A Short Review on the Phase Structures, Oxidation Kinetics, and Mechanical Properties of Complex Ti-Al Alloys
Abstract
1. Introduction
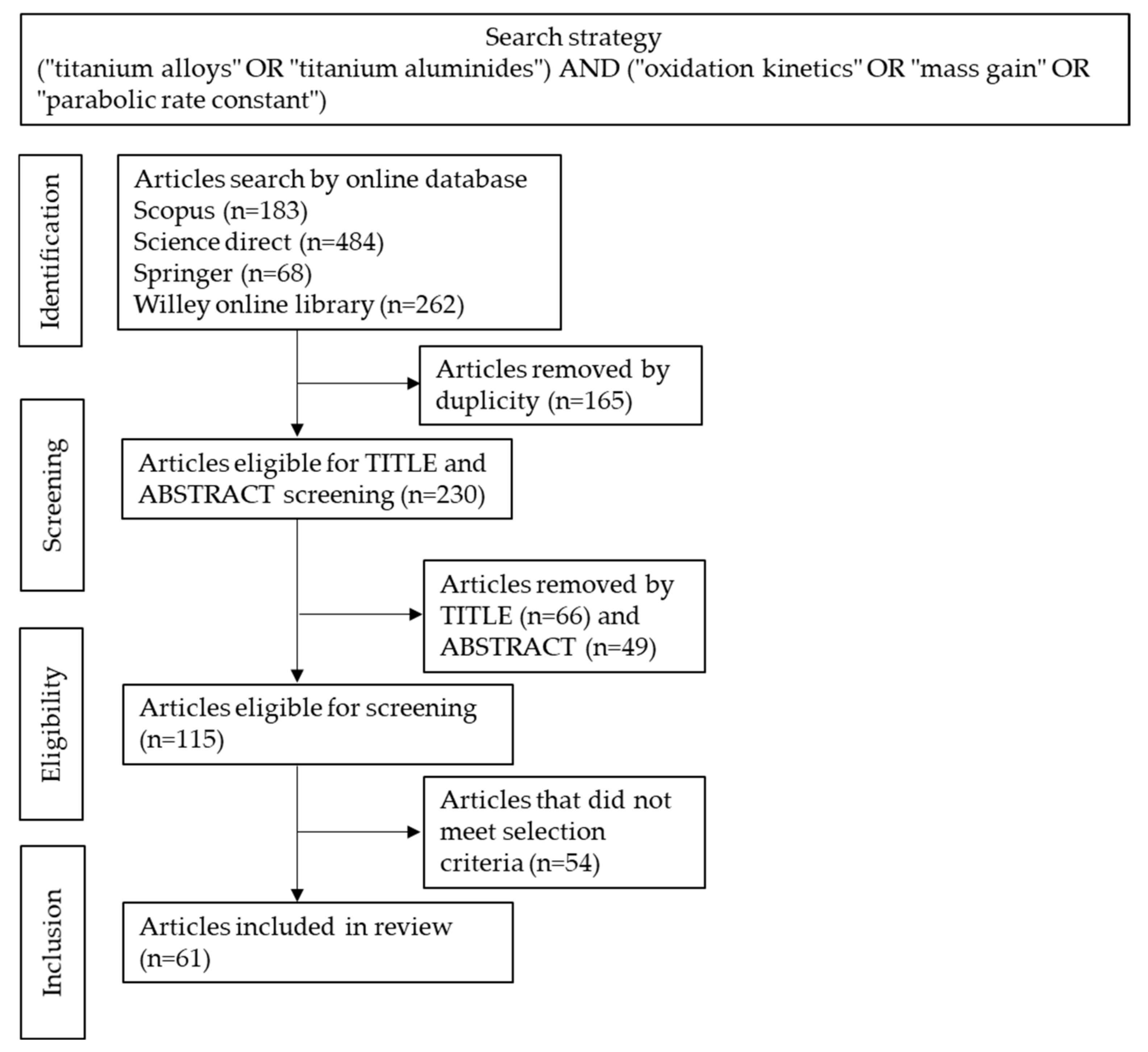
2. Review Methods
3. High-Temperature Oxidation of Metallic Materials
4. Oxidation Behaviour of Ti-Al Alloys
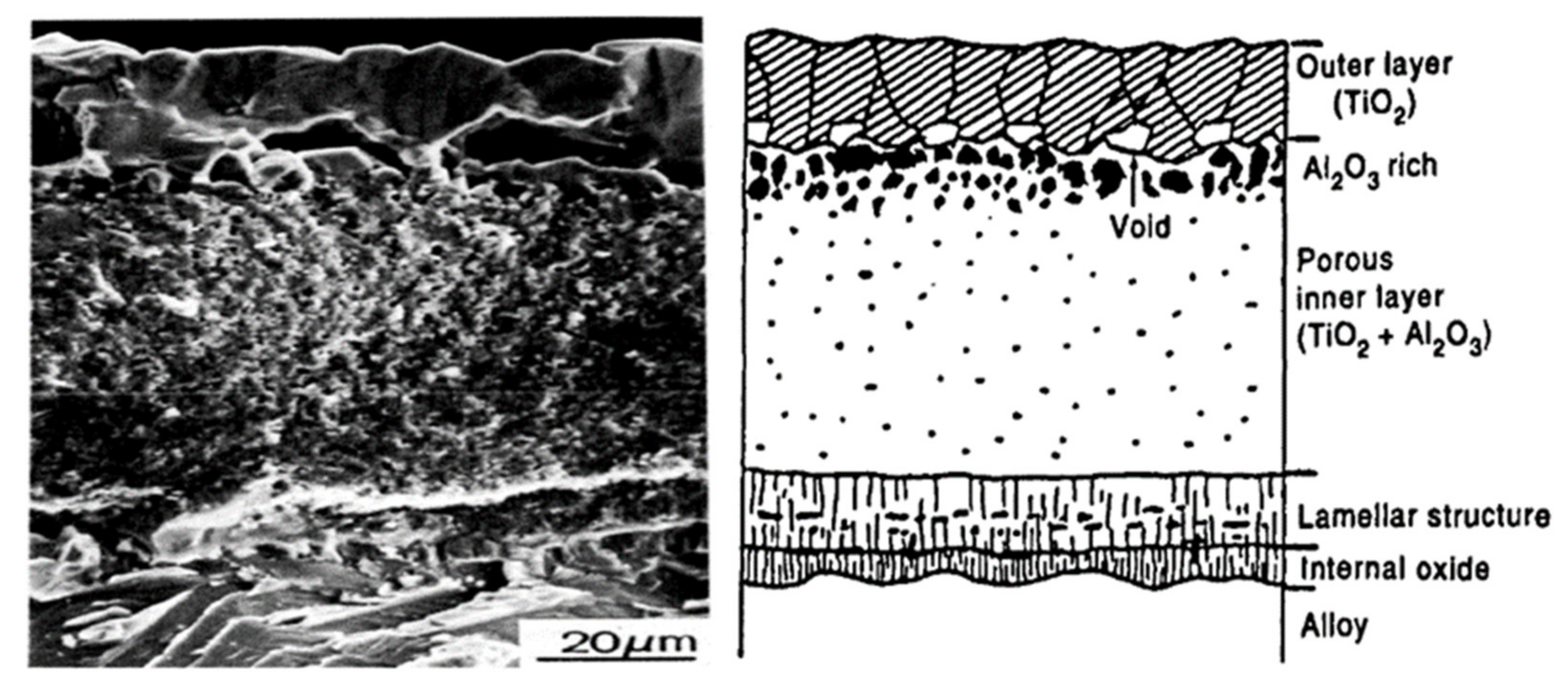
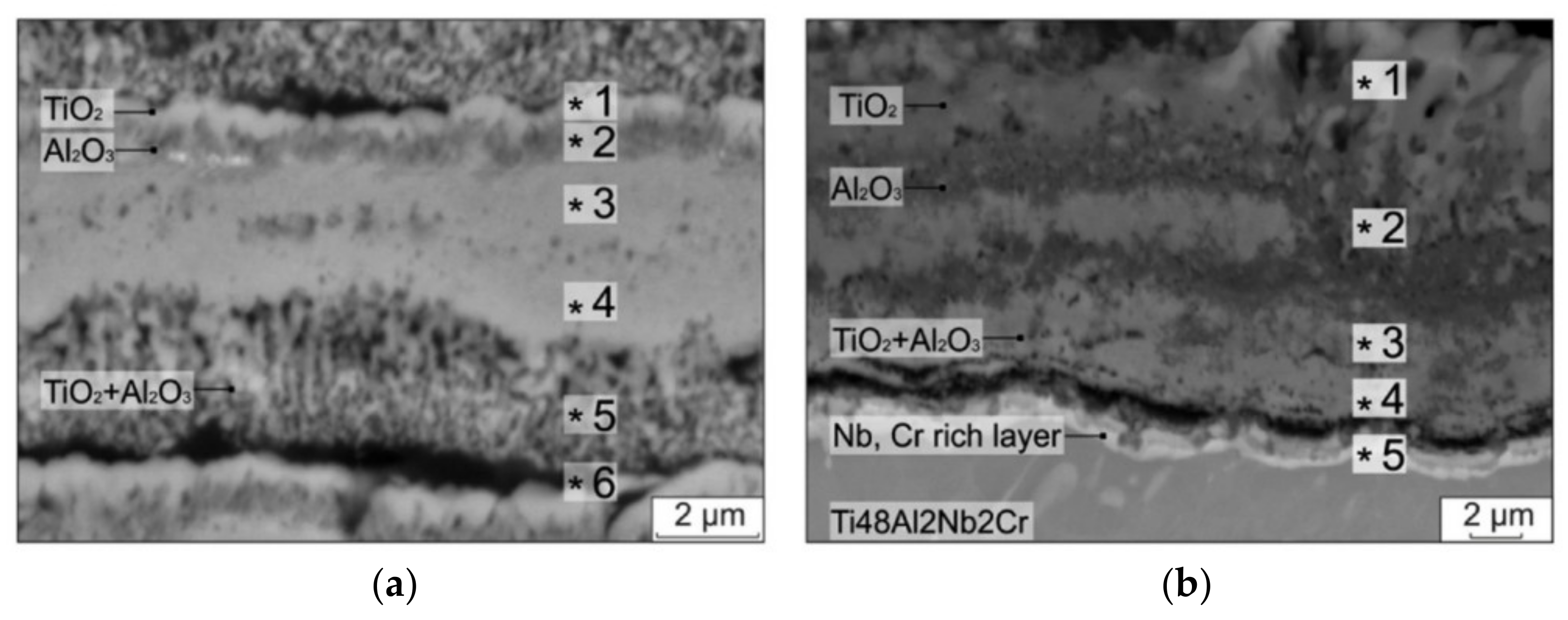
4.1. TiO2 Oxide Scale
4.2. Al2O3 Oxide Scale
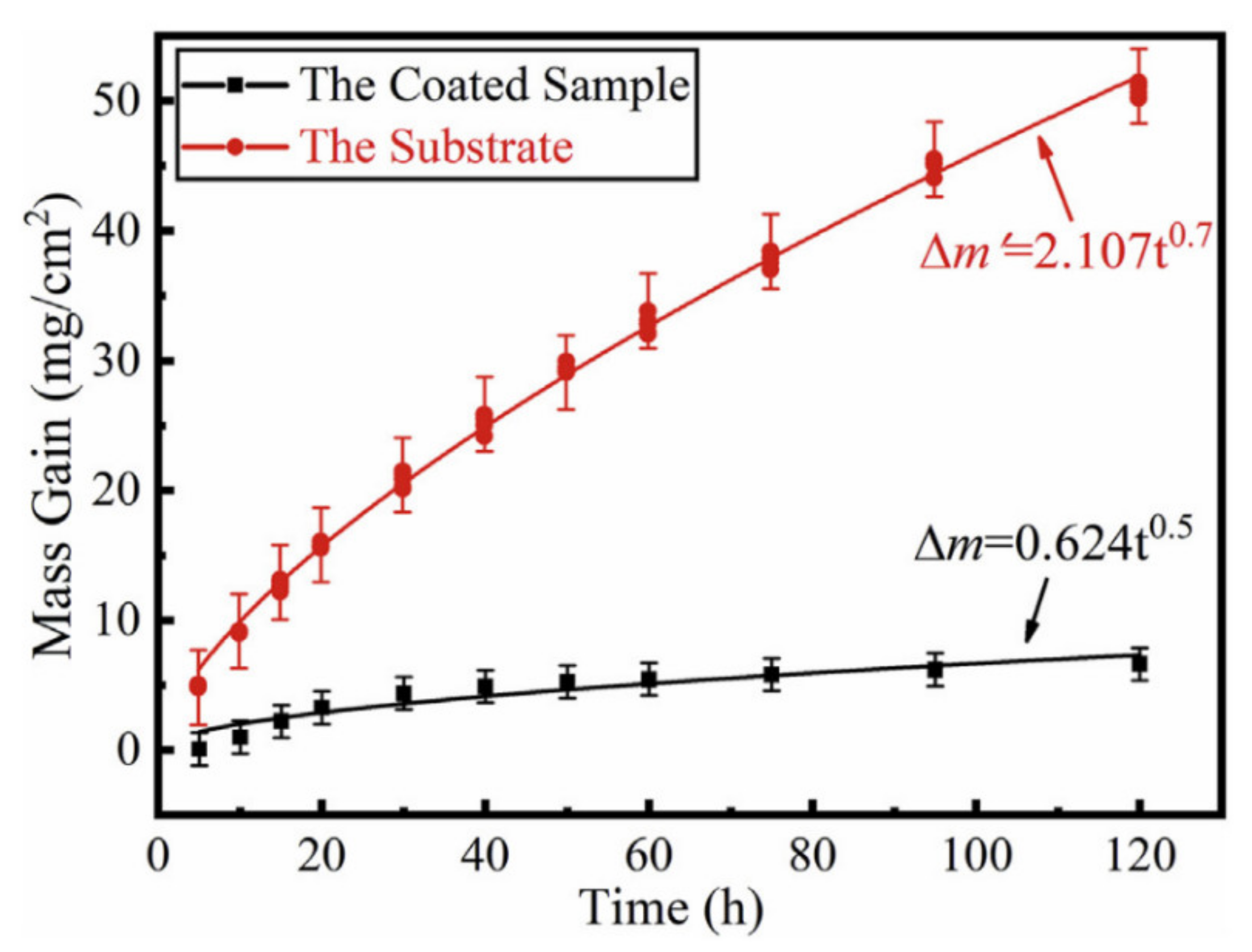
5. High-temperature Oxidation Kinetics of Ti-Al Alloys
5.1. Alloying Modification of Ti-Al Alloys
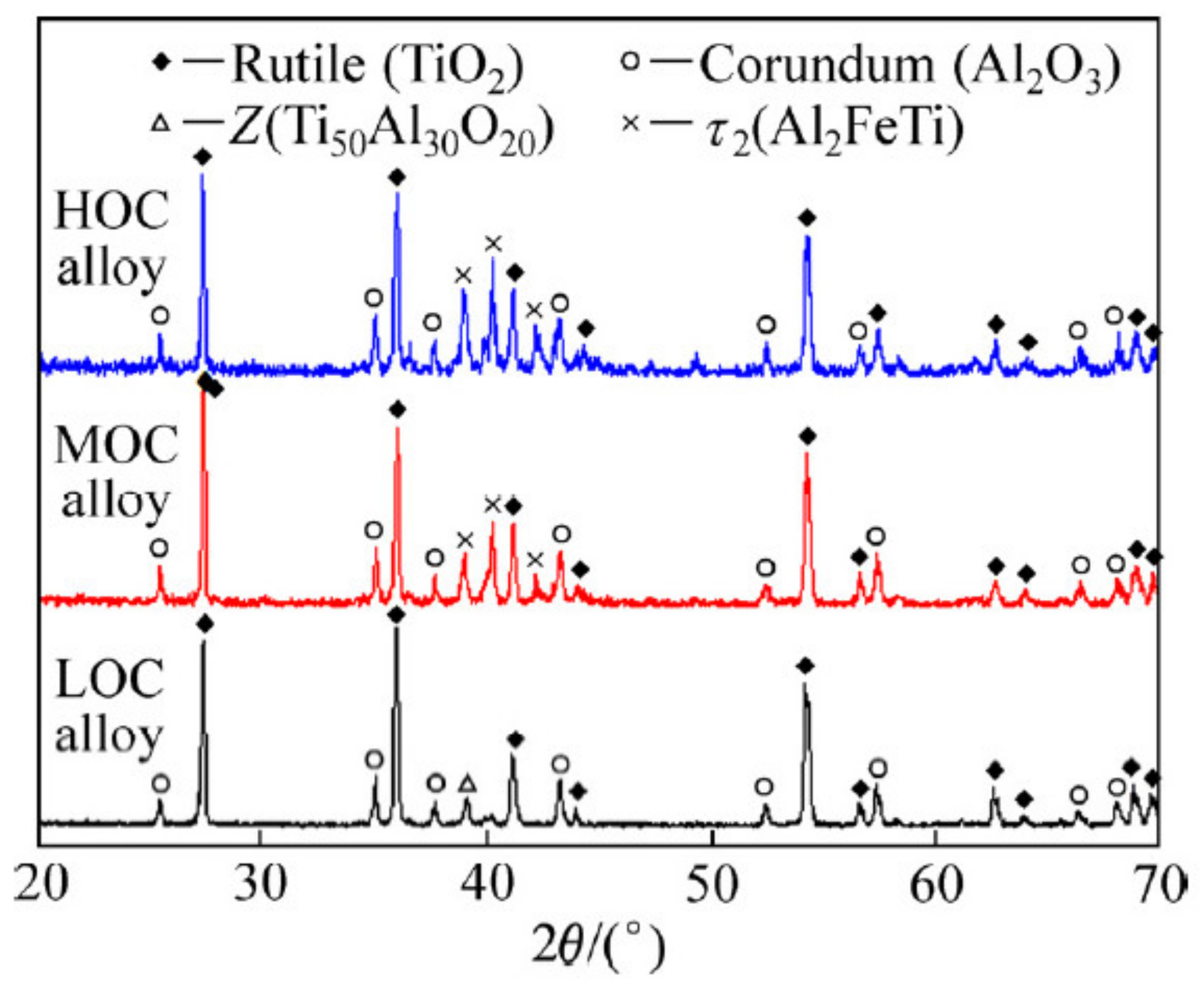
5.2. Phase Structures and Oxidation Kinetics of Ti-Al Alloys
5.2.1. Oxidation at 600–800 °C
5.2.2. Oxidation at 800–1000 °C
6. The Effect of Alloying Additions on Mechanical Properties
Mechanical Properties
7. Conclusions
- The oxidation behaviour of complex Ti-Al alloys closely follows parabolic kinetics at 600–1000 °C. The main oxidation products of these alloys are TiO2, Al2O3, TiO2 + Al2O3, and alloy enriched oxides such as Cr2O3, SiO2, Ta2O5, and AlTaO4. These alloy enriched oxides have been found to be beneficial in decreasing oxygen solubility and diffusivity, hence promoting the formation of protective Al2O3.
- The inclusion of alloying elements can improve the oxidation protection of Ti-Al alloys at high temperature by forming an oxygen-diffusion barrier upon oxidation that suppresses the interdiffusion between the oxygen and metal ions. Among all the investigated alloys, Si appears to be the most beneficial alloying addition, achieving excellent oxidation resistance at high temperatures. Si addition of about 5–13 at.% forms titanium silicide that promotes the formation of protective Al2O3 and inhibits the growth of TiO2. This strengthening silicide also improves the mechanical properties of Ti-Al alloys.
- The enhancement of hardness can be attributed to the formation of new phases, reduction in the grain size, and solid solution strengthening by element additions to Ti-Al alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motonishi, S.; Isoda, S.; Itoh, H.; Tsumori, Y.; Terada, Y. Study on machining of titanium and its alloys. Ann. CIRP 1983, 32, 65–69. [Google Scholar]
- Sharif, S.; Abd Rahim, E.; Sasahara, H. Machinability of titanium alloys in drilling. In Titanium Alloys towards Achieving Enhanced Properties for Diversified Applications; InTech: Rijeka, Croatia, 2012; pp. 118–138. [Google Scholar]
- Lipsitt, H.A.; Shechtman, D.; Schafrik, R.E. The deformation and fracture of TiAl at elevated temperatures. Metall. Trans. A 1975, 6A, 1991–1996. [Google Scholar] [CrossRef]
- Kawabata, T.; Kanai, T.; Izumi, O. Positive temperature dependence of the yield stress in TiAl L10 type superlattice intermetallic compound single crystals at 293–1273 K. Acta Metall. 1985, 33, 1355–1366. [Google Scholar] [CrossRef]
- Kim, Y.-W. Intermetallic alloys based on gamma titanium aluminide. JOM 1989, 41, 24–30. [Google Scholar] [CrossRef]
- Froes, F.H.; Suryanarayana, C.; Eliezer, D. Synthesis, properties and applications of titanium aluminides. J. Mater. Sci. 1992, 27, 5113–5140. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Zhuang, L.; Li, S. Effect of surface aluminizing on long-term high-temperature thermal stability of TC4 titanium alloy. Surf. Rev. Lett. 2016, 23, 1–7. [Google Scholar] [CrossRef]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of g-TiAl-based alloys. A review. Intermetallics 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Taniguchi, S.; Shibata, T. Influence of additional elements on the oxidation behaviour of TiAl. Intermetallics 1996, 522–523, 85–93. [Google Scholar] [CrossRef]
- Samal, S. High temperature oxidation of metals. In High Temperature Corrosion; InTech: Rijeka, Croatia, 2016; pp. 102–121. [Google Scholar]
- Haugsrud, R. On the high-temperature oxidation of nickel. Corros. Sci. 2003, 45, 211–235. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Cui, R.J.; Tang, X.X.; Gao, M.; Zhang, H.; Gong, S.K. Thermodynamic analysis of interactions between Ti-Al alloys and oxide ceramics. Trans. Nonferrous Met. Soc. China Engl. Ed. 2012, 22, 887–894. [Google Scholar] [CrossRef]
- Małecka, J. Oxidation behavior of Al2O3 coating on Ti-25Al-12.5Nb alloy. J. Mater. Eng. Perform. 2016, 25, 2951–2958. [Google Scholar] [CrossRef][Green Version]
- Luthra, K.L. Stability of protective oxide films on Ti-base alloys. Oxid. Met. 1991, 36, 475–490. [Google Scholar] [CrossRef]
- Adler, T.A.; Aylor, D.; Bray, A. Corrosion: Fundamentals, Testing, and Protection; ASM International: Novelty, OH, USA, 2003. [Google Scholar]
- Pedeferri, P. High temperature corrosion. In Corrosion Science and Engineering, Engineering Materials; Springer Nature Switzerland: Cham, Switzerland, 2018; pp. 589–610. [Google Scholar]
- Tang, Z.; Wang, F.; Wu, W. Effect of a sputtered TiAlCr coating on the oxidation resistance of TiAl intermetallic compound. Oxid. Met. 1997, 48, 511–525. [Google Scholar] [CrossRef]
- Wei, D.B.; Zhang, P.Z.; Yao, Z.J.; Liang, W.P.; Miao, Q.; Xu, Z. Oxidation of double-glow plasma chromising coating on TC4 titanium alloys. Corros. Sci. 2013, 66, 43–50. [Google Scholar] [CrossRef]
- Izumi, T.; Yoshioka, T.; Hayashi, S.; Narita, T. Oxidation behavior of sulfidation processed TiAl-2 at.%X (X=Si, Mn, Ni, Ge, Y, Zr, La, and Ta) alloys at 1173 K in air. Intermetallics 2005, 13, 694–703. [Google Scholar] [CrossRef]
- Panin, P.V.; Kochetkov, A.S.; Zavodov, A.V.; Lukina, E.A. Effect of Gd addition on phase composition, structure, and properties of beta-solidifying TiAl-based alloy with Zr and Cr content variability. Intermetallics 2020, 121, 106781. [Google Scholar] [CrossRef]
- Zhang, X.J.; Li, Q.; Zhao, S.Y.; Gao, C.X.; Wang, L.; Zhang, J. Improvement in the oxidation resistance of a γ-TiAl-based alloy by sol-gel derived Al2O3 film. Appl. Surf. Sci. 2008, 255, 1860–1864. [Google Scholar] [CrossRef]
- Gao, J.; He, Y.; Gao, W. Electro-codeposition of Al2O3-Y2O3 composite thin film coatings and their high-temperature oxidation resistance on γ-TiAl alloy. Thin Solid Films 2012, 520, 2060–2065. [Google Scholar] [CrossRef]
- Wu, L.; Wu, J.-J.; Wu, W.-Y.; Cao, F.; Jiang, M.-Y. Sol-gel-based coatings for oxidation protection of TiAl alloys. J. Mater. Sci. 2020, 55, 6330–6351. [Google Scholar] [CrossRef]
- Reddy, R.G.; Wen, X.; Divakar, M. Isothermal oxidation of TiAl alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2001, 32, 2357–2361. [Google Scholar] [CrossRef]
- Zhao, K.; OuYang, S.H.; Liu, Y.; Liu, B.; Liang, X.P.; Li, H.Z.; Wang, Y. Isothermal oxidation behavior of TiAl intermetallics with different oxygen contents. Trans. Nonferrous Met. Soc. China Engl. Ed. 2019, 29, 526–533. [Google Scholar] [CrossRef]
- Taniguchi, S.; Shibata, T.; Itoh, S. Oxidation behavior of TiAl at high temperatures in purified oxygen. Mater. Trans. JIM 1991, 32, 151–156. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, F.; Wang, A.; Yu, H.; Chen, C. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Garip, Y.; Ozdemir, O. A study of the cycle oxidation behavior of the Cr/Mn/Mo alloyed Ti–48Al–based intermetallics prepared by ECAS. J. Alloys Compd. 2020, 818, 152818. [Google Scholar] [CrossRef]
- McKee, D.W.; Huang, S.C. The oxidation behaviour of gamma-titanium aluminide alloys under thermal cycling conditions. Corros. Sci. 1992, 33, 1899–1914. [Google Scholar] [CrossRef]
- Wang, F.; Tang, Z.; Wu, W. Effect of chromium on the oxidation resistance of TiAl intermetallics. Oxid. Met. 1997, 48, 381–390. [Google Scholar] [CrossRef]
- Brady, M.P.; Brindley, W.J.; Smialek, J.L.; Locci, I.E. The oxidation and protection of gamma titanium aluminides. JOM 1996, 48, 46–50. [Google Scholar] [CrossRef]
- Wagner, C. Reaktionstypen bei der Oxydation von Legierungen. Z. Elektrochem. 1959, 63, 772–782. [Google Scholar]
- He, Y.; Li, Z.; Qi, H.; Gao, W. Standard free energy change of formation per unit volume: A new parameter for evaluating nucleation and growth of oxides, sulphides, carbides and nitrides. Mater. Res. Innov. 1997, 1, 157–160. [Google Scholar] [CrossRef]
- David, R.L. CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Becker, S.; Rahmel, A.; Schorr, M.; Schütze, M. Mechanism of isothermal oxidation of the intermetallic TiAl and of TiAl alloys. Oxid. Met. 1992, 38, 425–464. [Google Scholar] [CrossRef]
- Kim, D.J.; Seo, D.Y.; Saari, H.; Sawatzky, T.; Kim, Y.W. Isothermal oxidation behavior of powder metallurgy beta gamma TiAl-2Nb-2Mo alloy. Intermetallics 2011, 19, 1509–1516. [Google Scholar] [CrossRef]
- Hu, X.; Li, F.; Shi, D.; Xie, Y.; Li, Z.; Yin, F. A design of self-generated Ti–Al–Si gradient coatings on Ti–6Al–4V alloy based on silicon concentration gradient. J. Alloys Compd. 2020, 830, 154670. [Google Scholar] [CrossRef]
- Ostrovskaya, O.; Badini, C.; Baudana, G.; Padovano, E.; Biamino, S. Thermogravimetric investigation on oxidation kinetics of complex Ti-Al alloys. Intermetallics 2018, 93, 244–250. [Google Scholar] [CrossRef]
- Ebach-Stahl, A.; Eilers, C.; Laska, N.; Braun, R. Cyclic oxidation behaviour of the titanium alloys Ti-6242 and Ti-17 with Ti-Al-Cr-Y coatings at 600 and 700°C in air. Surf. Coat. Technol. 2013, 223, 24–31. [Google Scholar] [CrossRef]
- Mitoraj-Królikowska, M.; Godlewska, E. Silicide coatings on Ti-6Al-1Mn (at.%) alloy and their oxidation resistance. Surf. Coat. Technol. 2018, 334, 491–499. [Google Scholar] [CrossRef]
- Li, X.T.; Huang, L.J.; Jiang, S.; Gao, Y.N.; An, Q.; Wang, S.; Zhang, R.; Geng, L. Microstructure and super oxidation resistance of the network structured Ti-Al-Si coating. J. Alloys Compd. 2019, 807, 151679. [Google Scholar] [CrossRef]
- Maliutina, I.N.; Si-Mohand, H.; Sijobert, J.; Bertrand, P.; Lazurenko D, V.; Bataev, I.A. Structure and oxidation behavior of γ-TiAl coating produced by laser cladding on titanium alloy. Surf. Coat. Technol. 2017, 319, 136–144. [Google Scholar] [CrossRef]
- Song, J.; Zhang, P.Z.; Wei, D.B.; Wei, X.F.; Wang, Y. Isothermal oxidation behavior and microstructure of plasma surface Ta coating on γ-TiAl. Mater. Charact. 2014, 98, 54–59. [Google Scholar] [CrossRef]
- Zambrano Carrullo, J.C.; Pereira Falcón, J.C.; Amigó Borrás, V. Influence of process parameters and initial microstructure on the oxidation resistance of Ti48Al2Cr2Nb coating obtained by laser metal deposition. Surf. Coat. Technol. 2019, 358, 114–124. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.; Shen, Y. Oxidation behavior of a high Si content Al–Si composite coating fabricated on Ti–6Al–4V substrate by mechanical alloying method. J. Alloys Compd. 2017, 701, 27–36. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Zhang, H.; Yu, H.; Chen, C.; Li, Y. Microstructure and high-temperature oxidation resistance of Ti-Al-Nb coatings on a Ti-6Al-4V alloy fabricated by laser surface alloying. Surf. Coat. Technol. 2018, 344, 479–488. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, N.; Wang, A.; Zhang, H.; Chen, C. Microstructure and high temperature oxidation behavior of Ti-Al-Nb-Si coatings on Ti-6Al-4V alloy. J. Alloys Compd. 2018, 765, 46–57. [Google Scholar] [CrossRef]
- Liu, X.; You, K.; Wang, Z.; Zhang, M.; He, Z. Effect of Mo-alloyed layer on oxidation behavior of TiAl-based alloy. Vacuum 2013, 89, 209–214. [Google Scholar] [CrossRef]
- Laska, N.; Braun, R.; Knittel, S. Oxidation behavior of protective Ti-Al-Cr based coatings applied on the γ-TiAl alloys Ti-48-2-2 and TNM-B1. Surf. Coat. Technol. 2018, 349, 347–356. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Laptev, I.S.; Golkovsky, M.G.; Stark, A.; Paul, J.; Bataev, I.; Ruktuev, A.A.; Song, L.; Gollwitzer, C.; Pyczak, F. Influence of the Ti/Al/Nb ratio on the structure and properties on intermetallic layers obtained on titanium by non-vacuum electron beam cladding. Mater. Charact. 2020, 163, 110246. [Google Scholar] [CrossRef]
- Fröhlich, M.; Ebach-Stahi, A.; Braun, R.; Leyens, C. Oxidation protective coatings for c-TiAl–recent trends. Materwiss. Werksttech. 2007, 38, 667–673. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Y.; Gong, S.; Xu, H. Effect of Ti-Al-Cr coatings on the high temperature oxidation behavior of TiAl alloys. Mater. Sci. Eng. A 2001, 307, 182–187. [Google Scholar] [CrossRef]
- Oukati Sadeq, F.; Sharifitabar, M.; Shafiee Afarani, M. Synthesis of Ti–Si–Al coatings on the surface of Ti–6Al–4V alloy via hot dip siliconizing route. Surf. Coat. Technol. 2018, 337, 349–356. [Google Scholar] [CrossRef]
- Tkachenko, S.; Datskevich, O.; Dvořák, K.; Spotz, Z.; Kulak, L.; Čelko, L. Isothermal oxidation behavior of experimental Ti−Al−Si alloys at 700 °C in air. J. Alloys Compd. 2017, 694, 1098–1108. [Google Scholar] [CrossRef]
- Lee, D.B.; Park, K.B.; Nakamura, M. Effects of Cr and Nb on the high temperature oxidation of TiAl. Met. Mater. Int. 2002, 8, 319–326. [Google Scholar] [CrossRef]
- Neelam, N.S.; Banumathy, S.; Bhattacharjee, A. The effect of Cr and Mo addition on the oxidation behaviour of Ti-46.5Al-3.5Nb-2Cr-0.3B. Mater. Today Proc. 2019, 15, 30–35. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, X.; Hayat, M.D.; Yang, F.; Liu, C.C.; Li, Y.; Li, X.Y.; Xu, W.; Qu, X.H.; Cao, P. Effect of Sn addition on the high-temperature oxidation behavior of high Nb-containing TiAl alloys. Corros. Sci. 2020, 166, 108449. [Google Scholar] [CrossRef]
- Shen, Y.; Ding, X.; Wang, F.; Tan, Y.; Yang, J.M. High temperature oxidation behavior of Ti-Al-Nb ternary alloys. J. Mater. Sci. 2004, 39, 6583–6589. [Google Scholar] [CrossRef]
- Novák, P.; Kříž, J.; Průša, F.; Kubásek, J.; Marek, I.; Michalcová, A.; Voděrová, M.; Vojtěch, D. Structure and properties of Ti-Al-Si-X alloys produced by SHS method. Intermetallics 2013, 39, 11–19. [Google Scholar] [CrossRef]
- Shida, Y.; Anada, H. Oxidation behavior of binary Ti-Al alloys in high temperature air environment. Mater. Trans. JIM 1993, 34, 236–242. [Google Scholar] [CrossRef]
- Smialek, J.L. Oxidation behaviour of TiAl3 coatings and alloys. Corros. Sci. 1993, 35, 1199–1208. [Google Scholar] [CrossRef]
- Munro, T.C.; Gleeson, B. The deposition and oxidation resistance of aluminide coatings on γ-TiAl. Mater. Sci. Forum 1997, 251–254, 753–760. [Google Scholar] [CrossRef]
- Knaislová, A.; Novák, P.; Cabibbo, M.; Průša, F.; Paoletti, C.; Jaworska, L.; Vojtěch, D. Combination of reaction synthesis and spark plasma sintering in production of Ti-Al-Si alloys. J. Alloys Compd. 2018, 752, 317–326. [Google Scholar] [CrossRef]
- Imayev, R.M.; Imayev, V.M.; Oehring, M.; Appel, F. Alloy design concepts for refined gamma titanium aluminide based alloys. Intermetallics 2007, 15, 451–460. [Google Scholar] [CrossRef]
- Anderson, P.M.; Li, C. Hall-Petch relations for multilayered materials. Nanostruct. Mater. 1995, 5, 349–362. [Google Scholar] [CrossRef]
- Guan, Z.Q.; Pfullmann, T.; Oehring, M.; Bormann, R. Phase formation during ball milling and subsequent thermal decomposition of Ti-Al-Si powder blends. J. Alloys Compd. 1997, 252, 245–251. [Google Scholar] [CrossRef]
- Gao, T.; Li, P.; Li, Y.; Liu, X. Influence of Si and Ti contents on the microstructure, microhardness and performance of TiAlSi intermetallics in Al-Si-Ti alloys. J. Alloys Compd. 2011, 509, 8013–8017. [Google Scholar] [CrossRef]
- Cabibbo, M.; Knaislová, A.; Novák, P.; Průša, F.; Paoletti, C. Role of Si on lamellar formation and mechanical response of two SPS Ti–15Al–15Si and Ti–10Al–20Si intermetallic alloys. Intermetallics 2021, 131, 107099. [Google Scholar] [CrossRef]
- Shao, Z.J.; Li, Y.; Zhou, B.; He, X.C.; Zhang, S.Z.; Xu, L. Effect of phase transition caused by different treatment process on mechanical properties of powder metallurgy Ti-22Al-24Nb-0.5Mo (at.%) alloys. Mater. Charact. 2020, 159, 110022. [Google Scholar] [CrossRef]
- Ismaeel, A.; Wang, C.S. Effect of Nb additions on microstructure and properties of γ-TiAl based alloys fabricated by selective laser melting. Trans. Nonferrous Met. Soc. China Engl. Ed. 2019, 29, 1007–1016. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, H.; Wang, Q.; Chen, R.; Guo, J.; Ding, H.; Su, Y. Improvement of microstructure and mechanical properties of TiAl−Nb alloy by adding Fe element. Trans. Nonferrous Met. Soc. China Engl. Ed. 2020, 30, 1315–1324. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, X.; Liu, C.; Hui, T.; Zhang, C.; Qu, X. Sintering densification, microstructure and mechanical properties of Sn-doped high Nb-containing TiAl alloys fabricated by pressureless sintering. Intermetallics 2020, 125, 106891. [Google Scholar] [CrossRef]
- Mogale, N.; Matizamhuka, W.; Machaka, R.; Shongwe, M.; Yamabe-Mitarai, Y. The effect of Cr additions and oxidation on densification, microstructure, phase constituents and mechanical properties of TiAlCr alloys produced by SPS. Mater. Today Proc. 2021, 38, 621–627. [Google Scholar] [CrossRef]
- Knaislová, A.; Novák, P.; Kopecek, J.; Pruša, F. Properties comparison of Ti-Al-Si alloys produced by various metallurgy methods. Materials 2019, 12, 3084. [Google Scholar] [CrossRef]
- He, Z.Y.; Wang, Z.X.; Liu, X.P.; Han, P.D. Preparation of TiAl-Cr surface alloy by plasma-surface alloying technique. Vacuum 2013, 89, 280–284. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B.; Liu, Y.; Liu, Y. Processing map and hot deformation behavior of Ta-particle reinforced TiAl composite. Trans. Nonferr. Met. Soc. China Engl. Ed. 2020, 30, 657–667. [Google Scholar] [CrossRef]


| Oxide | Formula | Pilling-Bedworth Ratio (RPB) | Reference |
|---|---|---|---|
| Potassium oxide | K2O | 0.45 | [13] |
| Calcium oxide | CaO | 0.64 | [17] |
| Barium oxide | BaO | 0.67 | [17] |
| Magnesium oxide | MgO | 0.81 | [17] |
| Sodium oxide | Na2O | 0.97 | [13] |
| Aluminium oxide | Al2O3 | 1.28 | [17] |
| Lead (II) oxide | PbO | 1.28 | [17] |
| Zirconium (IV) oxide | ZrO2 | 1.56 | [17] |
| Zinc oxide | ZnO | 1.58 | [17] |
| Nickel (II) oxide | NiO | 1.65 | [17] |
| Iron (II) oxide | FeO | 1.70 | [18] |
| Copper (II) oxide | CuO | 1.70 | [18] |
| Titanium (IV) oxide | TiO2 | 1.78 | [13] |
| Manganese (II) oxide | MnO | 1.80 | [18] |
| Chromium (III) oxide | Cr2O3 | 2.07 | [17] |
| Iron (III) oxide | Fe2O3 | 2.14 | [17] |
| Silicon dioxide | SiO2 | 2.15 | [17] |
| Tantalum (V) oxide | Ta2O5 | 2.47 | [17] |
| Niobium (V) oxide | Nb2O5 | 2.69 | [17] |
| Vanadium (V) oxide | V2O5 | 3.25 | [17] |
| Molybdenum (VI) oxide | MoO3 | 3.30 | [17] |
| Tungsten (VI) oxide | WO3 | 3.30 | [17] |
| Exposure | Nominal Composition of Coating (wt.%) | Nominal Composition of the Substrate (wt.%) | Mass Gain of Coating (mg/cm2) | Mass Gain of the Substrate (mg/cm2) | Parabolic Rate Constant of Coating (g2∙cm−4∙s−1) | Parabolic Rate Constant of the Substrate (g2∙cm−4∙s−1) | Deposition Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| 600 °C; C | Ti-49.4Al-19.5Cr-0.6Y * | Ti-5Al-2Sn-2Zr-4Mo-4Cr | <0.10 | <0.30 | 1.3 × 10−13 | Magnetron sputtering | [41] | |
| Ti-49.4Al-19.5Cr-0.6Y * | Ti-6Al-2Sn-4Zr-2Mo | <0.15 | <0.25 | 6.2 × 10−14 | ||||
| 700 °C; C | Ti-49.4Al-19.5Cr-0.6Y * | Ti-5Al-2Sn-2Zr-4Mo-4Cr | <0.25 | <2.25 | 4.9 × 10−12 | |||
| Ti-49.4Al-19.5Cr-0.6Y * | Ti-6Al-2Sn-4Zr-2Mo | <0.25 | <1 | 9.1 × 10−13 | ||||
| 700 °C; C | TiSi2 (Silicide) | Ti-6Al-1Mn * | <0.1 | <5 | 2.3 × 10−14 | 1.6 × 10−11 | Pack cementation | [42] |
| 700 °C; C | Ti-61Al-14Si * | TiB whiskers reinforced Ti-6Al-4V (TiBw/Ti-6Al-4V) | <0.36 | <4.01 | 3.6 × 10−12 | Hot-dip siliconising | [43] | |
| 800 °C; C | Ti-61Al-14Si * | TiBw/Ti-6Al-4V | <1.33 | <22.18 | 4.9 × 10−11 | |||
| 900 °C; C | Ti-61Al-14Si * | TiBw/Ti-6Al-4V | <3.98 | <54.21 | 1.1 × 10−10 | |||
| 700 °C; I | Ti-48Al-2Cr-2Nb | Ti-6Al-2Sn-4Zr-2Mo | <0.3 | <0.3 | Laser cladding | [44] | ||
| 800 °C; I | Ti-48Al-2Cr-2Nb | Ti-6Al-2Sn-4Zr-2Mo | <0.8 | <4.8 | ||||
| 900 °C; I | Ti-48Al-2Cr-2Nb | Ti-6Al-2Sn-4Zr-2Mo | <3.5 | <31.4 | ||||
| 750 °C; I | Ta | Ti-46.5Al-2.5Cr-1V | <2 | <4 | Double glow plasma surface alloying treatment | [45] | ||
| 850 °C; I | Ta | Ti-46.5Al-2.5Cr-1V | <12 | <28 | ||||
| 800 °C; I | Ti-48Al-2Cr-2Nb | Ti-6Al-4V | <2.5 | <20 | Laser cladding | [46] | ||
| 800 °C; I | Ti-41.6Al-4.8Si | Ti-6Al-4V | <0.25 | <5 | Laser surface alloying | [29] | ||
| Ti-38.1Al-9.5Si | <0.15 | |||||||
| Ti-27.5Al-10.3Si | <0.27 | |||||||
| 800 °C; I | Ti-59.5Al-13.9Si | Ti-6Al-4V | <5 | <45 | Self-generated gradient hot-dipping infiltration | [39] | ||
| 850 °C; C | Al-Si | Ti-6Al-4V | <2 | <8 | Mechanical alloying | [47] | ||
| 800 °C; I | Ti-54.67Al-4.43Nb * | Ti-6Al-4V | <6 | <5 | Laser surface alloying | [48] | ||
| Ti-50.88Al-5.61Nb * | <5.5 | |||||||
| Ti-48.11Al-7.08Nb * | <5 | |||||||
| Ti-44.65Al-9.31Nb * | <4 | |||||||
| Ti-40.15Al-12.34Nb * | <8 | |||||||
| 800 °C; I | Ti-Al-5.46Nb-5.30Si | Ti-6Al-4V | <2.5 | <5 | 2.8 × 10−11 | Laser surface alloying | [49] | |
| Ti-Al-10.84Nb-5.79Si | <2 | 1.5 × 10−11 | ||||||
| Ti-Al-16.47Nb-5.93Si | <2 | 1.4 × 10−11 | ||||||
| Ti-Al-5.46Nb-11.01Si | <2 | 1.1 × 10−11 | ||||||
| Ti-Al-9.59Nb-10.52Si | <1.5 | 5.7 × 10−12 | ||||||
| 850 °C; C | Mo | Ti-46.5Al-2.5V-1Cr * | <3 | <14 | Plasma surface metallurgy | [50] | ||
| 850 °C; C | Ti-46Al-36Cr-4Zr * | Ti-48Al-2Cr-2Nb * | <0.125 | <1.75 | Magnetron sputtering | [51] | ||
| Ti-58Al-14Cr-1Y * | Ti-48Al-2Cr-2Nb * | <0.25 | <1.75 | |||||
| Ti-46Al-39Cr-4Zr * | Ti-43.5Al-4Nb-1Mo-0.1B * | <0.125 | <1.50 | |||||
| Ti-59Al-14Cr-2Y * | Ti-43.5Al-4Nb-1Mo-0.1B * | <0.25 | <1.50 | |||||
| 900 °C; C | Ti-46Al-36Cr-4Zr * | Ti-48Al-2Cr-2Nb * | <0.25 | <1.25 | ||||
| Ti-58Al-14Cr-1Y * | Ti-48Al-2Cr-2Nb * | <0.5 | <1.25 | |||||
| Ti-46Al-39Cr-4Zr * | Ti-43.5Al-4Nb-1Mo-0.1B * | <0.5 | <1.50 | |||||
| Ti-59Al-14Cr-2Y * | Ti-43.5Al-4Nb-1Mo-0.1B * | <0.5 | <1.50 | |||||
| 950 °C; C | Ti-46Al-36Cr-4Zr * | Ti-48Al-2Cr-2Nb * | <0.25 | - | ||||
| Ti-58Al-14Cr-1Y * | Ti-48Al-2Cr-2Nb * | <0.625 | - | |||||
| Ti-46Al-39Cr-4Zr * | Ti-43.5Al-4Nb-1Mo-0.1B * | <0.125 | - | |||||
| Ti-59Al-14Cr-2Y * | Ti-43.5Al-4Nb-1Mo-0.1B * | <0.25 | - | |||||
| 900 °C; C | Ti-33Al-5Nb | Ti-0.086Fe-0.017Cr-0.016Ni-0.012V-0.011C | <0.9 | <6 | Non-vacuum electron beam cladding | [52] | ||
| Ti-28Al-17Nb | <0.5 | |||||||
| Ti-24Al-27Nb | <1 | |||||||
| Ti-20Al-34Nb | <3 | |||||||
| 900 °C; C | Ti-51Al-12Cr * | Ti-45Al-8Nb * | <1.1 | <1.5 | Magnetron sputtering | [53] | ||
| 950 °C; C | Ti-51Al-12Cr * | Ti-45Al-8Nb * | <1.2 | - | ||||
| 950 °C; I | Ti-50Al-10Cr * | Ti-50Al * | <3.8 | - | 3.2 × 10−11 | Magnetron sputtering | [54] | |
| Ti-50Al-15Cr * | <0.9 | 1.1 × 10−12 | ||||||
| 1000 °C; I | Ti-50Al-10Cr * | <1.5 | 3.6 × 10−12 | |||||
| Ti-50Al-15Cr * | <0.6 | 8.3 × 10−13 | ||||||
| 950 °C; C | Ti-50Al-10Cr * | <1.2 | ||||||
| Ti-50Al-15Cr * | <0.4 | |||||||
| 1000 °C; C | Ti-50Al-10Cr * | <2 | ||||||
| Ti-50Al-15Cr * | <0.5 | |||||||
| 1000 °C; I | Ti-Al-Si | Ti-6Al-4V | <0.5 | <3.5 | Hot-dip siliconising | [55] |
| Exposure | Nominal Composition (wt.%) | Mass Gain (mg/cm2) | Parabolic Rate Constant (g2∙cm−4∙s−1) | Preparation Method | Reference |
|---|---|---|---|---|---|
| 700 °C; I | Ti-6Al-2Sn-4Zr-2Mo-0.1Si | <1 | 2.14 × 10−12 | Argon arc melting | [56] |
| Ti-6Al-1.4Si | <0.50 | 8.29 × 10−13 | |||
| Ti-6Al-1.4Si-3Zr | <0.40 | 4.53 × 10−13 | |||
| Ti-6Al-1.2Si-2Zr-2Sn | <0.40 | 5.53 × 10−13 | |||
| Ti-6Al-1.2Si-2Zr-4Sn | <0.45 | 5.84 × 10−13 | |||
| 800 °C; I | Ti-51Al | <0.5 | Vacuum arc melting and induction skull melting | [57] | |
| Ti-47Al-4Cr | <0.8 | ||||
| Ti-48Al-2Cr-2Nb | <0.7 | ||||
| 900 °C; I | Ti-51Al | <1.7 | |||
| Ti-47Al-4Cr | <9.5 | ||||
| Ti-48Al-2Cr-2Nb | <2 | ||||
| 1000 °C; I | Ti-51Al | <10 | |||
| Ti-47Al-4Cr | <27.5 | ||||
| Ti-48Al-2Cr-2Nb | <2.5 | ||||
| 850 °C; I | Ti-46.5Al-3.5Nb-2Cr-0.3B | <1.75 | Vacuum arc melting | [58] | |
| Ti-46.5Al-3.5Nb-1Cr-1Mo-0.3B | <1.5 | ||||
| Ti-46.5Al-3.5Nb-2Mo-0.3B | <1.25 | ||||
| 1000 °C; I | Ti-45Al-8.5Nb-Sn * | <4.0 | Simple press and sinter route | [59] | |
| Ti-45Al-8.5Nb-3Sn * | <3.2 | ||||
| 1000 °C; I | Ti-47.5Al-5Nb * | <5 | Arc-melting | [60] | |
| Ti-42.8Al-14.2Nb * | <2 | ||||
| Ti-40Al-20Nb * | <4 | ||||
| Ti-30Al-40Nb * | <8 | ||||
| 1000 °C; I | Ti-15Al-15Si | <20 | Self-propagating high- temperature synthesis | [61] | |
| Ti-15Al-15Si-15Co | <18 | ||||
| Ti-15Al-15Si-15Cr | <5 | ||||
| Ti-15Al-15Si-15Cu | <20 | ||||
| Ti-15Al-15Si-15Fe | <18 | ||||
| Ti-15Al-15Si-15Mo | <1 | ||||
| Ti-15Al-15Si-15Ni | <18 |
| Nominal Composition (wt.%) | Grain Size (nm) | Microhardness (GPa) | Young’s Modulus (GPa) | Compressive Stress (MPa) | Reference |
|---|---|---|---|---|---|
| Annealed at 800 °C | [68] | ||||
| Ti-28.2Al-18.5Si | 25 | ||||
| Ti-40Al-9.5Si | 40 | ||||
| Ti-44.7Al-5.6Si | 55 | ||||
| Ti-23.5Al-6.5Si | 56 | ||||
| Ti-38.4Al-5Si | 50 | ||||
| Ti-45.6Al-1.2Si | 35 | ||||
| Ti-28Al-7Si | 95 | ||||
| Ti-28Al-10Si | 50 | ||||
| Ti-20Al-13.5Si | 53 | ||||
| Ti-20Al-15Si | 40 | ||||
| Ti-52Al-2.5Si | 56 | ||||
| Annealed at 900 °C | |||||
| Ti-28.2Al-18.5Si | 72 | ||||
| Ti-40Al-9.5Si | 96 | ||||
| Ti-44.7Al-5.6Si | 120 | ||||
| Ti-23.5Al-6.5Si | 168 | ||||
| Ti-38.4Al-5Si | 96 | ||||
| Ti-45.6Al-1.2Si | 64 | ||||
| Ti-28Al-7Si | 160 | ||||
| Ti-28Al-10Si | 104 | ||||
| Ti-20Al-13.5Si | 96 | ||||
| Ti-20Al-15Si | 94 | ||||
| Ti-52Al-2.5Si | 144 | ||||
| Annealed at 1100 °C | |||||
| Ti-28.2Al-18.5Si | 160 | ||||
| Ti-40Al-9.5Si | 220 | ||||
| Ti-44.7Al-5.6Si | 170 | ||||
| Ti-23.5Al-6.5Si | 340 | ||||
| Ti-38.4Al-5Si | 190 | ||||
| Ti-45.6Al-1.2Si | 220 | ||||
| Ti-28Al-7Si | 180 | ||||
| Ti-28Al-10Si | 240 | ||||
| Ti-20Al-13.5Si | 200 | ||||
| Ti-20Al-15Si | 160 | ||||
| Ti-52Al-2.5Si | 280 | ||||
| Al-3Ti-2Ti | 0.5884 | [69] | |||
| Al-6Si-2Ti | 0.6375 | ||||
| Al-10Si-2Ti | 0.6865 | ||||
| Al-12Si-2Ti | 0.9317 | ||||
| Al-14Si-2Ti | 1.324 | ||||
| Al-18Si-2Ti | 1.618 | ||||
| Al-30Si-2Ti | 2.059 | ||||
| Al-60Si-2Ti | 2.991 | ||||
| Ti-10Al-20Si | dTiAl = 950 | 1.02 | 57 | 340 | [70] |
| dTi5Si3 = 160 | |||||
| Ti-15Al-15Si | dTiAl = 320 | 1.0 | 33 | 330 | |
| dTi5Si3 = 140 | |||||
| Ti-22Al-24Nb-0.5Mo * | 50 × 103 | 2.707 | [71] | ||
| After rolling | 20–50 × 103 | 3.236 | |||
| Heat treated 980 °C; aged 830 °C | 10–40 × 103 | 3.158 | |||
| Heat treated 980 °C; aged 800 °C | 10–40 × 103 | 3.354 | |||
| Heat treated 960 °C; aged 830 °C | 10–40 × 103 | 3.138 | |||
| Heat treated 960 °C; aged 800 °C | 10–40 × 103 | 3.315 | |||
| Ti-48Al-2Mn-3Nb * | 19.37 | 1295 | [72] | ||
| Ti-48Al-2Mn-4Nb * | 19.53 | 1320 | |||
| Ti-48Al-2Mn-5Nb * | 19.09 | 1340 | |||
| Ti-48Al-2Mn-6Nb * | 19.24 | 1360 | |||
| Ti-48Al-2Mn-7Nb * | 19.58 | 1390 | |||
| Ti-46Al-5Nb-0.1B * | 1750 | [73] | |||
| Ti-46Al-5Nb-0.1B-0.3Fe * | 1869.5 | ||||
| Ti-46Al-5Nb-0.1B-0.5Fe * | 1830 | ||||
| Ti-46Al-5Nb-0.1B-0.7Fe * | 1710 | ||||
| Ti-46Al-5Nb-0.1B-0.9Fe * | 1520 | ||||
| Ti-46Al-5Nb-0.1B-1.1Fe * | 1450 | ||||
| TiAl * | 2870 | [74] | |||
| TiAl-1Sn * | 3029 | ||||
| TiAl-2Sn * | 2960 | ||||
| TiAl-3Sn * | 2634 | ||||
| TiAl-5Sn * | 1501 | ||||
| Ti-47.5Al-1Cr * | 700 | [75] | |||
| Ti-47.5Al-1.5Cr * | 781 | ||||
| Ti-47.5Al-3Cr * | 615 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.P.; Liew, W.Y.H.; Melvin, G.J.H.; Jiang, Z.-T. A Short Review on the Phase Structures, Oxidation Kinetics, and Mechanical Properties of Complex Ti-Al Alloys. Materials 2021, 14, 1677. https://doi.org/10.3390/ma14071677
Lim HP, Liew WYH, Melvin GJH, Jiang Z-T. A Short Review on the Phase Structures, Oxidation Kinetics, and Mechanical Properties of Complex Ti-Al Alloys. Materials. 2021; 14(7):1677. https://doi.org/10.3390/ma14071677
Chicago/Turabian StyleLim, Hooi Peng, Willey Yun Hsien Liew, Gan Jet Hong Melvin, and Zhong-Tao Jiang. 2021. "A Short Review on the Phase Structures, Oxidation Kinetics, and Mechanical Properties of Complex Ti-Al Alloys" Materials 14, no. 7: 1677. https://doi.org/10.3390/ma14071677
APA StyleLim, H. P., Liew, W. Y. H., Melvin, G. J. H., & Jiang, Z.-T. (2021). A Short Review on the Phase Structures, Oxidation Kinetics, and Mechanical Properties of Complex Ti-Al Alloys. Materials, 14(7), 1677. https://doi.org/10.3390/ma14071677






