Structure and Properties of Zr-Mo-Si-B-(N) Hard Coatings Obtained by d.c. Magnetron Sputtering of ZrB2-MoSi2 Target
Abstract
1. Introduction
2. Materials and Methods
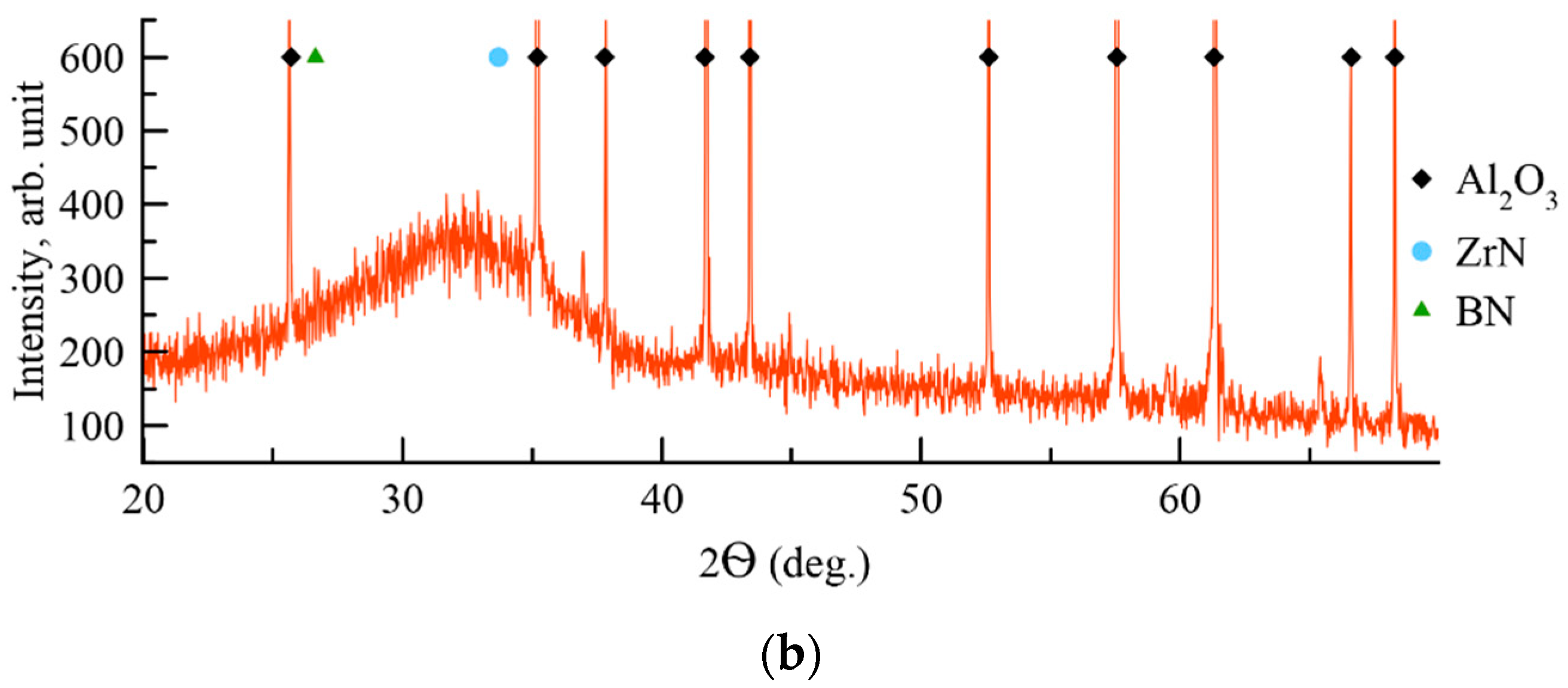
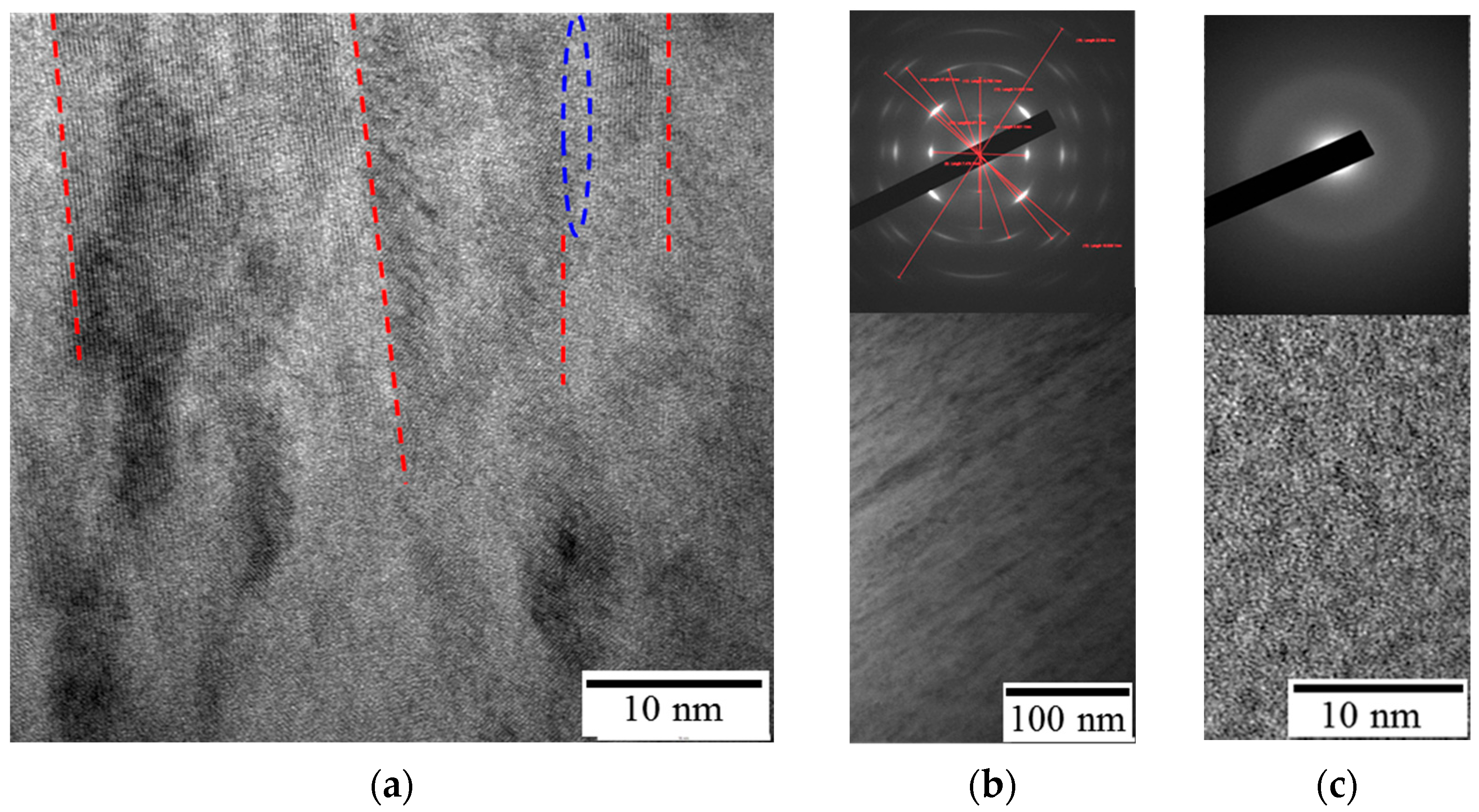
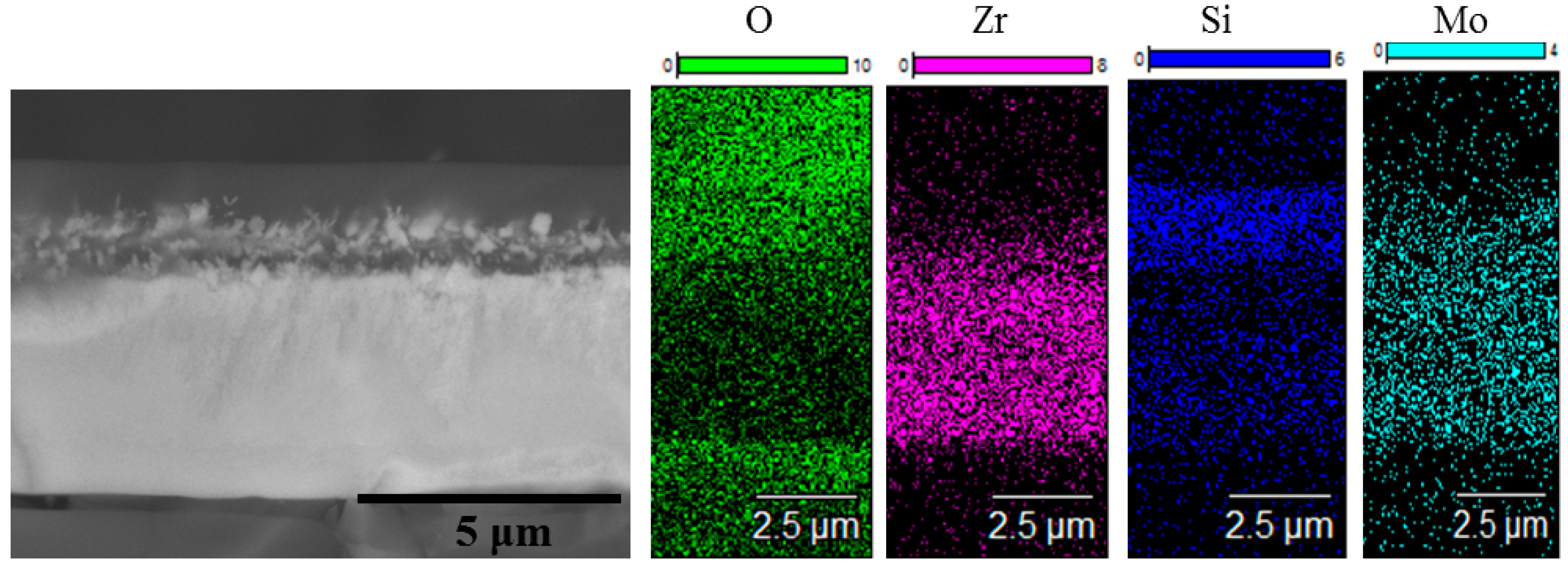
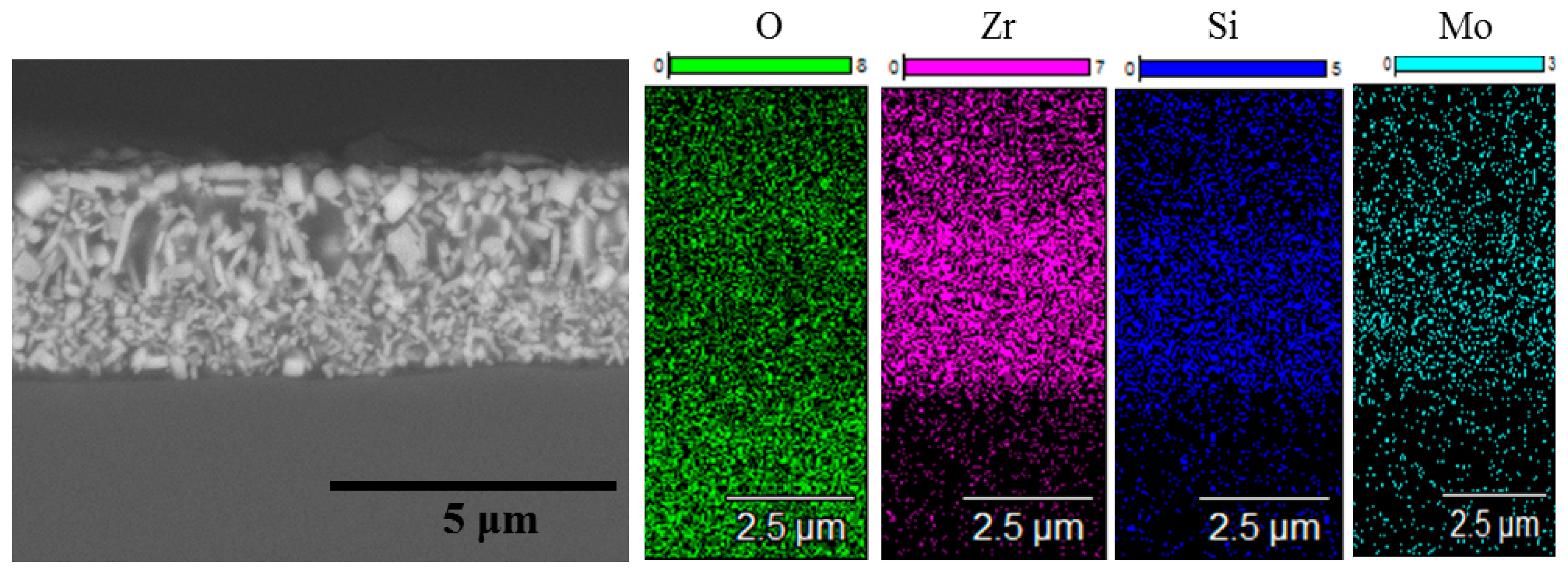
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonber, J.K.; Murthy, T.S.R.C.; Subramanian, C.; Kumar, S.; Fotedar, R.K.; Suri, A.K. Investigations on synthesis of ZrB2 and development of new composites with HfB2 and TiSi2. Int. J. Refract. Met. Hard Mater. 2011, 29, 21–30. [Google Scholar] [CrossRef]
- Saunders, T.; Grasso, S.; Reece, M.J. Limiting oxidation of ZrB2 by application of an electric field across its oxide scale. J. Alloys Compd. 2015, 653, 629–635. [Google Scholar] [CrossRef]
- Wang, T.G.; Liu, Y.; Zhang, T.; Kim, D.I.; Kim, K.H. Influence of Nitrogen Flow Ratio on the Microstructure, Composition, and Mechanical Properties of DC Magnetron Sputtered Zr-B-O-N Films. J. Mater. Sci. Technol. 2012, 28, 981–991. [Google Scholar] [CrossRef]
- Macías, H.A.; Yate, L.; Coy, L.E.; Aperador, W.; Olaya, J.J. Influence of Si-addition on wear and oxidation resistance of TiWSixN thin films. Ceram. Int. 2019, 45, 17363–17375. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Andreev, S.O.; Shvyndina, N.V.; Levashov, E.A.; Timofeev, A.N.; Shtansky, D.V. The influence of Si concentrations on the oxidation resistance of Mo-Si-B-(N) coatings. Russ. J. Non-Ferrous Met. 2014, 55, 645–651. [Google Scholar] [CrossRef]
- Xin, L.; Chen, Q.; Teng, Y.; Wang, W.; Sun, A.; Zhu, S.; Wang, F. Effects of silicon and multilayer structure of TiAl(Si)N coatings on the oxidation resistance of Ti6Al4V. Surf. Coat. Technol. 2013, 228, 48–58. [Google Scholar] [CrossRef]
- Bae, K.E.; Chae, K.W.; Park, J.K.; Lee, W.S.; Baik, Y.J. Oxidation behavior of amorphous boron carbide-silicon carbide nano-multilayer thin films. Surf. Coat. Technol. 2015, 276, 55–58. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, F.V.; Lemesheva, M.V.; Shvyndina, N.V.; Levashov, E.A.; Potanin, A.Y. Structure, Mechanical Properties, and Oxidation Resistance of ZrB2, ZrSiB, and ZrSiB/SiBC Coatings. Prot. Met. Phys. Chem. Surf. 2018, 54, 1147–1156. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Chu, Y.; Li, K.; Fu, Q. ZrB2-SiC gradient oxidation protective coating for carbon/carbon composites. Ceram. Int. 2014, 40, 7171–7176. [Google Scholar] [CrossRef]
- Ren, Y.; Qian, Y.; Xu, J.; Zuo, J.; Li, M. Ultra-high temperature oxidation resistance of ZrB2-20SiC coating with TaSi2 addition on siliconized graphite. Ceram. Int. 2019, 45, 15366–15374. [Google Scholar] [CrossRef]
- Yang, X.; Feng, C.; Qing, W. ZrB2 -SiC as a protective coating for C/SiC composites: Effect of high temperature oxidation on thermal shock property and protection mechanism. J. Asian Ceram. Soc. 2016, 4, 159–163. [Google Scholar] [CrossRef]
- Aliasgarian, R.; Naderi, M.; Mirsalehi, S.E. Ablation mechanism of ZrB2-SiC coating for SiC-coated graphite under an oxyacetylene flame. Surf. Coat. Technol. 2018, 350, 511–518. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Liu, Y.; Guo, Y.; Zhang, M.; Zhang, L.; Jian, X.; Yin, L.; Xie, J.; Deng, L. Oxidation behaviour of plasma-sprayed ZrB2-SiC coatings. Ceram. Int. 2019, 45, 2385–2392. [Google Scholar] [CrossRef]
- Krishnarao, R.V.; Alam, M.Z.; Das, D.K. In-situ formation of SiC, ZrB2-SiC and ZrB2-SiC-B4C-YAG coatings for high temperature oxidation protection of C/C composites. Corros. Sci. 2018, 141, 72–80. [Google Scholar] [CrossRef]
- Hu, D.; Fu, Q.; Liu, T.; Tong, M. Structural design and ablation performance of ZrB2/MoSi2 laminated coating for SiC coated carbon/carbon composites. J. Eur. Ceram. Soc. 2020, 40, 212–219. [Google Scholar] [CrossRef]
- Liu, X.; Deng, C.; Deng, C.; Liu, M.; Zhou, K. Mullite-modified ZrB2-MoSi2 coating for carbon/carbon composites to withstand long term ablation. Ceram. Int. 2018, 44, 4330–4337. [Google Scholar] [CrossRef]
- Yanjiao, Y.; Mingjiang, D.; Chunbei, W.; Huijun, H.; Songsheng, L. Microstructure and Anti-oxidation Properties of SiC/MoSi2-ZrB2 Coating for Carbon/Carbon Composites Prepared by Magnetron Sputtering Method. Rare Met. Mater. Eng. 2017, 46, 3663–3668. [Google Scholar] [CrossRef]
- Jinyuan, M.; Min, L.; Chunming, D.; Jie, M.; Dechang, Z. A Comparative Study of Spray-dried and Mechanically-mixed ZrB2-MoSi2 Composite Coatings Fabricated by Low Pressure Plasma Spray. Rare Met. Mater. Eng. 2016, 45, 1386–1390. [Google Scholar] [CrossRef]
- Cheng, S.; Geng, L.; Liu, X.; Wang, Y. Laser ablation behavior and mechanism of C/SiC coated with ZrB2 –MoSi2–SiC/Mo prepared by HVOF. Ceram. Int. 2020, 46, 17752–17762. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, H.; Li, H.; Zheng, X.; Ding, C. Dense ZrB2-MoSi2 composite coating fabricated by low pressure plasma spray (LPPS). Ceram. Int. 2013, 39, 9773–9777. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, Y.; Hu, C.; Li, H.; Zeng, Y.; Zheng, X.; Ren, M.; Sun, J. High temperature oxidation resistance of metal silicide incorporated ZrB2 composite coatings prepared by vacuum plasma spray. Ceram. Int. 2015, 41, 14868–14875. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, H.; Liu, Z.; Hu, C.; Wang, X.; Zheng, X.; Ding, C. Microstructure evolution of ZrB2-MoSi2 composite coatings at middle and high temperatures. Surf. Coat. Technol. 2015, 273, 30–38. [Google Scholar] [CrossRef]
- Yang, T.; Guo, X. Oxidation behavior of Zr-Y alloyed Mo-Si-B based alloys. Int. J. Refract. Met. Hard Mater. 2020, 88, 105200. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Li, R.; Chen, X.; Wang, T.; Zhang, G. Unprecedented oxidation resistance at 900 °C of Mo–Si–B composite with addition of ZrB2. Ceram. Int. 2020, 46, 14632–14639. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Iatsyuk, I.V.; Shvindina, N.V.; Levashov, E.A.; Shtansky, D.V. Comparative investigation of structure, mechanical properties, and oxidation resistance of Mo-Si-B and Mo-Al-Si-B coatings. Corros. Sci. 2017, 123, 319–327. [Google Scholar] [CrossRef]
- Sossaman, T.; Perepezko, J.H. Viscosity control of borosilica by Fe doping in Mo-Si-B environmentally resistant alloys. Corros. Sci. 2015, 98, 406–416. [Google Scholar] [CrossRef]
- Dong, Z.H.; Peng, X.; Wang, F.H. Oxidation of a ZrB2 coating fabricated on Ta-W alloy by electrophoretic deposition and laser melting. Mater. Lett. 2015, 148, 76–78. [Google Scholar] [CrossRef]
- Gu, S.-c.; Zhu, S.-z.; Ma, Z.; Han, S.-p.; Liu, Y.-b. Preparation and properties of ZrB2-MoSi2-glass composite powders for plasma sprayed high temperature oxidation resistance coating on C/SiC composites. Powder Technol. 2019, 345, 544–552. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, D.; Ru, H.; Wang, W.; Zhang, C. Oxidation protective ZrB2-MoSi2-SiC-Si coating for graphite materials prepared by slurry dipping and vapor silicon infiltration. Surf. Coat. Technol. 2018, 339, 91–100. [Google Scholar] [CrossRef]
- Lange, A.; Braun, R. Magnetron-sputtered oxidation protection coatings for Mo-Si-B alloys. Corros. Sci. 2014, 84, 74–84. [Google Scholar] [CrossRef]
- Riedl, H.; Vieweg, A.; Limbeck, A.; Kalaš, J.; Arndt, M.; Polcik, P.; Euchner, H.; Bartosik, M.; Mayrhofer, P.H. Thermal stability and mechanical properties of boron enhanced Mo-Si coatings. Surf. Coat. Technol. 2015, 280, 282–290. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, F.V.; Yatsyuk, I.V. Study of ZrSiB Coatings Obtained by Magnetron Sputtering of ZrB2-20%Si and ZrB2-50%ZrSi2 Cathodes. Phys. At. Nucl. 2019, 82, 1437–1440. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, F.V.; Novikov, A.V.; Sagalova, T.B.; Petrzhik, M.I.; Levashov, E.A.; Shtansky, D.V. A comparative study of microstructure, oxidation resistance, mechanical, and tribological properties of coatings in Mo–B–(N), Cr–B–(N) and Ti–B–(N) systems. Phys. Met. Metallogr. 2017, 118, 1136–1146. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Pierson, J.F.; Bychkova, M.Y.; Manakova, O.S.; Levashov, E.A.; Shtansky, D.V. Comparative Study of Sliding, Scratching, and Impact-Loading Behavior of Hard CrB2 and Cr–B–N Films. Tribol. Lett. 2016, 63, 1–11. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Sheveyko, A.N.; Sorokin, D.I.; Lev, L.C.; Mavrin, B.N.; Kiryukhantsev-Korneev, P.V. Structure and properties of multi-component and multilayer TiCrBN/WSex coatings deposited by sputtering of TiCrB and WSe2 targets. Surf. Coat. Technol. 2008, 202, 5953–5961. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, F.V. Possibilities of glow discharge optical emission spectroscopy in the investigation of coatings. Russ. J. Non-Ferrous Met. 2014, 55, 494–504. [Google Scholar] [CrossRef]
- Levashov, E.A.; Shtansky, D.V.; Kiryukhantsev-Korneev, P.V.; Petrzhik, M.I.; Tyurina, M.Y.; Sheveiko, A.N. Multifunctional nanostructured coatings: Formation, structure, and the uniformity of measuring their mechanical and tribological properties. Russ. Metall. 2010, 2010, 917–935. [Google Scholar] [CrossRef]
- Tengdelius, L.; Samuelsson, M.; Jensen, J.; Lu, J.; Hultman, L.; Forsberg, U.; Janzén, E.; Högberg, H. Direct current magnetron sputtered ZrB2 thin films on 4H-SiC(0001) and Si(100). Thin Solid Films 2014, 550, 285–290. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, G.; Zhang, L.; Zhang, Y.; Yin, J.; Ma, X.; Wen, J.; Dai, L.; Wang, X.; Chen, H.; et al. Application of ZrB2 thin film as a low emissivity film at high temperature. Appl. Surf. Sci. 2020, 527, 146763. [Google Scholar] [CrossRef]
- Samuelsson, M.; Jensen, J.; Helmersson, U.; Hultman, L.; Högberg, H. ZrB2 thin films grown by high power impulse magnetron sputtering from a compound target. Thin Solid Films 2012, 526, 163–167. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Pierson, J.F.; Kuptsov, K.A.; Shtansky, D.V. Hard Cr-Al-Si-B-(N) coatings deposited by reactive and non-reactive magnetron sputtering of CrAlSiB target. Appl. Surf. Sci. 2014, 314, 104–111. [Google Scholar] [CrossRef]
- Forniés, E.; Escobar Galindo, R.; Sánchez, O.; Albella, J.M. Growth of CrNx films by DC reactive magnetron sputtering at constant N2/Ar gas flow. Surf. Coat. Technol. 2006, 200, 6047–6053. [Google Scholar] [CrossRef]
- Mendizabal, L.; Bayón, R.; G-Berasategui, E.; Barriga, J.; Gonzalez, J.J. Effect of N2 flow rate on the microstructure and electrochemical behavior of TaNx films deposited by modulated pulsed power magnetron sputtering. Thin Solid Films 2016, 610, 1–9. [Google Scholar] [CrossRef]
- Bujak, J.; Walkowicz, J.; Kusiński, J. Influence of the nitrogen pressure on the structure and properties of (Ti,Al)N coatings deposited by cathodic vacuum arc PVD process. Surf. Coat. Technol. 2004, 180–181, 150–157. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Lapitskaya, V.; Khabarava, A.; Chizhik, S.; Warcholinski, B.; Gilewicz, A. The influence of nitrogen on the morphology of ZrN coatings deposited by magnetron sputtering. Appl. Surf. Sci. 2020, 522, 146508. [Google Scholar] [CrossRef]
- Houska, J.; Mares, P.; Simova, V.; Zuzjakova, S.; Cerstvy, R.; Vlcek, J. Dependence of characteristics of MSiBCN (M=Ti, Zr, Hf) on the choice of metal element: Experimental and ab-initio study. Thin Solid Films 2016, 616, 359–365. [Google Scholar] [CrossRef]
- Houska, J.; Kohout, J.; Vlcek, J. Effect of N and Zr content on structure, electronic structure and properties of ZrBCN materials: An ab-initio study. Thin Solid Films 2013, 542, 225–231. [Google Scholar] [CrossRef]
- Pleva, M.; Grančič, B.; Mikula, M.; Truchlý, M.; Roch, T.; Satrapinskyy, L.; Gregor, M.; Ďurina, P.; Girman, V.; Švec, P.; et al. Thermal stability of amorphous Ti-B-Si-N coatings with variable Si/B concentration ratio. Surf. Coat. Technol. 2018, 333, 52–60. [Google Scholar] [CrossRef]
- Lin, J.; Moore, J.J.; Mishra, B.; Pinkas, M.; Sproul, W.D. The structure and mechanical and tribological properties of TiBCN nanocomposite coatings. Acta Mater. 2010, 58, 1554–1564. [Google Scholar] [CrossRef]
- Tengdelius, L.; Broitman, E.; Lu, J.; Eriksson, F.; Birch, J.; Nyberg, T.; Hultman, L.; Högberg, H. Hard and elastic epitaxial ZrB2 thin films on Al2O3 substrates deposited by magnetron sputtering from a ZrB2 compound target. Acta Mater. 2016, 111, 166–172. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wearing 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Liu, Z.; Huang, J.; Kong, J.; Xiong, D. Mechanical and tribological properties of Hf1-xMoxNy thin films as a function of Mo contents. Surf. Coat. Technol. 2019, 375, 589–599. [Google Scholar] [CrossRef]
- Levashov, E.A.; Petrzhik, M.I.; Shtansky, D.V.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Valiev, R.Z.; Gunderov, D.V.; Prokoshkin, S.D.; Korotitskiy, A.V.; Smolin, A.Y. Nanostructured titanium alloys and multicomponent bioactive films: Mechanical behavior at indentation. Mater. Sci. Eng. A 2013, 570, 51–62. [Google Scholar] [CrossRef]
- Wang, J.; Munroe, P.; Zhou, Z.; Xie, Z. Nanostructured molybdenum nitride-based coatings: Effect of nitrogen concentration on microstructure and mechanical properties. Thin Solid Films 2019, 682, 82–92. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, T.G.; Yan, B.; Qi, H.J.; Guo, Y.Y.; Xu, S.S. Study on the microstructure and mechanical properties of Zr-B-(N) tool coatings prepared by hybrid coating system. Procedia Manuf. 2018, 26, 806–817. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Zeng, Q.; Wang, J.; Cheng, L.; Ren, H.; Guan, K. Crystal structure and elastic properties of ZrB compared with ZrB2: A first-principles study. Comput. Mater. Sci. 2010, 49, 814–819. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Sytchenko, A.D. The Influence of H, W, H/E, H3/E2, Structure and Chemical Composition on the Resistance of Ti–B–(N), Mo–B–(N), Cr–B–(N), and Zr–B–(N) Coatings to Cyclic Impact Loading. Prot. Met. Phys. Chem. Surf. 2020, 56, 1190–1200. [Google Scholar] [CrossRef]
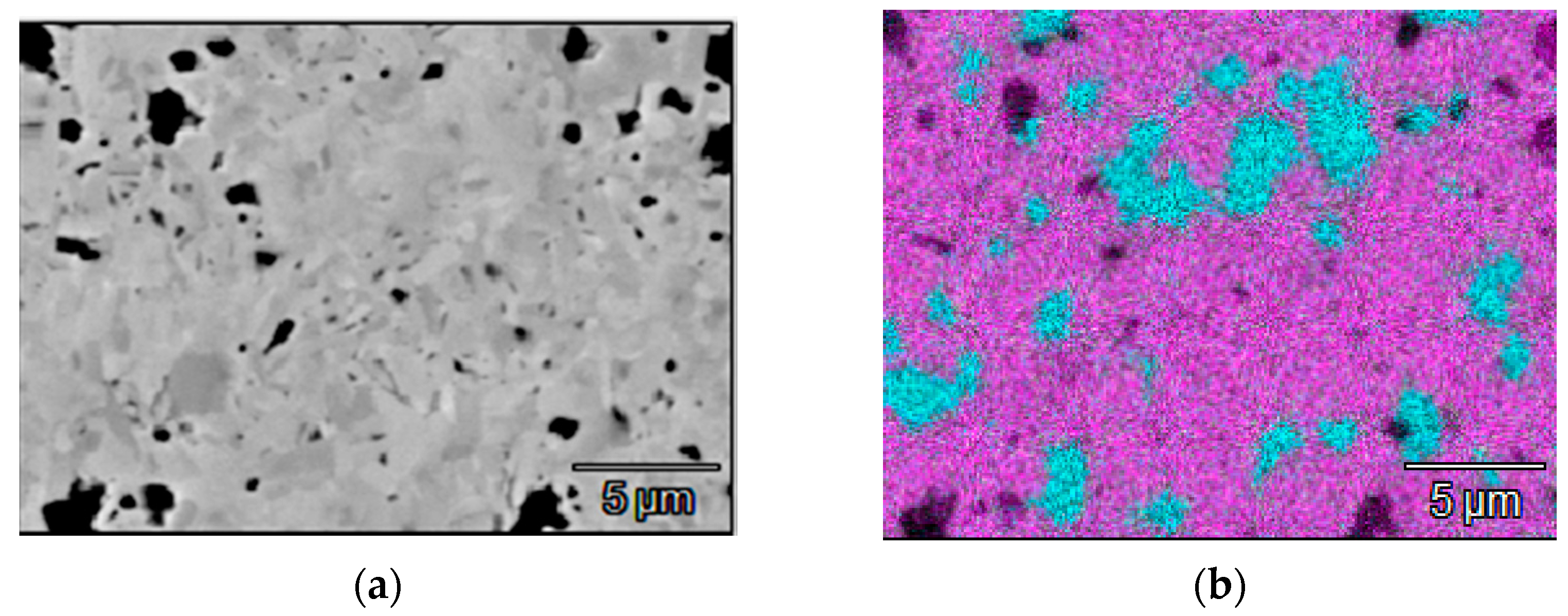
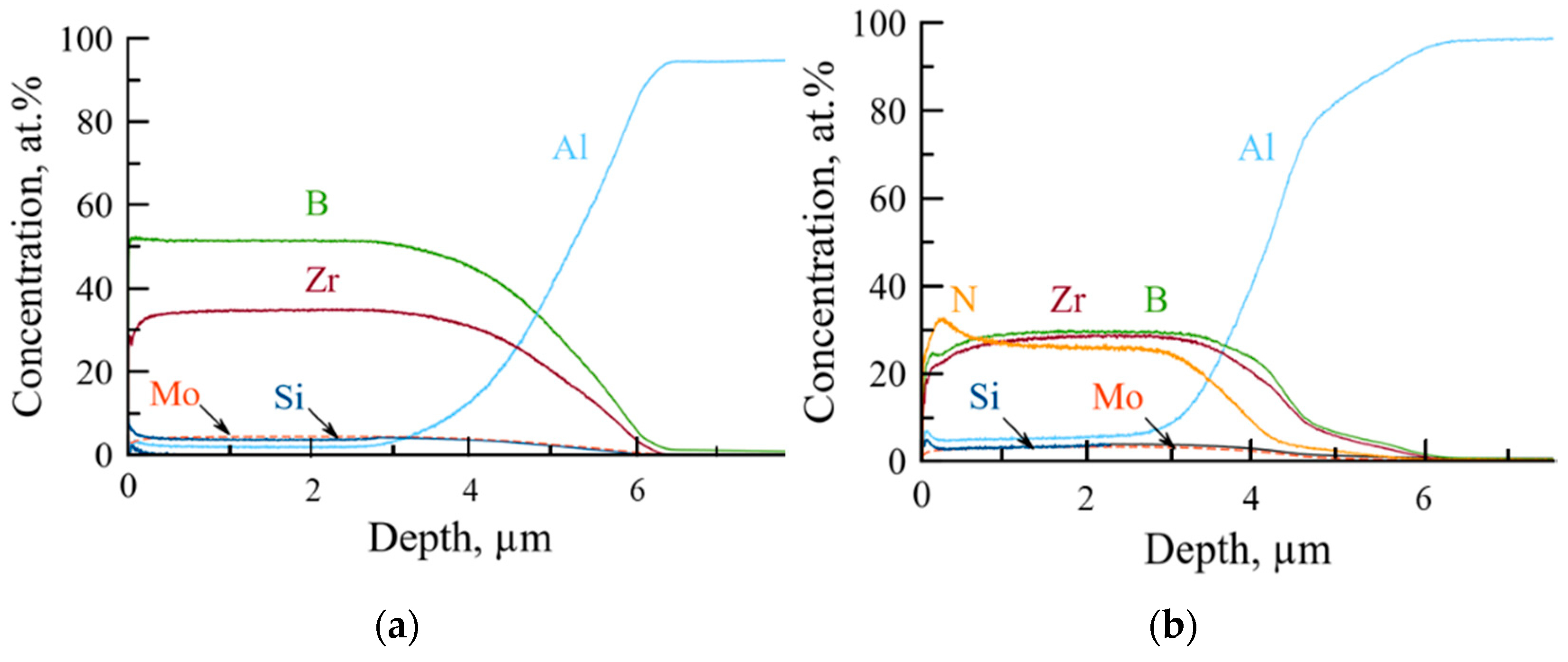
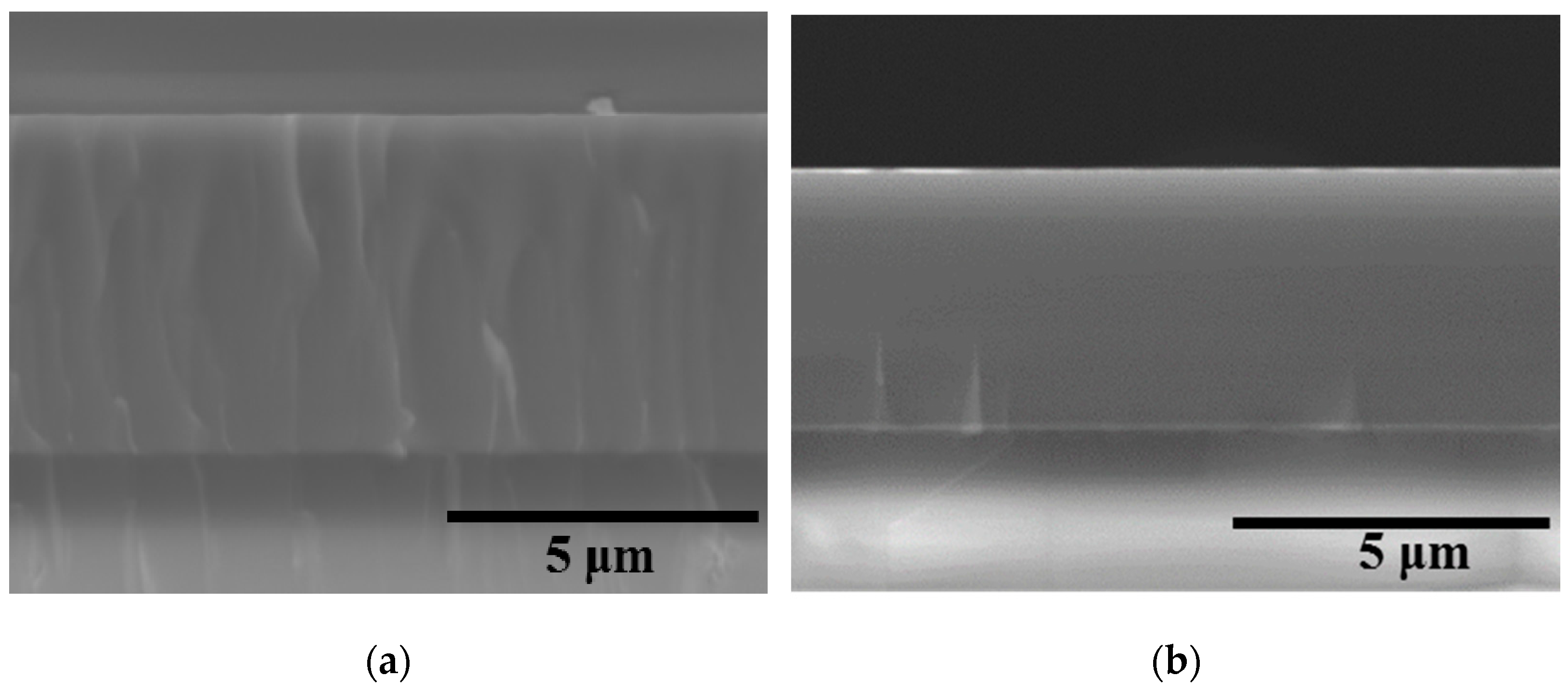
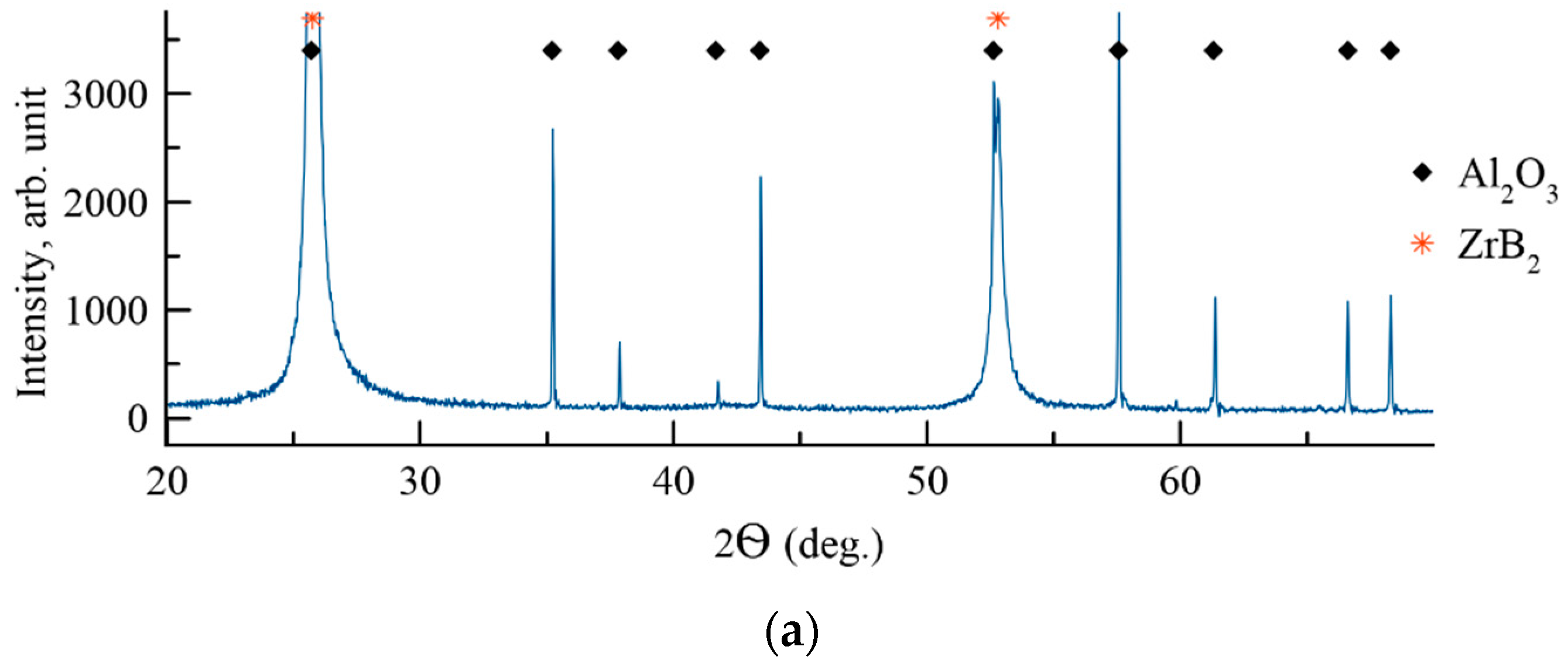


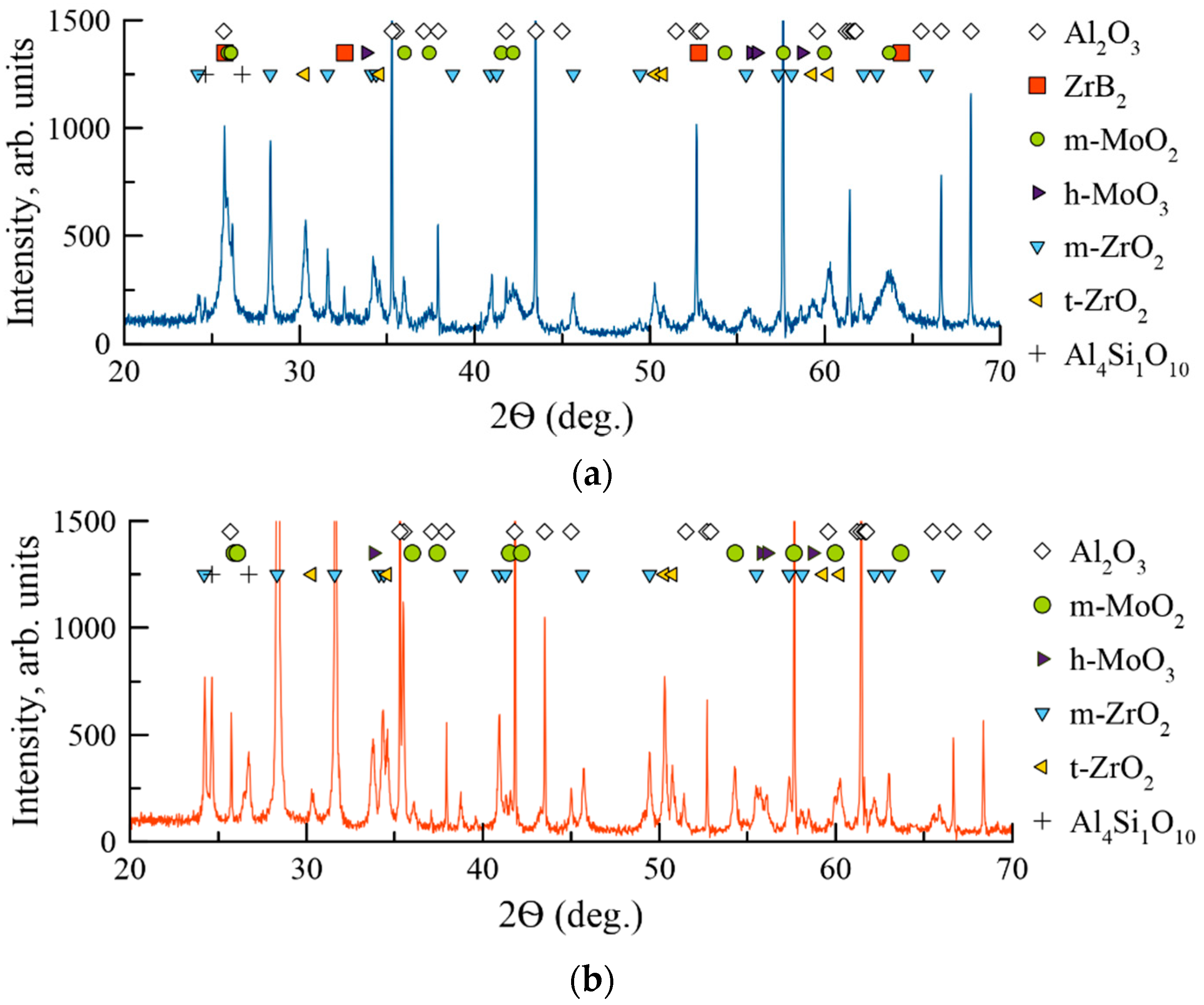
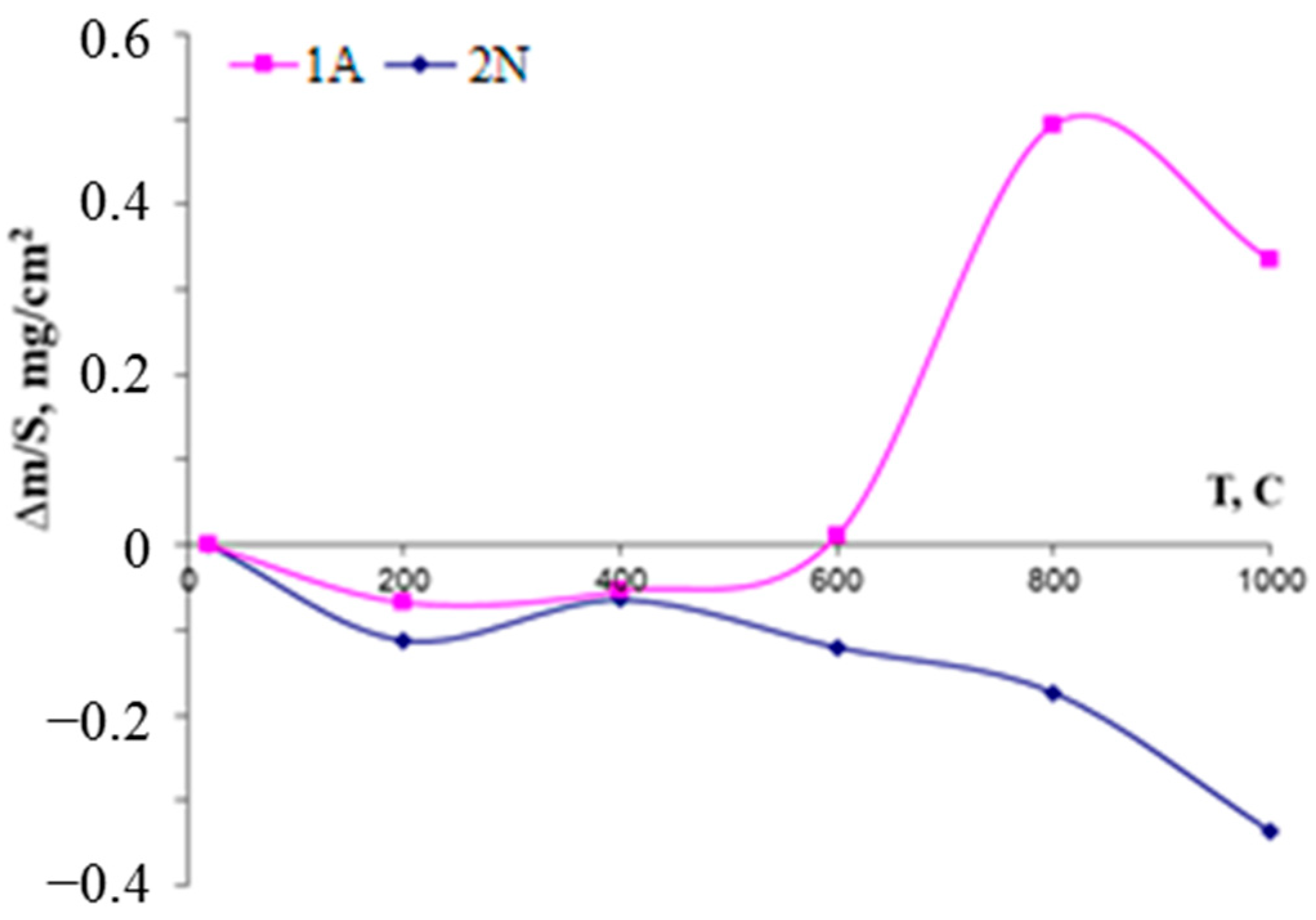

| № | Deposition Medium | Composition, at.% | H, GPa | E, GPa | H/E | H3/E2, GPa | W, % | Vw, Mm3/(Nm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr | Mo | Si | B | N | ||||||||
| 1A | Ar | 37 | 4 | 4 | 55 | 0 | 36 | 415 | 0.087 | 0.271 | 84 | 4.2 × 10−7 |
| 2N | N2 | 31 | 3 | 5 | 32 | 29 | 14 | 160 | 0.088 | 0.107 | 64 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiryukhantsev-Korneev, P.; Sytchenko, A.; Pogozhev, Y.; Vorotilo, S.; Orekhov, A.; Loginov, P.; Levashov, E. Structure and Properties of Zr-Mo-Si-B-(N) Hard Coatings Obtained by d.c. Magnetron Sputtering of ZrB2-MoSi2 Target. Materials 2021, 14, 1932. https://doi.org/10.3390/ma14081932
Kiryukhantsev-Korneev P, Sytchenko A, Pogozhev Y, Vorotilo S, Orekhov A, Loginov P, Levashov E. Structure and Properties of Zr-Mo-Si-B-(N) Hard Coatings Obtained by d.c. Magnetron Sputtering of ZrB2-MoSi2 Target. Materials. 2021; 14(8):1932. https://doi.org/10.3390/ma14081932
Chicago/Turabian StyleKiryukhantsev-Korneev, Philipp, Alina Sytchenko, Yuriy Pogozhev, Stepan Vorotilo, Anton Orekhov, Pavel Loginov, and Evgeny Levashov. 2021. "Structure and Properties of Zr-Mo-Si-B-(N) Hard Coatings Obtained by d.c. Magnetron Sputtering of ZrB2-MoSi2 Target" Materials 14, no. 8: 1932. https://doi.org/10.3390/ma14081932
APA StyleKiryukhantsev-Korneev, P., Sytchenko, A., Pogozhev, Y., Vorotilo, S., Orekhov, A., Loginov, P., & Levashov, E. (2021). Structure and Properties of Zr-Mo-Si-B-(N) Hard Coatings Obtained by d.c. Magnetron Sputtering of ZrB2-MoSi2 Target. Materials, 14(8), 1932. https://doi.org/10.3390/ma14081932








