New Insights into the Crystal Chemistry of Elpidite, Na2Zr[Si6O15]·3H2O and (Na1+YCax□1−X−Y)Σ=2Zr[Si6O15]·(3−X)H2O, and Ab Initio Modeling of IR Spectra
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Description
2.2. Chemical and Structural Analysis
2.3. Calculation Details
3. Results
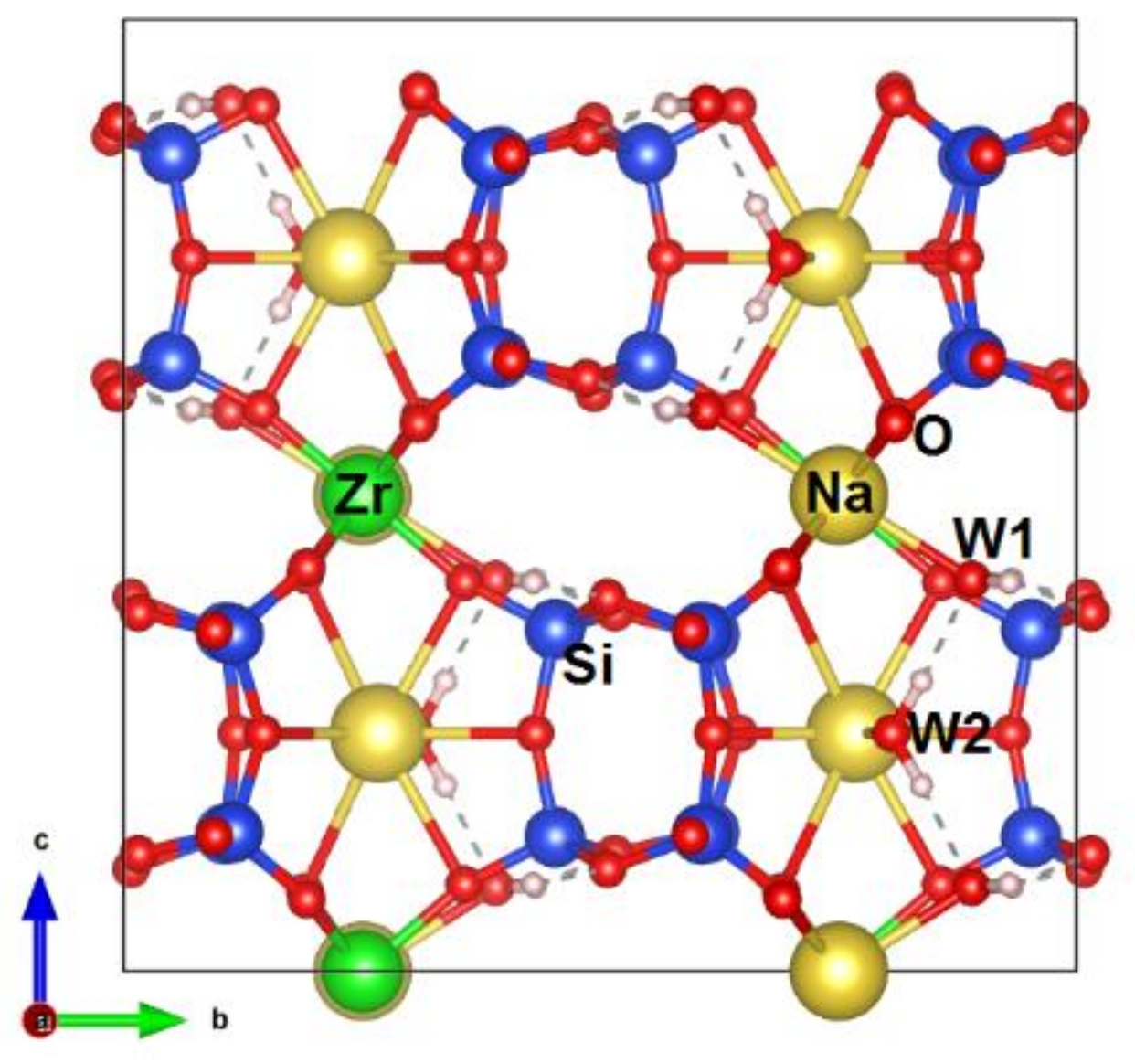
3.1. Chemical Composition and Structure Description
- (Na1.86K0.01)(Zr0.94Hf0.02REE0.02)[Si6O14.79(OH)0.21]·3.10H2O and (Na1.82K0.02Cu0.01)(Zr0.93Hf0.02REE0.01)[Si6O14.71(OH)0.29]·3.06H2O for elpidite samples from Burpala massif;
- (Na1.21Ca0.31Y0.03Fe0.03K0.01Cu0.01Ti0.01Mn0.01)(Zr0.94REE0.02Hf0.01)[Si6O14.94(OH)0.06]·2.94H2O and (Na1.04Ca0.40Y0.01Fe0.01K0.01)(Zr0.95REE0.01Hf0.01)[Si6O14.79(OH)0.21]·2.79H2O for elpidite samples from the Khan-Bogdo massif.
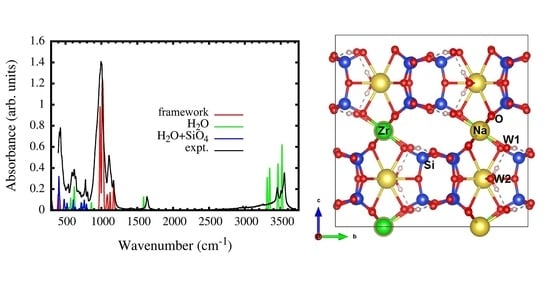
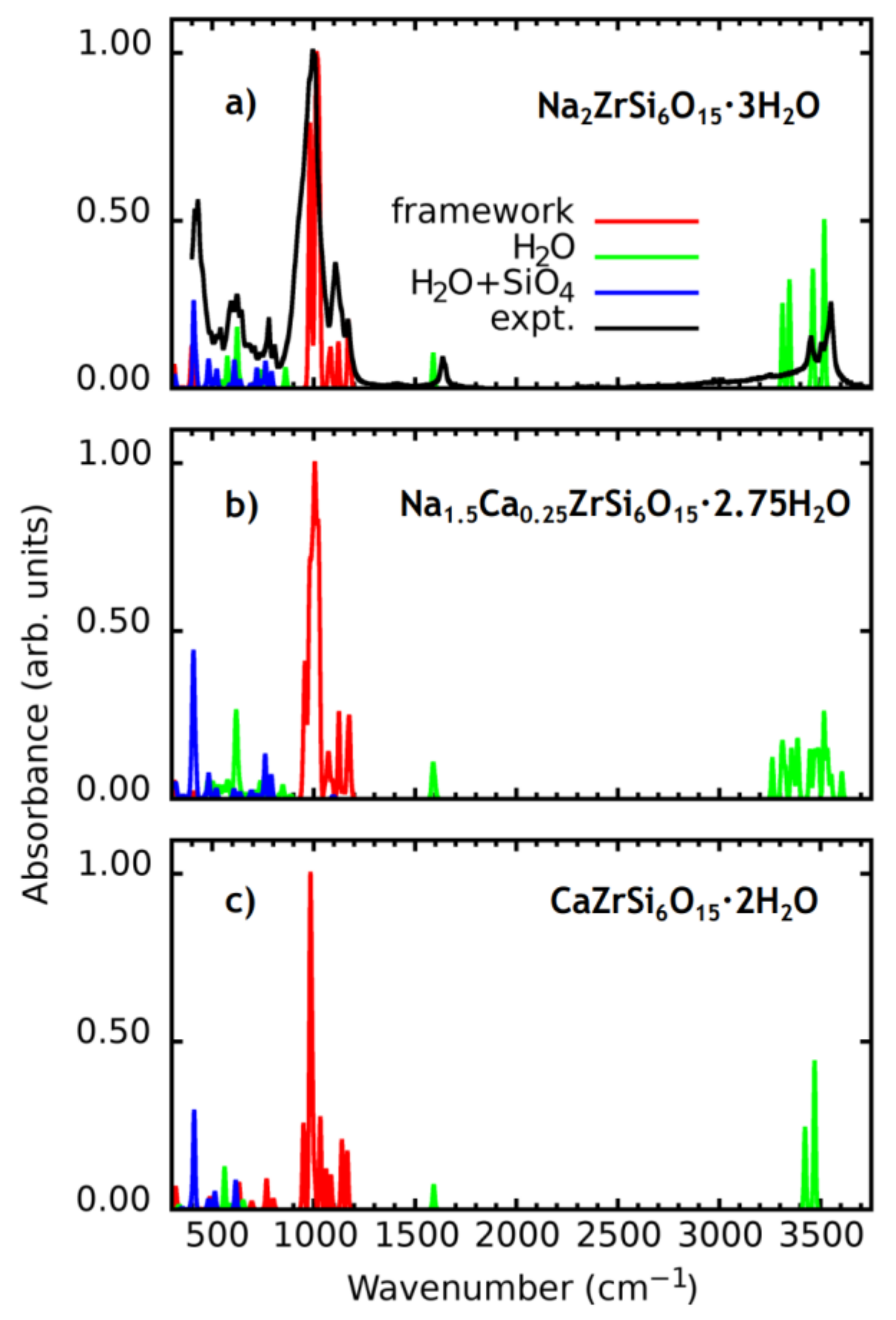
3.2. IR Spectra Simulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K. Crystal chemistry, properties and synthesis of microporous silicates containing transition elements. Russ. Chem. Rev. 2004, 73, 227–246. [Google Scholar] [CrossRef]
- Rocha, J.; Lin, Z. Microporous mixed octahedral-pentahedral-tetrahedral framework silicates. Rev. Mineral. Geochem. 2005, 57, 173–201. [Google Scholar] [CrossRef]
- Day, M.; Hawthorne, F.C. A structure hierarchy for silicate minerals: Chain, ribbon, and tube silicates. Mineral. Mag. 2020, 84, 165–244. [Google Scholar] [CrossRef]
- Gatta, G.D.; Rotiroti, N.; McIntyre, G.J.; Guastoni, A.; Nestola, F. New insights into the crystal chemistry of epididymite and eudidymite from Malosa, Malawi: A single-crytal neutron diffraction study. Am. Mineral. 2008, 93, 1158–1165. [Google Scholar] [CrossRef]
- Agakhanov, A.A.; Pautov, L.A.; Karpenko, V.Y.; Sokolova, E.; Abdu, Y.A.; Hawthorne, F.C.; Pekov, I.V.; Siidra, O.I. Yusupovite, Na2Zr(Si6O15)(H2O)3, a new mineral species from Darai-Pioz alkaline massif and its implications as a new microporous filter for large ions. Am. Min. 2015, 100, 1502–1508. [Google Scholar] [CrossRef]
- Grigor’eva, A.A.; Zubkova, N.V.; Pekov, I.V.; Kolitsch, U.; Pushcharovsky, D.Y.; Vigasina, M.F.; Giester, G.; Dordević, T.; Tillmanns, E.; Chukanov, N.V. Crystal chemistry of elpidite from Khan Bogdo (Mongolia) and its K- and Rb-exchanged forms. Crystallogr. Rep. 2011, 56, 832–841. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Nikolova, R.P.; Chukanov, N.V.; Kostov-Kytin, V.V.; Pekov, I.V.; Varlamov, D.A.; Larikova, T.S.; Kazheva, O.N.; Chervonnaya, N.A.; Shilov, G.V.; et al. Crystal Chemistry and Properties of Elpidite and Its Ag-Exchanged Forms. Minerals 2019, 9, 420. [Google Scholar] [CrossRef]
- Mesto, E.; Kaneva, E.; Schingaro, E.; Vladykin, N.; Lacalamita, M.; Scordari, F. Armstrongite from Khan Bogdo (Mongolia): Crystal structure determination and implications for zeolite-like cation exchange properties. Am. Mineral. 2014, 99, 2424–2432. [Google Scholar] [CrossRef]
- Jeffery, A.J.; Gertisser, R.; Jackson, R.A.; O’Driscoll, B.; Kronz, A. On the compositional variability of dalyite, K2ZrSi6O15: A new occurrence from Terceira, Azores. Mineral. Mag. 2016, 80, 547–565. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Uvarova, Y.A.; Sokolova, E. A structure hierarchy for silicate minerals: Sheet silicates. Mineral. Mag. 2019, 83, 3–55. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.V.; Kerestedjian, T.N. Rietveld analysis of elpidite framework flexibility using in situ powder XRD data of thermally treated samples. Minerals 2020, 10, 639. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V. Heterosilicates with tetrahedral-octahedral frameworks: Mineralogical and crystal-chemical aspects. Rev. Mineral. Geochem. 2005, 57, 105–143. [Google Scholar] [CrossRef]
- Nedel’ko, V.V.; Chukanov, N.V.; Pekov, I.V. Dehydration kinetics of the microporous zirconosilicate elpidite. Inorg. Mater. 2011, 47, 502–505. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Ksenofontov, D.A.; Kabalov, Y.K.; Chukanov, N.V.; Nedel’ko, V.V. Dehydration-induced structural transformations of the microporous zirconosilicate elpidite. Inorg. Mater. 2011, 47, 506–512. [Google Scholar] [CrossRef]
- Cametti, G.; Armbruster, T.; Nagashima, M. Dehydration and thermal stability of elpidite: An in-situ single crystal X-ray diffraction study. Microporous Mesoporous Mater. 2016, 227, 81–87. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Bakakin, V.V.; Pekov, I.V. Structural evolution of microporous zirconosilicate elpidite under high pressure. J. Struct. Chem. 2014, 55, 1252–1259. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Pekov, I.V.; Chukanov, N.V.; Yapaskurt, V.O.; Turchkova, A.G.; Larikova, T.S.; Pushcharovsky, D.Y. A highly hydrated variety of elpidite from the Khibiny alkaline complex, Kola Peninsula, Russia. Mineral. Mag. 2020, 1–7. [Google Scholar] [CrossRef]
- Bruker APEX2, version 2.0-2; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Bruker SAINT, version 6.0; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- Sheldrick, G.M. XPREP, version 2008/2; Bruker-AXS: Madison, WI, USA, 2008.
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. Crystals version 12: Software for guided crystal structure analysis. J. App. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Ungaretti, L.; Oberti, R. Site populations in minerals: Terminology and presentation of results of crystal-structure refinement. Can. Mineral. 1995, 33, 907–911. [Google Scholar]
- Cannillo, E.; Rossi, G.; Ungaretti, L. The crystal structure of elpidite. Am. Mineral. J. Earth Planet. Mater. 1973, 58, 106–109. [Google Scholar]
- Kaneva, E.; Shendrik, R.Y.; Radomskaya, T.A.; Suvorova, L.A. Fedorite from Murun alkaline complex (Russia): Spectroscopy and crystal chemical features. Minerals 2020, 10, 702. [Google Scholar] [CrossRef]
- Gagnè, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. 2015, B71, 562–578. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condenc. Matter. 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed]
- Kaneva, E.; Bogdanov, A.; Shendrik, R. Structural and vibrational properties of agrellite. Sci. Rep. 2020, 10, 15569. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.; Tanaka, I. First-principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. 2006, B73, 045112. [Google Scholar] [CrossRef]
- Skelton, J.M.; Burton, L.A.; Jackson, A.J.; Oba, F.; Parker, S.C.; Walsh, A. Lattice dynamics of the tin sulphides SnS2, SnS and Sn2S3: Vibrational spectra and thermal transport. Phys. Chem. Chem. Phys. 2017, 19, 12452–12465. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Grice, J.D.; Rowe, R.; Poirier, G. Hydroterskite: A new mineral species from the Saint-Amable sill, Quebec, and comparison with terskite and elpidite. Can. Mineral. 2015, 53, 821–832. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Kashaev, A.A. Features of the crystal structure of calcium-containing elpidite. Sov. Phys. Crystallogr. 1978, 23, 24–27. [Google Scholar]
- Chukanov, N.V. Infrared Spectra of Mineral Species; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Salinas-Sanchez, A.; Garcia-Muñoz, J.L.; Rodriguez-Carvajal, J.; Saez-Puche, R.; Martinez, J.L. Structural characterization of R2BaCuO5 (R = Y, Lu, Yb, Tm, Er, Ho, Dy, Gd, Eu and Sm) oxides by X-ray and neutron diffraction. J. Solid State Chem. 1992, 100, 201–211. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond In Inorganic Chemistry: The Bond Valence Model; Oxford University Press: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Pekov, I.V.; Krivovichev, S.V.; Zolotarev, A.A.; Yakovenchuk, V.N.; Armbruster, T.; Pakhomovsky, Y.A. Crystal chemistry and nomenclature of the lovozerite group. Eur. J. Miner. 2009, 21, 1061–1071. [Google Scholar] [CrossRef]
- Kaneva, E.V.; Vladykin, N.V.; Mesto, E.; Lacalamita, M.; Scordari, F.; Schingaro, E. Refinement of the crystal structure of vlasovite from Burpala Massif (Russia). Crystallogr. Rep. 2018, 63, 1092–1098. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef]
- Renner, B.; Lehmann, G. Correlation of angular and bond length distortions in TO4 units in crystals. Z. Kristallogr. 1986, 175, 43–59. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Vicković, I. IVTON—Program for the calculation of geometrical aspects of crystal structures and some crystal chemical applications. J. Appl. Cryst. 1996, 29, 305–306. [Google Scholar] [CrossRef]
| Crystal Data | ElB-1 | ElB-2 | ElKhB-1 | ElKhB-2 |
|---|---|---|---|---|
| a (Å) b (Å) c (Å) | 7.1183(2) 14.6968(5) 14.6032(5) | 7.1299(5) 14.7131(10) 14.6276(9) | 7.1303(1) 14.6532(2) 14.6303(2) | 7.1296(1) 14.6437(2) 14.6270(2) |
| V (Å3) | 1527.73(5) | 1534.48(9) | 1528.60(2) | 1527.11(2) |
| Z | 4 | 4 | 4 | 4 |
| Crystal dimensions (mm) | 0.15 × 0.10 × 0.09 | 0.18 × 0.11 × 0.09 | 0.13 × 0.10 × 0.10 | 0.16 × 0.09 × 0.08 |
| Structural formula | Na2Zr1.03[Si6O15]·3.02H2O | Na1.98Zr1.04[Si6O15]·2.91H2O | Na1.38Ca0.33Zr1.02[Si6O15]·2.64H2O | Na1.09Ca0.47Zr1.02[Si6O15]·2.52H2O |
| Data Collection | ||||
| Independent reflections | 3014 | 4431 | 4058 | 4869 |
| Rmerging (R(int)) (%) | 6.90 | 4.70 | 4.90 | 7.00 |
| hmin, hmax | −10, 10 | −12, 12 | −10, 11 | −12, 12 |
| kmin, kmax | −22, 22 | −25, 17 | −24, 24 | −26, 26 |
| lmin, lmax | −21, 22 | −24, 25 | −24, 24 | −25, 26 |
| Refinement | ||||
| Space group | Pbcm | Pbcm | Pbcm | Pbcm |
| Reflections used in the refinement (I > 3σ(I)) | 1672 | 2151 | 2147 | 2533 |
| N. of refined parameters | 148 | 148 | 149 | 149 |
| Ra [on F] (%) | 2.47 | 3.00 | 2.12 | 2.78 |
| Rwb [on F] (%) | 2.41 | 3.12 | 2.92 | 3.42 |
| Goof c | 1.1174 | 1.1013 | 1.0560 | 1.0177 |
| Δρmin/Δρmax (e−/Å3) | −0.50/0.50 | −0.87/0.66 | −0.52/0.46 | −1.12/0.86 |
| Constituent | This Study | ElMSHil(a) | ElMSHil(b) | ElLov | ElKhB(c) | |||
|---|---|---|---|---|---|---|---|---|
| ElB-1 | ElB-2 | ElKhB-1 | ElKhB-2 | |||||
| SiO2 | 60.2(6) | 60.7(2) | 60.4(7) | 61.1(7) | 59.82 | 58.45(53) | 59.02 | 61.05 |
| Al2O3 | 0.02(2) | 0.01(1) | 0.03(3) | 0.04(2) | 0.08 | 0.04(6) | 0.15 | — |
| Na2O | 9.6(5) | 9.5(6) | 6.3(7) | 5.5(5) | 7.82 | 9.9(1) | 10.17 | 8.64 |
| MgO | 0.02(1) | 0.01(1) | b.d.l. | 0.02(2) | 0.01 | 0.01(1) | — | — |
| K2O | 0.06(4) | 0.12(3) | 0.08(3) | 0.06(4) | 0.07 | 0.02(3) | 0.04 | 0.08 |
| CaO | 0.03(1) | 0.02(2) | 2.9(3) | 3.8(2) | 0.62 | 0.01(1) | 0.04 | 1.41 |
| TiO2 | 0.01(1) | 0.02(1) | 0.07(5) | 0.07(2) | 1.15 | 0.1(1) | 0.04 | — |
| V2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | — | 0.03(5) | — | — |
| Cr2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | — | 0.06(8) | — | — |
| MnO | b.d.l. | 0.02(2) | 0.08(5) | b.d.l. | 0.71 | 0.06(6) | — | — |
| FeO | 0.04(4) | 0.05(5) | 0.36(5) | 0.14(4) | 0.89 | — | — | — |
| Fe2O3 | n.d. | n.d. | n.d. | n.d. | — | 0.08(9) | 0.02 | — |
| NiO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | — | 0.03(6) | — | — |
| CuO | 0.05(3) | 0.08(7) | 0.12(6) | 0.06(4) | — | — | — | — |
| SrO | 0.06(6) | 0.02(2) | b.d.l. | b.d.l. | — | 0.03(4) | — | — |
| ZrO2 | 19.3(6) | 19.2(4) | 19.4(5) | 19.8(6) | 15.00 | 20.0(4) | 20.89 | 20.94 |
| Nb2O5 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 1.12 | 0.2(2) | 0.62 | — |
| BaO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | — | 0.2(2) | — | — |
| La2O3 | b.d.l. | 0.04(4) | b.d.l. | b.d.l. | 0.12 | — | — | — |
| Ce2O3 | 0.09(9) | 0.06(6) | 0.08(7) | b.d.l. | 0.24 | — | — | — |
| Pr2O3 | 0.04(4) | 0.10(6) | b.d.l. | b.d.l. | — | — | — | — |
| Nd2O3 | 0.06(6) | 0.03(3) | 0.06(6) | b.d.l. | — | — | — | — |
| Sm2O3 | 0.04(4) | b.d.l. | b.d.l. | 0.09(9) | — | — | — | — |
| Eu2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | — | — | — | — |
| Gd2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | — | — | — | — |
| Dy2O3 | 0.1(1) | 0.05(5) | b.d.l. | b.d.l. | — | — | — | — |
| Ho2O3 | b.d.l. | b.d.l. | 0.06(6) | 0.12(9) | — | — | — | — |
| Er2O3 | b.d.l. | b.d.l. | 0.07(7) | 0.07(7) | — | — | — | — |
| Yb2O3 | 0.08(8) | 0.09(9) | 0.2(1) | 0.08(8) | — | — | — | — |
| Lu2O3 | 0.08(8) | b.d.l. | 0.11(7) | b.d.l. | — | — | — | — |
| HfO2 | 0.8(1) | 0.6(2) | 0.5(1) | 0.4(1) | 0.11 | — | 0.43 | — |
| F | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.11 | 0.04(7) | — | — |
| Cl | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.01 | 0.01(2) | — | — |
| P2O5 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | — | 0.01(3) | — | — |
| Total | 90.68 | 90.72 | 91.12 | 91.49 | 88.05 | 89.38 | 91.42 | 92.12 |
| Wavenumber (cm−1) | Absorbance (arb. Units) | Peak Attribution | Wavenumber (cm−1) | Absorbance (arb. Units) | Peak Attribution | Wavenumber (cm−1) | Absorbance (arb. Units) | Peak Attribution |
|---|---|---|---|---|---|---|---|---|
| Na2ZrSi6O15·3H2O Model | Na1.5Ca0.25ZrSi6O15·2.75H2O Model | CaZrSi6O15·2H2O Model | ||||||
| 401–420 | 0.12–0.39 | Si1 + Si2 + Si3 + W1 | 406–409 | 0.33–0.72 | Si1 + Si2 + Si3 + W1 + W2 | 411 | 0.35 | framework + W1 |
| 480 | 0.11 | framework + W1 + W2 | 419 | 0.16 | framework +W1 | 561 | 0.15 | W1 |
| 574 | 0.14 | W1 + W2 | 482 | 0.17 | framework + W1 + W2 | 615 | 0.1 | framework + W1 |
| 609 | 0.12 | framework + W1 + W2 | 501–620 | 0.10–0.45 | W1 + W2 | 632–768 | 0.09–0.11 | framework |
| 623 | 0.26 | W1 + W2 | 761 | 0.34 | Si1 + Si2 + Si3 + W1 + W2 | 950–1005 | 0.12–1.00 | framework |
| 762 | 0.12 | Si1 + Si2 + Si3 + W1 + W2 | 790 | 0.14 | Si1 + Si3 + W1 + W2 | 1032 | 0.33 | Si1 + Si2 |
| 976 | 0.3 | Si2 + Si3 | 948 | 0.22 | Si1 + Si3 | 1061 | 0.14 | framework + W1 |
| 982 | 0.73 | framework | 953 | 0.42 | Si1 + Si2 + Si3 | 1085 | 0.12–1.00 | Si1 + Si3 |
| 986–999 | 0.13–0.5 | Zr + Si1 + Si3 | 960 | 0.73 | Si1 + Si3 | 1139 | 0.25 | Si1 + Si2 + Si3 |
| 1010 | 1 | framework | 966 | 0.19 | Si2 + Si3 | 1166 | 0.21 | Si1 + Si3 |
| 1015 | 0.47 | Zr + Si1 + Si3 | 973–978 | 0.33–0.66 | Si1 + Si2 + Si3 | 1592 | 0.08 | W1 |
| 1021–1022 | 0.33–0.70 | Si1 + Si2 + Si3 | 981 | 0.65 | Si1 + Si3 | 3420–3424 | 0.06–0.25 | W1 |
| 1030 | 0.75 | framework | 985–998 | 0.17–0.84 | framework | 3471 | 0.53 | W1 |
| 1074–1084 | 0.11–0.17 | framework + W1 | 1000 | 0.16 | Zr + Si1 + Si3 | |||
| 1122 | 0.21 | Si2 | 1000–1029 | 0.11–1.00 | framework | |||
| 1171–1182 | 0.10–0.29 | framework | 1031 | 0.36 | Si1 + Si2 | |||
| 1589 | 0.13 | W1 + W2 | 1054–1094 | 0.08–0.21 | framework + W1 | |||
| 1592 | 0.01 | W2 | 1125 | 0.67 | Si2 | |||
| 1594 | 0.02 | W1 + W2 | 1165 | 0.11 | framework | |||
| 3312–3347 | 0.08–0.50 | W2 | 1167–1170 | 0.10–0.16 | Si1 + Si3 | |||
| 3459–3465 | 0.16–0.24 | W1 + W2 | 1173 | 0.13 | Si1 + Si2 + Si3 | |||
| 3517–3519 | 0.03–0.50 | W1 | 1178 | 0.45 | framework | |||
| 1584–1589 | 0.01–0.19 | W1 + W2 | ||||||
| 1591 | 0.02 | W2 | ||||||
| 1597 | 0.05 | W1 + W2 | ||||||
| 1599 | 0.06 | W1 | ||||||
| 1604 | 0.01 | W1 + W2 | ||||||
| 3262–3308 | 0.31–0.34 | W2 | ||||||
| 3315 | 0.34 | W1 + W2 | ||||||
| 3327–3367 | 0.23–0.40 | W2 | ||||||
| 3385–3467 | 0.16–0.40 | W1 + W2 | ||||||
| 3473 | 0.28 | W1 | ||||||
| 3482–3487 | 0.06–0.24 | W1 + W2 | ||||||
| 3491–3517 | 0.18–0.32 | W1 | ||||||
| 3519 | 0.27 | W2 | ||||||
| 3532–3605 | 0.20–0.28 | W1 | ||||||
| Sample | GII (%) Zr | GII (%) Na | GII (%) Si | GII (%) O | GII (%) Total |
|---|---|---|---|---|---|
| ElB-1 | 2.60 | 4.77 | 11.02 | 9.82 | 9.26 |
| ElB-2 | 1.40 | 5.81 | 9.02 | 9.47 | 8.65 |
| ElKhB-1 | 1.20 | 6.20 | 13.13 | 10.80 | 10.48 |
| ElKhB-2 | 0.40 | 12.79 | 12.92 | 10.80 | 11.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, A.; Kaneva, E.; Shendrik, R. New Insights into the Crystal Chemistry of Elpidite, Na2Zr[Si6O15]·3H2O and (Na1+YCax□1−X−Y)Σ=2Zr[Si6O15]·(3−X)H2O, and Ab Initio Modeling of IR Spectra. Materials 2021, 14, 2160. https://doi.org/10.3390/ma14092160
Bogdanov A, Kaneva E, Shendrik R. New Insights into the Crystal Chemistry of Elpidite, Na2Zr[Si6O15]·3H2O and (Na1+YCax□1−X−Y)Σ=2Zr[Si6O15]·(3−X)H2O, and Ab Initio Modeling of IR Spectra. Materials. 2021; 14(9):2160. https://doi.org/10.3390/ma14092160
Chicago/Turabian StyleBogdanov, Alexander, Ekaterina Kaneva, and Roman Shendrik. 2021. "New Insights into the Crystal Chemistry of Elpidite, Na2Zr[Si6O15]·3H2O and (Na1+YCax□1−X−Y)Σ=2Zr[Si6O15]·(3−X)H2O, and Ab Initio Modeling of IR Spectra" Materials 14, no. 9: 2160. https://doi.org/10.3390/ma14092160
APA StyleBogdanov, A., Kaneva, E., & Shendrik, R. (2021). New Insights into the Crystal Chemistry of Elpidite, Na2Zr[Si6O15]·3H2O and (Na1+YCax□1−X−Y)Σ=2Zr[Si6O15]·(3−X)H2O, and Ab Initio Modeling of IR Spectra. Materials, 14(9), 2160. https://doi.org/10.3390/ma14092160