Failure Load and Fatigue Behavior of Monolithic Translucent Zirconia, PICN and Rapid-Layer Posterior Single Crowns on Zirconia Implants
Abstract
1. Introduction
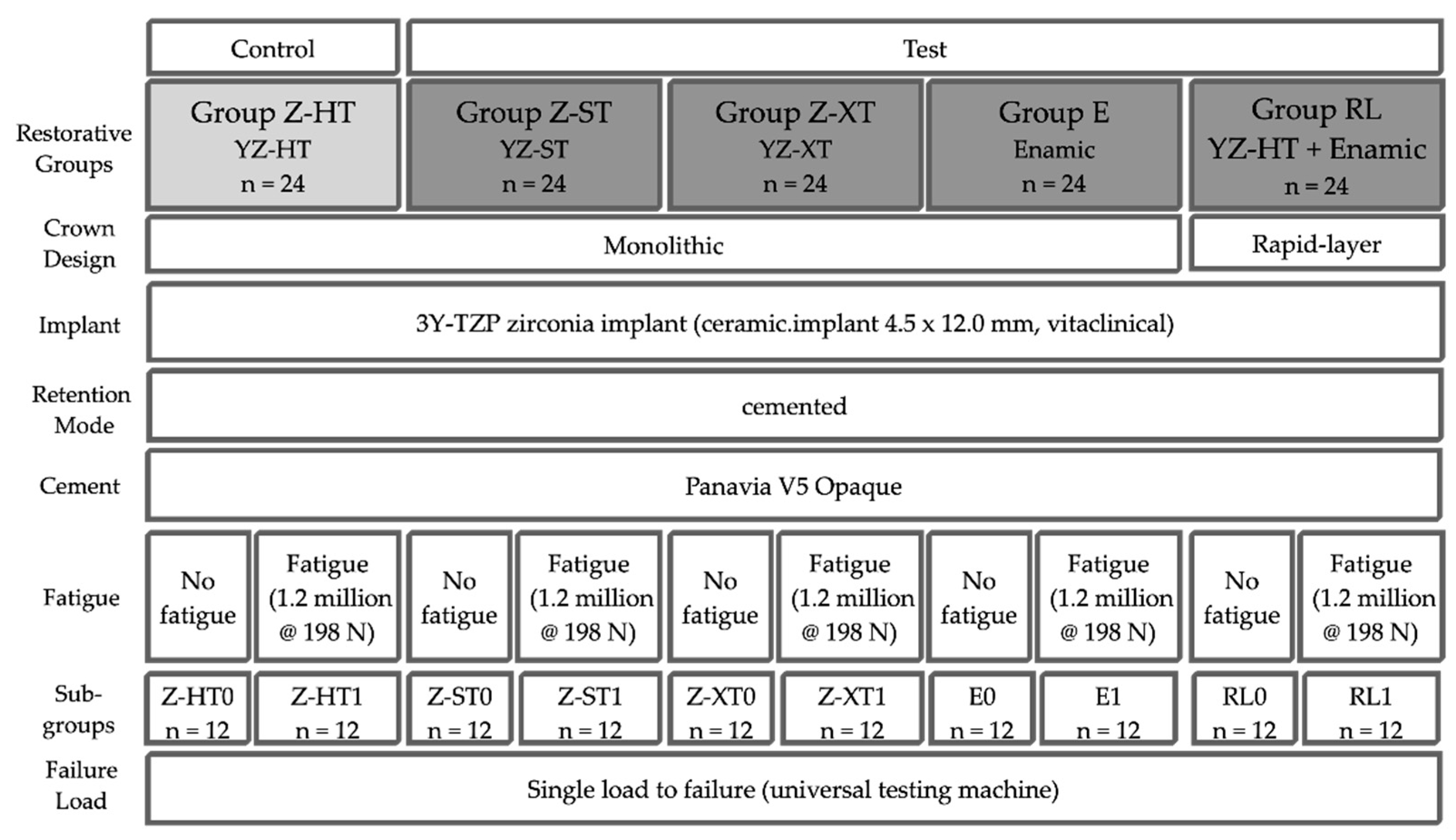
2. Materials and Methods
- Control group Z-HT: 3Y-TZP monolithic “high translucent” zirconia crown (Vita YZ-HT, Vita Zahnfabrik, Bad Säckingen, Germany);
- Test group Z-ST: 4Y-TZP monolithic “super translucent” zirconia crown (Vita YZ-ST, Vita Zahnfabrik);
- Test group Z-XT: 5Y-TZP monolithic “extra translucent” zirconia crown (Vita YZ-XT, Vita Zahnfabrik);
- Test group E: monolithic polymer-infiltrated ceramic network crown (PICN, Vita Enamic, Vita Zahnfabrik);
- Test group RL (rapid layer): polymer-infiltrated ceramic network (PICN Vita Enamic, Vita Zahnfabrik) “table top” adhesively bonded to a 3Y-TZP framework (Vita YZ-HT, Vita Zahnfabrik).
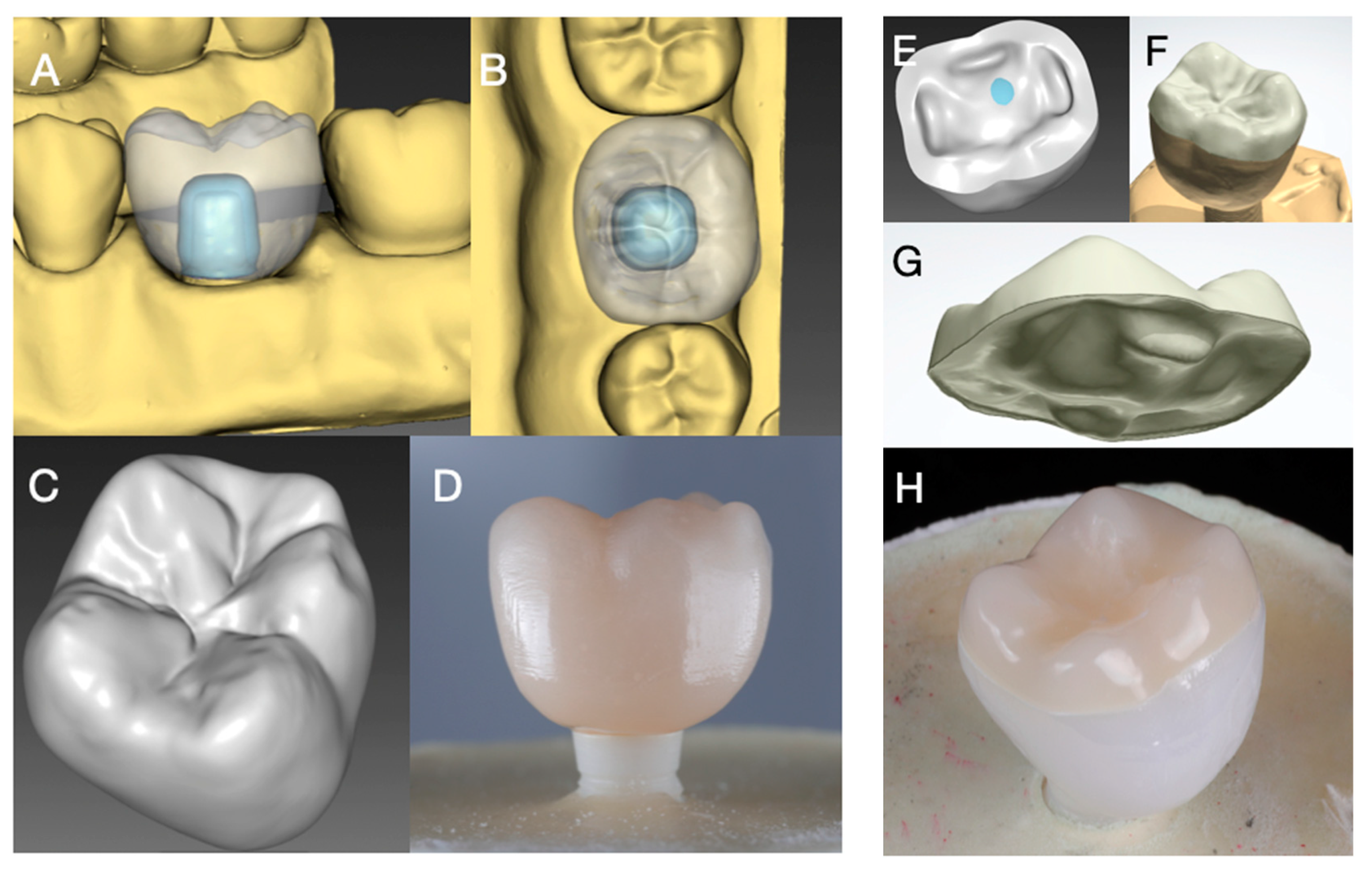
2.1. Fabrication of Implant Crowns
2.2. Preparation of Specimens
2.3. Adhesive Cementation
2.4. Fatigue Analysis
2.5. Load to Failure
2.6. Fractographic Analysis
2.7. Statistical Analysis
3. Results
3.1. Fatigue Exposure
3.2. Single Load to Failure
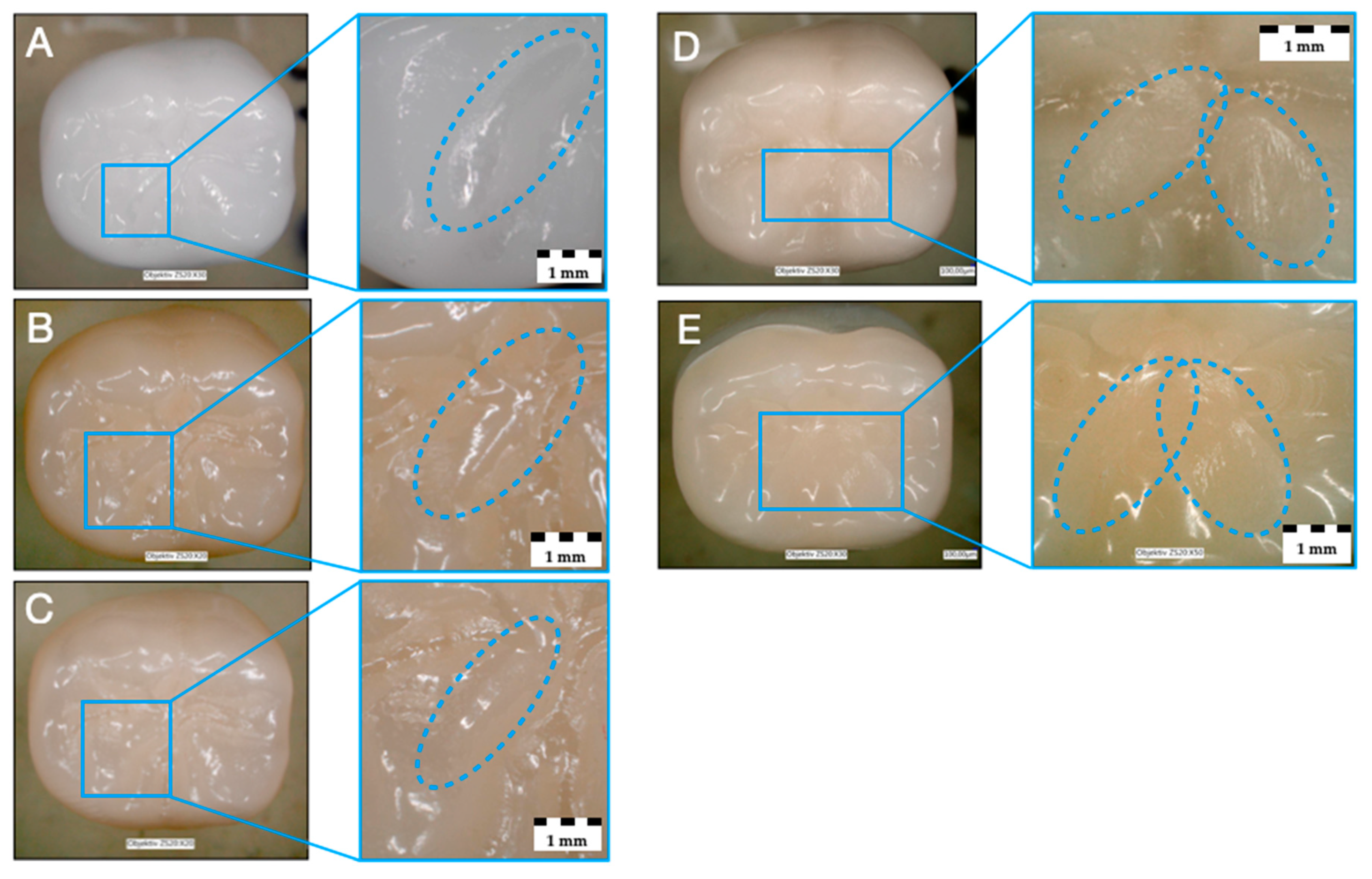
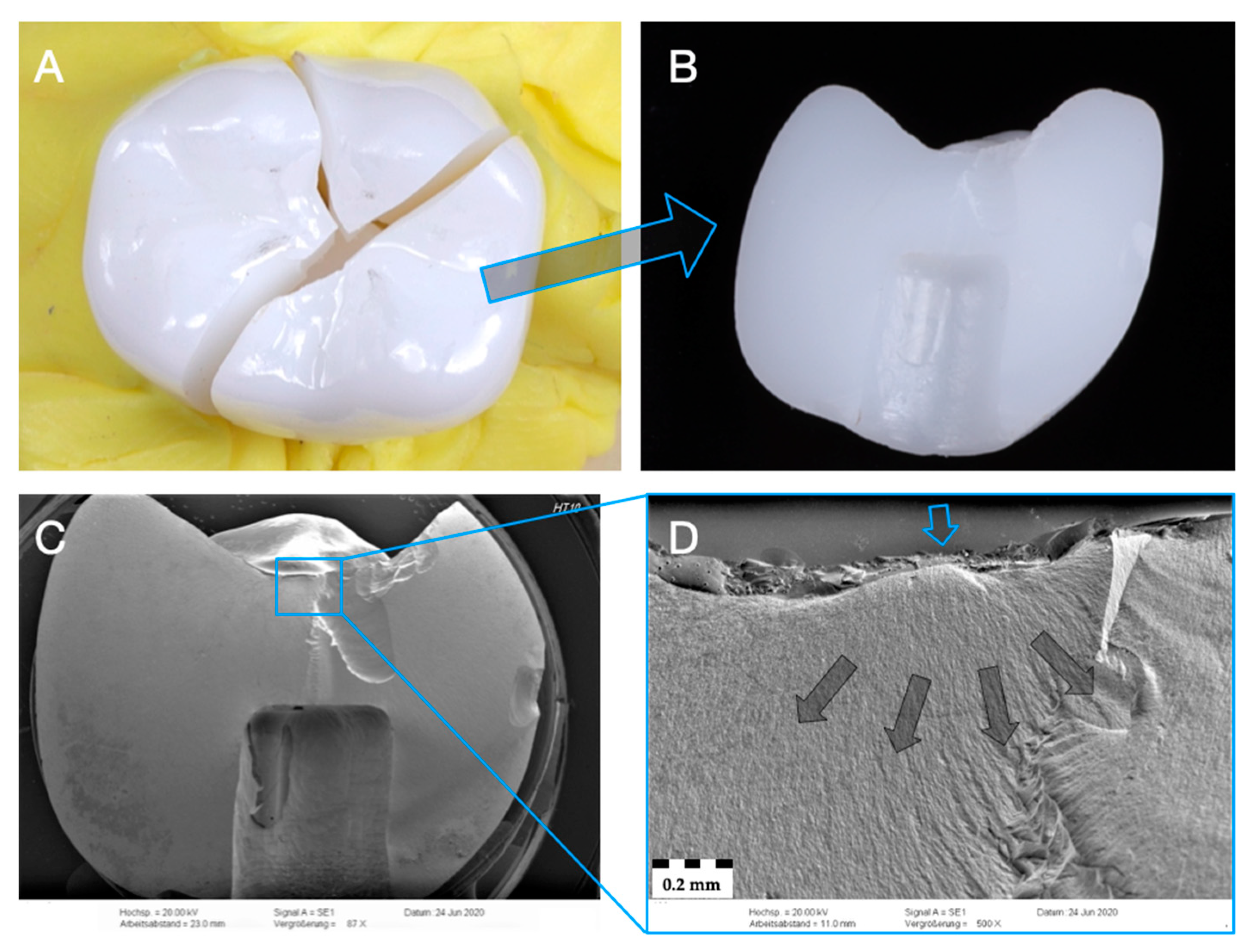
3.3. Failure and Fractographic Analysis after Single-Load-To-Failure Testing
4. Discussion
5. Conclusions
- Fatigue did only influence the failure load of the restorative concept RL (higher failure loads with fatigue).
- All tested materials showed higher failure loads (>1750 N) than normal physiological occlusal loads in the posterior region (200–900N) and can be recommended for clinical use.
- Irrespective of the material used in monolithic application, zirconia (Z-HT, Z-ST, Z-XT) and PICN restorations failed mostly from bulk fractures. When PICN was used as a table top, fracture was limited to PICN, whereas the 3Y-TZP framework remained intact.
- PICN materials, especially in the proposed rapid-layer design, might be an interesting restorative treatment concept for zirconia implants due to their facilitated replaceability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Zirconia compared to titanium dental implants in preclinical studies—A systematic review and meta-analysis. Clin. Oral Implant. Res. 2019, 30, 365–395. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 135–153. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.; Spies, B.C.; Kohal, R.J.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-Year results of a prospective cohort investigation. Clin. Oral Implant. Res. 2020, 31, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Giulini, N.; Hölscher, W.; Schwiertz, A.; Schwarz, F.; Sader, R. Prospective controlled clinical study investigating long-term clinical parameters, patient satisfaction, and microbial contamination of zirconia implants. Clin. Implant. Dent. Relat. Res. 2019, 21, 263–271. [Google Scholar] [CrossRef]
- Bienz, S.P.; Hilbe, M.; Hüsler, J.; Thoma, D.S.; Hämmerle, C.H.F.; Jung, R.E. Clinical and histological comparison of the soft tissue morphology between zirconia and titanium dental implants under healthy and experimental mucositis conditions—A randomized controlled clinical trial. J. Clin. Periodontol. 2020. [Google Scholar] [CrossRef]
- Thoma, D.S.; Benic, G.I.; Munoz, F.; Kohal, R.; Sanz Martin, I.; Cantalapiedra, A.G.; Hämmerle, C.H.; Jung, R.E. Histological analysis of loaded zirconia and titanium dental implants: An experimental study in the dog mandible. J. Clin. Periodontol. 2015, 42, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Clever, K.; Schlegel, K.A.; Kniha, H.; Conrads, G.; Rink, L.; Modabber, A.; Holzle, F.; Kniha, K. Experimental peri-implant mucositis around titanium and zirconia implants in comparison to a natural tooth: Part 2—Clinical and microbiological parameters. Int. J. Oral Maxillofac. Surg. 2019, 48, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Noguerol, B.; Sanz-Sanchez, I.; Hämmerle, C.H.F.; Schliephake, H.; Renouard, F.; Sicilia, A.; Steering, C.; Cordaro, L.; Jung, R.; et al. European Association for Osseointegration Delphi study on the trends in implant dentistry in Europe for the year 2030. Clin. Oral Implant. Res. 2019, 30, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Dennison, D.K. Clinical longevity of zirconia implants with the focus on biomechanical and biological outcome. Curr. Oral Health Rep. 2020, 7, 344–351. [Google Scholar] [CrossRef]
- Spies, B.C.; Pieralli, S.; Vach, K.; Kohal, R.J. CAD/CAM-Fabricated ceramic implant-supported single crowns made from lithium disilicate: Final results of a 5-year prospective cohort study. Clin. Implant. Dent. Relat. Res. 2017, 19, 876–883. [Google Scholar] [CrossRef]
- Spies, B.C.; Balmer, M.; Jung, R.E.; Sailer, I.; Vach, K.; Kohal, R.J. All-Ceramic single crowns supported by zirconia implants: 5-Year results of a prospective multicenter study. Clin. Oral Implant. Res. 2019, 30, 466–475. [Google Scholar] [CrossRef]
- Spies, B.C.; Balmer, M.; Patzelt, S.B.; Vach, K.; Kohal, R.J. Clinical and patient-reported outcomes of a zirconia oral implant: Three-Year results of a prospective cohort investigation. J. Dent. Res. 2015, 94, 1385–1391. [Google Scholar] [CrossRef]
- Spies, B.C.; Witkowski, S.; Vach, K.; Kohal, R.J. Clinical and patient-reported outcomes of zirconia-based implant fixed dental prostheses: Results of a prospective case series 5 years after implant placement. Clin. Oral Implant. Res. 2018, 29, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, F.A.; Boldt, J.; Gierthmuehlen, P.C. CAD/CAM ceramic restorative materials for natural teeth. J. Dent. Res. 2018, 97, 1082–1091. [Google Scholar] [CrossRef]
- Rabel, K.; Spies, B.C.; Pieralli, S.; Vach, K.; Kohal, R.J. The clinical performance of all-ceramic implant-supported single crowns: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. 18), 196–223. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 199–214. [Google Scholar] [CrossRef]
- Güth, J.F.; Stawarczyk, B.; Edelhoff, D.; Liebermann, A. Zirconia and its novel compositions: What do clinicians need to know? Quintessence Int. 2019, 50, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lawn, B.R. Novel zirconia materials in dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Isler, S. Microstructural, physical, and optical characterization of high-translucency zirconia ceramics. J. Prosthet. Dent. 2020, 123, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Inokoshi, M.; Batuk, M.; Hadermann, J.; Naert, I.; Van Meerbeek, B.; Vleugels, J. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent. Mater. 2016, 32, e327–e337. [Google Scholar] [CrossRef]
- Coldea, A.; Swain, M.V.; Thiel, N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent. Mater. 2013, 29, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Awada, A.; Nathanson, D. Mechanical properties of resin-ceramic CAD/CAM restorative materials. J. Prosthet. Dent. 2015, 114, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Pitta, J.; Hicklin, S.P.; Fehmer, V.; Boldt, J.; Gierthmuehlen, P.C.; Sailer, I. Mechanical stability of zirconia meso-abutments bonded to titanium bases restored with different monolithic all-ceramic crowns. Int. J. Oral Maxillofac. Implant. 2019, 34, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, P.; Kirsten, H.; Haak, R.; Olms, C. Biomechanical properties of polymer-infiltrated ceramic crowns on one-piece zirconia implants after long-term chewing simulation. Int. J. Implant. Dent. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical outcomes of zirconia dental implants: A systematic review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Strub, J.R.; Lu, X.Y. Wear of composite resin veneering materials in a dual-axis chewing simulator. J. Oral Rehabil. 1999, 26, 372–378. [Google Scholar] [CrossRef]
- Delong, R.; Sakaguchi, R.L.; Douglas, W.H.; Pintado, M.R. The wear of dental amlgam in an artifical mouth: A clinical correlation. Dent. Mater. 1985, 1, 238–242. [Google Scholar] [CrossRef]
- Rosentritt, M.; Behr, M.; van der Zel, J.M.; Feilzer, A.J. Approach for valuating the influence of laboratory simulation. Dent. Mater. 2009, 25, 348–352. [Google Scholar] [CrossRef]
- Strub, J.R.; Gerds, T. Fracture strength and failure mode of five different single-tooth implant-abutment combinations. Int. J. Prosthodont. 2003, 16, 167–171. [Google Scholar]
- Rohr, N.; Balmer, M.; Müller, J.A.; Märtin, S.; Fischer, J. Chewing simulation of zirconia implant supported restorations. J. Prosthodont. Res. 2019, 63, 361–367. [Google Scholar] [CrossRef]
- Zaugg, L.K.; Meyer, S.; Rohr, N.; Zehnder, I.; Zitzmann, N.U. Fracture behavior, marginal gap width, and marginal quality of vented or pre-cemented CAD/CAM all-ceramic crowns luted on Y-TZP implants. Clin. Oral Implant. Res. 2018, 29, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Joos, M.; Sailer, I.; Filippi, A.; Mukaddam, K.; Rosentritt, M.; Kühl, S. Stability of screw-retention in two-piece zirconia implants: An in vitro study. Clin. Oral Implant. Res. 2020, 31, 607–614. [Google Scholar] [CrossRef]
- Varga, S.; Spalj, S.; Lapter Varga, M.; Anic Milosevic, S.; Mestrovic, S.; Slaj, M. Maximum voluntary molar bite force in subjects with normal occlusion. Eur. J. Orthod. 2011, 33, 427–433. [Google Scholar] [CrossRef]
- Turgut, S. Optical properties of currently used zirconia-based esthetic restorations fabricated with different techniques. J. Esthet. Restor. Dent. 2020, 32, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rohr, N.; Coldea, A.; Zitzmann, N.U.; Fischer, J. Loading capacity of zirconia implant supported hybrid ceramic crowns. Dent. Mater. 2015, 31, e279–e288. [Google Scholar] [CrossRef]
- Rohr, N.; Märtin, S.; Fischer, J. Correlations between fracture load of zirconia implant supported single crowns and mechanical properties of restorative material and cement. Dent. Mater. J. 2018, 37, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Nueesch, R.; Conejo, J.; Mante, F.; Fischer, J.; Märtin, S.; Rohr, N.; Blatz, M.B. Loading capacity of CAD/CAM-fabricated anterior feldspathic ceramic crowns bonded to one-piece zirconia implants with different cements. Clin. Oral Implant. Res. 2019, 30, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Vonderheide, M.; Conejo, J. The effect of resin bonding on long-term success of high-strength ceramics. J. Dent. Res. 2018, 97, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kern, M. Bonding to oxide ceramics—Laboratory testing versus clinical outcome. Dent. Mater. 2015, 31, 8–14. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, W.; Jiang, F.; Wang, Z.; Zhao, J.; Zhou, C.; Wu, J. Effects of air-abrasion pressure on mechanical and bonding properties of translucent zirconia. Clin. Oral Investig. 2020, 25, 1979–1988. [Google Scholar] [CrossRef]
- Franco-Tabares, S.; Wardecki, D.; Nakamura, K.; Ardalani, S.; Hjalmarsson, L.; Franke Stenport, V.; Johansson, C.B. Effect of airborne-particle abrasion and polishing on novel translucent zirconias: Surface morphology, phase transformation and insights into bonding. J. Prosthodont. Res. 2020, 65, 97–105. [Google Scholar] [CrossRef]
- Blumer, L.; Schmidli, F.; Weiger, R.; Fischer, J. A systematic approach to standardize artificial aging of resin composite cements. Dent. Mater. 2015, 31, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Kruzic, J.J.; Arsecularatne, J.A.; Tanaka, C.B.; Hoffman, M.J.; Cesar, P.F. Recent advances in understanding the fatigue and wear behavior of dental composites and ceramics. J. Mech. Behav. Biomed. Mater. 2018, 88, 504–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sailer, I.; Lawn, B.R. Fatigue of dental ceramics. J. Dent. 2013, 41, 1135–1147. [Google Scholar] [CrossRef]
- Mörmann, W.H.; Stawarczyk, B.; Ender, A.; Sener, B.; Attin, T.; Mehl, A. Wear characteristics of current aesthetic dental restorative CAD/CAM materials: Two-Body wear, gloss retention, roughness and Martens hardness. J. Mech. Behav. Biomed. Mater. 2013, 20, 113–125. [Google Scholar] [CrossRef]
- Zhang, F.; Spies, B.C.; Vleugels, J.; Reveron, H.; Wesemann, C.; Müller, W.D.; van Meerbeek, B.; Chevalier, J. High-Translucent yttria-stabilized zirconia ceramics are wear-resistant and antagonist-friendly. Dent. Mater. 2019, 35, 1776–1790. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.C.; Janyavula, S.; Syklawer, S.; McLaren, E.A.; Burgess, J.O. Wear of enamel opposing zirconia and lithium disilicate after adjustment, polishing and glazing. J. Dent. 2014, 42, 1586–1591. [Google Scholar] [CrossRef]
- Preis, V.; Grumser, K.; Schneider-Feyrer, S.; Behr, M.; Rosentritt, M. Cycle-Dependent in vitro wear performance of dental ceramics after clinical surface treatments. J. Mech. Behav. Biomed. Mater. 2016, 53, 49–58. [Google Scholar] [CrossRef]
- Esquivel-Upshaw, J.F.; Kim, M.J.; Hsu, S.M.; Abdulhameed, N.; Jenkins, R.; Neal, D.; Ren, F.; Clark, A.E. Randomized clinical study of wear of enamel antagonists against polished monolithic zirconia crowns. J. Dent. 2018, 68, 19–27. [Google Scholar] [CrossRef]
- Bonfante, E.A.; Coelho, P.G. A critical perspective on mechanical testing of implants and prostheses. Adv. Dent. Res. 2016, 28, 18–27. [Google Scholar] [CrossRef]
- Kelly, J.R.; Benetti, P.; Rungruanganunt, P.; Bona, A.D. The slippery slope: Critical perspectives on in vitro research methodologies. Dent. Mater. 2012, 28, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.V.; Coldea, A.; Bilkhair, A.; Guess, P.C. Interpenetrating network ceramic-resin composite dental restorative materials. Dent. Mater. 2016, 32, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Van Brakel, R.; Cune, M.S.; Van Winkelhoff, A.J.; De Putter, C.; Verhoeven, J.W.; Van der Reijden, W. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: An in vivo study in man. Clin. Oral Implant. Res. 2011, 22, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential behavior of fibroblasts and epithelial cells on structured implant abutment materials: A comparison of materials and surface topographies. Clin. Implant. Dent. Relat. Res. 2015, 17, 1237–1249. [Google Scholar] [CrossRef] [PubMed]

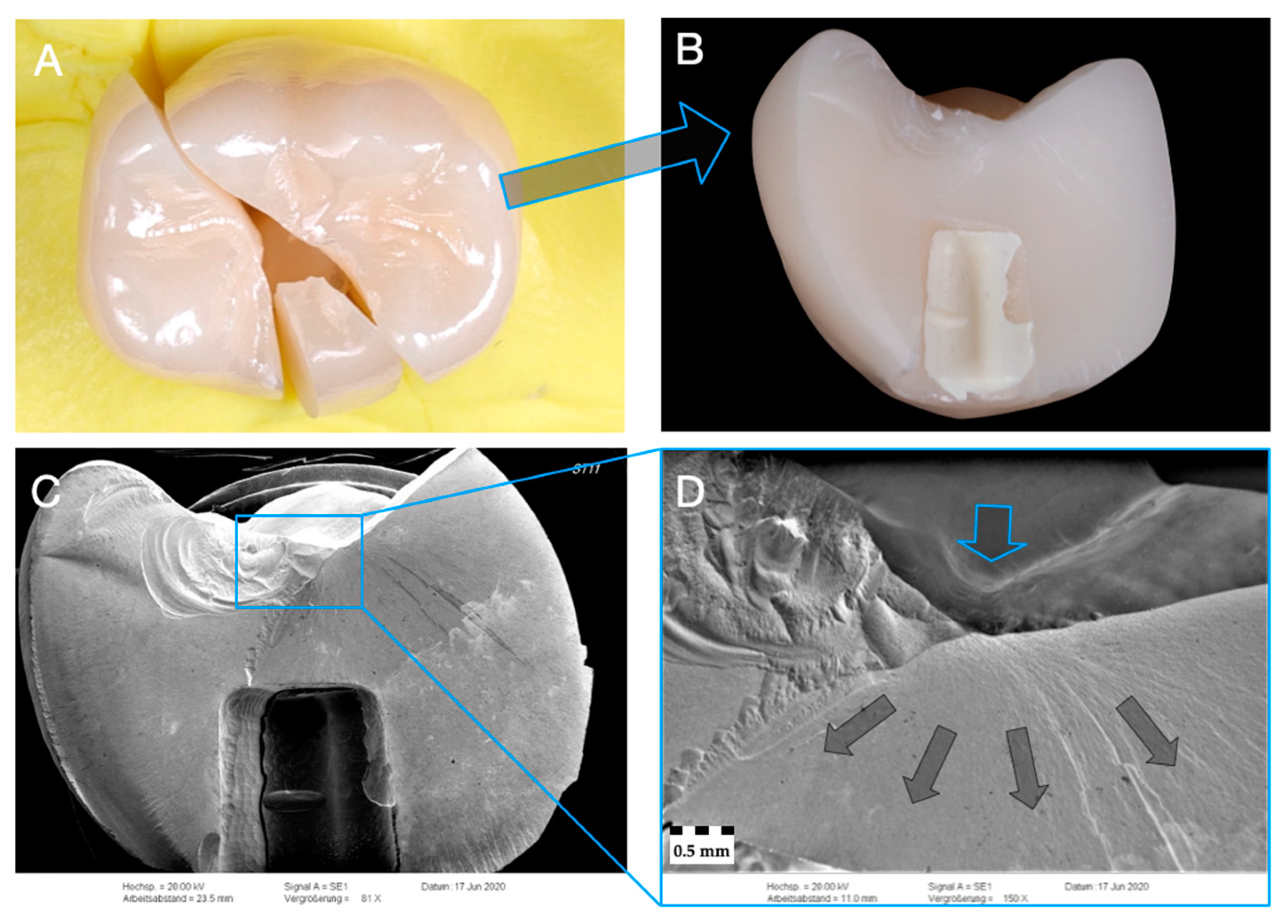
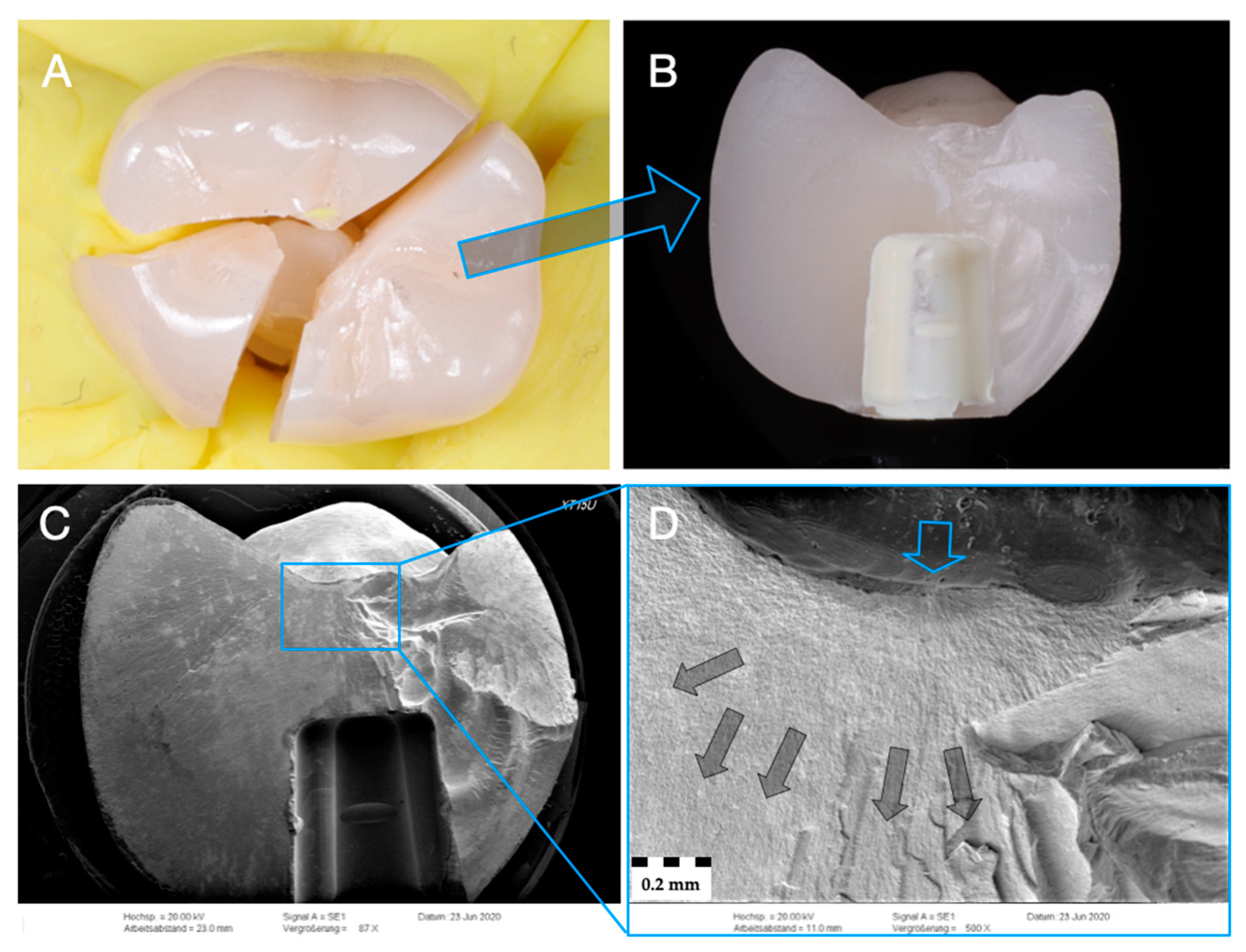
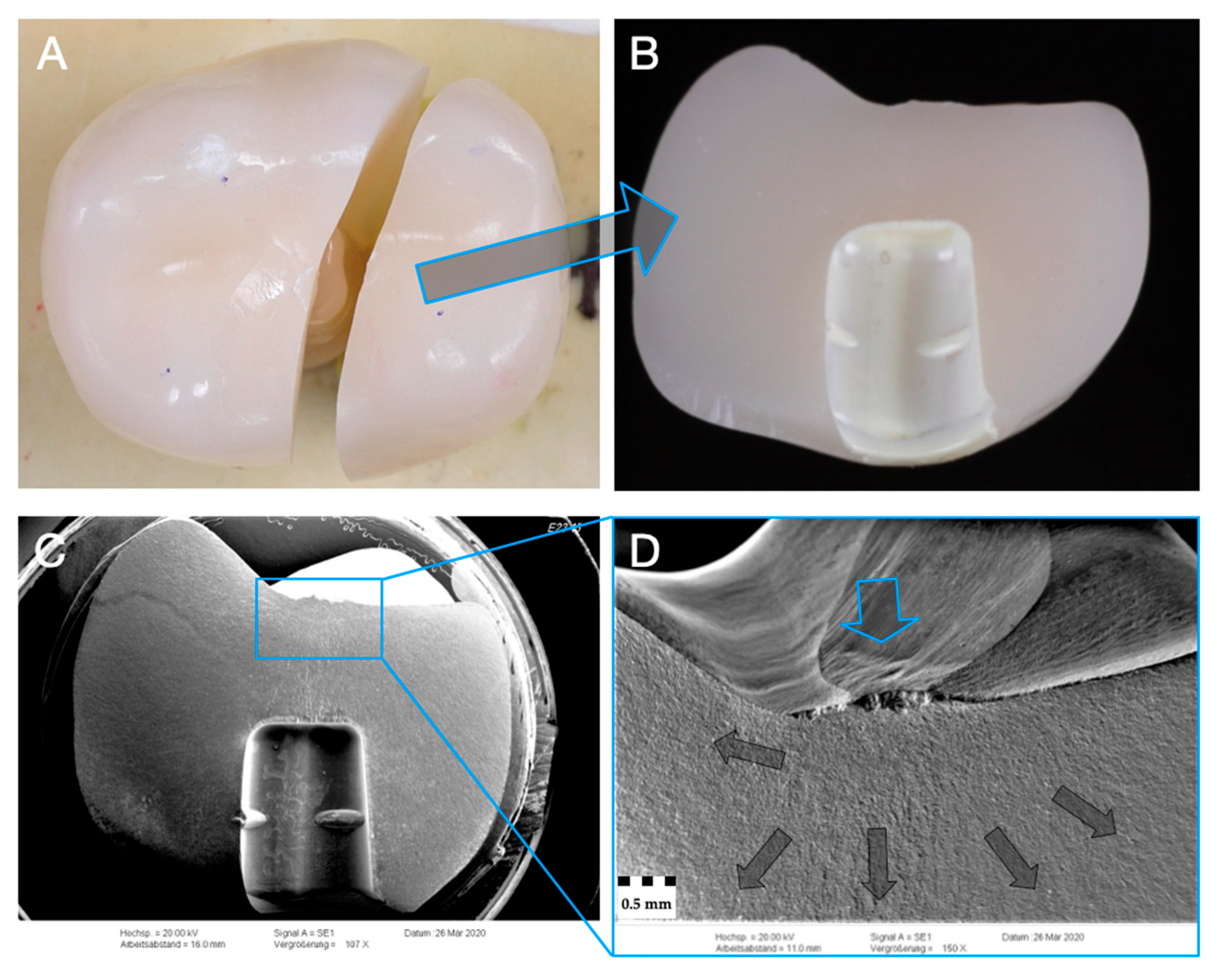
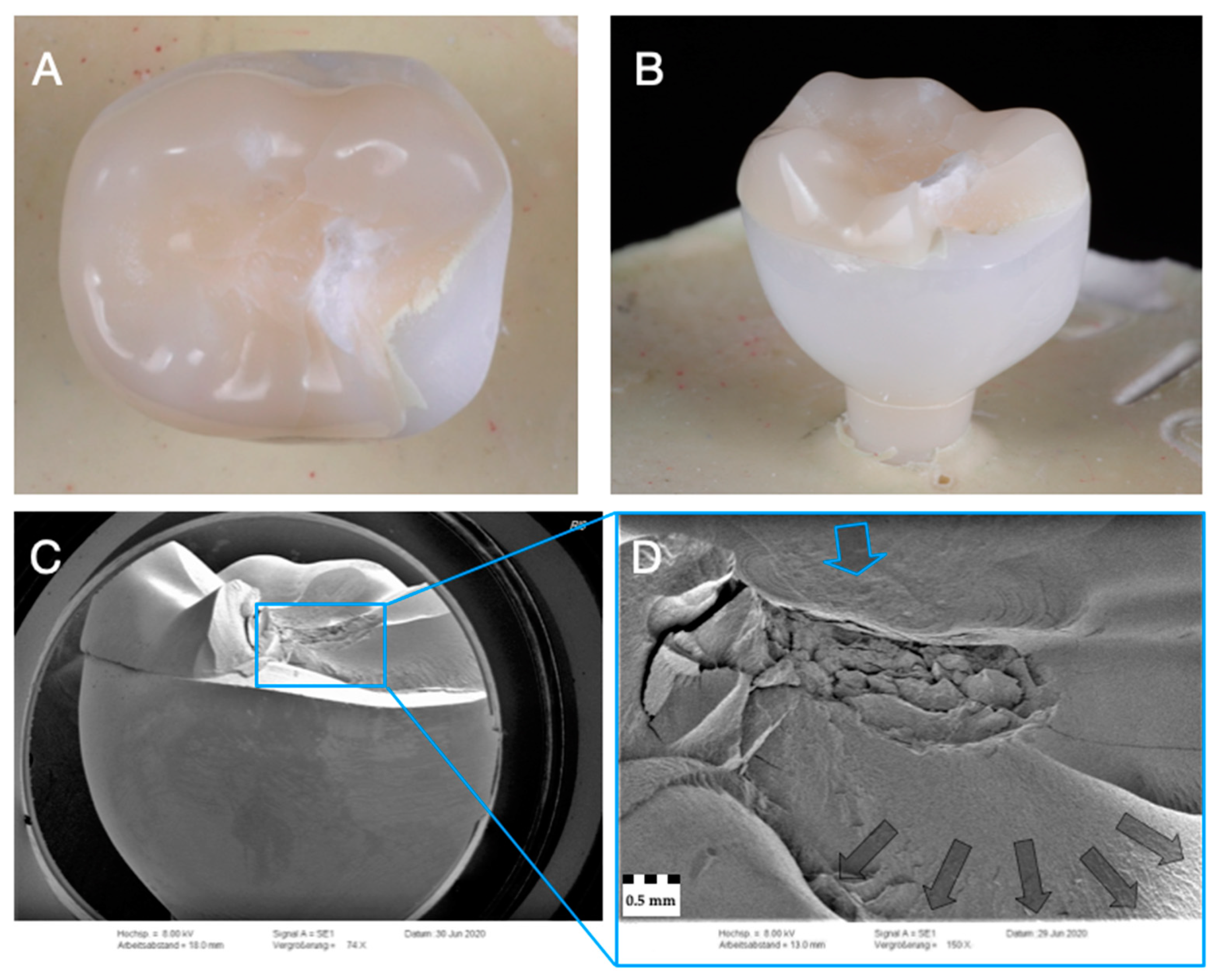
| Group (n = 24) | Crown/ Implant Design | Type | Name | Y2O3 (Weight%) | Flexural Strength (MPa) | Manufacturer |
|---|---|---|---|---|---|---|
| Z-HT | Monolithic Crown | 3Y-TZP Zirconia | Vita YZ HT | 4–6 | 1200 | Vita Zahnfabrik, Bad Säckingen, Germany |
| Z-ST | Monolithic Crown | 4Y-TZP Zirconia | Vita YZ ST | 6–8 | >850 | Vita Zahnfabrik, Bad Säckingen, Germany |
| Z-XT | Monolithic Crown | 5Y-TZP Zirconia | Vita YZ XT | 8–10 | >600 | Vita Zahnfabrik, Bad Säckingen, Germany |
| E | Monolithic Crown | PICN | Vita Enamic | - | 150–160 | Vita Zahnfabrik, Bad Säckingen, Germany |
| RL | Rapid- Layer Crown | 3Y-TZP Zirconia | Vita YZ HT | 4–6 | 1200 | Vita Zahnfabrik, Bad Säckingen, Germany |
| PICN | Vita Enamic | - | 150–160 | |||
| All groups | One-piece Implant | 3Y-TZP Zirconia | ceramic. implant | 5 | 1400 | vitaclinical, Bad Säckingen, Germany |
| Zirconia Crowns | Polymer-Infiltrated Ceramic Crown/Table Top | Zirconia Implant | |
|---|---|---|---|
| Group | Z-HT, Z-ST, Z-XT, RL (framework) | E, RL | All |
| Surface Treatment | Air-particle abrasion with 50 μm Al2O3 at 2 bar, Ultrasonic cleaning with 70% ethanol for 3 min | Cleaning with 70% Ethanol, Etching with 5% hydrofluoric acid for 60 s (Vita Ceramics Etch, Vita Zahnfabrik), rinsed with air–water spray (30 s), ultrasonic cleaning with distilled water (5 min) | - |
| Primer | Clearfil Ceramic Primer Plus (Kuraray Noritake) | ||
| Adhesive Cement | Panavia V5 opaque (Kuraray Noritake) | ||
| Group | Without Fatigue | With Fatigue | Influence of Fatigue | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t-Value | p-Value | |
| Z-HT | 8450 ± 1443 a | 8145 ± 1426 A | 0.522 | 0.607 |
| Z-ST | 8161 ± 1024 a | 8557 ± 1288 A | −0.833 | 0.414 |
| Z-XT | 6747 ± 795 b | 6110 ± 683 B | 2.103 | 0.047 |
| E | 1889 ± 278 c | 1769 ± 413 C | 0.834 | 0.413 |
| RL | 3938 ± 795 d | 5881 ± 862 B | −5.736 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spitznagel, F.A.; Röhrig, S.; Langner, R.; Gierthmuehlen, P.C. Failure Load and Fatigue Behavior of Monolithic Translucent Zirconia, PICN and Rapid-Layer Posterior Single Crowns on Zirconia Implants. Materials 2021, 14, 1990. https://doi.org/10.3390/ma14081990
Spitznagel FA, Röhrig S, Langner R, Gierthmuehlen PC. Failure Load and Fatigue Behavior of Monolithic Translucent Zirconia, PICN and Rapid-Layer Posterior Single Crowns on Zirconia Implants. Materials. 2021; 14(8):1990. https://doi.org/10.3390/ma14081990
Chicago/Turabian StyleSpitznagel, Frank A., Sara Röhrig, Robert Langner, and Petra C. Gierthmuehlen. 2021. "Failure Load and Fatigue Behavior of Monolithic Translucent Zirconia, PICN and Rapid-Layer Posterior Single Crowns on Zirconia Implants" Materials 14, no. 8: 1990. https://doi.org/10.3390/ma14081990
APA StyleSpitznagel, F. A., Röhrig, S., Langner, R., & Gierthmuehlen, P. C. (2021). Failure Load and Fatigue Behavior of Monolithic Translucent Zirconia, PICN and Rapid-Layer Posterior Single Crowns on Zirconia Implants. Materials, 14(8), 1990. https://doi.org/10.3390/ma14081990






