Microstructure Development and Properties of the Two-Component Melt-Spun Ni55Fe20Cu5P10B10 Alloy at Elevated Temperatures
Abstract
1. Introduction
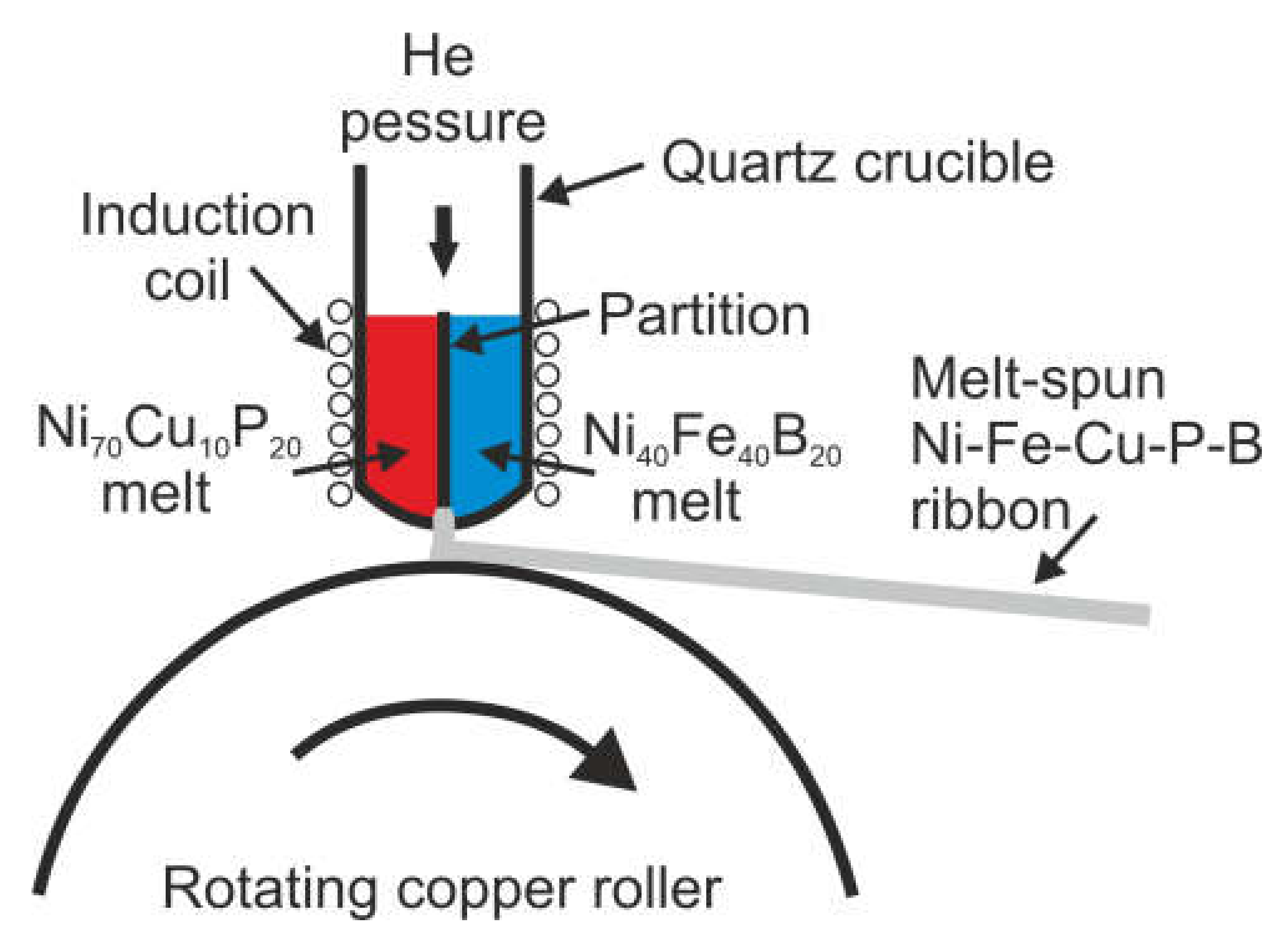
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
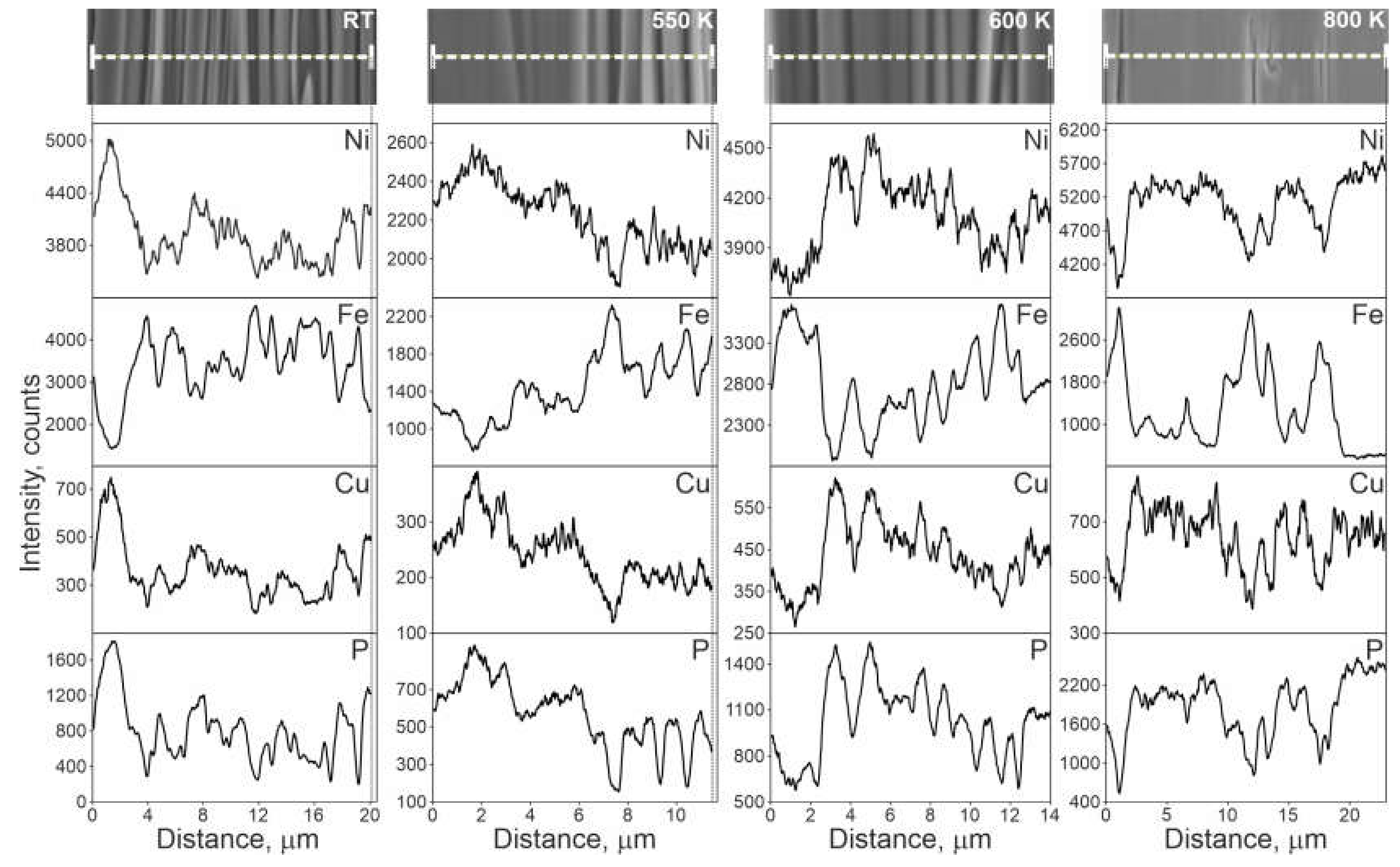
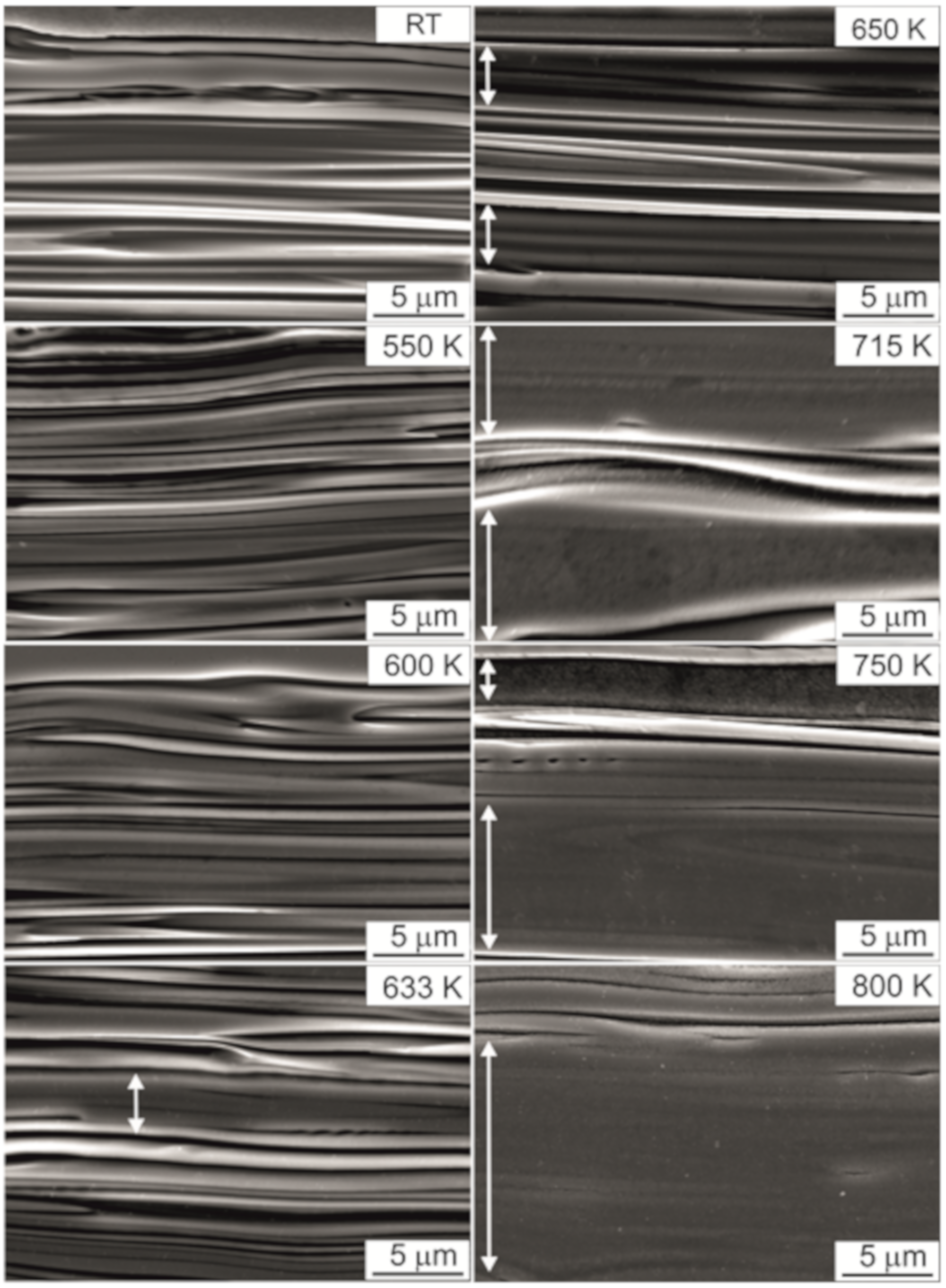
- In the TCMS samples, two kinds of band-like areas are observed: the ones rich in Ni, Cu, and P, and the other is consisting mainly of Fe, Ni, and B. The composite crystallization takes place in two stages: the first stage consists the crystallization of the Ni-Cu-P bands, the second stage is due to the crystallization of the Fe-Ni-B bands. The coarsened band-like morphology is observed at elevated temperatures even at 800 K.
- (2)
- The beginning of coarsening is observable at the temperature of 633 and 650 K. This overlaps with the crystallization of Ni-Cu-P bands, during which Ni-FCC phases, Ni3P tetragonal nickel phosphide and CuNi-FCC are formed from the amorphous matrix. At higher temperatures where the crystallization of Fe-Ni-B bands occurrs and crystalline phases are formed (i.e.,: FeNi-FCC, FeNi3-FCC, and tetragonal Fe1.5Ni1.5B), the effect of coarsening becomes more pronouced and the thickness of chemically homogeneous bands significantly increases.
- (3)
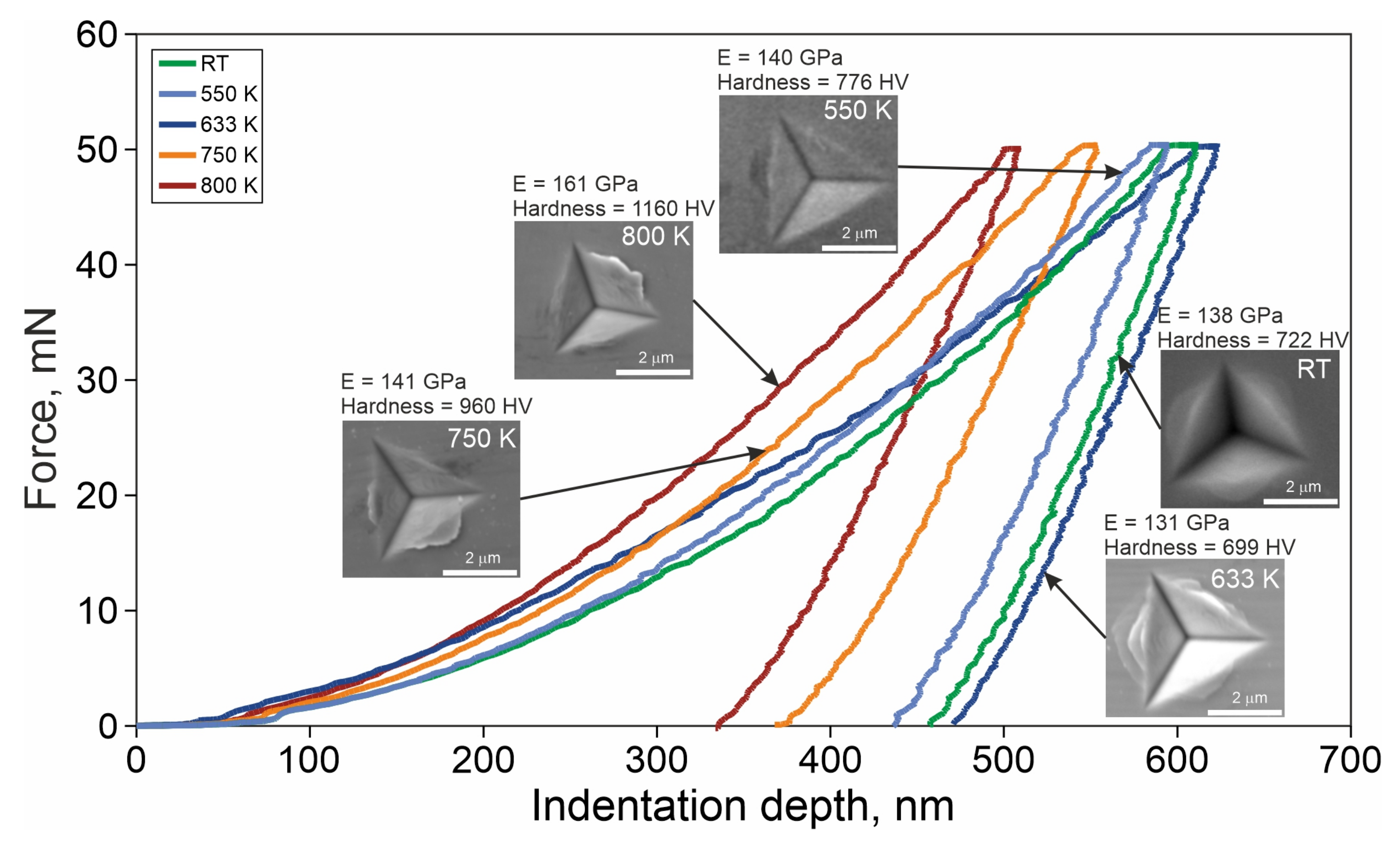
- For the TCMS samples heated to 600, 633, and 650 K, multiple slip-steps near the indentation were observed, which suggests the formation of multiple shear bands and plastic deformation of the composite during the test after heating to this temperature range.
- (4)
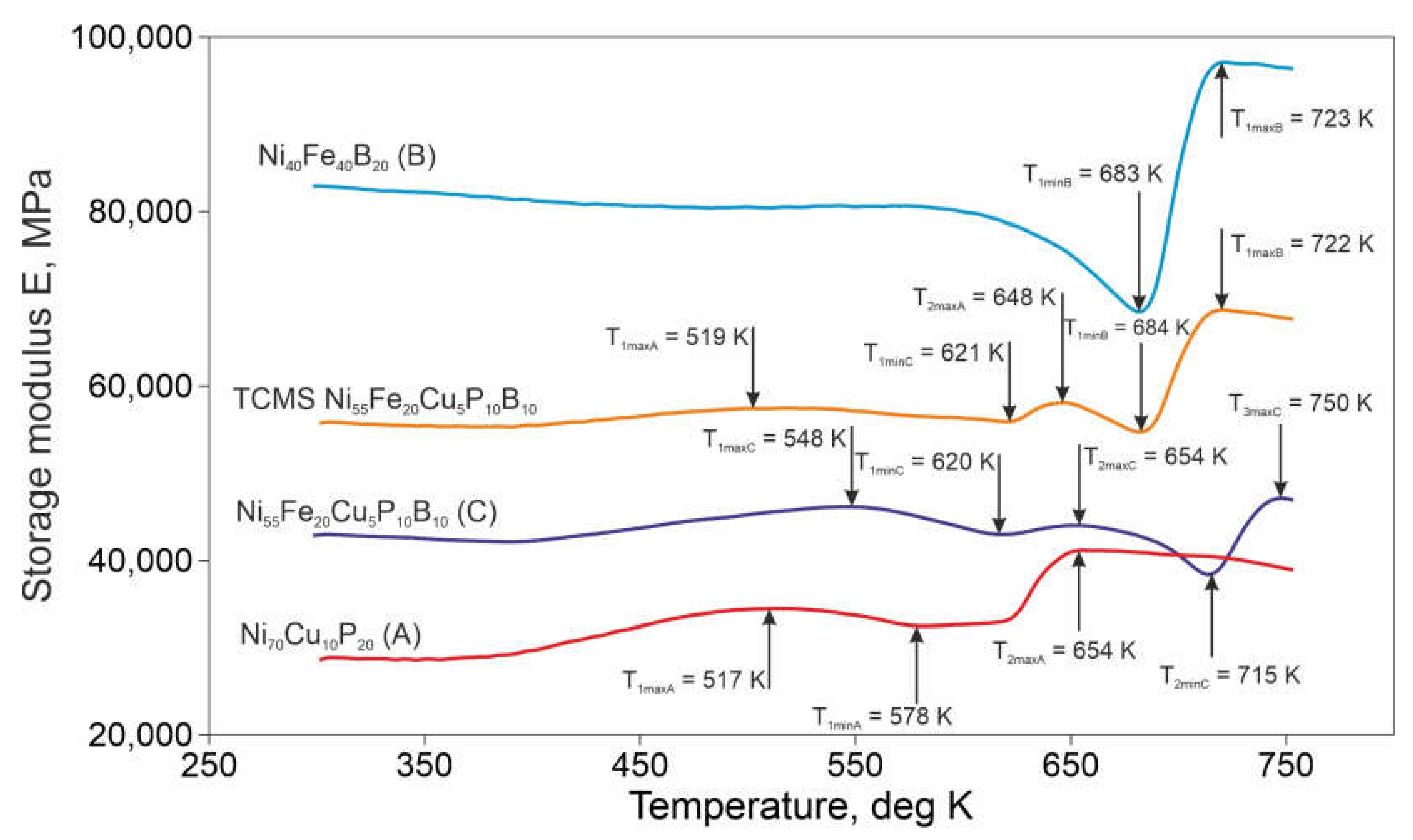
- The transformations within the amorphous phase of the Ni-Fe-B regions cause temporary decrease in the mechanical properties such as HV number, Young modulus, and storage modulus of the TCMS composite in the range of temperature ca 600 and 684 K, despite the presence of the crystalline phases occurring within Cu-Ni-P regions of the TCMS alloy.
- (5)
- The increase of HV number and Young modulus in the TCMS sample heated to temperature as high as 800 K, and increase of storage modulus at T1maxB = 722 K, can be due to the crystallization of amorhpous part of the composite enriched Ni, Fe, and B and formation of the FCC FeNi, FCC FeNi3, and Fe1.5Ni1.5B phases. This transformation can be attributed to the DSC peaks at T1B and T2C.
- (6)
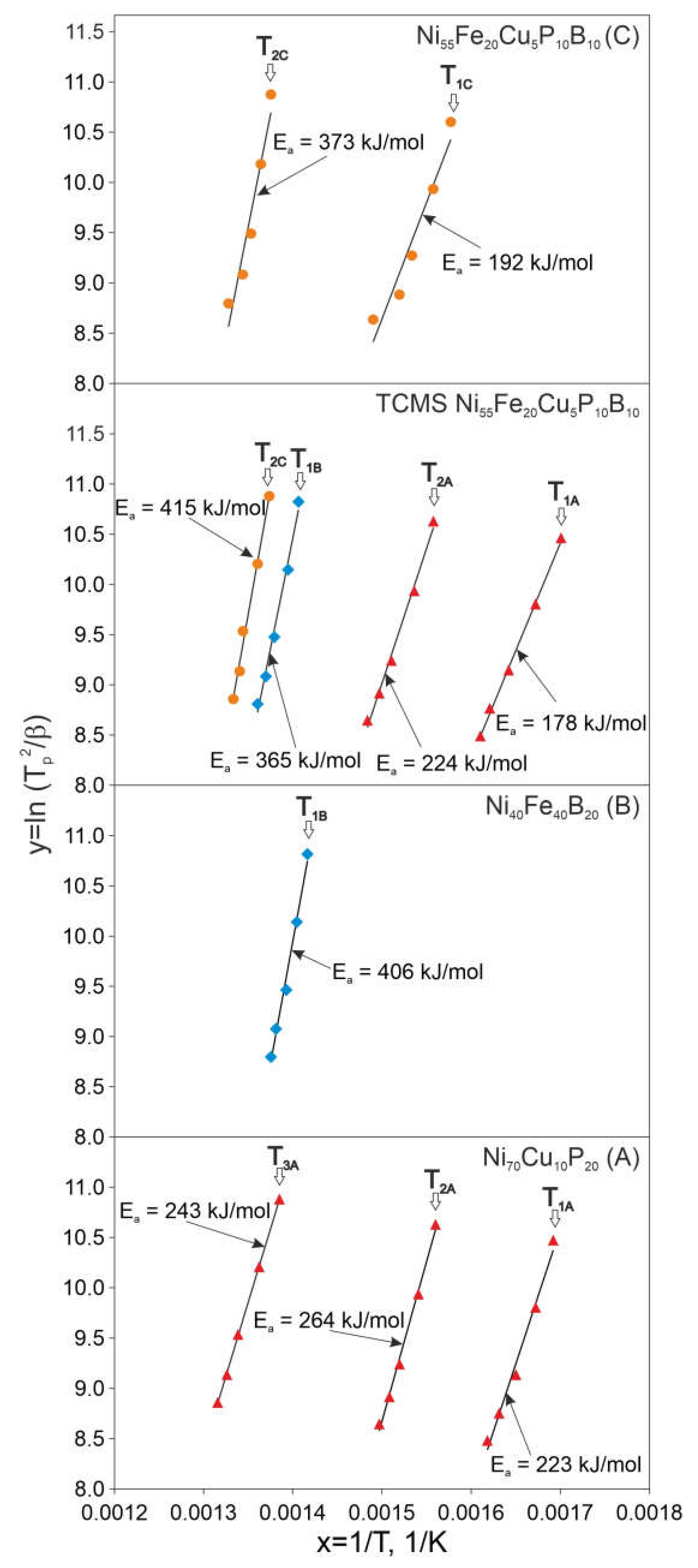
- The transformations observed in the DSC study for the TCMS alloy, are very close to these obtained for A and B alloys melt-spun from a single-chamber crucible. Moreover, there are also transformations in this composite alloy similar to those that are observed in the homogeneous melt-spun C alloy of the average nominal composition of TCMS alloy. The occurence of these transformations can be attributed to the fact that there is a small volume fraction of the TCMS alloy, which corresponds to the compositions A and B.
- (7)
- The activation energies of the transformations occurring in the TCMS alloy are lower than the corresponding values obtained for A and B alloys (i.e.,: E1A, E2A, E1B) melt-spun from a single-chamber crucible. Activation energy for 2 C transformation in TCMS alloy is related to the crystallization of the compositions that contain locally more boron or more phosphorus than it is in an average composition of alloy C. These observations may be due to the fact that there are areas with a chemical composition similar to the alloys A and B as well as with intermediate concentrations, similar to the homogeneous alloy C but with local concentrations of phosphorus and boron higher than in alloy C.
- (8)
- In comparison with the alloy C of the same nominal composition, TCMS alloy is characterized by a higher storage modulus in the entire studied temperature range. Similarly, TCMS alloy has a higher storage modulus than alloy A. At lower temperatures, changes in the storage modulus in TCMS alloy show similarity to alloy A, while from 684 K, changes in E’ modulus are similar to material B. Small influence of areas with averaged composition C on the course of changes of storage modulus E’ in TCMS alloy was found.
- (9)
- This work shows the unique properties of the TCMS composite in comparison with the melt-spun alloy of the same nominal chemical composition that was produced traditionally. The mechanical and the thermal properties of this composite are obviously inherited from the amorphous state of the alloys that are precursors for the two-component melt spinning.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Chen, H.; Feng, H.; Fang, Q.; Liu, Y.; Liu, F.; Wu, H.; Liaw, P.K. Microstructure evolution and deformation mechanism of amorphous/crystalline high-entropy-alloy composites. J. Mater. Sci. Technol. 2020, 54, 14–19. [Google Scholar] [CrossRef]
- Li, S.; Wang, F.; Li, J.-L.; Huang, P. Length scale dependent plasticity of amorphous/amorphous NiNb/ZrCuNiALSI nanolaminates. J. Non-Cryst. Solids 2020, 535, 119996. [Google Scholar] [CrossRef]
- Park, S.-Y.; Gwak, E.-J.; Huang, M.; Ruoff, R.S.; Kim, J.-Y. Nanolaminate of metallic glass and graphene with enhanced elastic modulus, strength, and ductility in tension. Scr. Mater. 2017, 139, 63–66. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.W.; Wang, A.M.; Fu, H.M.; Li, H.; Zhang, H.W.; Zhang, H.F. New ductile laminate structure of Ti-alloy/Ti-based metallic glass composite with high specific strength. J. Mater. Sci. Technol. 2018, 34, 708–712. [Google Scholar] [CrossRef]
- Song, K.K.; Han, X.L.; Pauly, S.; Qin, Y.S.; Kosiba, K.; Peng, C.X.; Gong, J.H.; Chen, X.; Wang, L.; Sarac, B.; et al. Deformation behavior of designed dual-phase CuZr metallic glasses. Mater. Des. 2018, 139, 132–140. [Google Scholar] [CrossRef]
- Saini, S.; Srivastava, A.P.; Neogy, S. The effect of Ag addition on the crystallization kinetics and glass forming ability of Zr-(CuAg)-Al bulk metallic glass. J. Alloys Compd. 2019, 772, 961–967. [Google Scholar] [CrossRef]
- Permyakova, I.; Glezer, A. Amorphous-Nanocrystalline Composites Prepared by High-Pressure Torsion. Metals 2020, 10, 511. [Google Scholar] [CrossRef]
- Wei, R.; Tao, J.; Sun, H.; Chen, C.; Sun, G.W.; Li, F.S. Soft magnetic Fe26.7Co26.7Ni26.6Si9B11 high entropy metallic glass with good bending ductility. Mater. Lett. 2017, 197, 87–89. [Google Scholar] [CrossRef]
- Hin, S.; Bernard, C.; Doquet, V.; Yokoyama, Y.; Magueresse, A.; Keryvin, V. Influence of as-cast spherulites on the fracture toughness of a Zr55Cu30Al10Ni5 bulk metallic glass. Mater. Sci. Eng. 2019, 740–741, 137–147. [Google Scholar] [CrossRef]
- Liu, D.; Lin, S.; Zhu, Z.; Zhang, B.; Li, Z.; Zhang, L.; Fu, H.; Wang, A.; Li, H.; Zhang, H.; et al. The design and mechanical behaviors of in-situ formed ductile dendrite Ti-based bulk metallic glass composites with tailored composition and mechanisms. Mater. Sci. Eng. 2018, 732, 148–156. [Google Scholar] [CrossRef]
- Rezaei, M.R.; Shabestari, S.G.; Razavi, S.H. Effect of ECAP consolidation temperature on the microstructure and mechanical properties of Al-Cu-Ti metallic glass reinforced aluminum matrix composite. J. Mater. Sci. Technol. 2017, 33, 1031–1038. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, H.J.; Kim, T.S.; Kim, Y.C.; Bae, J.C. Consolidation behavior of Cu- and Ni-based bulk metallic glass composites. J. Alloy. Compd. 2007, 434–435, 336–339. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Ali, N.; Saeed, M. Glass-Forming Ability and Soft Magnetic Properties (Co75Ti25)100−xFex (x; 0–20 at.%) Systems Fabricated by SPS of Mechanically Alloyed Nanopowders. Nanomaterials 2020, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Z.; Sun, F.; Li, X.; Ma, J. Forming of metallic glasses: Mechanisms and processes. Mater. Today Adv. 2020, 7, 100077. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, M.; Wang, C.; Yang, S.; Han, J.; Xie, G.; Liu, X. Formation of two-glassy-phase bulk metallic glass in Zr-Co-Al-Y immiscible system. J. Alloy. Compd. 2019, 781, 8–12. [Google Scholar] [CrossRef]
- Varshneya, A.K.; Mauro, J.C. Fundamentals of Inorganic Glasses, 3rd ed.; Elsevier: Amsterdam, Holland, The Netherlands, 2019; pp. 71–100. [Google Scholar]
- Ziewiec, K.; Prusik, K.; Bryła, K.; Ziewiec, A. Microstructure of the Fe-Ni-P Melt-Spun Ribbons Produced From the Single-Chamber and From the Double-Chamber Crucibles. Solid State Phenom. 2013, 203–204, 361–367. [Google Scholar] [CrossRef]
- Ziewiec, K.; Błachowski, A.; Ruebenbauer, K.; Ziewiec, A.; Prusik, K.; Latuch, J.; Zięba, M.; Bryła, K. Microstructure of the Ni–Fe–Cu–P melt-spun ribbons produced from the single-chamber and from the double-chamber crucibles. J. Alloys Compd. 2014, 615, S29–S34. [Google Scholar] [CrossRef]
- PDF4+, version 2007, Powder Diffraction Files; International Center for Diffraction Data: Newtown Square, PA, USA, 2007.
- Hu, Y.; Yang, L.; Shi, C.; Tang, W. Microstructural evolution and phase transformation kinetics of pulse-electroplated Ni–Cu–P alloy film during annealing. Mater. Chem. Phys. 2013, 141, 944–950. [Google Scholar] [CrossRef]
- Van Veldhuizen, H.B.; Koebrugge, G.W.; Sietsma, J.; Van den Beukel, A. The crystallization kinetics of amorphous Fe40Ni40B20 measured by several physical properties. J. Non-Cryst. Solids 1990, 117–118, 240–243. [Google Scholar] [CrossRef]
- Xie, C.; Li, W.; Shen, F.; Liu, Y.; Xie, L.; Liao, Z.; Zhong, S.Y. Plastic deformation behavior of a novel Fe-based metallic glass underdifferent mechanical testing techniques. J. Non-Cryst. Solids 2018, 499, 58–61. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.L.; Yavari, A.R.; Greer, A.L. Mechanical properties of Fe-based bulk glassy alloys in Fe-B-Si-Nb and Fe-Ga-P-C-B-Si systems. J. Mater. Res. 2003, 18, 1487–1492. [Google Scholar] [CrossRef]
- Miura, H.; Isa, S. Activation energies for crystallization of amorphous Fe-Ni-P-B alloys. J. Non-Cryst. Solids 1984, 68, 255–260. [Google Scholar] [CrossRef]
- Brechtl, J.; Agarwal, S.; Crespillo, M.L.; Salasin, J.; Yang, T.; Bei, H.; Zinkle, S.J. Investigation of the mechanical and microstructural evolution of a Cu based bulk metallic glass during ion irradiation. Intermet 2020, 116, 106655. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, P.; Wang, W.; Shi, Q.; Yang, H.; Wang, D.; Hong, Y.; Wang, L.; Guo, C.; Lin, S.; et al. AlCoCrNiMo high-entropy alloy as diffusion barrier between NiAlHf coating and Ni-based single crystal superalloy. Surf. Coat. Technol. 2021. [Google Scholar] [CrossRef]
- Li, W.H.; Wei, B.C.; Sun, Y.F.; Xia, L.; Wang, Y.R.; Dong, Y.D. Effect of Fe content on the thermal stability and dynamic mechanical behavior of NdAlNiCu bulk metallic glasses. Mater. Sci. Eng. A 2004, 385, 397–401. [Google Scholar] [CrossRef]
- Ziewiec, K. Properties characterization of the Cu68.5Ni12P19.5 alloy at elevated temperatures. J. Non-Cryst. Solids 2008, 354, 4019–4023. [Google Scholar] [CrossRef]
- Ziewiec, K.; Bryła, K.; Błachowski, A.; Ruebenbauer, K.; Prusik, K.; Kąc, S.; Kozieł, T. Properties and microstructure of the (Fe,Ni)-Cu-(P,Si,B) melt-spun alloys. J. Microsc. 2010, 237, 232–236. [Google Scholar] [CrossRef]
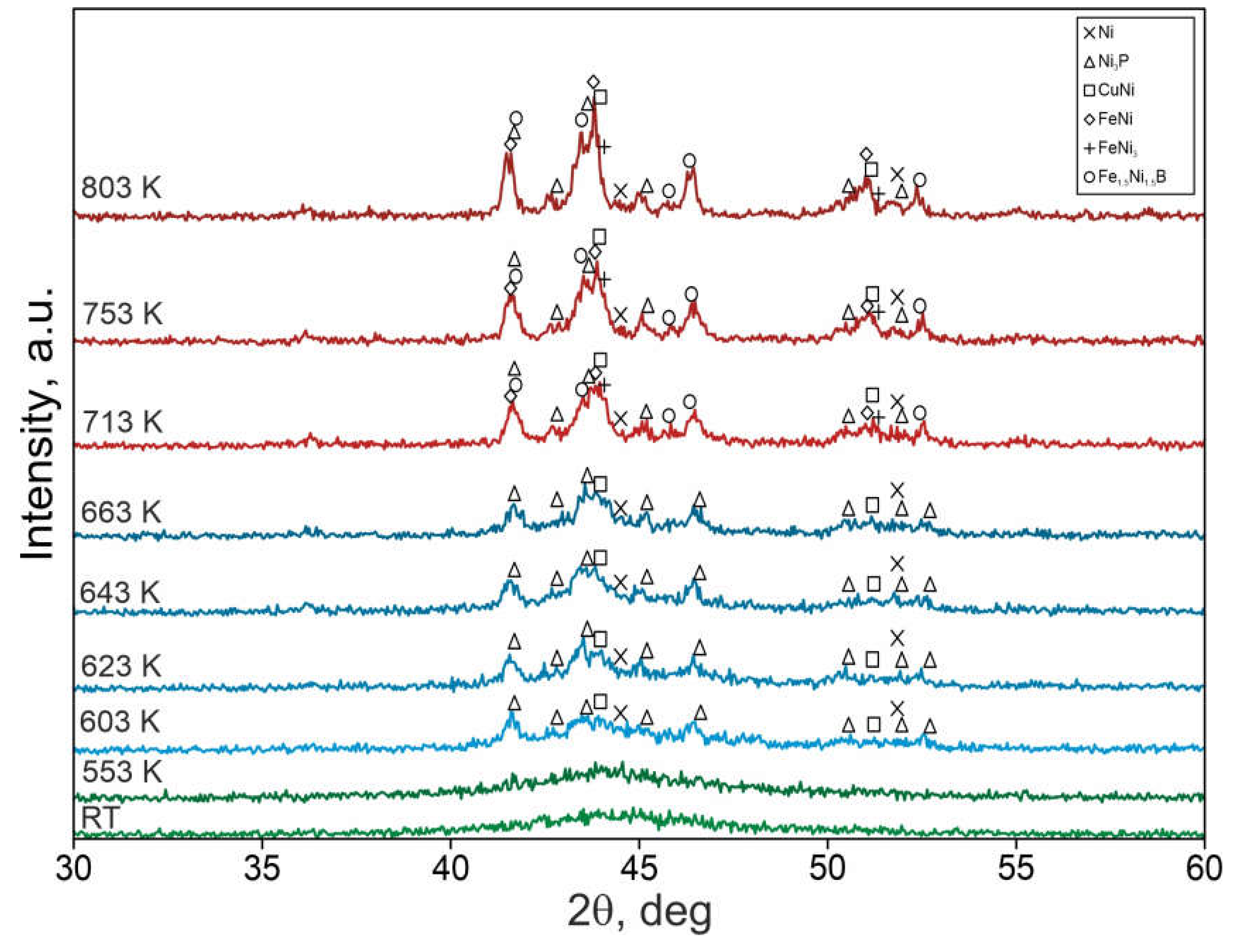


| Temperature (K) | Phases | |||||
|---|---|---|---|---|---|---|
| Ni | Ni3P | CuNi | FeNi | FeNi3 | Fe1.5Ni1.5B | |
| 293 | - | - | - | - | - | - |
| 553 | - | - | - | - | - | - |
| 603 | ● | ● | ● | - | - | - |
| 623 | ● | ● | ● | - | - | - |
| 643 | ● | ● | ● | - | - | - |
| 663 | ● | ● | ● | - | - | - |
| 713 | ● | ● | ● | ● | ● | ● |
| 753 | ● | ● | ● | ● | ● | ● |
| 803 | ● | ● | ● | ● | ● | ● |
| β, K/min | T1A | T1C | T2A | T1B | T2C | T3A |
|---|---|---|---|---|---|---|
| Ni55Fe20Cu5P10B10 (C) | ||||||
| 10 | - | 634 | - | - | 727 | - |
| 20 | - | 642 | - | - | 733 | - |
| 40 | - | 652 | - | - | 739 | - |
| 60 | - | 658 | - | - | 744 | - |
| 80 | - | 671 | - | - | 753 | - |
| TCMS Ni55Fe20Cu5P10B10 | ||||||
| 10 | 588 | - | 642 | 711 | 728 | - |
| 20 | 598 | - | 651 | 717 | 735 | - |
| 40 | 609 | - | 662 | 725 | 744 | - |
| 60 | 617 | - | 668 | 730 | 746 | - |
| 80 | 621 | - | 674 | 735 | 750 | - |
| Ni40Fe40B20 (B) | ||||||
| 10 | - | - | - | 706 | - | - |
| 20 | - | - | - | 712 | - | - |
| 40 | - | - | - | 718 | - | - |
| 60 | - | - | - | 724 | - | - |
| 80 | - | - | - | 727 | - | - |
| Ni70Cu10P20 (A) | ||||||
| 10 | 591 | - | 641 | - | - | 722 |
| 20 | 598 | - | 649 | - | - | 734 |
| 40 | 606 | - | 658 | - | - | 747 |
| 60 | 613 | - | 663 | - | - | 754 |
| 80 | 618 | - | 668 | - | - | 760 |
| E1A | E1C | E2A | E1B | E2C | E3A |
|---|---|---|---|---|---|
| Ni55Fe20Cu5P10B10 (C) | |||||
| - | 192 | - | - | 373 | - |
| TCMS Ni55Fe20Cu5P10B10 | |||||
| 178 | - | 224 | 365 | 415 | - |
| Ni40Fe40B20 (B) | |||||
| - | - | - | 406 | - | - |
| Ni70Cu10P20 (A) | |||||
| 223 | - | 264 | - | - | 243 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziewiec, K.; Wojciechowska, M.; Jankowska-Sumara, I.; Ziewiec, A.; Kąc, S. Microstructure Development and Properties of the Two-Component Melt-Spun Ni55Fe20Cu5P10B10 Alloy at Elevated Temperatures. Materials 2021, 14, 1741. https://doi.org/10.3390/ma14071741
Ziewiec K, Wojciechowska M, Jankowska-Sumara I, Ziewiec A, Kąc S. Microstructure Development and Properties of the Two-Component Melt-Spun Ni55Fe20Cu5P10B10 Alloy at Elevated Temperatures. Materials. 2021; 14(7):1741. https://doi.org/10.3390/ma14071741
Chicago/Turabian StyleZiewiec, Krzysztof, Mirosława Wojciechowska, Irena Jankowska-Sumara, Aneta Ziewiec, and Sławomir Kąc. 2021. "Microstructure Development and Properties of the Two-Component Melt-Spun Ni55Fe20Cu5P10B10 Alloy at Elevated Temperatures" Materials 14, no. 7: 1741. https://doi.org/10.3390/ma14071741
APA StyleZiewiec, K., Wojciechowska, M., Jankowska-Sumara, I., Ziewiec, A., & Kąc, S. (2021). Microstructure Development and Properties of the Two-Component Melt-Spun Ni55Fe20Cu5P10B10 Alloy at Elevated Temperatures. Materials, 14(7), 1741. https://doi.org/10.3390/ma14071741








