Change of 0.34Cr-1Ni-Mo-Fe Steel Dislocation Structure in Plasma Electrolyte Hardening
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Structure of the Initial State of 0.34Cr-1Ni-Mo-Fe Steel
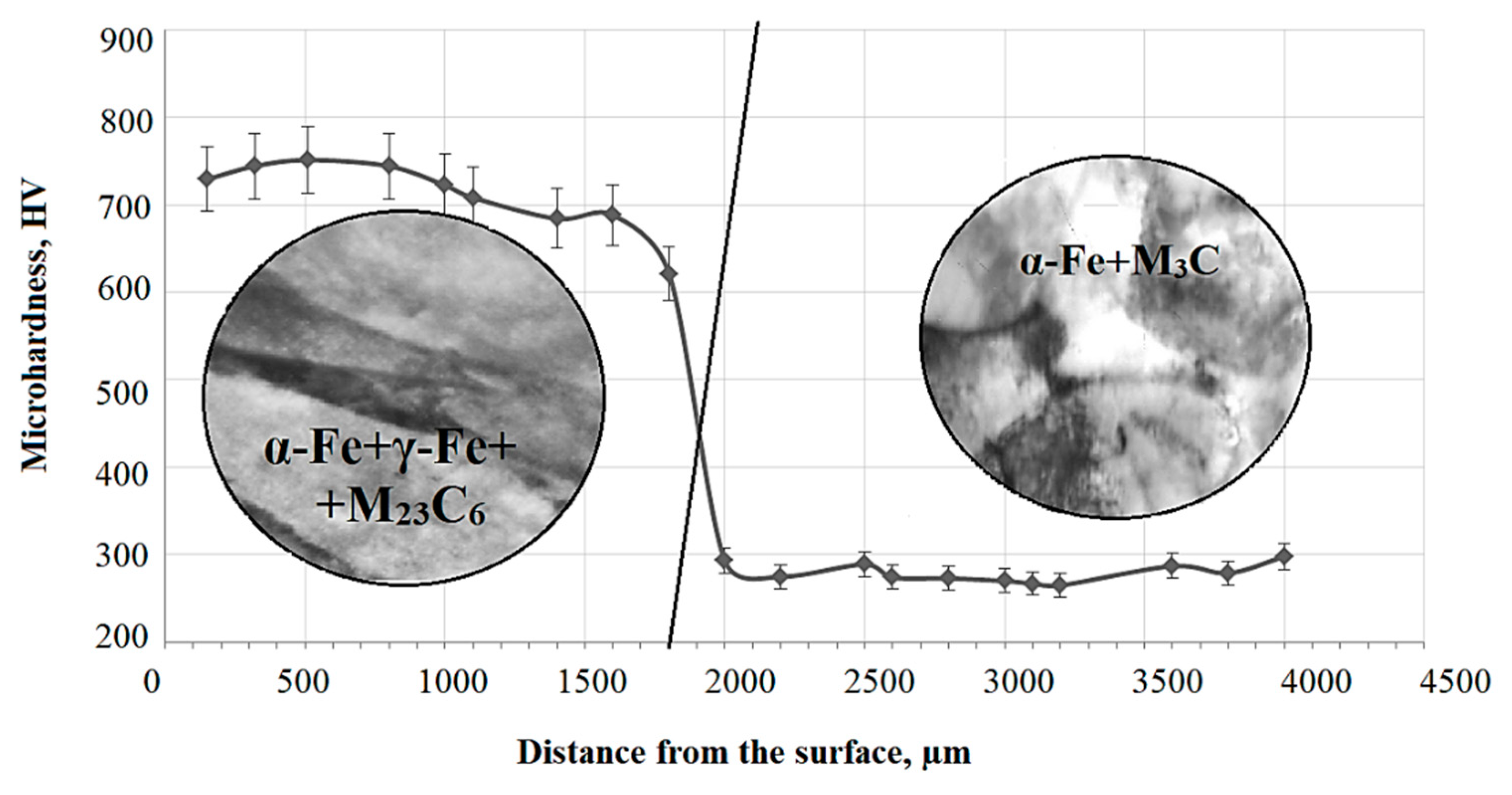
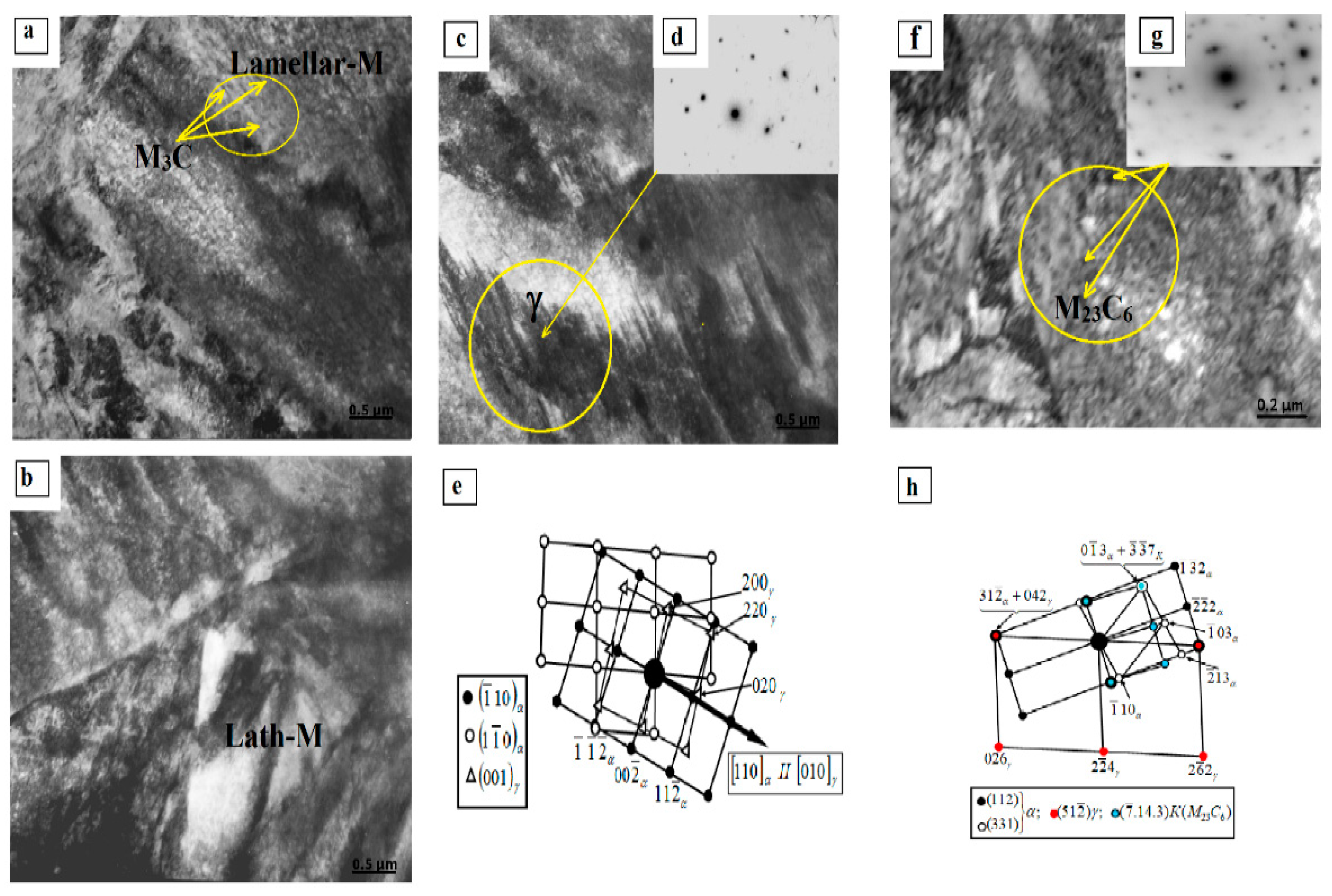
3.2. Structure of 0.34Cr-1Ni-Mo-Fe Steel after PEH
4. Conclusions
- (1)
- Surface hardening led to the formation of packet–lamellar martensite with volume fractions of 60% and 40%, respectively, and γ-phase in the form of residual austenite with fractions of 5% and 7% in packet–lamellar martensite crystals, respectively, as well as particles of the carbide phase, namely, cementite with fractions in crystals of lamellar martensite of 0.6% and 1.5%, respectively, and complex carbide M23C6 with fractions in crystals of packet–lamellar martensite of 0.15% and 0.35%, respectively;
- (2)
- The scalar dislocation density (ρ) of packet and lamellar martensite after PEH are equal to 3.78 × 1010 cm−2 and 3.0 × 1010 cm−2, respectively, which is 1.5 times higher than in the initial state; the amplitude of the long-range internal stresses of the packet and lamellar martensite are σd = 285 MPa and σd = 270 MPa, and the amplitude of the shear stresses of the packet and lamellar martensite have the values σL = 390 MPa and σL = 345 MPa, that is, the condition σL > σd is met, which in turn confirms the plastic nature of the bending-torsion of the crystal lattice.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duraji, V.N. Chemical-thermal treatment of metals with heating in electrolytic plasma. Actual Conf. Surf. Treat. Technol. M 2010, 6, 59–61. [Google Scholar]
- Tyurin, Y.N.; Pogrebnyak, A.D. Electric heating using a liquid electrode. Surf. Coat. Technol. 2001, 142–144, 293–299. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Agureev, L.; Savushkina, S.; Ashmarin, A.; Borisov, A.; Apelfeld, A.; Anikin, K.; Tkachenko, N.; Gerasimov, M.; Shcherbakov, A.; Ignatenko, V.; et al. Study of plasma electrolytic oxidation coatings on aluminum composites. Metals 2018, 8, 459. [Google Scholar] [CrossRef]
- Belkin, P.N.; Kusmanov, S.A. Plasma electrolytic hardening of steels: Review. Surf. Eng. Appl. Electrochem. 2016, 52, 531–546. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Smirnov, A.A.; Silkin, S.A.; Belkin, P.N. Increasing wear and corrosion resistance of low-alloy steel by anode plasma electrolytic nitriding. Surf. Coat. Technol. 2016. [Google Scholar] [CrossRef]
- Tarakci, M.; Korkmaz, K.; Gencer, Y.; Usta, M. Plasma electrolytic surface carburizing and hardening of pure iron. Surf. Coat. Technol. 2005, 199, 205–212. [Google Scholar] [CrossRef]
- Shadrin, S.Y.; Belkin, P.N. Analysis of models for calculation of temperature of anode plasma electrolytic heating. Int. J. Heat Mass Transf. 2012, 55, 179–186. [Google Scholar] [CrossRef]
- Dayança, A.; Karacaa, B.; Kumruoğlub, L.C. The cathodic electrolytic plasma hardening of steel and cast iron based automotive camshafts. Acta Phys. Pol. A 2017, 131, 374–378. [Google Scholar] [CrossRef]
- Belkin, P.N.; Kusmanov, S.A.; Smirnov, A.A. Plasma electrolytic hardening and nitrohardening of medium carbon steels. Mater. Sci. Forum 2016, 844, 146–152. [Google Scholar] [CrossRef]
- Luk, S.F.; Leung, T.P.; Miu, W.S.; Pashby, I. A study of the effect of average preset voltage on hardness during electrolytic surface-hardening in aqueous solution. J. Mat. Proc. Technol 1999, 91, 245–249. [Google Scholar] [CrossRef]
- Bejar, M.A.; Henriquez, R. Surface hardening of steel by plasma-electrolysis boronizing. Mater. Des. 2009, 30, 1726–1728. [Google Scholar] [CrossRef]
- Popova, N.A.; Nikonenko, E.L.; Nikonenko, A.V.; Gromov, V.E. Effect of electrolytic-plasma nitrocarburizing on the structural and phase state of ferrite-pearlitic steels. Steel Transl. 2019, 49, 671–677. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Satbayeva, Z.A.; Bayatanova, L.B.; Kilyshkanov, M.K.; Kalibayev, K.A.; Kochneva, A.K. Influence of electrolyte-plasma surface hardening on the structure and properties of steel 40KhN. J. Phys. Conf. Ser. 2019, 1393, 012119. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Satbayeva, Z.; Baizhan, D. Effect of electrolytic-plasma surface strengthening on the structure and properties of steel 40KhN. METAL 2019, 950–955. [Google Scholar] [CrossRef]
- Satbayeva, Z.A.; Baizhan, D.R.; Kenesbekov, A.B. Features of structure formation in 40KhN steel during electrolyte-plasma surface hardening. In Proceedings of the 11 International Symposium “Powder Metallurgy: Surface Engineering, New Powder Composite Materials, Welding”, Minsk, Belarus, 10–12 April 2019; pp. 76–86. [Google Scholar]
- Safonov, E.N. Plasma Hardening of Machine Parts: Monograph; NTI (Branch) UrFU: Nizhny Tagil, Russia, 2014; p. 116. [Google Scholar]
- Korotkov, V.A. Surface Plasma Hardening; NTI (branch) UrFU: Nizhny Tagil, Russia, 2012; p. 64. [Google Scholar]
- Rakhadilov, B.K.; Skakov, M.K.; Sagdoldina, Z.B.; Kenesbekov, A.B. Method of Surface Hardening of Steel Products: Patent for Invention of the Republic of Kazakhstan; IPC C21D 1/09; Bul. No. 20; Patent law of the Republic of Kazakhstan: Almaty, Kazakhstan, 2018. [Google Scholar]
- Rakhadilov, B.K.; Zhurerova, L.; Pavlov, A. Method of electrolyte-plasma surface hardening of 65G and 20GL low-alloy steels samples. IOP Conf. Ser. Mater. Sci. Eng. 2016, 142, 1–7. [Google Scholar] [CrossRef]
- Saltykov, S.A. Stereometric Metallography. M. Metallurgyl. 1976. Available online: https://www.twirpx.com/file/201942/ (accessed on 10 April 2021).
- Hirsch, P.; Howie, A.; Nicholson, P.; Pashley, D.; Whelan, M. Electron microscopy of thin crystals. Mosc. Mir 1968. Available online: https://www.twirpx.com/file/911407/ (accessed on 10 April 2021).
- Utevsky, L.M. Diffraction electron microscopy in metal science. Mosc. Metall. 1973. Available online: https://www.studmed.ru/utevskiy-lm-difrakcionnaya-elektronnaya-mikroskopiya-v-metallovedenii_b449ce8d495.html (accessed on 10 April 2021).
- Koneva, N.A.; Lychagin, D.V.; Teplyakova, L.A. Crystal lattice reversals and stages of plastic deformation//Experimental research and theoretical description of disclinations. Leningr. FTI 1984, 161–164. [Google Scholar]
- Koneva, N.A.; Lychagin, D.V.; Zhukovsky, S.P.; Kozlov, E.V. Evolution of dislocation structure and stages of plastic flow of polycrystalline iron-nickel alloy. FMM 1985, 60, 171–179. [Google Scholar]
- Koneva, N.A.; Kozlov, E.V. The nature of substructural hardening. Izv. Vuzov. Phys. 1982, 8, 3–14. [Google Scholar] [CrossRef]
- Gromov, V.Y.; Kozlov, E.V.; Bazaykin, V.I.; Zellermaer, V.Y.; Ivanov, Y.F.; Ignatenko, L.N.; Zakirov, D.M. Physics and mechanics of drawing and volumetric stamping. Mosc. Nedra 1997, 293. [Google Scholar]
- Gulyaev, A.P. Metallurgy. M. Metall. 1977. Available online: https://www.twirpx.com/file/85832/ (accessed on 10 April 2021).
- Popova, N.A.; Nikonenko, Y.L.; Nikonenko, A.V.; Gromov, V.Y.; Peregudov, O.A. Influence of electrolyte plasma nitrocementation on the structural-phase state of ferrite-pearlite steels. Proceedings of higher educational institutions. Chernaya Metall. 2019, 62, 782–789. [Google Scholar]
- Popova, N.; Erygina, L.; Nikonenko, E.; Kalashnikov, M.; Skakov, M. Structure and phase transformations in 0.34C-1Cr-1Ni-1Mo-Fe steel after electrolytic-plasma treatment. AIP Conf. Proc. 2017, 1909, 020179. [Google Scholar]
- Popova, N.; Erygina, L.; Nikonenko, E.; Skakov, M. Phase composition of perlite steel modified by electrolyte plasma nitriding. AIP Conf. Proc. 2017, 1899, 030002. [Google Scholar]
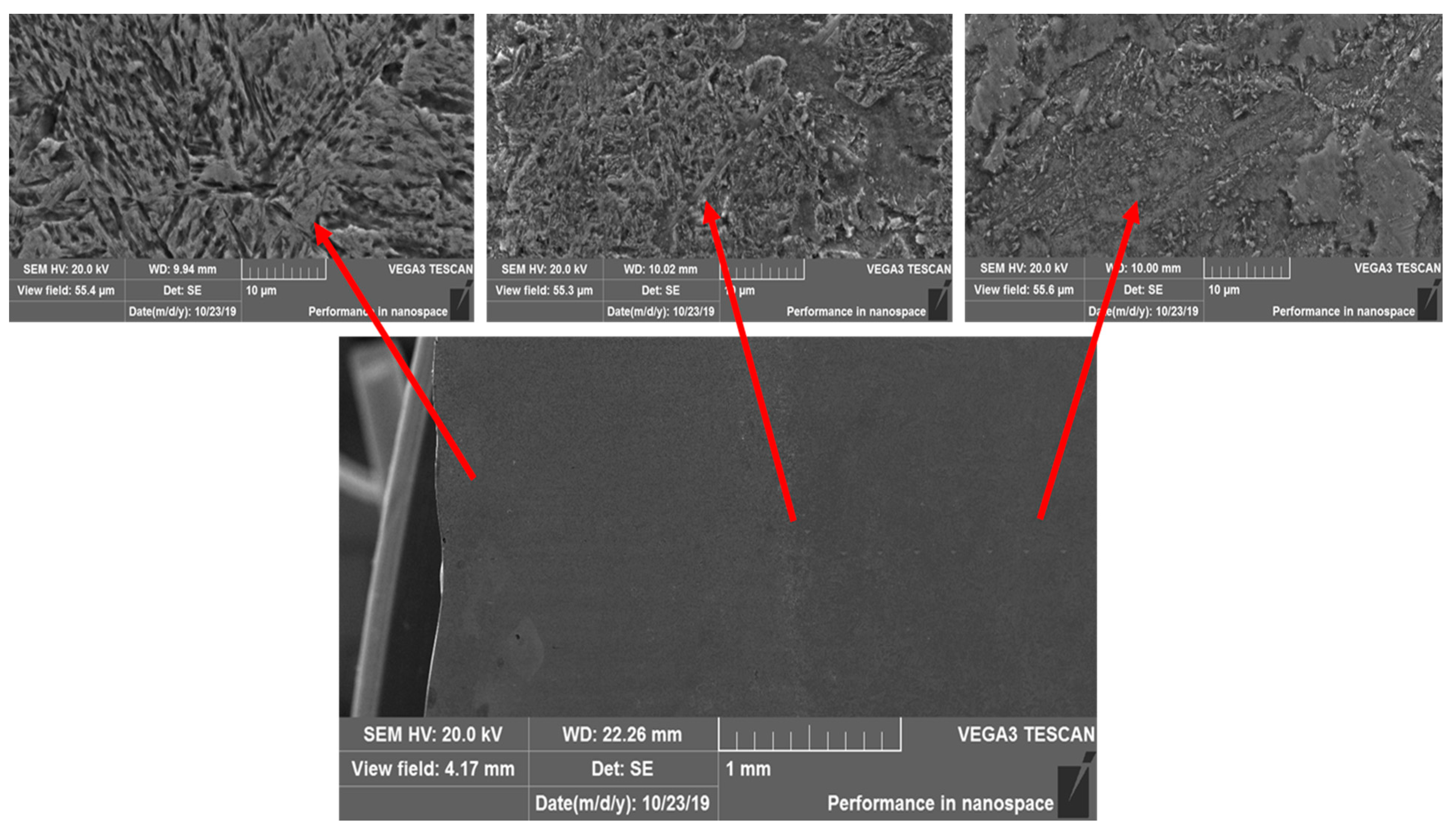

| Material State | Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Phase State | Hµ, HV | f | j, 10−4 mm3/Nm | V, µm3 | ∆m, mg | Ra, μm | |
| The Initial State | α-Fe, M3C | 283 | 0.44 | 1.08 | 1.05 | 36.03 | 0.199 |
| After PEH | α-Fe, γ-Fe, M23C6 | 742 | 0.37 | 0.32 | 0.6 | 20.63 | 0.446 |
| Phase | Crystal Lattice Type | Space Group | Crystal Lattice Parameters, nm | ||
|---|---|---|---|---|---|
| a | b | c | |||
| α-Fe | BCC | Im3m | 0.28664 | ||
| γ-Fe | FCC | Fm3m | 0.3573 | ||
| M3C | Orthorhombic | Pnma | 0.5080 | 0.6774 | 0.4520 |
| M23(C)6 | FCC | Fm3m | 1.0585 | ||
| Morphological Component of the Steel Matrix | Volume Fraction in the Steel Matrix, PV ± 5, % | Average Scalar Dislocation Density, ρ × 1010 ± 0.01 × 1010, cm−2 |
|---|---|---|
| Sample surface | ||
| Lamellar perlite | 35.00 | 1.50 |
| Ferrite–carbide mixture | 45.00 | 2.25 |
| Fragmented ferrite | 20.00 | 2.95 |
| In the material | 100.00 | 2.20 |
| Quantitative Characteristics of the Dislocation Structure | Packet Martensite | Lamellar Martensite |
|---|---|---|
| Volume fraction in the steel matrix, PV, % | 60.00 | 40.00 |
| Average size of packs or plates of martensite, d, µm | 0.25 | 1.10 |
| Volume fraction of cementite, δ,% | 0.60 | 1.50 |
| Average particle size of cementite, d, nm | 12 × 32 | 20 × 80 |
| Fraction of retained austenite in crystals of the α-phase, δ,% | 5.00 | 7.00 |
| Volume fraction of M23C6-type carbides, δ,% | 0.15 | 0.35 |
| Average particle size of M23C6-type carbides, d, nm | 8.00 | 16.00 |
| Phase | Average Quantitative Parameters of the Dislocation Structure | |||||
|---|---|---|---|---|---|---|
| PV ± 5% | ρ± 0.1 × 1010, cm−2 | ρ±, cm−2 | χ, cm−1 | σL, MPa | σd, MPa | |
| After Surface Hardening | ||||||
| Packet martensite | 60% | 3.78 × 1010 | 2.06 × 1010 | 515 | 390 | 285 |
| Lamellar martensite | 40% | 3.00 × 1010 | 1.84 × 1010 | 460 | 345 | 270 |
| In the material | 100% | 3.47 × 1010 | 1.97 × 1010 | 495 | 370 | 280 |
| Initial State | ||||||
| In the material | 100% | 2.20 × 1010 | ~0 | ~0 | 295 | ~0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhadilov, B.; Satbayeva, Z.; Ramankulov, S.; Shektibayev, N.; Zhurerova, L.; Popova, N.; Uazyrkhanova, G.; Sagdoldina, Z. Change of 0.34Cr-1Ni-Mo-Fe Steel Dislocation Structure in Plasma Electrolyte Hardening. Materials 2021, 14, 1928. https://doi.org/10.3390/ma14081928
Rakhadilov B, Satbayeva Z, Ramankulov S, Shektibayev N, Zhurerova L, Popova N, Uazyrkhanova G, Sagdoldina Z. Change of 0.34Cr-1Ni-Mo-Fe Steel Dislocation Structure in Plasma Electrolyte Hardening. Materials. 2021; 14(8):1928. https://doi.org/10.3390/ma14081928
Chicago/Turabian StyleRakhadilov, Bauyrzhan, Zarina Satbayeva, Sherzod Ramankulov, Nurdaulet Shektibayev, Laila Zhurerova, Natalya Popova, Gulzhaz Uazyrkhanova, and Zhuldyz Sagdoldina. 2021. "Change of 0.34Cr-1Ni-Mo-Fe Steel Dislocation Structure in Plasma Electrolyte Hardening" Materials 14, no. 8: 1928. https://doi.org/10.3390/ma14081928
APA StyleRakhadilov, B., Satbayeva, Z., Ramankulov, S., Shektibayev, N., Zhurerova, L., Popova, N., Uazyrkhanova, G., & Sagdoldina, Z. (2021). Change of 0.34Cr-1Ni-Mo-Fe Steel Dislocation Structure in Plasma Electrolyte Hardening. Materials, 14(8), 1928. https://doi.org/10.3390/ma14081928





