Assessment of Demineralization Inhibition Effects of Dentin Desensitizers Using Swept-Source Optical Coherence Tomography
Abstract
1. Introduction
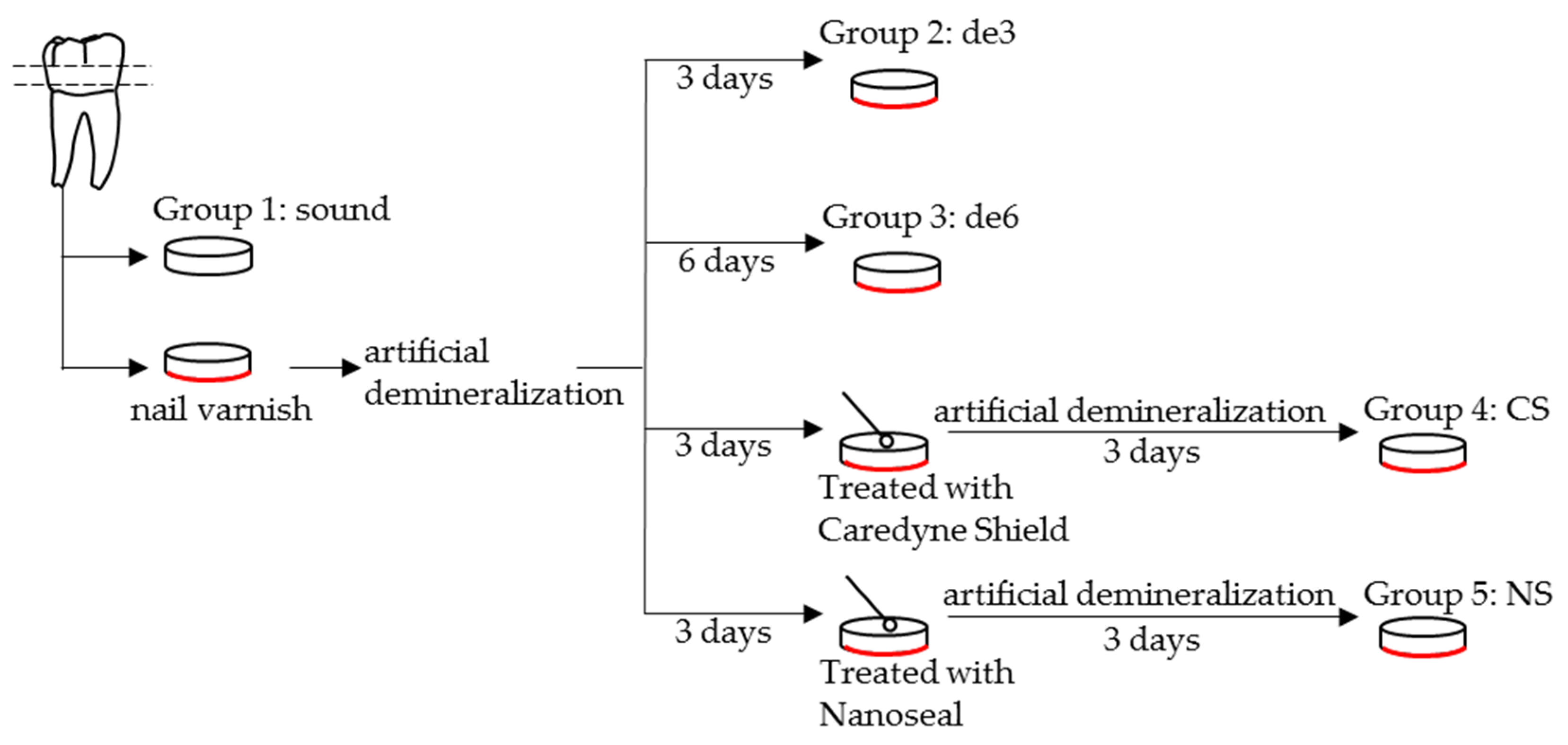
2. Materials and Methods
2.1. Materials
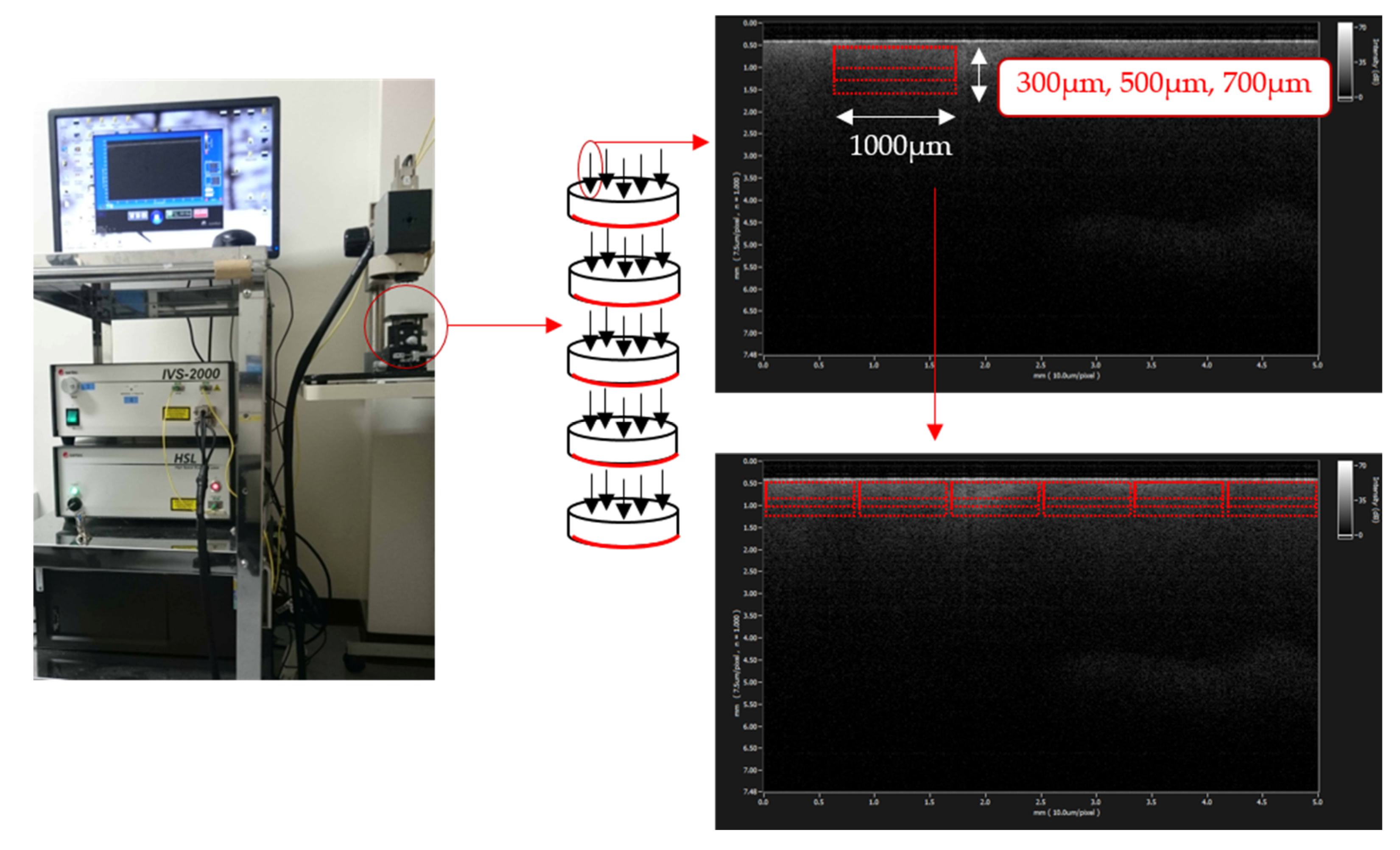
2.2. SS-OCT Analysis
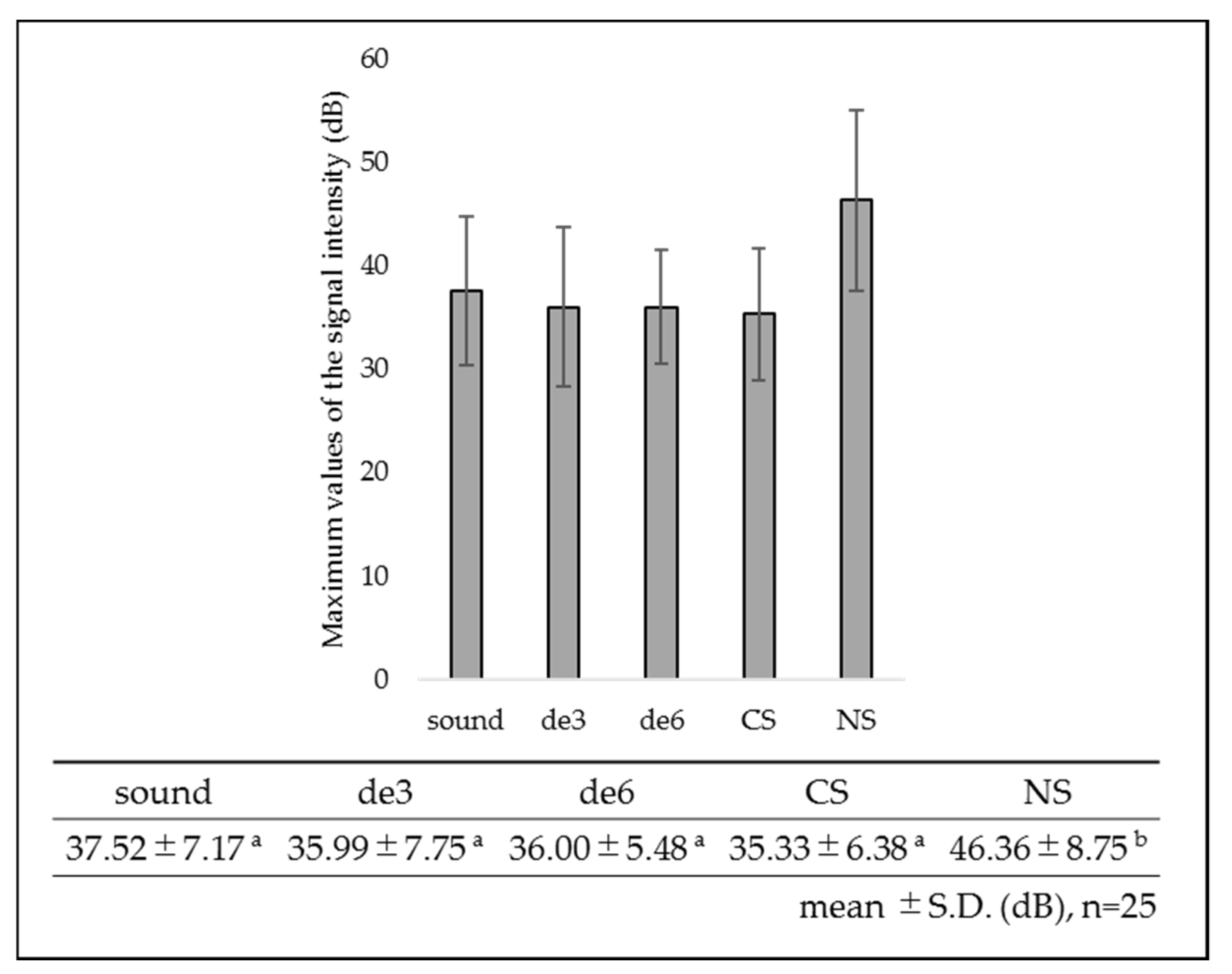
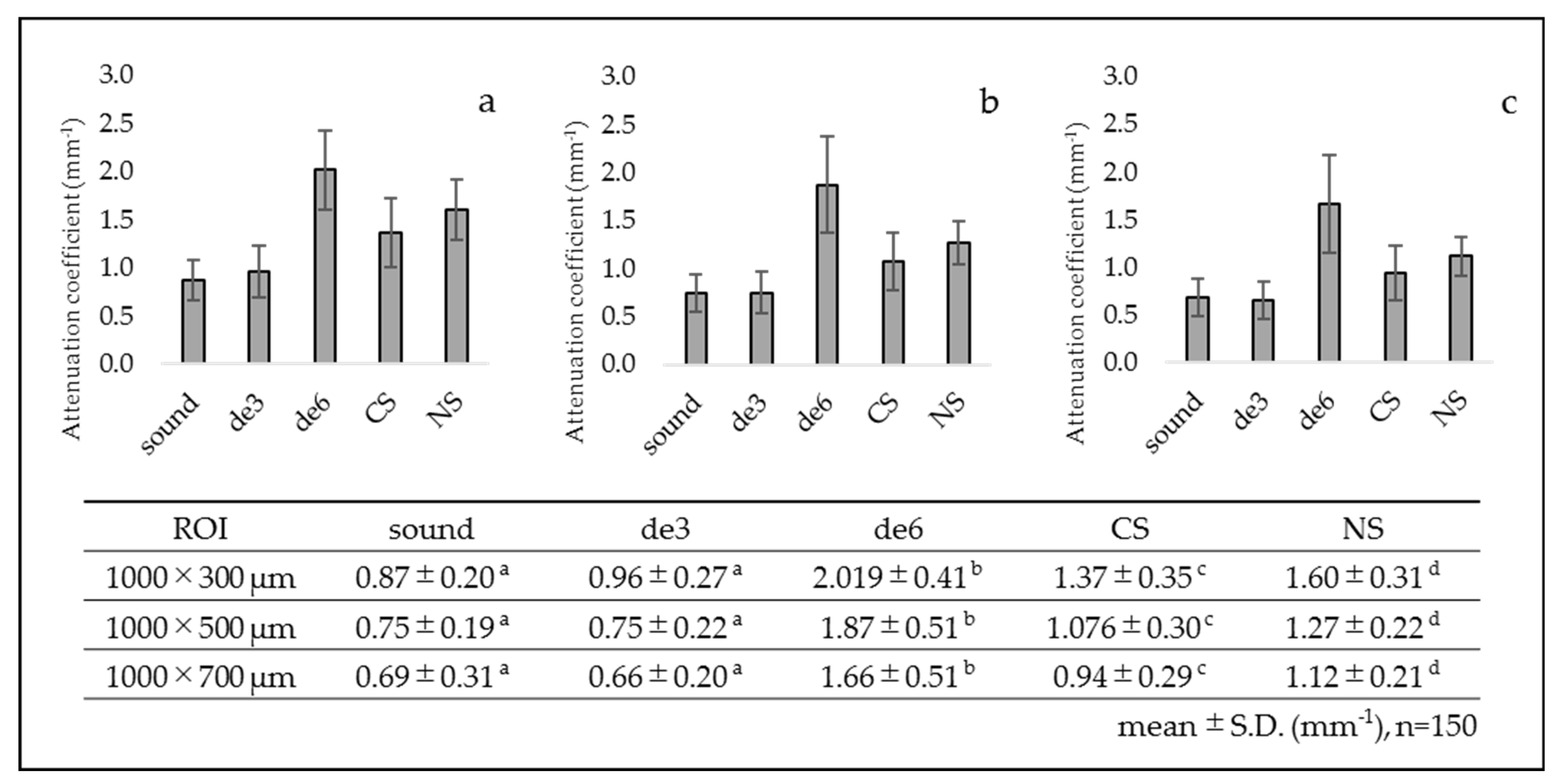
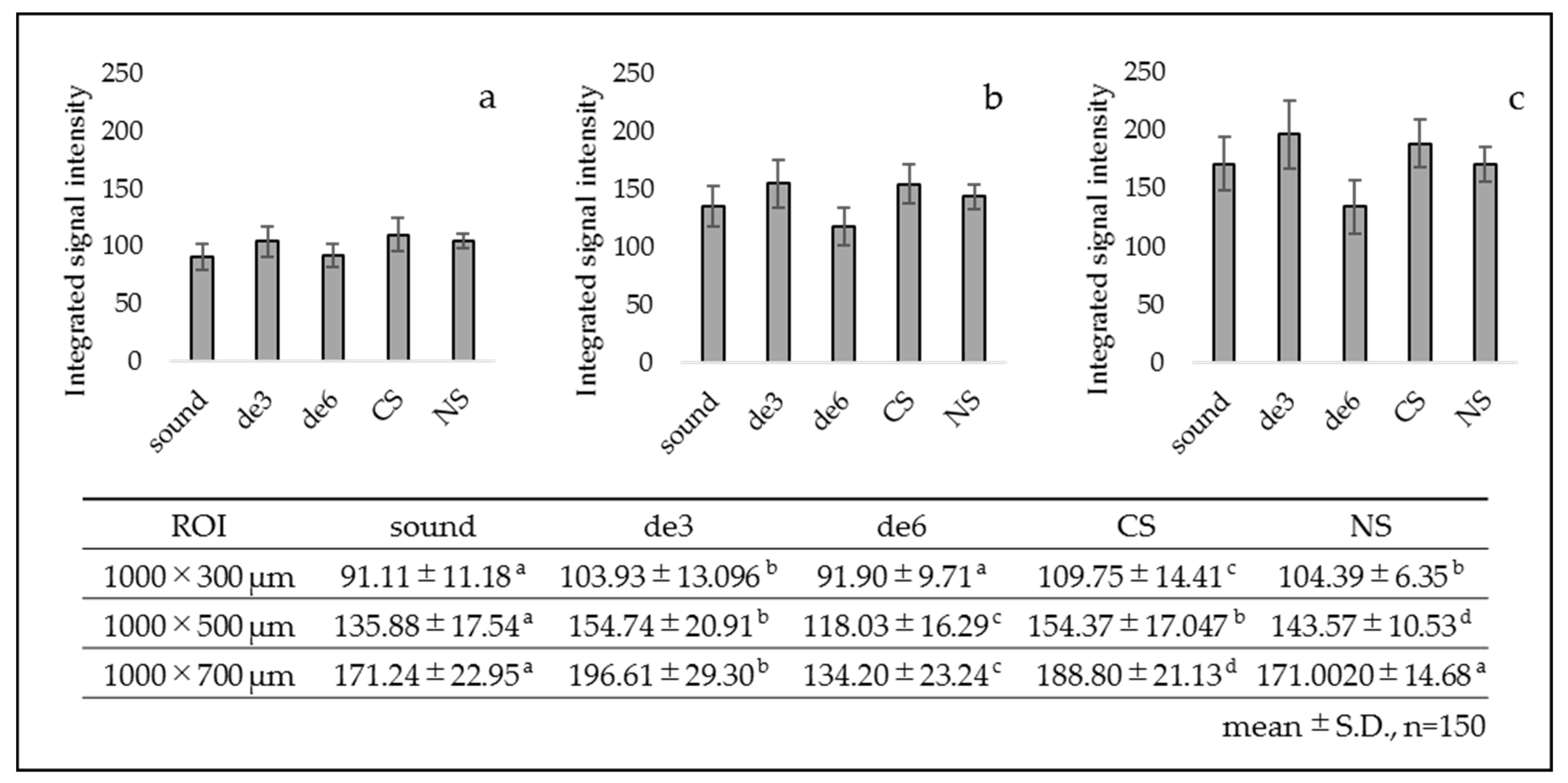
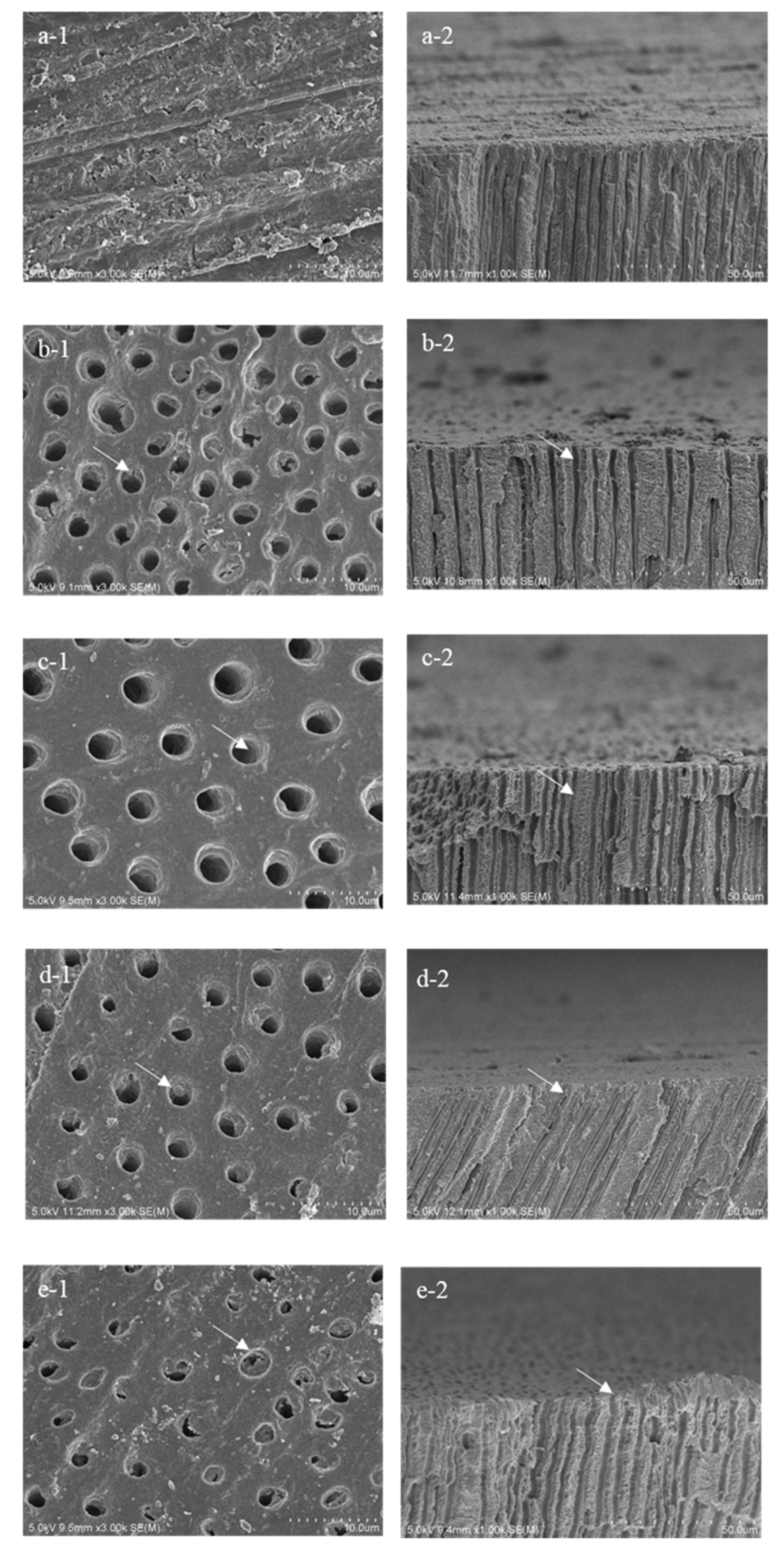
- Group 1: sound dentin without any treatment as a control (sound).
- Group 2: demineralized dentin for three days (de3).
- Group 3: demineralized dentin for six days (de6).
- Group 4: demineralized dentin for six days with Caredyne Shield. Caredyne Shield was applied to the demineralized dentin after three days of demineralization, and the surface was further demineralized for three days (CS).
- Group 5: demineralized dentin for six days with Nanoseal. Nanoseal was applied to the demineralized dentin after three days of demineralization, and the surface was further demineralized for three days (NS).
2.3. SEM/EDX Analysis
2.4. Statistical Analysis
3. Results
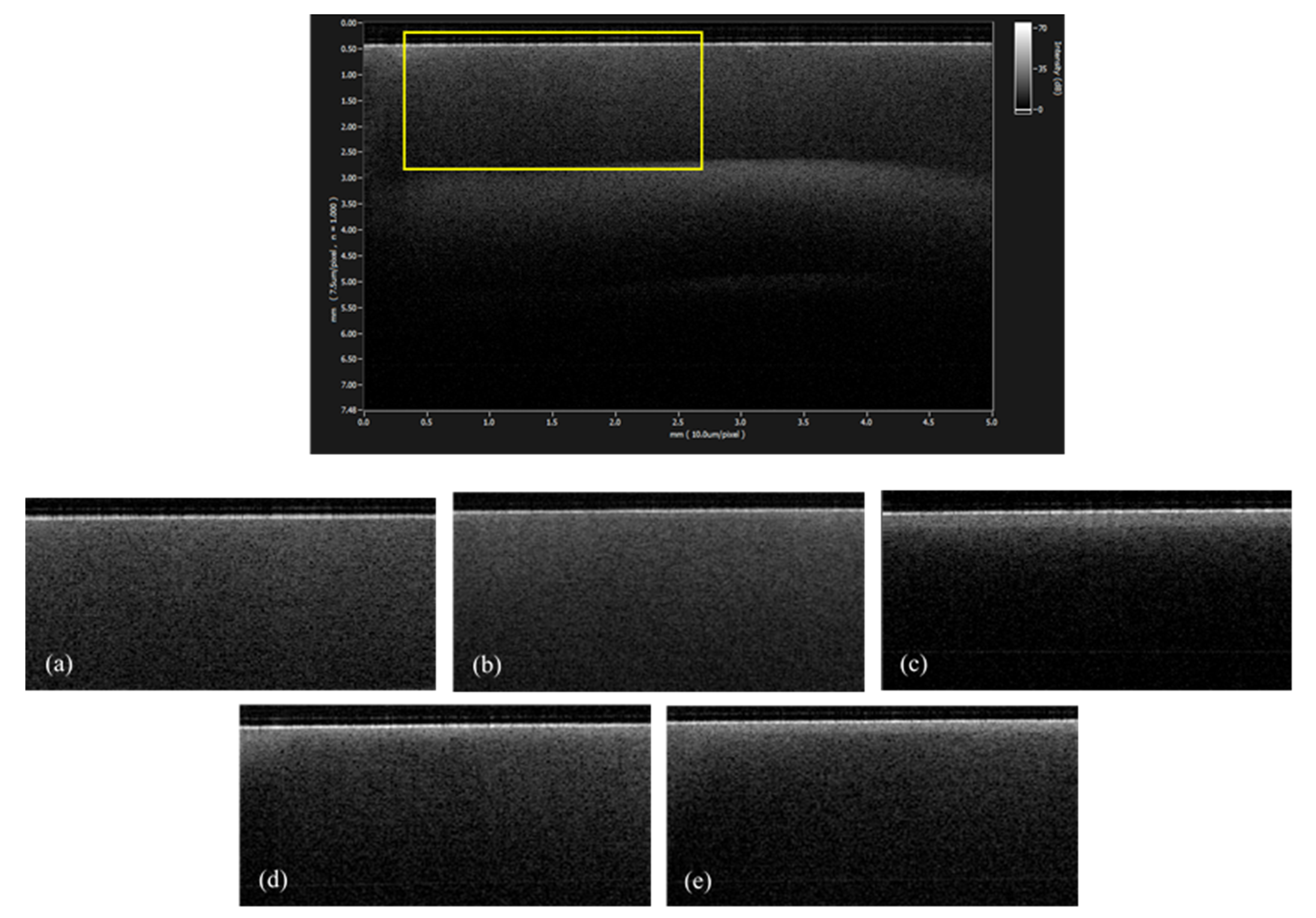
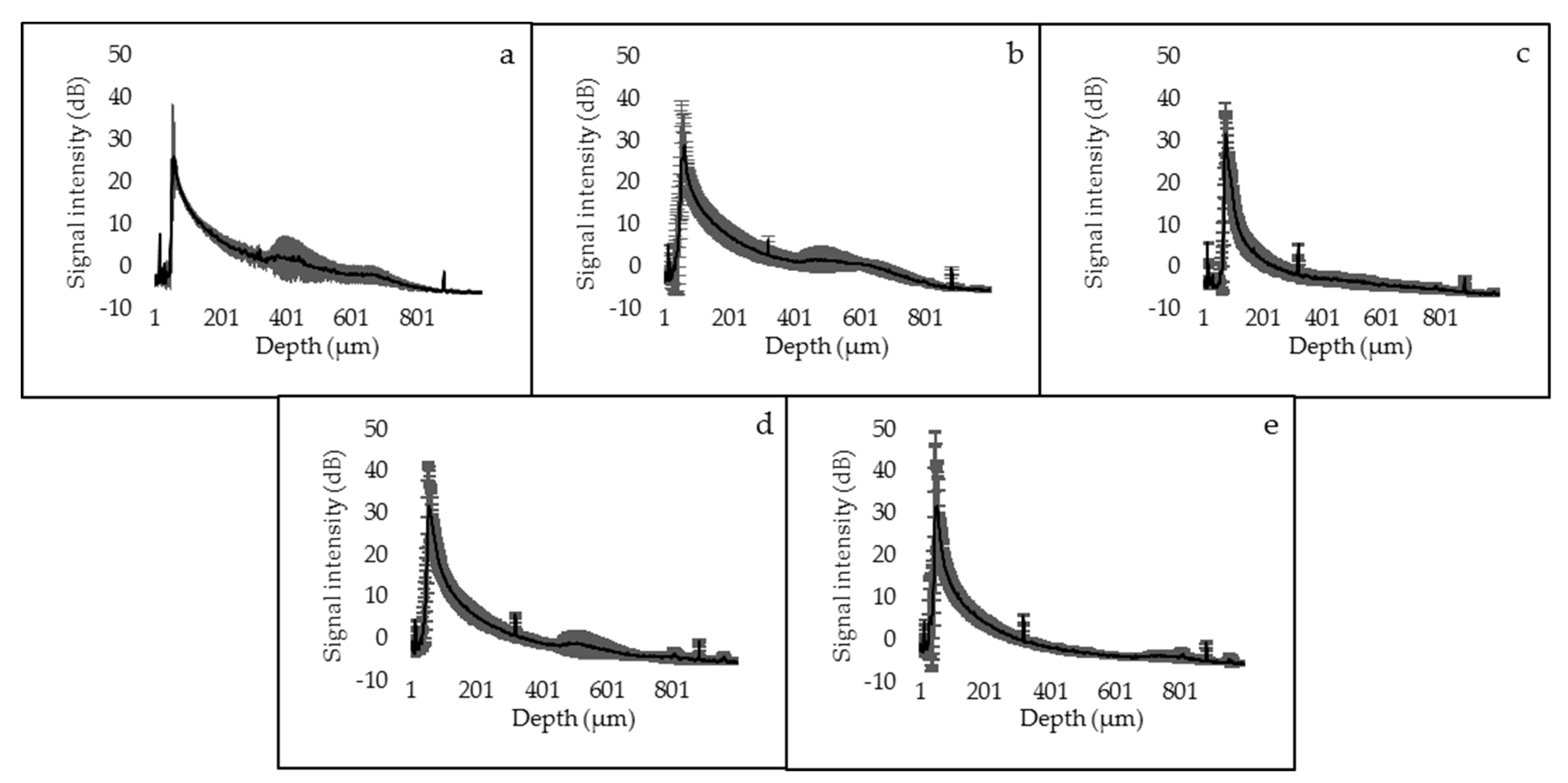
3.1. SS-OCT Analysis
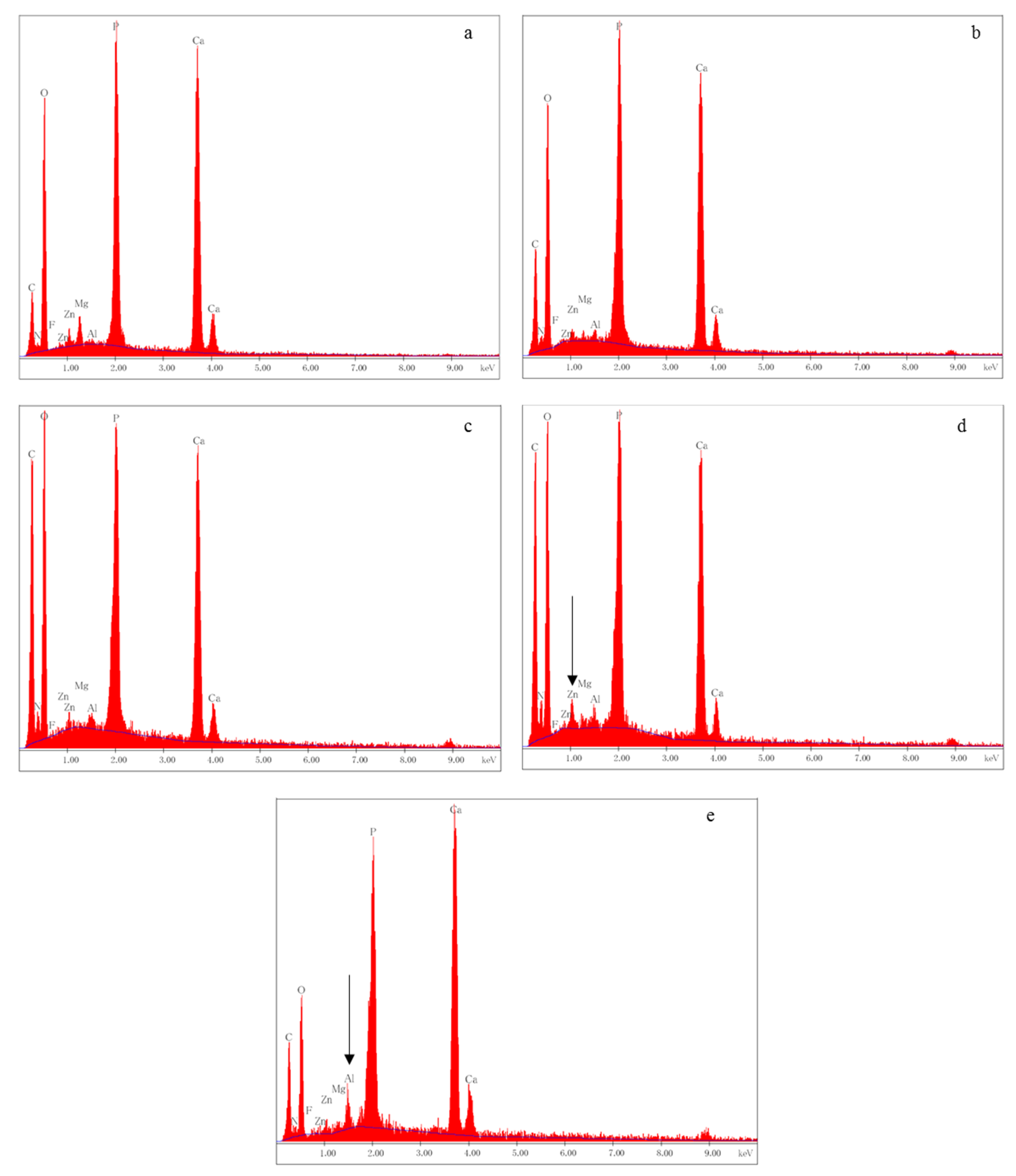
3.2. SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, A.-N.; Jang, I.-S.; Son, S.-A.; Jung, K.-H.; Park, J.-K. Effect of erosive and abrasive stress on sealing ability of different desensitizers: In-Vitro study. PLoS ONE 2019, 14, e0220823. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.R.; Borges, R.; Marchi, J.; Eduardo, C.D.P.; Marques, M.M. Bioactive glass and high-intensity lasers as a promising treatment for dentin hypersensitivity: An in vitro study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.S.; Attin, T.; Sener, B.; Sahrmann, P. Dentin tubule obturation of a bioglass-based dentin desensitizer under repeated exposure to lactid acid and brushing. BMC Oral Health 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Liu, S.; Lv, Y.; Liu, W.; Ma, W.; Xu, P. Effect of a dentifrice containing different particle sizes of hydroxyapatite on dentin tubule occlusion and aqueous Cr (VI) sorption. Int. J. Nanomed. 2019, 14, 5243–5256. [Google Scholar] [CrossRef] [PubMed]
- Kanehira, M.; Ishihata, H.; Araki, Y.; Takahashi, H.; Sasaki, K.; Finger, W.J. Effect of artificial saliva on permeability of dentin treated with phosphate containing desensitizer measured by digital flow meter. Dent. Mater. J. 2019, 38, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.R.; Narhi, M.N.; Addy, M.; Gangarosa, L.; Orchardson, R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J. Clin. Periodontol. 1997, 24, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Splieth, C.H.; Tachou, A. Epidemiology of dentin hypersensitivity. Clin. Oral Investig. 2012, 17, 3–8. [Google Scholar] [CrossRef]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin Hypersensitivity: Etiology, Diagnosis and Treatment; A Literature Review. J. Dent. 2013, 14, 136–145. [Google Scholar]
- Yoshizaki, K.T.; Francisconi-Dos-Rios, L.F.; Sobral, M.A.P.; Aranha, A.C.C.; Mendes, F.M.; Scaramucci, T. Clinical features and factors associated with non-carious cervical lesions and dentin hypersensitivity. J. Oral Rehabil. 2017, 44, 112–118. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Noiri, Y.; Ozaki, K.; Uchida, A.; Ishikawa, Y.; Ishida, H. Transmission Electron Microscopic Characterization of Hypersensitive Human Radicular Dentin. J. Dent. Res. 1990, 69, 1293–1297. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Masada, J.; Uchida, A.; Ishida, H. Scanning Electron Microscopic Characterization of Sensitive vs. Insensitive Human Radicular Dentin. J. Dent. Res. 1989, 68, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Bordea, I.R.; Candrea, S.; Alexescu, T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Han, L.; Okiji, T. Effects of a novel fluoride-containing aluminocalciumsilicate-based tooth coating material (Nanoseal) on enamel and dentin. Am. J. Dent. 2013, 26, 191–195. [Google Scholar]
- Lodha, E.; Hamba, H.; Nakashima, S.; Sadr, A.; Nikaido, T.; Tagami, J. Effect of different desensitizers on inhibition of bovine dentin demineralization: Micro-computed tomography assessment. Eur. J. Oral Sci. 2014, 122, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Okiji, T. Dentin tubule occluding ability of dentin desensitizers. Am. J. Dent. 2015, 28, 90–94. [Google Scholar] [PubMed]
- Miyajima, H.; Ishimoto, T.; Ma, S.; Chen, J.; Nakano, T.; Imazato, S. In vitro assessment of a calcium-fluoroaluminosilicate glass-based desensitizer for the prevention of root surface demineralization. Dent. Mater. J. 2016, 35, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Nikaido, T.; Abdou, A.; Matin, K.; Burrow, M.F.; Tagami, J. Inhibitory effect of zinc-containing desensitizer on bacterial biofilm formation and root dentin demineralization. Dent. Mater. J. 2019, 38, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, T.; Tanaka, K.; Iijima, Y. Inhibition of dentine demineralization by zinc oxide: In vitro and in situ studies. Dent. Mater. 2005, 21, 1170–1177. [Google Scholar] [CrossRef]
- Osorio, R.; Cabello, I.; Toledano, M. Bioactivity of zinc-doped dental adhesives. J. Dent. 2014, 42, 403–412. [Google Scholar] [CrossRef]
- Thanatvarakorn, O.; Islam, S.; Nakashima, S.; Sadr, A.; Nikaido, T.; Tagami, J. Effects of zinc fluoride on inhibiting dentin demineralization and collagen degradation in vitro: A comparison of various topical fluoride agents. Dent. Mater. J. 2016, 35, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Cabello, I.; Osorio, E.; Aguilera, F.S.; Medina-Castillo, A.L.; Toledano-Osorio, M.; Osorio, R. Zn-containing polymer nanogels promote cervical dentin remineralization. Clin. Oral Investig. 2018, 23, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, A.; Sherief, D.; Fawzy, A.S. Resin-based dental composites for tooth filling. In Advanced Dental Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 127–173. [Google Scholar] [CrossRef]
- Khosravani, M.R. Mechanical behavior of restorative dental composites under various loading conditions. J. Mech. Behav. Biomed. Mater. 2019, 93, 151–157. [Google Scholar] [CrossRef]
- Tian, L.; Peng, C.; Shi, Y.; Guo, X.; Zhong, B.; Qi, J.; Wang, G.; Cai, Q.; Cui, F. Effect of mesoporous silica nanoparticles on dentinal tubule occlusion: An in vitro study using SEM and image analysis. Dent. Mater. J. 2014, 33, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Ueno, T.; Shimada, Y.; Matin, K.; Zhou, Y.; Wada, I.; Sadr, A.; Sumi, Y.; Tagami, J. Optical analysis of enamel and dentin caries in relation to mineral density using swept-source optical coherence tomography. J. Med. Imaging 2016, 3, 035507. [Google Scholar] [CrossRef]
- Ei, T.Z.; Shimada, Y.; Nakashima, S.; Romero, M.J.R.H.; Sumi, Y.; Tagami, J. Comparison of resin-based and glass ionomer sealants with regard to fluoride-release and anti-demineralization efficacy on adjacent unsealed enamel. Dent. Mater. J. 2018, 37, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; Higham, S.M.; Podoleanu, A.G.; Rogers, J.A.; Jackson, D.A. Use of optical coherence tomography for assessment of dental caries: Quantitative procedure. J. Oral Rehabil. 2001, 28, 1092–1093. [Google Scholar] [CrossRef] [PubMed]
- Sowa, M.G.; Popescu, D.P.; Friesen, J.R.; Hewko, M.D.; Choo-Smith, A.L.-P. A comparison of methods using optical coherence tomography to detect demineralized regions in teeth. J. Biophotonics 2011, 4, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Sadr, A.; Sumi, Y.; Tagami, J. Application of Optical Coherence Tomography (OCT) for Diagnosis of Caries, Cracks, and Defects of Restorations. Curr. Oral Health Rep. 2015, 2, 73–80. [Google Scholar] [CrossRef]
- Wefel, J.; Heilman, J.; Jordan, T. Comparisons of in vitro Root Caries Models. Caries Res. 1995, 29, 204–209. [Google Scholar] [CrossRef]
- Addy, M. Tooth brushing, tooth wear and dentine hypersensitivity—Are they associated? Int. Dent. J. 2005, 55 (Suppl. S4), 261–267. [Google Scholar] [CrossRef]
- Hariri, I.; Sadr, A.; Shimada, Y.; Tagami, J.; Sumi, Y. Effects of structural orientation of enamel and dentine on light attenuation and local refractive index: An optical coherence tomography study. J. Dent. 2012, 40, 387–396. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Knuttel, A.; Yadlowsky, M.; Eckhaus, M. Optical-coherence tomography of a dense tissue: Statistics of attenuation and backscattering. Phys. Med. Biol. 1994, 39, 1705–1720. [Google Scholar] [CrossRef]
- Mandurah, M.M.; Sadr, A.; Shimada, Y.; Kitasako, Y.; Nakashima, S.; Bakhsh, T.A.; Tagami, J.; Sumi, Y. Monitoring remineralization of enamel subsurface lesions by optical coherence tomography. J. Biomed. Opt. 2013, 18, 046006. [Google Scholar] [CrossRef] [PubMed]
- Mandurah, M.M.; Sadr, A.; Bakhsh, T.A.; Shimada, Y.; Sumi, Y.; Tagami, J. Characterization of transparent dentin in attrited teeth using optical coherence tomography. Lasers Med. Sci. 2014, 30, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.; Tay, F. Aggressiveness of contemporary self-etching adhesives part II. Etching effects on unground enamel. J. Esthet. Restor. Dent. 2004, 16, 70–71. [Google Scholar] [CrossRef]
- Zhou, J.; Chiba, A.; Scheffel, D.L.S.; Hebling, J.; Agee, K.; Niu, L.-N.; Tay, F.R.; Pashley, D.H. Effects of a Dicalcium and Tetracalcium Phosphate-Based Desensitizer on In Vitro Dentin Permeability. PLoS ONE 2016, 11, e0158400. [Google Scholar] [CrossRef]
- West, N.X.; Lussi, A.; Seong, J.; Hellwig, E. Dentin hypersensitivity: Pain mechanisms and aetiology of exposed cervical dentin. Clin. Oral Investig. 2012, 17 (Suppl. S1), S9–S19. [Google Scholar] [CrossRef]
- Wada, I.; Shimada, Y.; Ikeda, M.; Sadr, A.; Nakashima, S.; Tagami, J.; Sumi, Y. Clinical assessment of non-carious cervical lesion using swept-source optical coherence tomography. J. Biophotonics 2014, 8, 846–854. [Google Scholar] [CrossRef]
- Hariri, I.; Sadr, A.; Nakashima, S.; Shimada, Y.; Tagami, J.; Sumi, Y. Estimation of the Enamel and Dentin Mineral Content from the Refractive Index. Caries Res. 2013, 47, 18–26. [Google Scholar] [CrossRef]
- Liu, Y.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Acidity-induced release of zinc ion from BioUnion(TM) filler and its inhibitory effects against Streptococcus mutans. Dent. Mater. J. 2020, 39, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Freund, I. Optical second-harmonic scattering in rat-tail tendon. Biopolym. Orig. Res. Biomol. 1981, 20, 1271–1290. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, H.; Kato, A.; Tanaka, S. Suppression of root caries progression by application of Nanoseal®: A single-blind randomized clinical trial. Dent. Mater. J. 2020, 39, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.-J.; Larmas, M.; Salo, T. The Activation and Function of Host Matrix Metalloproteinases in Dentin Matrix Breakdown in Caries Lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Charone, S.; Tjäderhane, L. Role of Host-Derived Proteinases in Dentine Caries and Erosion. Caries Res. 2015, 49, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Osorio, E.; Román, J.S.; Toledano, M. Zinc-doped dentin adhesive for collagen protection at the hybrid layer. Eur. J. Oral. Sci. 2011, 119, 401–410. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Osorio, E.; Medina-Castillo, A.L.; Toledano-Osorio, M.; Aguilera, F.S. Ions-modified nanoparticles affect functional remineralization and energy dissipation through the resin-dentin interface. J. Mech. Behav. Biomed. Mater. 2017, 68, 62–79. [Google Scholar] [CrossRef]
- Niu, L.-N.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.-H.; Tay, F.R. Biomimetic remineralization of dentin. Dent. Mater. 2014, 30, 77–96. [Google Scholar] [CrossRef]
| Material | Manufacturer | Composition | Method of Application | Lot No. |
|---|---|---|---|---|
| Caredyne Shield (CS) | GC, Tokyo, Japan | Liquid A: Fluoro-zinc silicate glass Liquid B: Phosphoric acid | Liquid A and B are mixed equally and apply to the dentin surface for 5 to 20 s and rinse with water. | 1804121 |
| Nanoseal (NS) | Nippon Shika Yakuhin, Yamaguchi, Japan | Liquid A: Fluoro-alumino-calcium silicate glass Liquid B: Phosphoric acid | J1P |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, K.; Shimada, Y.; Shinno, Y.; Ono, S.; Yamaji, K.; Ohara, N.; Sadr, A.; Sumi, Y.; Tagami, J.; Yoshiyama, M. Assessment of Demineralization Inhibition Effects of Dentin Desensitizers Using Swept-Source Optical Coherence Tomography. Materials 2021, 14, 1876. https://doi.org/10.3390/ma14081876
Matsuzaki K, Shimada Y, Shinno Y, Ono S, Yamaji K, Ohara N, Sadr A, Sumi Y, Tagami J, Yoshiyama M. Assessment of Demineralization Inhibition Effects of Dentin Desensitizers Using Swept-Source Optical Coherence Tomography. Materials. 2021; 14(8):1876. https://doi.org/10.3390/ma14081876
Chicago/Turabian StyleMatsuzaki, Kumiko, Yasushi Shimada, Yasuo Shinno, Serina Ono, Kozo Yamaji, Naoko Ohara, Alireza Sadr, Yasunori Sumi, Junji Tagami, and Masahiro Yoshiyama. 2021. "Assessment of Demineralization Inhibition Effects of Dentin Desensitizers Using Swept-Source Optical Coherence Tomography" Materials 14, no. 8: 1876. https://doi.org/10.3390/ma14081876
APA StyleMatsuzaki, K., Shimada, Y., Shinno, Y., Ono, S., Yamaji, K., Ohara, N., Sadr, A., Sumi, Y., Tagami, J., & Yoshiyama, M. (2021). Assessment of Demineralization Inhibition Effects of Dentin Desensitizers Using Swept-Source Optical Coherence Tomography. Materials, 14(8), 1876. https://doi.org/10.3390/ma14081876








