Study of the Hydrolytic Stability of Fine-Grained Ceramics Based on Y2.5Nd0.5Al5O12 Oxide with a Garnet Structure under Hydrothermal Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlova, A.I.; Ojovan, M.I. Ceramic mineral waste-forms for nuclear waste immobilization. Materials 2019, 12, 2638. [Google Scholar] [CrossRef] [PubMed]
- Ewing, R.C.; Webert, W.J.; Clinard, F.W. Radiation effects in nuclear waste forms for high-level radioactive waste. Prog. Nucl. Energy 1995, 29, 63–127. [Google Scholar] [CrossRef]
- Potanina, E.; Golovkina, L.; Orlova, A.; Nokhrin, A.; Boldin, M.; Sakharov, N. Lanthanide (Nd, Gd) compounds with garnet and monazite structures. Powders synthesis by “wet” chemistry to sintering ceramics by Spark Plasma Sintering. J. Nucl. Mater. 2016, 473, 93–98. [Google Scholar] [CrossRef]
- Tomilin, S.V.; Lizin, A.A.; Lukinykh, A.N.; Livshits, T.S. Radiation resistance and chemical stability of yttrium aluminum garnet. Radiochemistry 2011, 53, 186–190. [Google Scholar] [CrossRef]
- Burakov, B.E.; Anderson, E.B. Experience of V.G. Khlopin Radium Institute on synthesis and investigation of Pu-doped ceramics. In Proceedings of the Plutonium Future—The Science, AIP Conference, Melville, NY, USA, 10–13 May 2000; pp. 159–160. [Google Scholar] [CrossRef]
- Yudintsev, S.V. A structural-chemical approach to selecting crystalline matrices for actinide immobilization. Geol. Ore Deposites 2003, 45, 151–165. [Google Scholar] [CrossRef]
- Livshits, T.S.; Lizin, A.A.; Zhang, J.M.; Ewing, R.C. Amorphization of rare earth aluminate garnets under ion irradiation and decay of 244Cm admixture. Geol. Ore Deposites 2010, 52, 267–278. [Google Scholar] [CrossRef]
- Livshits, T.S. Stability of artificial ferrite garnets with actinides and lanthanoids in water solutions. Geol. Ore Deposites 2008, 50, 470–481. [Google Scholar] [CrossRef]
- Lukinykh, A.N.; Tomilin, S.V.; Lizin, A.A.; Livshits, T.S. Radiation and chemical resistance of synthetic ceramics based on ferritic garnet. Radiochemistry 2008, 50, 432–437. [Google Scholar] [CrossRef]
- Laverov, N.P.; Yudintsev, S.V.; Livshits, T.S.; Stefanovsky, S.V.; Lukinykh, A.N.; Ewing, R.C. Synthetic Minerals with the Pyrochlore and Garnet Structures for Immobilization of Actinide-Containing Wastes. Geochem. Int. 2010, 48, 1–14. [Google Scholar] [CrossRef]
- Caporuscio, F.A.; Scott, B.L.; Xu, H.; Feller, R.K. Garnet nuclear waste forms—Solubility at repository conditions. Nucl. Eng. Des. 2014, 266, 180–185. [Google Scholar] [CrossRef]
- Utsunomiya, S.; Wang, L.; Yudintsev, S.; Ewing, R. Ion Irradiation Effects in Synthetic Garnets Incorporating Actinides. MRS Proc. 2011, 713. [Google Scholar] [CrossRef]
- Konovalov, E.E.; Lastov, A.I.; Nerozin, N.A. On immobilization of high-level waste in an Y-Al garnet-based cermet matrix in SHS conditions. Nucl. EnergyTech. 2015, 12, 103–106. [Google Scholar] [CrossRef]
- Weber, W.; Navrotsky, A.; Stefanovsky, S.; Vance, E.R.; Vernaz, E. Materials Science of High-Level Nuclear Waste Immobilization. MRS Bull. 2009, 34, 46–53. [Google Scholar] [CrossRef]
- Rák, Z.; Ewing, R.; Becker, U. Ferric garnet matrices for immobilization of actinides. J. Nucl. Mater. 2013, 436, 1–7. [Google Scholar] [CrossRef]
- Guo, X.; Rak, Z.; Tavakoli, A.H.; Becker, U.; Ewing, R.C.; Navrotsty, A. Thermodynamics of thorium substitution in yttrium iron garnet: Comparison of experimental and theoretical results. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 16945–16954. [Google Scholar] [CrossRef]
- Rák, Z.; Ewing, R.C.; Becker, U. Electronic structure and thermodynamic stability of uranium-doped yttrium iron garnet. J. Phys. Condens. Matter. 2013, 25, 495502. [Google Scholar] [CrossRef] [PubMed]
- Scheetz, B.E.; Agrawal, D.K.; Breval, E.; Roy, R. Sodium Zirconium-phosphate (NZP) as a Host Structure for Nuclear Waste Immobilization—A Review. Waste Manag. 1994, 14, 489–505. [Google Scholar] [CrossRef]
- Bykov, D.M.; Orlova, A.I.; Tomilin, S.V.; Lizin, A.A.; Lukinykh, A.N. Americium and plutonium in trigonal phosphates (NZP type) Am1/3[Zr2(PO4)3] and Pu1/4[Zr2(PO4)3]. Radiochemistry 2006, 48, 234–239. [Google Scholar] [CrossRef]
- Orlova, A.I.; Koryttseva, A.K.; Kanunov, A.E.; Chuvil’deev, V.N.; Moskvicheva, A.V.; Sakharov, N.; Boldin, M.; Nokhrin, A.V. Fabrication of NaZr2(PO4)3-type ceramic materials by spark plasma sintering. Inorg. Mater. 2012, 48, 313–317. [Google Scholar] [CrossRef]
- Gregg, D.J.; Karatchevtseva, I.; Triani, G.; Lumpkin, G.R.; Vance, E.R. The thermophysical properties of calcium and barium zirconium phosphate. J. Nucl. Mat. 2013, 441, 203–210. [Google Scholar] [CrossRef]
- Raison, P.E.; Haire, R.G.; Sato, T.; Ogawa, T. Fundamental and technological aspects of actinide oxide pyrochlores: Relevance for immobilization matrices. In Proceedings of the Sympos. “Sci. Basis for Nucl. Waste Management XXII”, Boston, MA, USA, 30 November–4 December 1998; MRS: Warrendale, PA, USA, 1999; Volume 556, pp. 3–10. [Google Scholar] [CrossRef]
- Ewing, R.C. The design and evaluation of nuclearwaste forms: Clues from mineralogy. Can. Mineral. 2001, 39, 697–715. [Google Scholar] [CrossRef]
- Strachan, D.M.; Scheele, R.D.; Icenhower, J.P.; Buck, E.C.; Kozelisky, A.E.; Sell, R.L.; Elovich, R.J.; Buchmiller, W.C. Radiation Damage Effects in Candidate Ceramics for Plutonium Immobilization: Final Report; Pacific North West National Laboratory: Richland, WA, USA, 2004. [Google Scholar]
- Strachan, D.M.; Scheele, R.D.; Buck, E.C.; Icenhower, J.P.; Kozelisky, A.E.; Sell, R.L.; Elovich, R.J.; Buchmiller, W.C. Radiation damage effects in candidate titanates for Pu disposition: Pyrochlore. J. Nucl. Mater. 2005, 345, 109–135. [Google Scholar] [CrossRef]
- Potanina, E.A.; Orlova, A.I.; Mikhailov, D.A.; Nokhrin, A.V.; Chuvil’deev, V.N.; Boldin, M.S.; Sakharov, N.V.; Lantcev, E.A.; Tokarev, M.G.; Murashov, A.A. Spark Plasma Sintering of fine-grained SrWO4 and NaNd(WO4)2tungstates ceramics with the scheelite structure for nuclear waste immobilization. J. Alloys Compd. 2019, 774, 182–190. [Google Scholar] [CrossRef]
- Saifulin, M.M.; O’Connell, J.H.; Janse van Vuuren, A.; Skuratov, V.A.; Kirilkin, N.S.; Zdorovets, M.V. Latent tracks in bulk yttrium-iron garnet crystals irradiated with low and high velocity krypton and xenon ions. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. Atoms 2019, 460, 98–103. [Google Scholar] [CrossRef]
- Tokita, M. Spark Plasma Sintering (SPS) Method, Systems, and Applications (Chapter 11.2.3). In Handbook of Advanced Ceramics, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1149–1177. [Google Scholar] [CrossRef]
- Olevsky, E.; Dudina, D. Field-Assisted Sintering; Springer Int. Publ.: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Mayorov, V.Y.; Kuryavyi, V.; Kaidalova, T.A.; Teplukhina, L.V.; Portnyagin, A.S.; Slobodyuk, A.; Belov, A.; Tananaev, I.G.; et al. SPS technique for ionizing radiation source fabrication based on dense cesium-containing core. J. Hazard. Mater. 2019, 369, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Papynov, E.K.; Shichalin, O.O.; Mayorov, V.Y.; Portnyagin, A.S.; Gridasova, E.; Agafonova, I.G.; Zakirova, A.E.; Tananaev, I.G.; Avramenko, V. Sol-gel and SPS combined synthesis of highly porous wollastonite ceramic materials with immobilized Au-NPs. Ceram. Int. 2017, 43, 8509–8516. [Google Scholar] [CrossRef]
- O’Brien, R.; Ambrosi, R.; Bannister, N.; Howe, S.; Atkinson, H. Spark Plasma Sintering of simulated radioisotope materials within tungsten cermets. J. Nucl. Mater. 2009, 393, 108–113. [Google Scholar] [CrossRef]
- Ryu, H.; Lee, Y.; Cha, S.; Hong, S. Sintering behaviour and microstructures of carbides and nitrides.for the inert matrix fuel by spark plasma sintering. J. Nucl. Mater. 2006, 352, 341–348. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Mironenko, A.Y.; Ryakov, A.; Manakov, I.; Makhrov, P.; Buravlev, I.; Tananaev, I.; Avramenko, V.; Sergienko, N. Synthesis of High-Density Pellets of Uranium Dioxide by Spark Plasma Sintering in Dies of Different Types. Radiochemistry 2018, 60, 362–370. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Maiorov, V.Y.; Belov, A.; Buravlev, I.; Azarova, Y.; Golub, A.; Gridasova, E.; Sukhorada, A.; Tananaev, I.; et al. Spark Plasma Sintering of Aluminosilicate Ceramic Matrices for Immobilization of Cesium Radionuclides. Radiochemistry 2019, 61, 185–191. [Google Scholar] [CrossRef]
- Chu, X.; Qing, Q.; Li, B.; Yang, Y.; Li, L.; Zhang, H.; Shao, D.; Lu, X. Rapid immobilization of complex simulated radionuclides by as-prepared Gd2Zr2O7 ceramics without structural design. J. Nucl. Mater. 2019, 526, 151782. [Google Scholar] [CrossRef]
- Wang, L.; Shu, X.; Yi, F.; Shao, D.; Zhang, K.; Zhang, H.; Xirui, L. Rapid fabrication and phase transition of Nd and Ce co-doped Gd2Zr2O7 ceramics by SPS. J. Eur. Ceram. Soc. 2018, 38, 2863–2870. [Google Scholar] [CrossRef]
- Karipbayev, Z.T.; Lisitsyn, V.M.; Mussakhanov, D.A.; Alpyssova, G.K.; Popov, A.; Polisadova, E.; Elsts, E.; Akilbekov, A.T.; Kukenova, A.B.; Kemere, M.; et al. Time-resolved luminescence of YAG:Ce and YAGG:Ce ceramics prepared by electron beam assisted synthesis. Nucl. Instrum. Methods Phys. Res. B. Beam Interact. Mater. Atoms 2020, 479, 222–228. [Google Scholar] [CrossRef]
- Golovkina, L.S.; Orlova, A.I.; Nokhrin, A.V.; Boldin, M.S.; Chuvil’deev, V.N.; Sakharov, N.V.; Belkin, O.A.; Shotin, S.V.; Zelenov, A.Y. Spark Plasma Sintering of fine-grain ceramic-metal composites based on garnet-structure oxide Y2.5Nd0.5Al5O12 for inert matrix fuel. Mater. Chem. Phys. 2018, 214, 516–526. [Google Scholar] [CrossRef]
- Golovkina, L.S.; Orlova, A.I.; Chuvil’deev, V.N.; Boldin, M.S.; Lantcev, E.A.; Nokhrin, A.V.; Sakharov, N.V.; Zelenov, A.Y. Spark Plasma Sintering of high-density fine-grained Y2.5Nd0.5Al5O12+SiC composite ceramics. Mater. Res. Bull. 2018, 103, 211–215. [Google Scholar] [CrossRef]
- De Groot, G.J.; Van der Sloot, H.A. Determination of leaching characteristics of waste minerals leading to environmental product certification. In Stabilization and Solidification of Hazardous, Radioactive and Mixed Wastes; Gilliam, T.M., Wiles, C.C., Eds.; ASTM: Philadelphia, PA, USA, 1992; Volume 2, pp. 149–170. [Google Scholar]
- Ikesue, A.; Kamata, K.; Yoshida, K. Effect of neodymium concentration on optical characteristics of polycrytstallineNd:YAG laser materials. J. Amer. Ceram. Soc. 1996, 79, 1921–1926. [Google Scholar] [CrossRef]
- Garanin, S.S.; Dmitryuk, A.V.; Mikhailov, M.D.; Zhilin, A.A.; Rukavishnikov, N.N. Laser ceramics. 2. Spectroscopic and lasing properties. J. Opt. Technol. 2011, 78, 393–399. [Google Scholar] [CrossRef]
- Hollingsworth, J.P.; Kuntz, J.D.; Ryerson, F.J.; Soules, T.F. Nd diffusion in YAG ceramics. Opt. Mater. 2011, 33, 592–595. [Google Scholar] [CrossRef]
- Cherniak, D.J. Rare earth element and gallium diffusion in yttrium aluminum garnet. Phys. Chem. Miner. 1998, 26, 156–163. [Google Scholar] [CrossRef]
- Boulesteix, R.; Maîte, A.; Baumard, J.-F.; Rabinovitch, Y.; Sallé, C.; Weber, S.; Kilo, M. The effect of silica doping on neodymium diffusion in yttrium aluminum garnet ceramics: Implications for sintering mechanisms. J. Eur. Ceram. Soc. 2009, 29, 2517–2526. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, B.; Jiang, Y.; Zhang, G.; Chen, S.; Fan, J.; Zhang, L. Spatial ions distribution on the binding interface in YAG/Nd:LuAG composite laser ceramic. J. Am. Ceram. Soc. 2017, 100, 5030–5037. [Google Scholar] [CrossRef]
- Boulesteix, R.; Maîte, A.; Baumard, J.-F.; Sallé, C.; Rabinovitch, Y. Mechanism of the liquid-phase sintering for Nd:YAG ceramics. Opt. Mater. 2009, 31, 711–715. [Google Scholar] [CrossRef]
- Jiménez-Melendo, M.; Haneda, H.; Nozawa, H. Ytterbium cation diffusion in yttrium aluminum garnet (YAG) — implications for creep mechanisms. J. Am. Ceram. Soc. 2001, 84, 2356–2360. [Google Scholar] [CrossRef]
- Torras, J.; Buj, I.; Rovira, M.; de Pablo, J. Semi-dynamic leaching tests of nickel containing wastes stabilized/solidified with magnesium potassium phosphate cements. J. Hazard. Mater. 2011, 186, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Wang, P.; Li, J.-S.; Zhang, T.-T.; Wang, S. Investigation of the leaching behavior of lead in stabilized/solidified waste using a two-year semi-dynamic leaching test. Chemosphere 2017, 166, 1–7. [Google Scholar] [CrossRef]
- Segal, V.M.; Beyerlein, I.J.; Tome, C.N.; Chuvil’deev, V.N.; Kopylov, V.I. Fundamentals and Engineering of Severe Plastic Deformation; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Pelleg, J. Diffusion in Ceramics; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
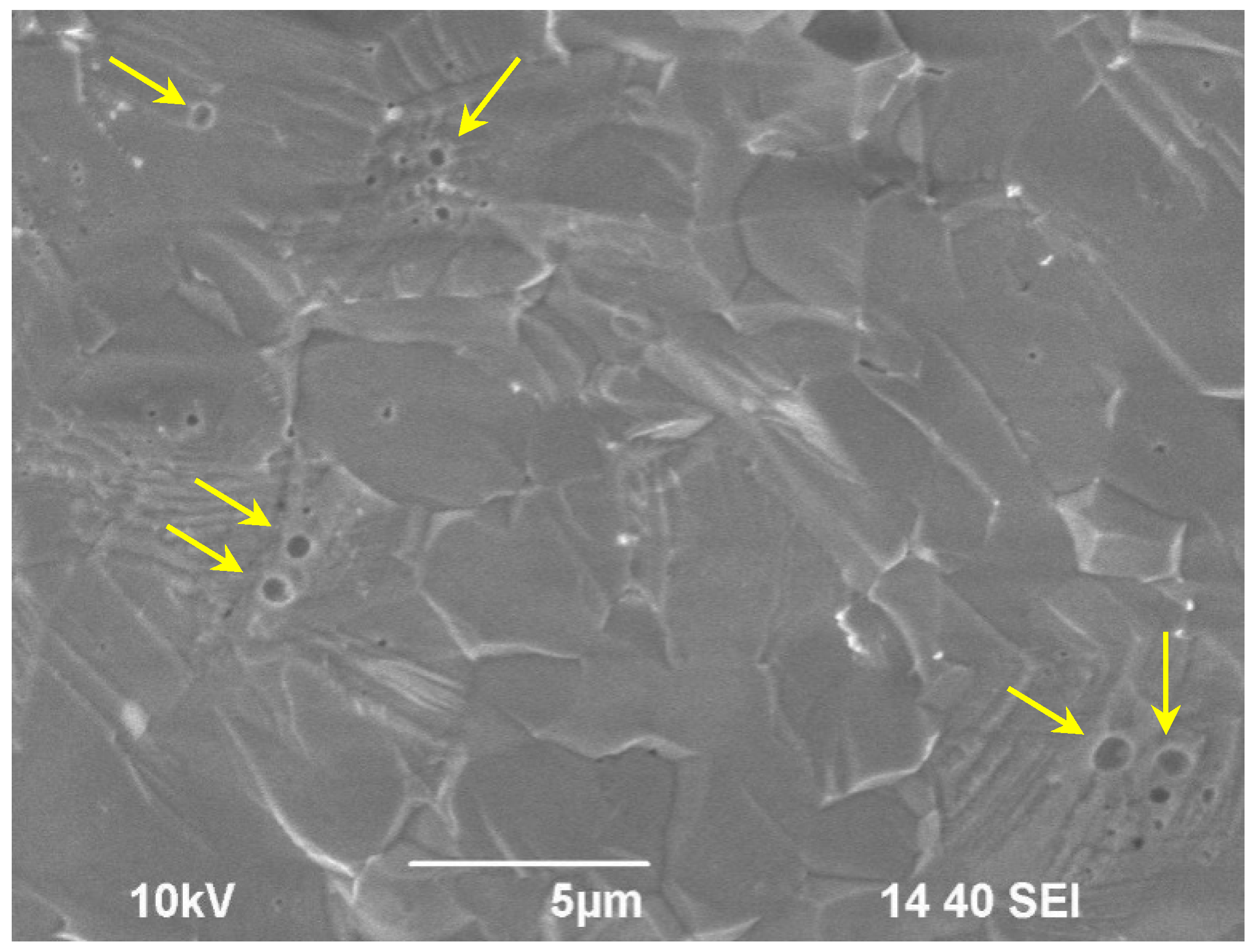
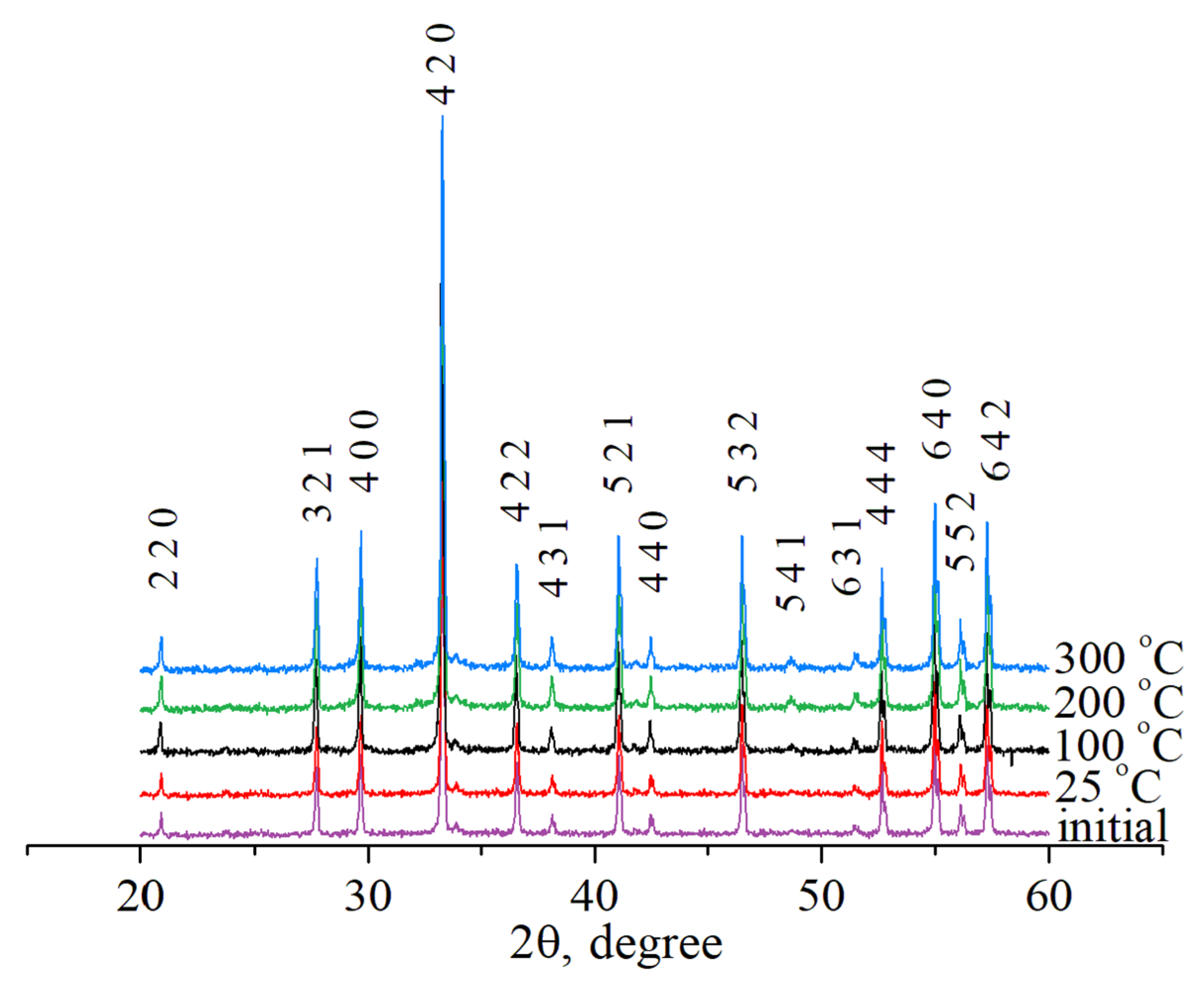

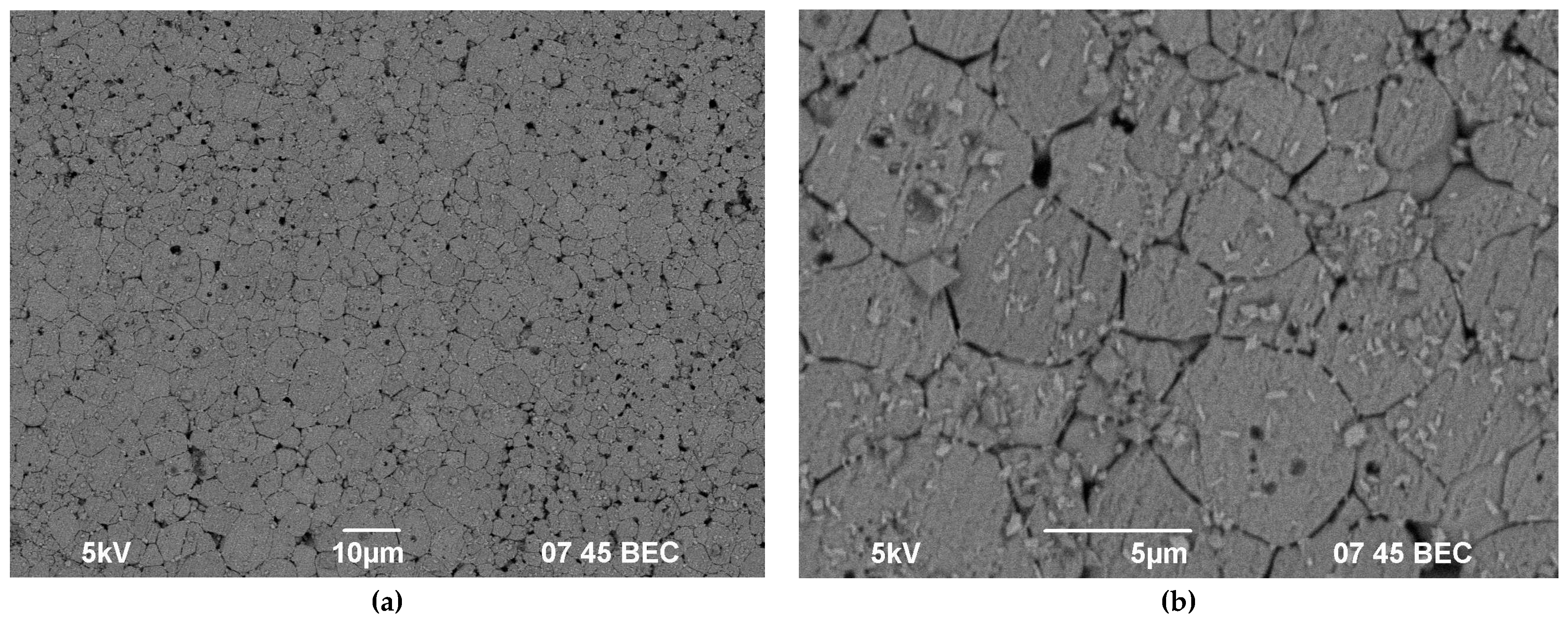
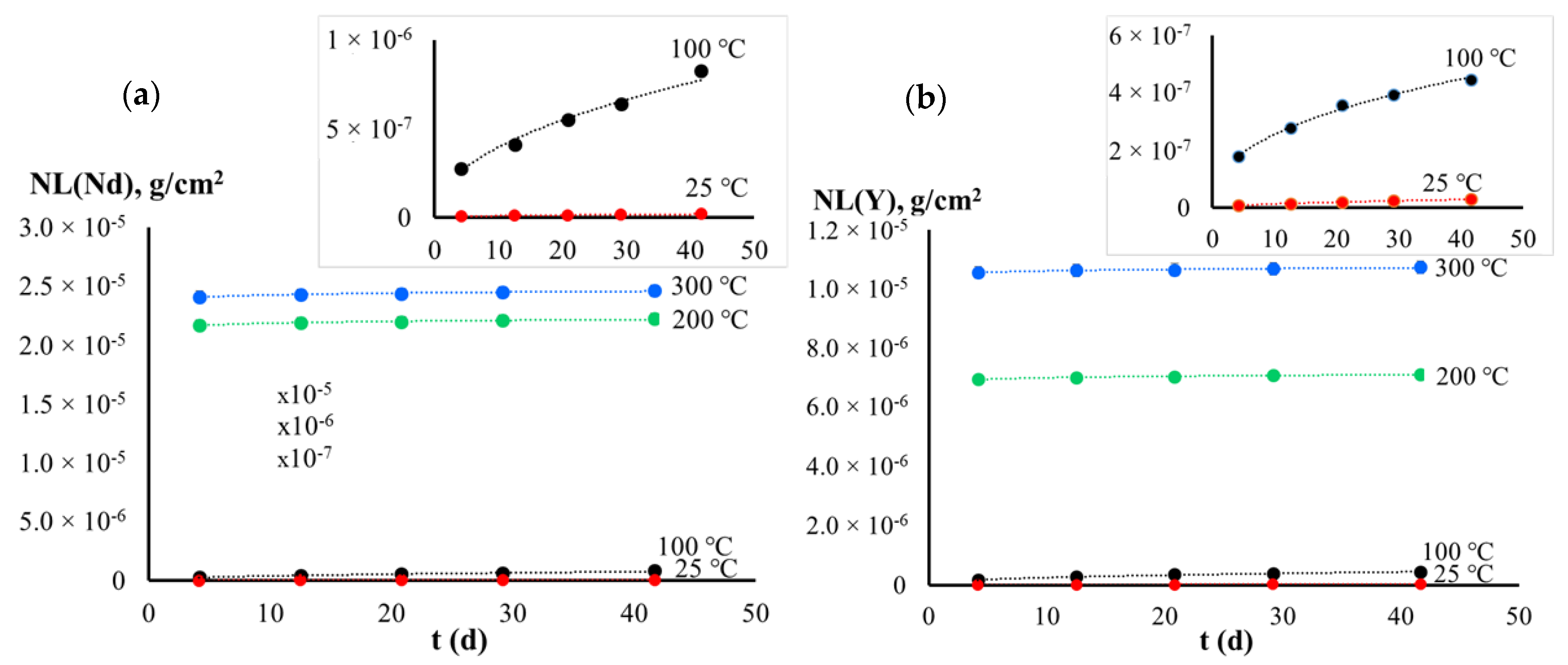
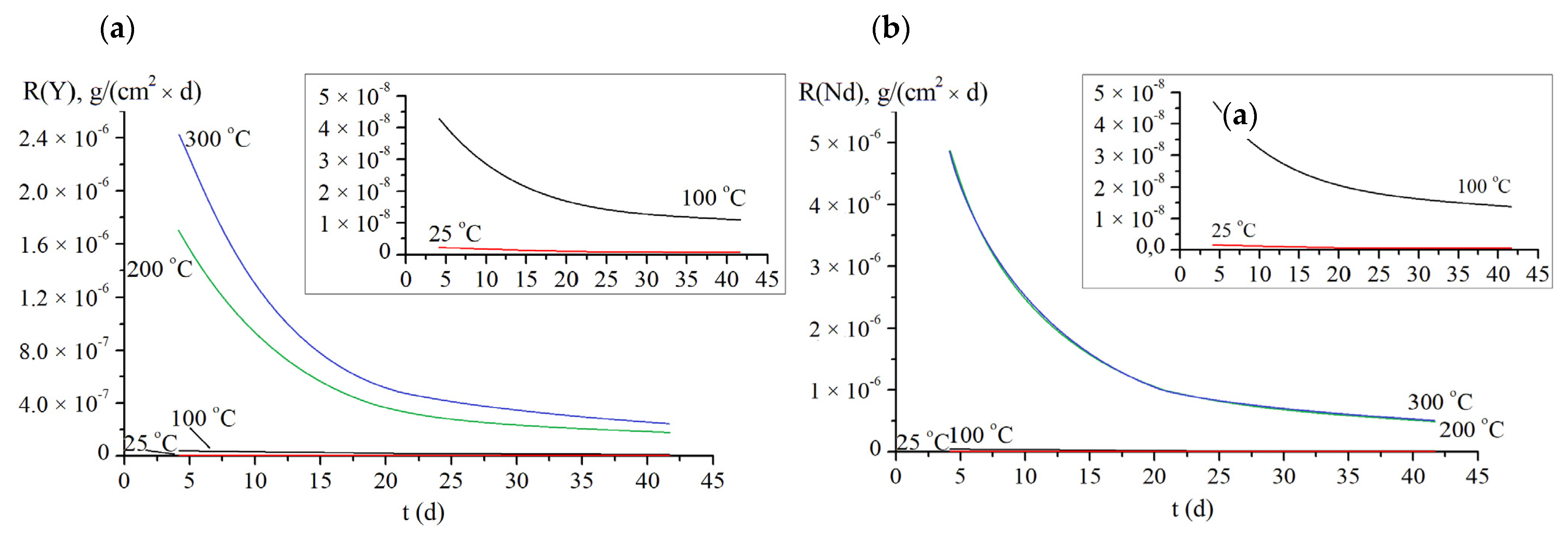
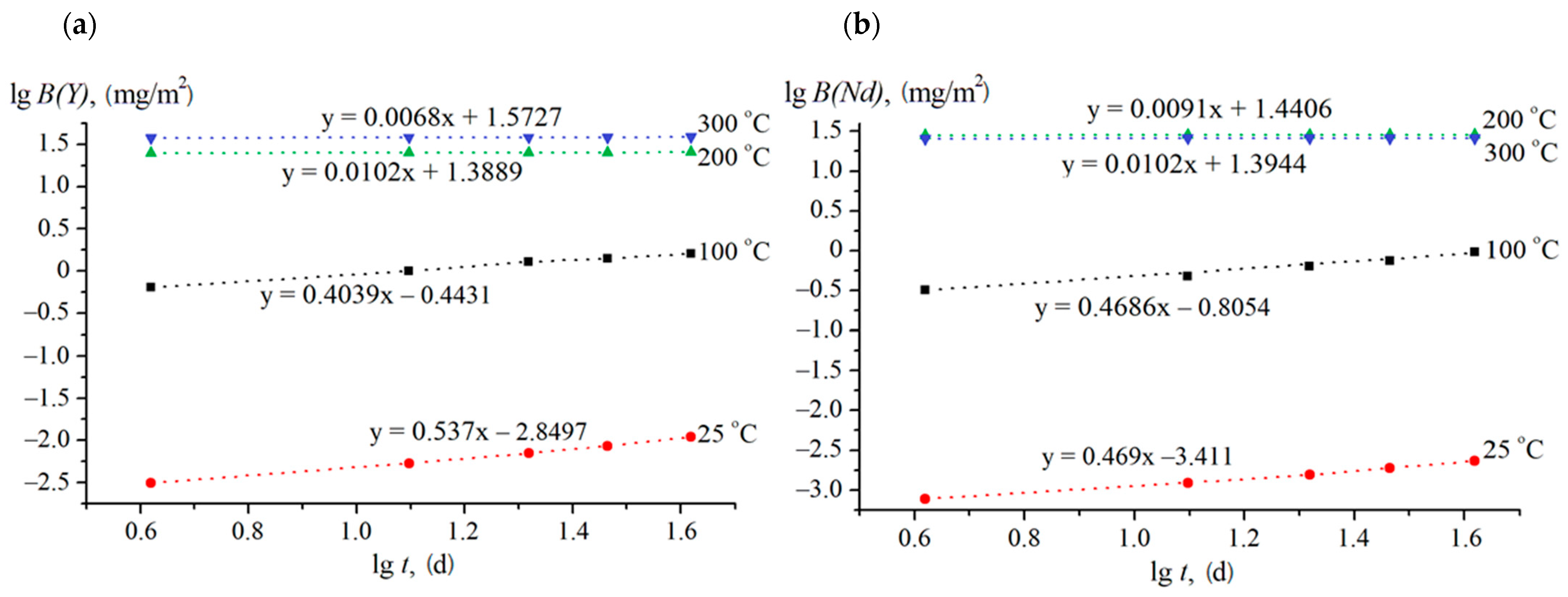
| Experimental Conditions | T (°C) | Leaching Rate Ri (at 1000 h), g/(cm2·d) | |
|---|---|---|---|
| Y | Nd | ||
| Static Mode | 25 | 7.11 × 10−10 | 4.14 × 10−10 |
| Hydrothermal Conditions | 100 | 1.08 × 10−8 | 1.38 × 10−8 |
| 200 | 1.75 × 10−7 | 4.99 × 10−7 | |
| 300 | 2.46 × 10−7 | 4.97 × 10−7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, L.; Nokhrin, A.; Boldin, M.; Lantsev, E.; Murashov, A.; Orlova, A.; Chuvil’deev, V. Study of the Hydrolytic Stability of Fine-Grained Ceramics Based on Y2.5Nd0.5Al5O12 Oxide with a Garnet Structure under Hydrothermal Conditions. Materials 2021, 14, 2152. https://doi.org/10.3390/ma14092152
Alekseeva L, Nokhrin A, Boldin M, Lantsev E, Murashov A, Orlova A, Chuvil’deev V. Study of the Hydrolytic Stability of Fine-Grained Ceramics Based on Y2.5Nd0.5Al5O12 Oxide with a Garnet Structure under Hydrothermal Conditions. Materials. 2021; 14(9):2152. https://doi.org/10.3390/ma14092152
Chicago/Turabian StyleAlekseeva, Liudmila, Aleksey Nokhrin, Maksim Boldin, Eugeniy Lantsev, Artem Murashov, Albina Orlova, and Vladimir Chuvil’deev. 2021. "Study of the Hydrolytic Stability of Fine-Grained Ceramics Based on Y2.5Nd0.5Al5O12 Oxide with a Garnet Structure under Hydrothermal Conditions" Materials 14, no. 9: 2152. https://doi.org/10.3390/ma14092152
APA StyleAlekseeva, L., Nokhrin, A., Boldin, M., Lantsev, E., Murashov, A., Orlova, A., & Chuvil’deev, V. (2021). Study of the Hydrolytic Stability of Fine-Grained Ceramics Based on Y2.5Nd0.5Al5O12 Oxide with a Garnet Structure under Hydrothermal Conditions. Materials, 14(9), 2152. https://doi.org/10.3390/ma14092152








