Structures and Dissolution Behaviors of Quaternary CaO-SrO-P2O5-TiO2 Glasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CaO-SrO-P2O5-TiO2 Glasses
2.2. Characterization of CaO-SrO-P2O5-TiO2 Glass Structures
2.3. Thermal Analysis of CaO-SrO-P2O5-TiO2 Glasses
2.4. Dissolution Behavior of CaO-SrO-P2O5-TiO2 Glasses
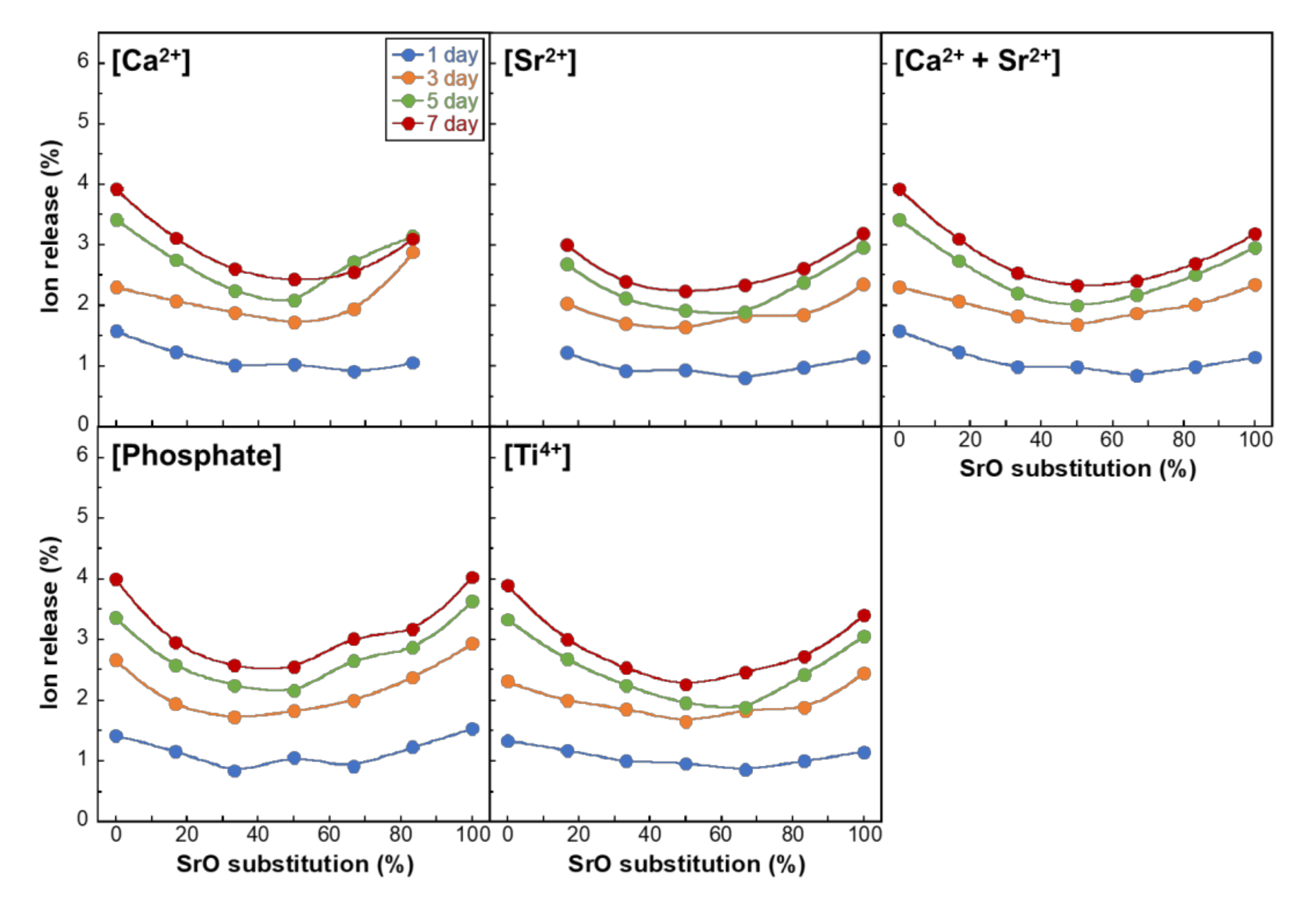
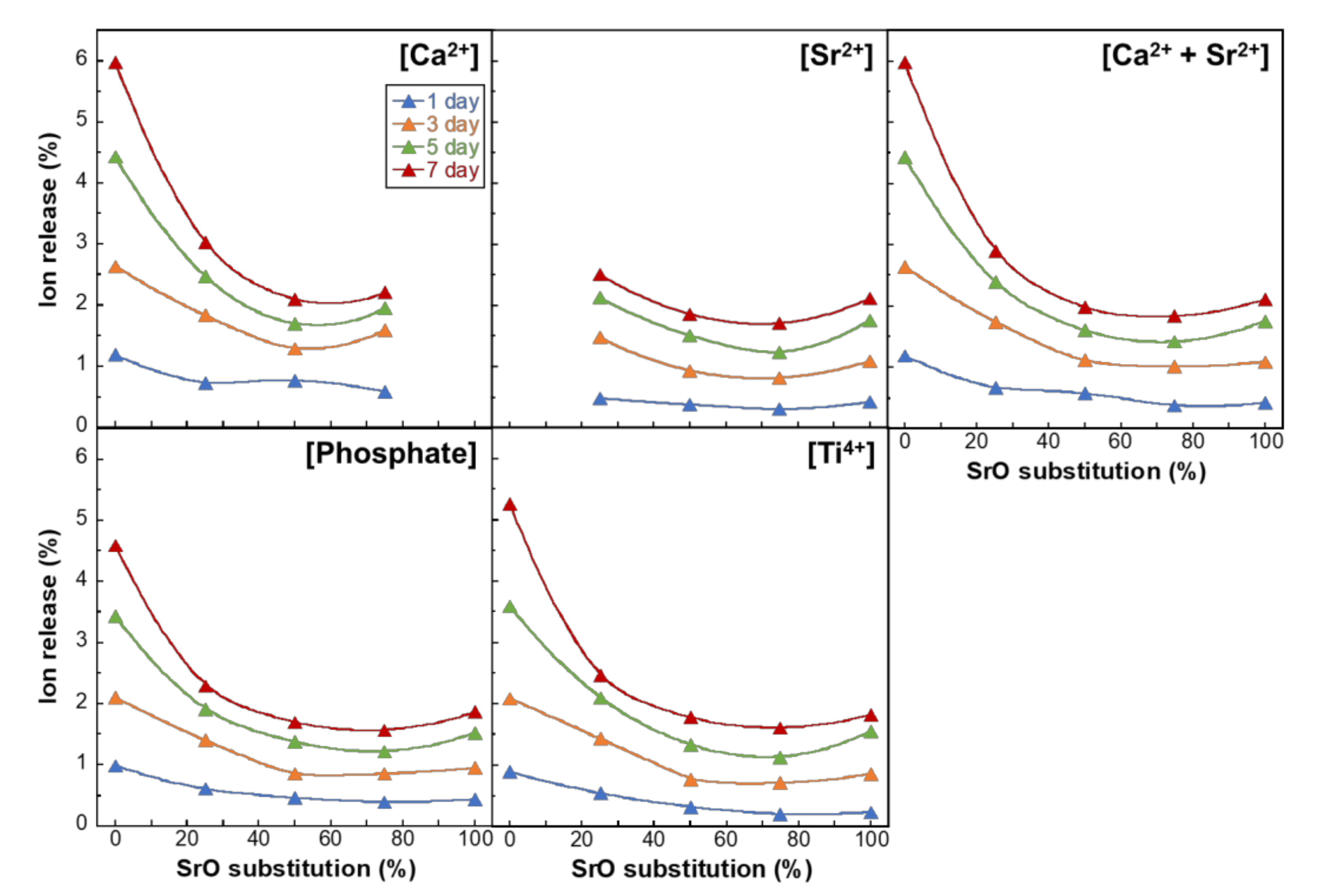
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Hoppe, A.; Mourino, V.; Boccaccini, A.R. Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci. 2013, 1, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef]
- Xynos, I.D.; Hukkanen, J.M.V.; Batten, J.J.; Buttery, D.L.; Hench, L.L.; Polak, M.J. Bioglass®45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-dependent regulation of MGP in osteoblasts: Role of ERK1/2 and Fra-1. J. Bone Miner. Res. 2009, 24, 1856–1868. [Google Scholar] [CrossRef]
- Yamada, S.; Ota, Y.; Obata, A.; Kasuga, T. Osteoblast-like cell responses to ion products released from magnesium- and silicate-containing calcium carbonates. Bio Med. Mater. Eng. 2017, 28, 47–56. [Google Scholar] [CrossRef]
- Marie, P.J. The calcium-sensing receptor in bone cells: A potential therapeutic target in osteoporosis. Bone 2010, 46, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Strontium ranelate: A physiological approach for optimizing bone formation and resorption. Bone 2006, 38, 10–14. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: New insights into its dual mode of action. Bone 2007, 40, S5–S8. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Quinn, S.J.; Kifor, O.; Ye, C.; Brown, E.M. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. 2007, 74, 438–447. [Google Scholar] [CrossRef]
- Barbara, A.; Delannoy, P.; Denis, B.G.; Marie, P.J. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism 2004, 53, 532–537. [Google Scholar] [CrossRef]
- Xue, W.; Moore, J.L.; Hosick, H.L.; Bose, S.; Bandyopadhyay, A.; Lu, W.W.; Cheung, K.M.C.; Luk, K.D.K. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J. Biomed. Mater. Res. A 2006, 79, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Zhao, X.J.; Wan, C.X.; Zhao, C.S.; Chen, Y.W. Effect of strontium ions on the growth of ROS17/2.8 cells on porous calcium polyphosphate scaffolds. Biomaterials 2006, 27, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Kim, Y.-J. Fabrication of strontium-substituted hydroxyapatite scaffolds using 3D printing for enhanced bone regeneration. J. Mater. Sci. 2021, 56, 1673–1684. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M.; Sedghi, A. A comparative study on the in vitro formation of hydroxyapatite, cytotoxicity and antibacterial activity of 58S bioactive glass substituted by Li and Sr. Mater. Sci. Eng. C 2018, 91, 349–360. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.; Cheng, L.; Lin, K. Enhancement of osteoporotic bone regeneration by strontium-substituted 45S5 bioglass via time-dependent modulation of autophagy and the Akt/mTOR signaling pathway. J. Mater. Chem. B 2021. [Google Scholar] [CrossRef]
- Knowles, J.C. Phosphate based glasses for biomedical applications. J. Mater. Chem. 2003, 13, 2395–2401. [Google Scholar] [CrossRef]
- Brauer, D.S. Phosphate Glasses. Bio Glasses 2012, 45–64. [Google Scholar] [CrossRef]
- Kasuga, T. Glass-based biomaterials design for generating advanced functions. J. Jpn. Soc. Biomater. 2016, 34, 66–70. [Google Scholar]
- Abou Neel, E.A.; Pickup, D.M.; Valappil, S.P.; Newport, R.J.; Knowles, J.C. Bioactive functional materials: A perspective on phosphate-based glasses. J. Mater. Chem. 2009, 19, 690–701. [Google Scholar] [CrossRef]
- Brow, R.K.; Tallant, D.R.; Warren, W.L.; McIntyre, A.; Day, D.E. Spectroscopic studies of the structure of titanophosphate and calcium titanophosphate glasses. Phys. Chem. Glasses 1997, 38, 300–306. [Google Scholar]
- Brauer, D.S.; Karpukhina, N.; Law, R.V.; Hill, R.G. Effect of TiO2 addition on structure, solubility and crystallisation of phosphate invert glasses for biomedical applications. J. Non Cryst. Solids 2010, 356, 2626–2633. [Google Scholar] [CrossRef]
- Kasuga, T.; Abe, Y. Calcium phosphate invert glasses with soda and titania. J. Non Cryst. Solids 1999, 243, 70–74. [Google Scholar] [CrossRef]
- Lee, S.; Maeda, H.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Structures and dissolution behaviors of CaO–P2O5–TiO2/Nb2O5 (Ca/P ≥ 1) invert glasses. J. Non Cryst. Solids 2015, 426, 35–42. [Google Scholar] [CrossRef]
- Morikawa, H.; Lee, S.; Kasuga, T.; Brauer, D.S. Effects of magnesium for calcium substitution in P2O5–CaO–TiO2 glasses. J. Non Cryst. Solids 2013, 380, 53–59. [Google Scholar] [CrossRef]
- Lee, S.; Maeda, H.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Structure and dissolution behavior of MgO-P2O5-TiO2/Nb2O5 (Mg/P ≥ 1) invert glasses. J. Ceram. Soc. Jpn. 2015, 123, 942–948. [Google Scholar] [CrossRef]
- Lee, S.; Nagata, F.; Kato, K.; Kasuga, T. Dissolution behavior of MgO-CaO-P2O5-TiO2 invert glasses. Phosphorus Res. Bull. 2020, 36, 10–14. [Google Scholar] [CrossRef]
- Lee, S.; Obata, A.; Kasuga, T. Ion release from SrO-CaO-TiO2-P2O5 glasses in Tris buffer solution. J. Ceram. Soc. Jpn. 2009, 117, 935–938. [Google Scholar] [CrossRef]
- Lee, S.; Obata, A.; Brauer, D.S.; Kasuga, T. Dissolution behavior and cell compatibility of alkali-free MgO-CaO-SrO-TiO2-P2O5 glasses for biomedical applications. Biomed. Glasses 2015, 1, 151–158. [Google Scholar] [CrossRef]
- Lee, S.; Uehara, H.; Maçon, A.L.B.; Maeda, H.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Preparation of antibacterial ZnO-CaO-P2O5-Nb2O5 invert glasses. Mater. Trans. 2016, 57, 2072–2076. [Google Scholar] [CrossRef]
- Lee, S.; Nakano, T.; Kasuga, T. Structure, dissolution behavior, cytocompatibility, and antibacterial activity of silver-containing calcium phosphate invert glasses. J. Biomed. Mater. Res. A 2017, 105, 3127–3135. [Google Scholar] [CrossRef]
- Obata, A.; Takahashi, Y.; Miyajima, T.; Ueda, K.; Narushima, T.; Kasuga, T. Effects of niobium ions released from calcium phosphate invert glasses containing Nb2O5 on osteoblast-like cell functions. Acs Appl. Mater. Interfaces 2012, 4, 5684–5690. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Lee, S.; Miyajima, T.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Structure and physicochemical properties of CaO–P2O5–Nb2O5–Na2O glasses. J. Non Cryst. Solids 2016, 432, 60–64. [Google Scholar] [CrossRef]
- Lee, S.; Maeda, H.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Structures and dissolution behaviors of MgO–CaO–P2O5–Nb2O5 glasses. J. Non Cryst. Solids 2016, 438, 18–25. [Google Scholar] [CrossRef]
- Meunier, P.J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J.E.; Spector, T.D.; Cannata, J.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S.; et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N. Engl. J. Med. 2004, 350, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Pors Nielsen, S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef]
- Sato, P.S.; Watanabe, T.; Maeda, H.; Obata, A.; Kasuga, T. Preparation of an antibacterial amorphous thin film by radiofrequency magnetron sputtering using a 65ZnO–30P2O5–5Nb2O5 glass. J. Non Cryst. Solids 2020, 528, 119724. [Google Scholar] [CrossRef]
- Bunker, B.C.; Arnold, G.W.; Wilder, J.A. Phosphate glass dissolution in aqueous solutions. J. Non Cryst. Solids 1984, 64, 291–316. [Google Scholar] [CrossRef]
- Ray, N.H. Composition—property relationships in inorganic oxide glasses. J. Non Cryst. Solids 1974, 15, 423–434. [Google Scholar] [CrossRef]
- Tylkowski, M.; Brauer, D.S. Mixed alkali effects in Bioglass® 45S5. J. Non Cryst. Solids 2013, 376, 175–181. [Google Scholar] [CrossRef]
- Ouchetto, M.; Elouadi, B.; Parke, S. Study of lanthanide zinc phosphate glasses by differential thermal analysis. Phys. Chem. Glasses 1991, 32, 22–28. [Google Scholar]
- Lee, S. Development of glass-related biomaterials for enhanced bone regeneration via stimulation of cell function. J. Ceram. Soc. Jpn. 2020, 128, 349–356. [Google Scholar] [CrossRef]
- Lee, S.; Maçon, A.L.B.; Kasuga, T. Structure and dissolution behavior of orthophosphate MgO–CaO–P2O5–Nb2O5 glass and glass-ceramic. Mater. Lett. 2016, 175, 135–138. [Google Scholar] [CrossRef]
- Lee, S.; Nakano, T.; Kasuga, T. Formation and structural analysis of 15MgO–15CaO–8P2O5–4SiO2 glass. J. Non Cryst. Solids 2017, 457, 73–76. [Google Scholar] [CrossRef]
- Karakassides, M.A.; Saranti, A.; Koutselas, I. Preparation and structural study of binary phosphate glasses with high calcium and/or magnesium content. J. Non Cryst. Solids 2004, 347, 69–79. [Google Scholar] [CrossRef]
- Brow, R.K.; Tallant, D.R.; Myers, S.T.; Phifer, C.C. The short-range structure of zinc polyphosphate glass. J. Non Cryst. Solids 1995, 191, 45–55. [Google Scholar] [CrossRef]
- Sakka, S.; Miyaji, F.; Fukumi, K. Structure of binary K2O-TiO2 and Cs2O-TiO2 glasses. J. Non Cryst. Solids 1989, 112, 64–68. [Google Scholar] [CrossRef]
- Mishra, A.; Rocherullé, J.; Massera, J. Ag-doped phosphate bioactive glasses: Thermal, structural and in-vitro dissolution properties. Biomed. Glasses 2016, 2, 38–48. [Google Scholar] [CrossRef]
- Ciceo Lucacel, R.; Hulpus, A.O.; Simon, V.; Ardelean, I. Structural characterization of phosphate glasses doped with silver. J. Non Cryst. Solids 2009, 355, 425–429. [Google Scholar] [CrossRef]
- Le, Q.H.; Calahoo, C.; Xia, Y.; Buchheim, J.; Bragatto, C.B.; Wondraczek, L. Optimization of electrical conductivity in the Na2O-P2O5-AlF3-SO3 glass system. J. Am. Ceram. Soc. 2020, 103, 4939–4956. [Google Scholar] [CrossRef]
- Vogel, W. Classical Theories of Glass Structure. In Glass Chemistry; Vogel, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 41–56. [Google Scholar]
- Brow, R.K. Review: The structure of simple phosphate glasses. J. Non Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Nelson, B.N.; Exarhos, G.J. Vibrational spectroscopy of cation-site interactions in phosphate glasses. J. Chem. Phys. 1979, 71, 2739–2747. [Google Scholar] [CrossRef]
- Rouse, G.B., Jr.; Miller, P.J.; Risen, W.M., Jr. Mixed alkali glass spectra and structure. J. Non Cryst. Solids 1978, 28, 193–207. [Google Scholar] [CrossRef]
- Maeda, H.; Tamura, T.; Kasuga, T. Experimental and theoretical investigation of the structural role of titanium oxide in CaO-P2O5–TiO2 invert glass. J. Phys. Chem. B 2017, 121, 5433–5438. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.B.; Correia, R.N.; Oliveira, J.M.M.; Fernandes, M.H.V. Structural characterization of TiO2–P2O5–CaO glasses by spectroscopy. J. Eur. Ceram. Soc. 2010, 30, 1253–1258. [Google Scholar] [CrossRef]
- Christie, J.K.; de Leeuw, N.H. Effect of strontium inclusion on the bioactivity of phosphate-based glasses. J. Mater. Sci. 2017, 52, 9014–9022. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Weng, W.; Santos, J.D.; Lopes, A.M. Structural studies of Na2O-TiO2-P2O5 system glasses investigated by FTIR and FT-Raman. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. B 2008, 49, 41–45. [Google Scholar]
- Nagarjuna, M.; Satyanarayana, T.; Gandhi, Y.; Veeraiah, N. Influence of Ag2O on some physical properties of LiF–TiO2–P2O5 glass system. J. Alloys Compd. 2009, 479, 549–556. [Google Scholar] [CrossRef]
- Mandlule, A.; Döhler, F.; van Wüllen, L.; Kasuga, T.; Brauer, D.S. Changes in structure and thermal properties with phosphate content of ternary calcium sodium phosphate glasses. J. Non Cryst. Solids 2014, 392–393, 31–38. [Google Scholar] [CrossRef]
- Dilmore, M.F.; Clark, D.E.; Hench, L.L. Chemical durability of Na2O-K2O-CaO-SiO2 glasses. J. Am. Ceram. Soc. 1978, 61, 439–443. [Google Scholar] [CrossRef]
- Zhifang, W.; Nai, Z.; Bo, M.; Zhongxin, S. Study of the mixed alkali effect on chemical durability of alkali silicate glasses. J. Non Cryst. Solids 1986, 84, 468–476. [Google Scholar] [CrossRef]
- Isard, J.O. The mixed alkali effect in glass. J. Non Cryst. Solids 1969, 1, 235–261. [Google Scholar] [CrossRef]
- Day, D.E. Mixed alkali glasses—Their properties and uses. J. Non Cryst. Solids 1976, 21, 343–372. [Google Scholar] [CrossRef]
- Swenson, J.; Adams, S. Mixed alkali effect in glasses. Phys. Rev. Lett. 2003, 90, 155507. [Google Scholar] [CrossRef] [PubMed]
- Kishioka, A.; Haba, M.; Amagasa, M. Glass formation in multicomponent phosphate systems containing TiO2. Bull. Chem. Soc. Jpn. 1974, 47, 2493–2496. [Google Scholar] [CrossRef]
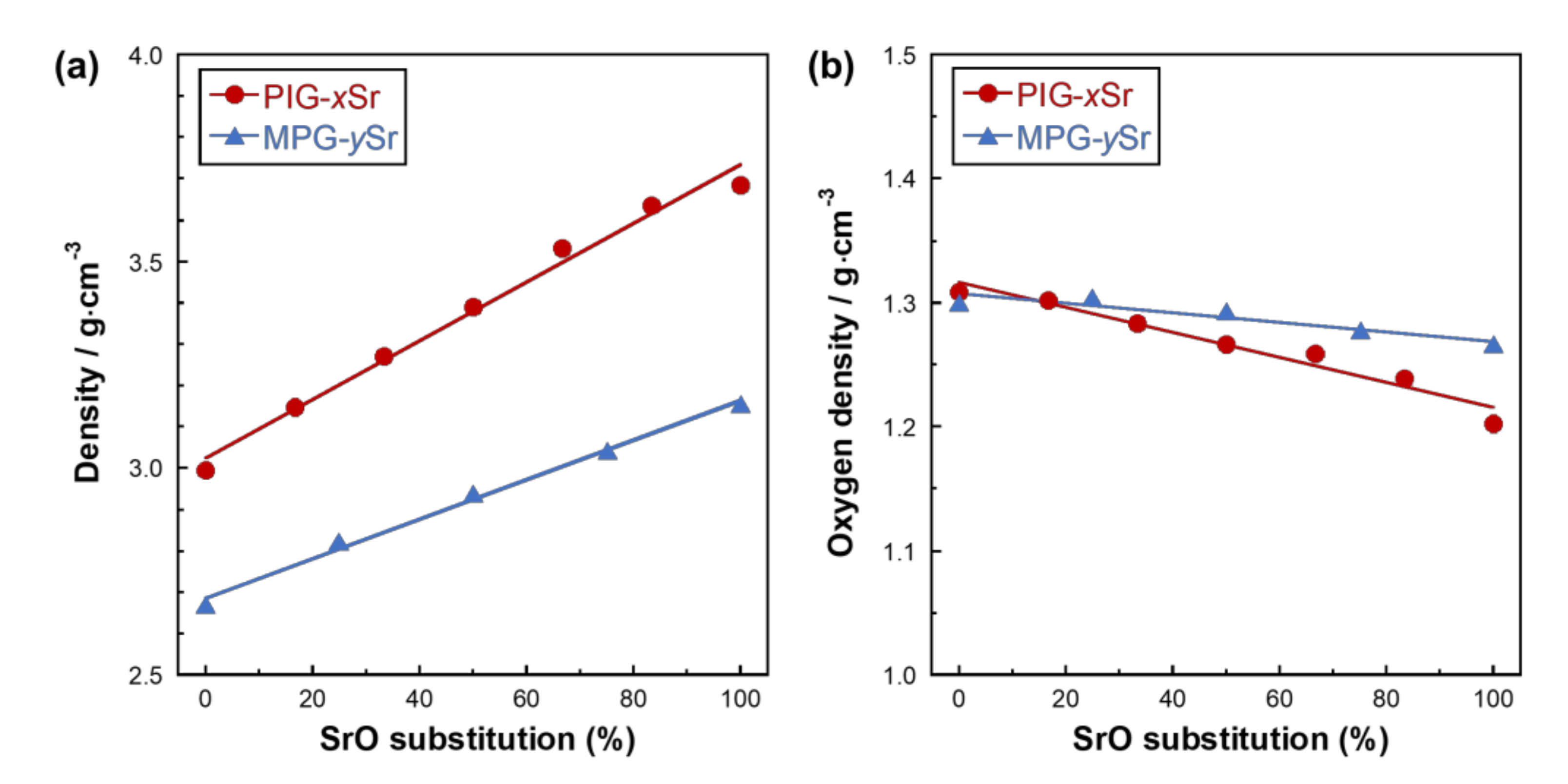
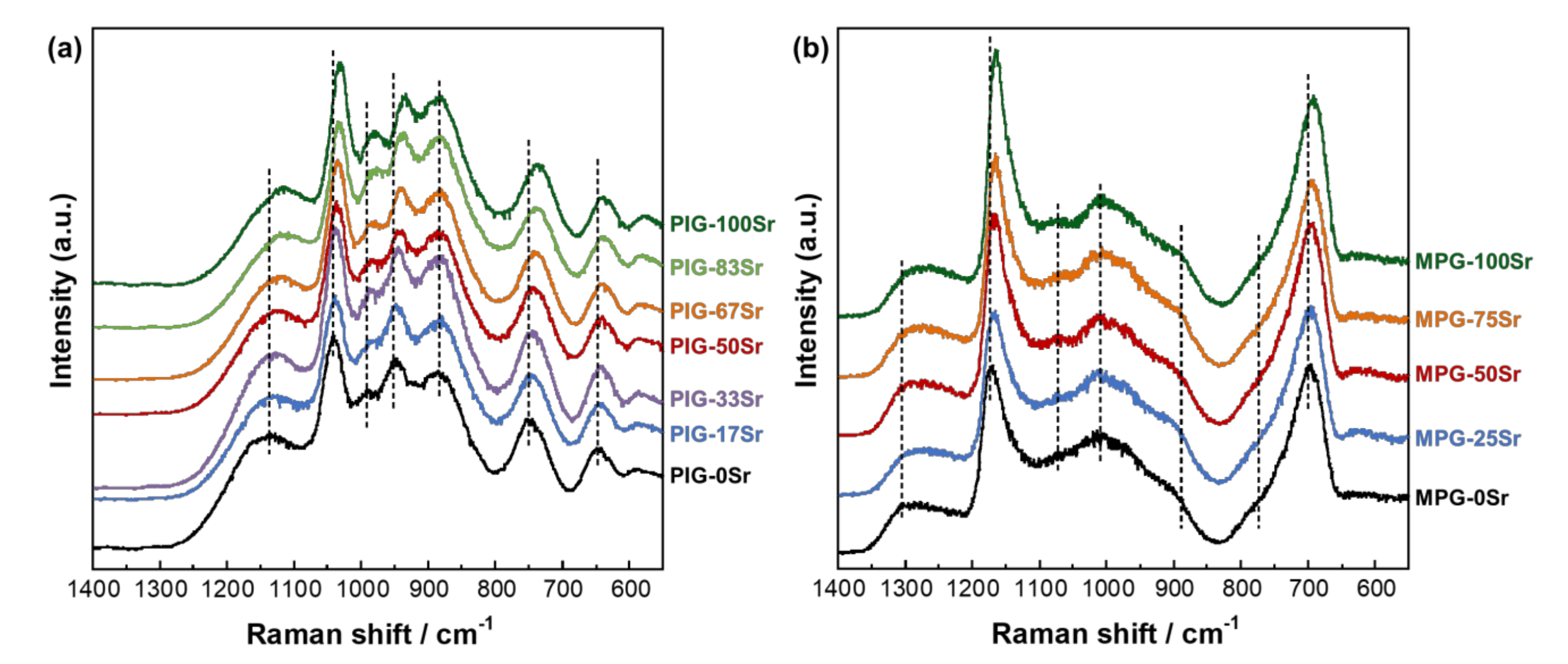
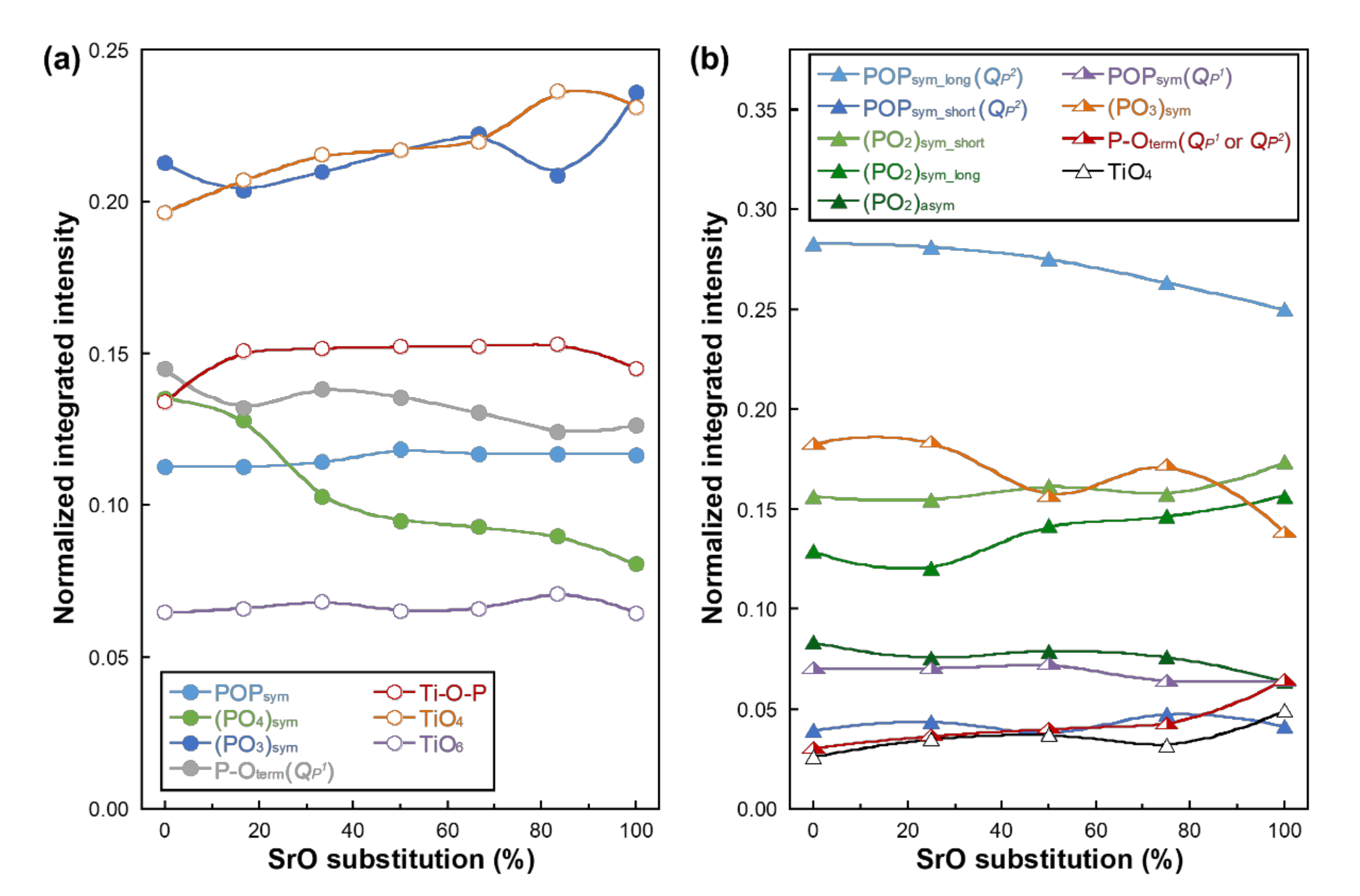
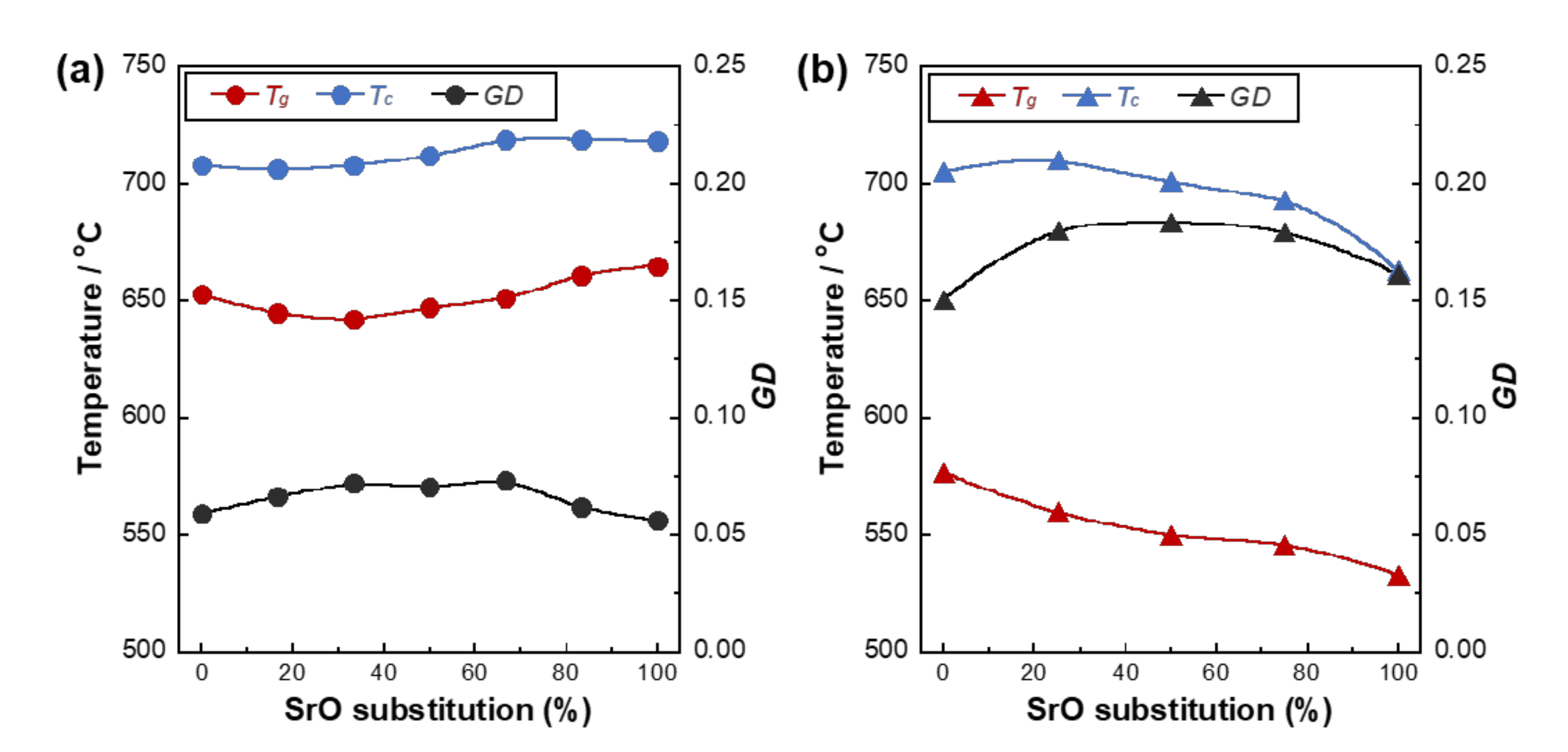
| Glass Code | CaO | SrO | P2O5 | TiO2 | SrO for CaO Substitution (%) |
|---|---|---|---|---|---|
| Phosphate invert glass series | |||||
| PIG-0Sr | 60 | - | 30 | 10 | 0 |
| PIG-17Sr | 50 | 10 | 30 | 10 | 16.7 |
| PIG-33Sr | 40 | 20 | 30 | 10 | 33.3 |
| PIG-50Sr | 30 | 30 | 30 | 10 | 50.0 |
| PIG-67Sr | 20 | 40 | 30 | 10 | 66.7 |
| PIG-83Sr | 10 | 50 | 30 | 10 | 83.3 |
| PIG-100Sr | - | 60 | 30 | 10 | 100 |
| Metaphosphate glass series | |||||
| MPG-0Sr | 45 | - | 50 | 5 | 0 |
| MPG-25Sr | 33.75 | 11.25 | 50 | 5 | 25 |
| MPG-50Sr | 22.5 | 22.5 | 50 | 5 | 50 |
| MPG-75Sr | 11.25 | 33.75 | 50 | 5 | 75 |
| MPG-100Sr | - | 45 | 50 | 5 | 100 |
| Raman Shift/cm−1 | Assignments |
|---|---|
| 585 | P-O symmetric stretching vibration mode of QP0 |
| 640 | Ti-O stretching vibration mode of TiO6 octahedra |
| 695 | POP symmetric stretching mode of bridging oxygen(QP2 long chain) |
| 740 | POP symmetric stretching mode of bridging oxygen(QP2 short chain) |
| 745, 770 | POP symmetric stretching mode of bridging oxygen (QP1) |
| 890 | Ti-O stretching vibration mode of TiO4 tetrahedra |
| 945 | PO4 symmetric stretching mode of non-bridging oxygen (QP0) |
| 990 | P-O-Ti bonds |
| 1005, 1035 | PO3 symmetric stretching mode of non-bridging oxygen (QP1) |
| 1095 | P-O vibration mode of the terminal for phosphate chains |
| 1125 | P-O stretching mode of the terminal QP1 |
| 1150 | PO2 symmetric stretching mode of non-bridging oxygen(QP2 long chain) |
| 1170 | PO2 symmetric stretching mode of non-bridging oxygen(QP2 short chain) |
| 1265 | PO2 asymmetric stretching mode of non-bridging oxygen (QP2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Nagata, F.; Kato, K.; Nakano, T.; Kasuga, T. Structures and Dissolution Behaviors of Quaternary CaO-SrO-P2O5-TiO2 Glasses. Materials 2021, 14, 1736. https://doi.org/10.3390/ma14071736
Lee S, Nagata F, Kato K, Nakano T, Kasuga T. Structures and Dissolution Behaviors of Quaternary CaO-SrO-P2O5-TiO2 Glasses. Materials. 2021; 14(7):1736. https://doi.org/10.3390/ma14071736
Chicago/Turabian StyleLee, Sungho, Fukue Nagata, Katsuya Kato, Takayoshi Nakano, and Toshihiro Kasuga. 2021. "Structures and Dissolution Behaviors of Quaternary CaO-SrO-P2O5-TiO2 Glasses" Materials 14, no. 7: 1736. https://doi.org/10.3390/ma14071736
APA StyleLee, S., Nagata, F., Kato, K., Nakano, T., & Kasuga, T. (2021). Structures and Dissolution Behaviors of Quaternary CaO-SrO-P2O5-TiO2 Glasses. Materials, 14(7), 1736. https://doi.org/10.3390/ma14071736







