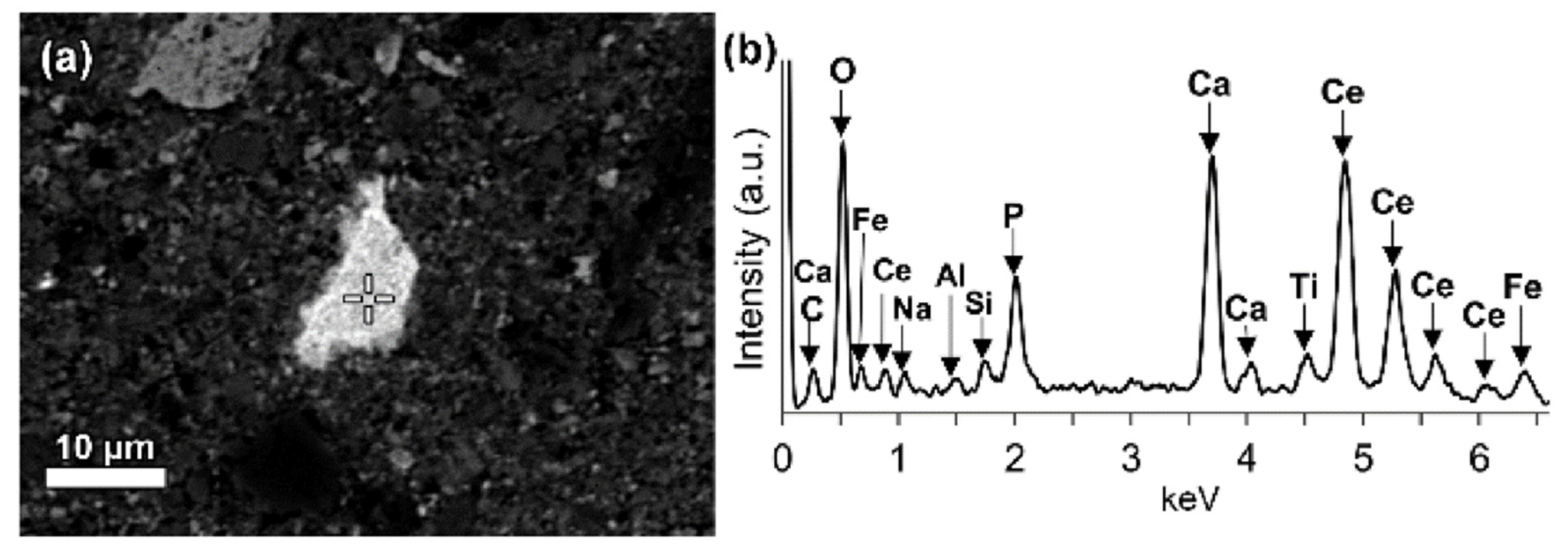
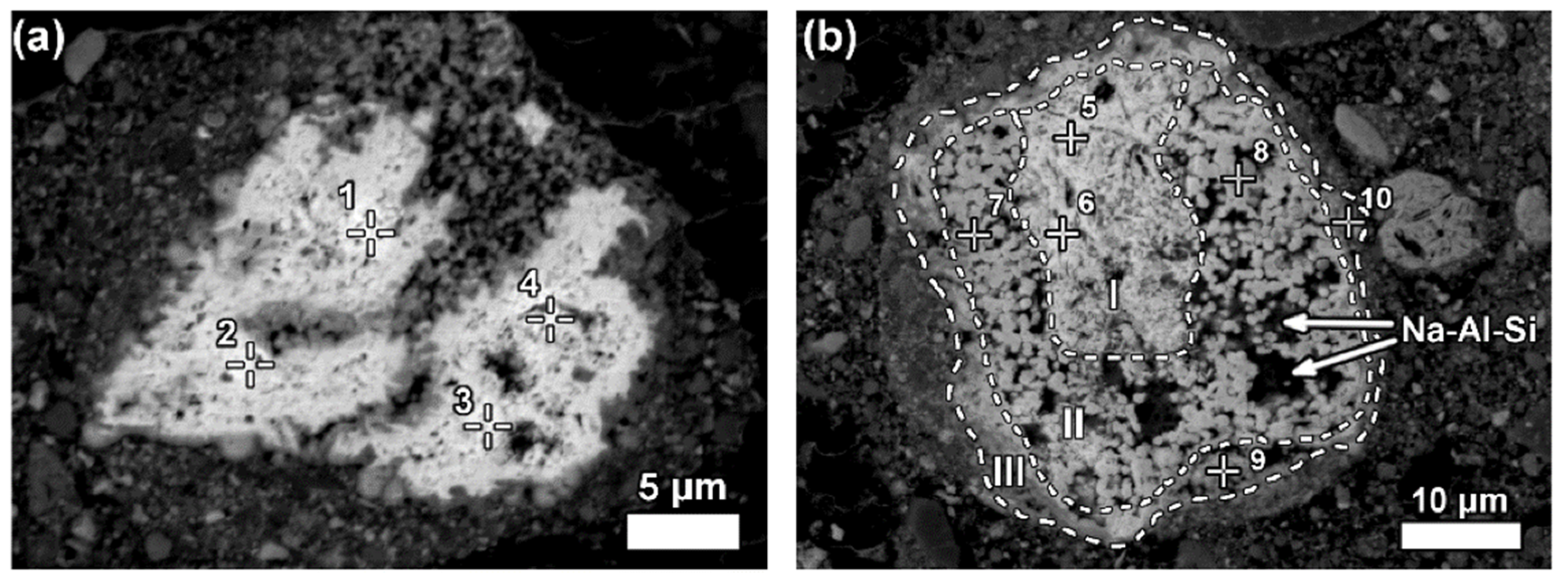
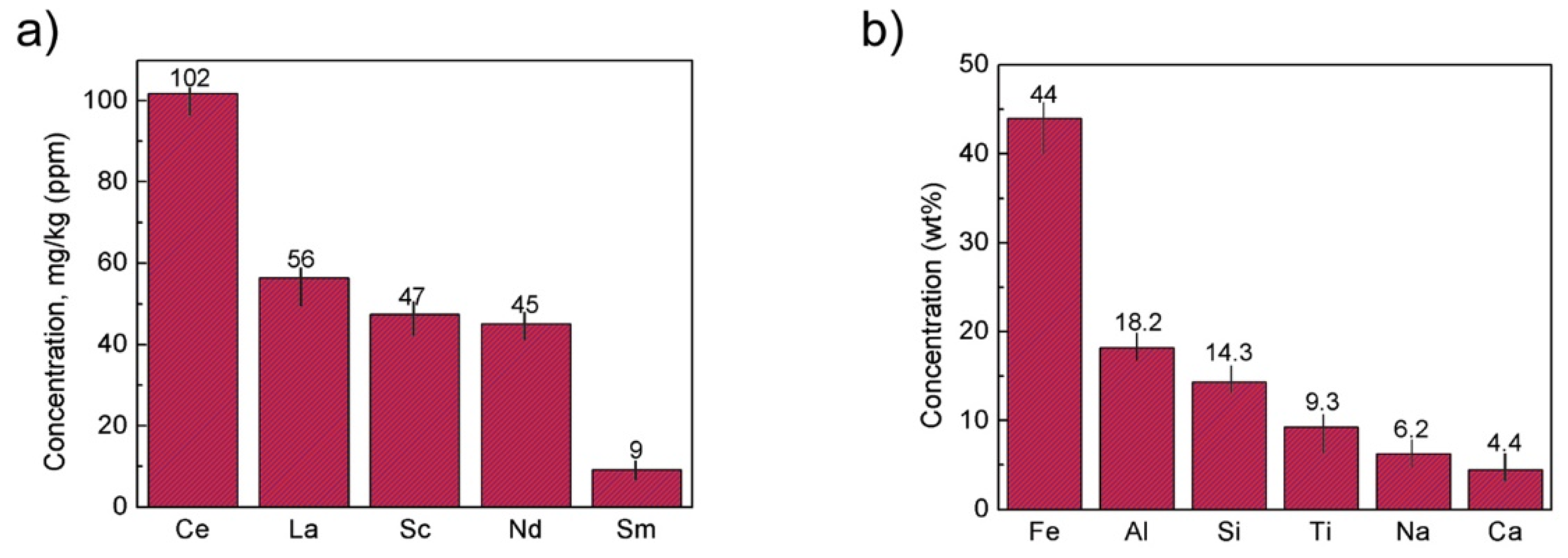
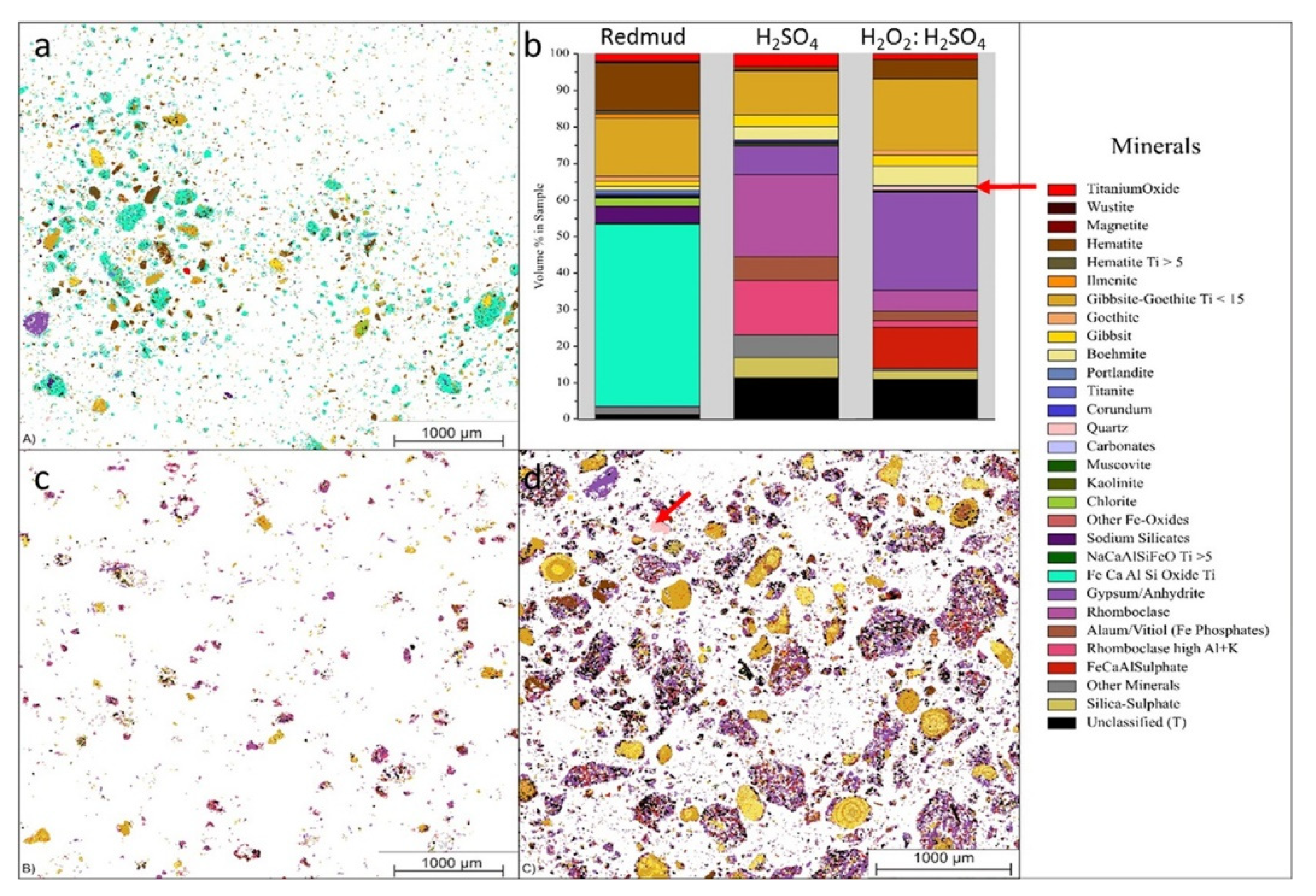
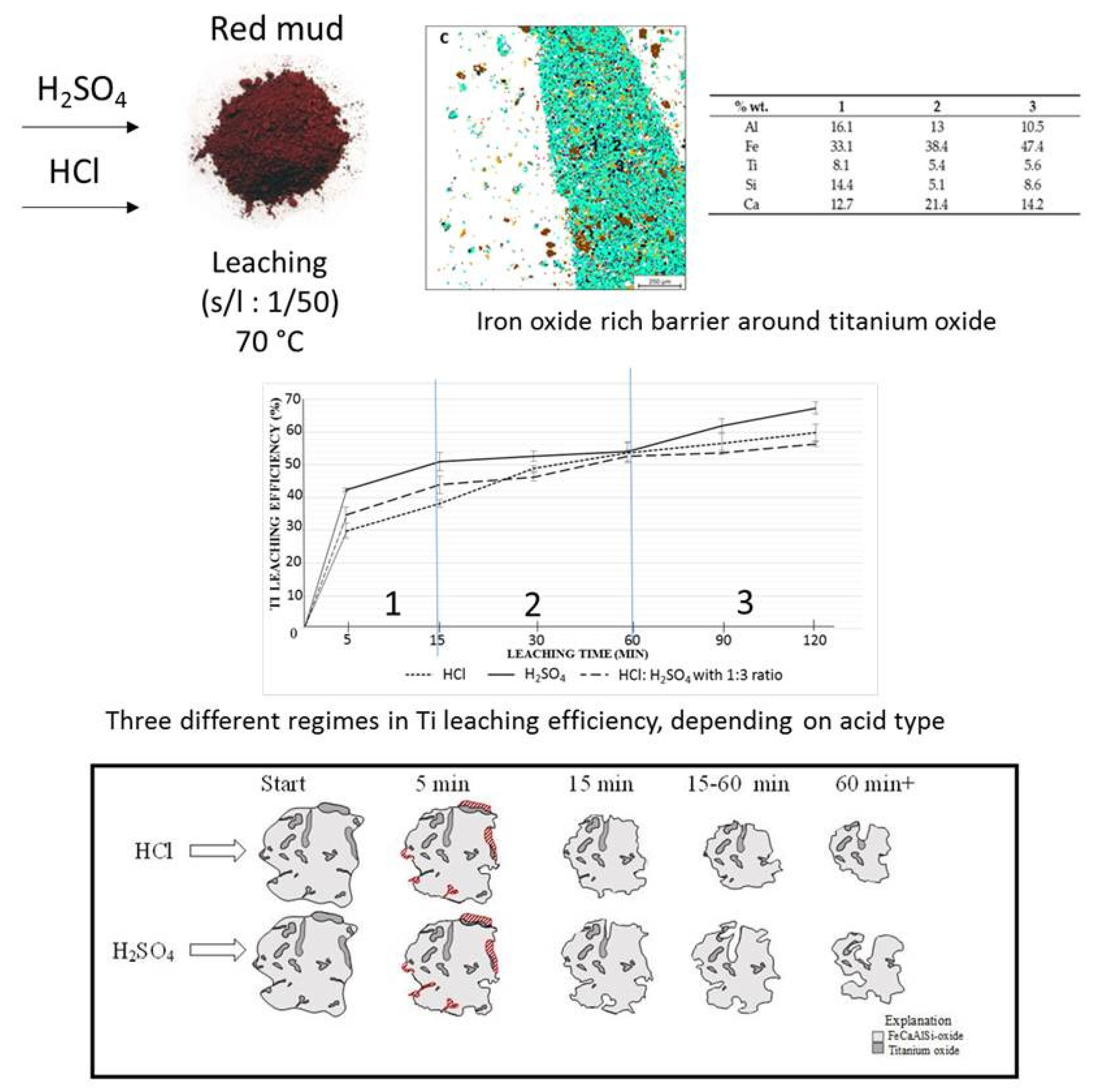
| Red Mud Compositions | ||||
|---|---|---|---|---|
| Major Elements | Wt.% | Minor Elements | Concentration (mg/kg) | Refs. |
| Fe2O3 | 30–60 | U | 50–60 | [31] |
| Al2O3 | 10–20 | Ga | 60–80 | [32,33] |
| SiO2 | 3–50 | V | 730 | [32,33,34,35] |
| Na2O | 2–10 | Zr | 1230 | [36,37] |
| CaO | 2–8 | Sc | 60–120 | [38] |
| TiO2 | 8.50 | Cr | 497 | [38] |
| P2O5 | 0.25 | Mn | 85 | [39] |
| MgO | 0.10 | Y | 60–150 | [39,40] |
| K2O | 0.06 | Ni | 31 | [38] |
| – | – | Zn | 20 | [38] |
| – | – | La | 0.1–1% | [41] |
| – | – | Th | 20–30 | [42] |
| Composition wt.% | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Al2O3 | Fe2O3 | SiO2 | TiO2 | CaO | Na2O | Mn | P2O5 | V2O5 | Gd2O3 | MgO | K2O | LOI | REFs |
| Ajka Aluminum Industry, Hungary | 16–18 | 33–48 | 9–15 | 4–6 | 0.5–3.5 | 8–12 | - | - | 0.2–0.3 | - | 0.3–1 | - | - | [35] |
| Aluminium Pechiney, Gardanne, France | 15.00 | 26.62 | 4.98 | 15.76 | 22.21 | 1.02 | - | -- | - | - | 0.95 | - | 12.10 | [37] |
| Bauxite ore refinery, Guinea | 26.60 | 48.40 | 5.50 | - | 1.30 | - | - | - | - | - | 0.90 | - | 14.60 | [36] |
| ALCOA factory, San Cibrao (Northwest of Spain) | 12.00 | 37.00 | 9.00 | 20.00 | 6.00 | 5.00 | - | - | - | - | - | - | - | [39] |
| Korea Chemical Co. | 23.70 | 16.60 | 22.90 | 6.70 | 6.70 | 11.60 | - | - | - | - | - | - | - | [40] |
| Shandong Aluminium Factory, China | 7.96 | 6.57 | 21.90 | - | 38.84 | 2.32 | - | - | - | - | 1.60 | 0.41 | 17.42 | [41] |
| Greek red mud, Greece | 15.60 | 42.50 | 9.20 | 5.90 | 19.70 | 2.40 | - | - | - | - | - | - | - | [42] |
| Slurry pond from Worsley Alumina, Australia | 15.00 | 60.00 | 5.00 | 5.00 | - | 16.00 | - | - | - | - | - | - | - | [43] |
| Alpart factory and the Alcan Ewartonred mud pond, Jamaica | 18.80 | 45.30 | 4.30 | 6.40 | 3.10 | 1.50 | - | - | - | - | - | - | [44] | |
| Shandong Aluminium Corporation, Shandong, China | 6.93 | 12.76 | 19.14 | 3.43 | 46.02 | 2.37 | - | - | - | - | 1.15 | 1.20 | 5.73 | [45] |
| Alumina-aluminio of San Ciprian, Lugo, Spain | 20.10 | 31.80 | 6.10 | 22.60 | 4.78 | 4.70 | - | - | - | - | 0.20 | 0.03 | [46] | |
| Etibank Seydiehir Aluminium Plant, Konya, Turkey | 20.39 | 36.94 | 15.74 | 4.98 | 2.23 | 10.10 | - | - | 0.05 | - | - | 8.19 | [47] | |
| Aluminium of Greece S.A. | 15.65 | 45.58 | 6.96 | 7.07 | 14.84 | 3.26 | - | - | - | - | - | 0.07 | - | [48] |
| Eurallumina alumina plant, Italy | 17.19 | 30.45 | 9.58 | 8.61 | 7.77 | 12.06 | - | - | - | - | 0.86 | 0.30 | 12.38 | [49] |
| Queensland Alumina Ltd. refinery, Gladstone, Australia | 25.45 | 34.05 | 17.06 | 4.90 | 3.69 | 2.74 | - | - | - | - | 1.86 | 0.20 | - | [50] |
| Seydiehir Aluminium Plant, Konya, Turkey | 14.10 | 38.30 | 2.50 | - | 4.10 | - | - | - | - | - | - | - | [51] | |
| HINDALCO Renukoot, India | 21.9 | 28.1 | 7.5 | 15.6 | 10.2 | 4.5 | – | – | – | – | - | - | 12.2 | [52] |
| IND ALMuri, India | 24.3 | 24.5 | 6.2 | 18.0 | – | 5.3 | – | – | – | – | - | - | – | [52] |
| BALCO Kobra, India | 19.4 | 27.9 | 7.3 | 16.4 | 11.8 | 3.3 | – | – | – | – | - | - | 12.6 | [52] |
| NALCo Damanjodi, India | 14.8 | 54.8 | 6.4 | 3.7 | 2.5 | 4.8 | 1.1 | 0.67 | 0.38 | 0.01 | - | - | 9.5 | [52] |
| INDALBelgam, India | 19.2 | 44.5 | 7.0 | 13.5 | 0.8 | 4.0 | – | – | – | – | -- | - | 10.0 | [52] |
| MALCO Mettur Dam, India | 14.0 | 18.0 | 56.0 | 50.0 | 2.0–4.0 | 6.0–9.0 | – | 1.0–2.0 | – | – | - | - | 12.60 | [52] |
| Country | Annual Output of Red Mud (Million Tons) | Cost (Million EUR)/Year |
|---|---|---|
| Australia | 30 | 373 |
| India | 10 | 125 |
| Brazil | 10.6 | 132 |
| China | 88 | 1100 |
| Greece | 0.7 | 8.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samal, S. Utilization of Red Mud as a Source for Metal Ions—A Review. Materials 2021, 14, 2211. https://doi.org/10.3390/ma14092211
Samal S. Utilization of Red Mud as a Source for Metal Ions—A Review. Materials. 2021; 14(9):2211. https://doi.org/10.3390/ma14092211
Chicago/Turabian StyleSamal, Sneha. 2021. "Utilization of Red Mud as a Source for Metal Ions—A Review" Materials 14, no. 9: 2211. https://doi.org/10.3390/ma14092211
APA StyleSamal, S. (2021). Utilization of Red Mud as a Source for Metal Ions—A Review. Materials, 14(9), 2211. https://doi.org/10.3390/ma14092211






