Investigation of the Possibilities of Wool Fiber Surface Modification with Copper Selenide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment Methods
2.3. Investigative Methods
3. Results and Discussion
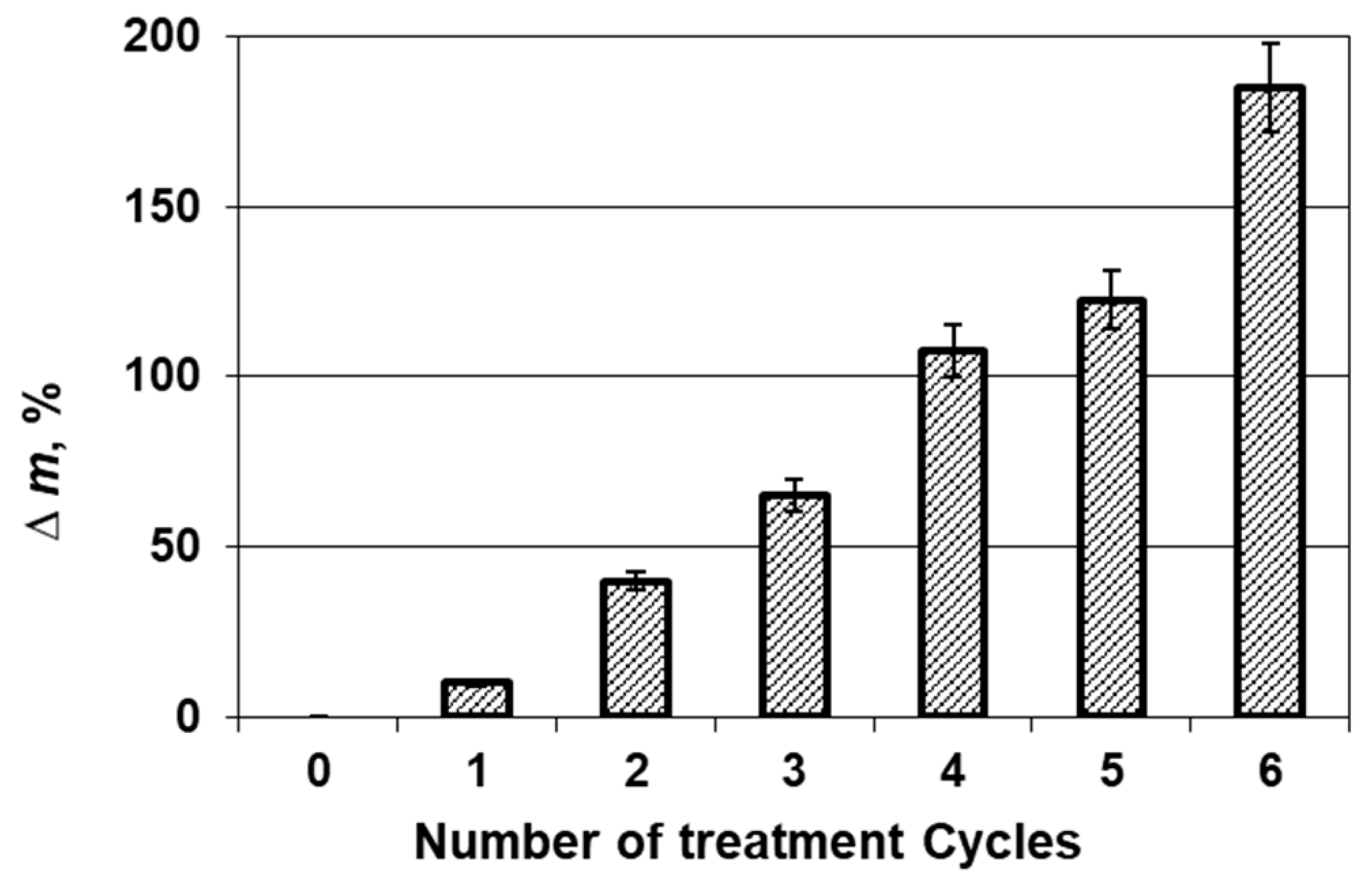
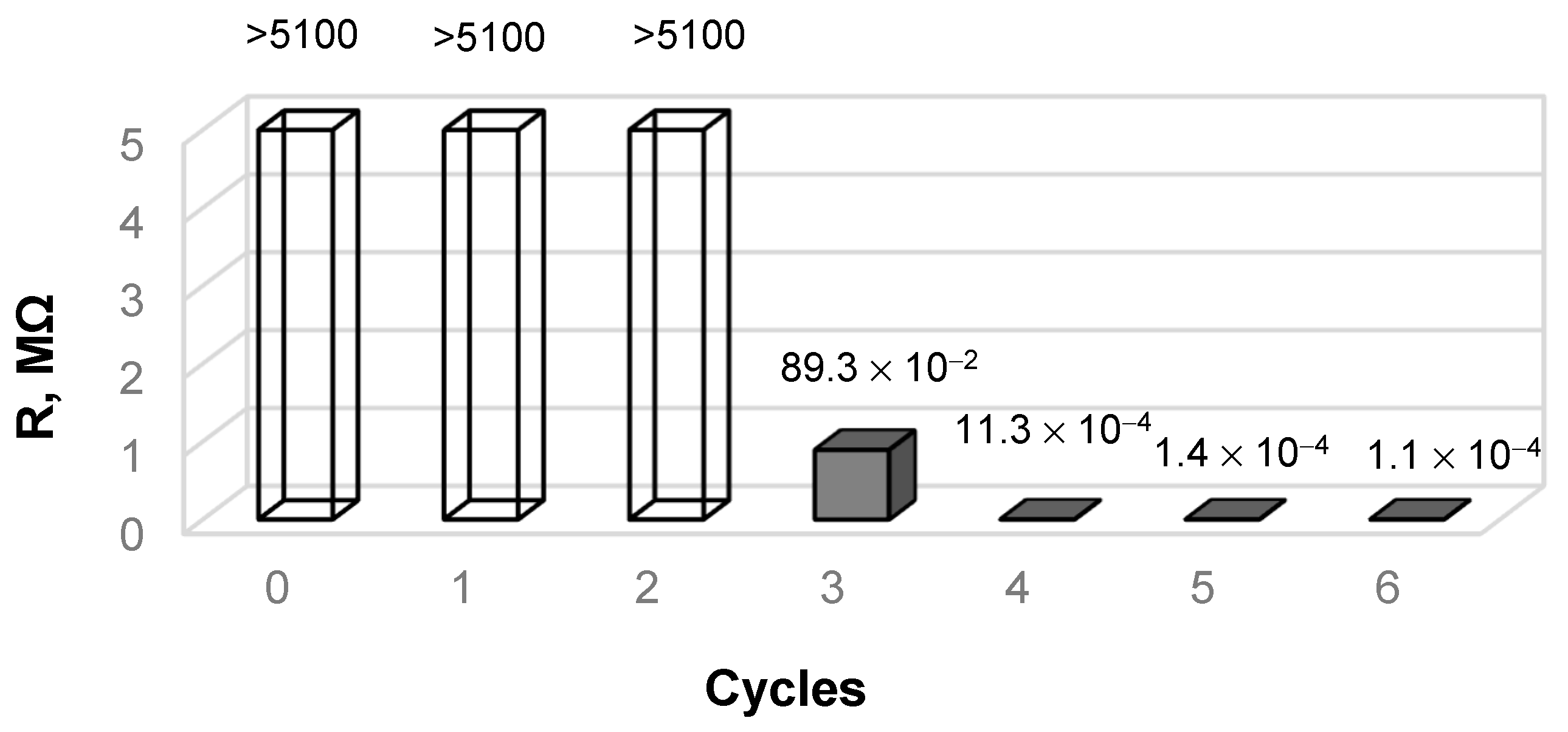
3.1. Physical and Mechanical Measurements
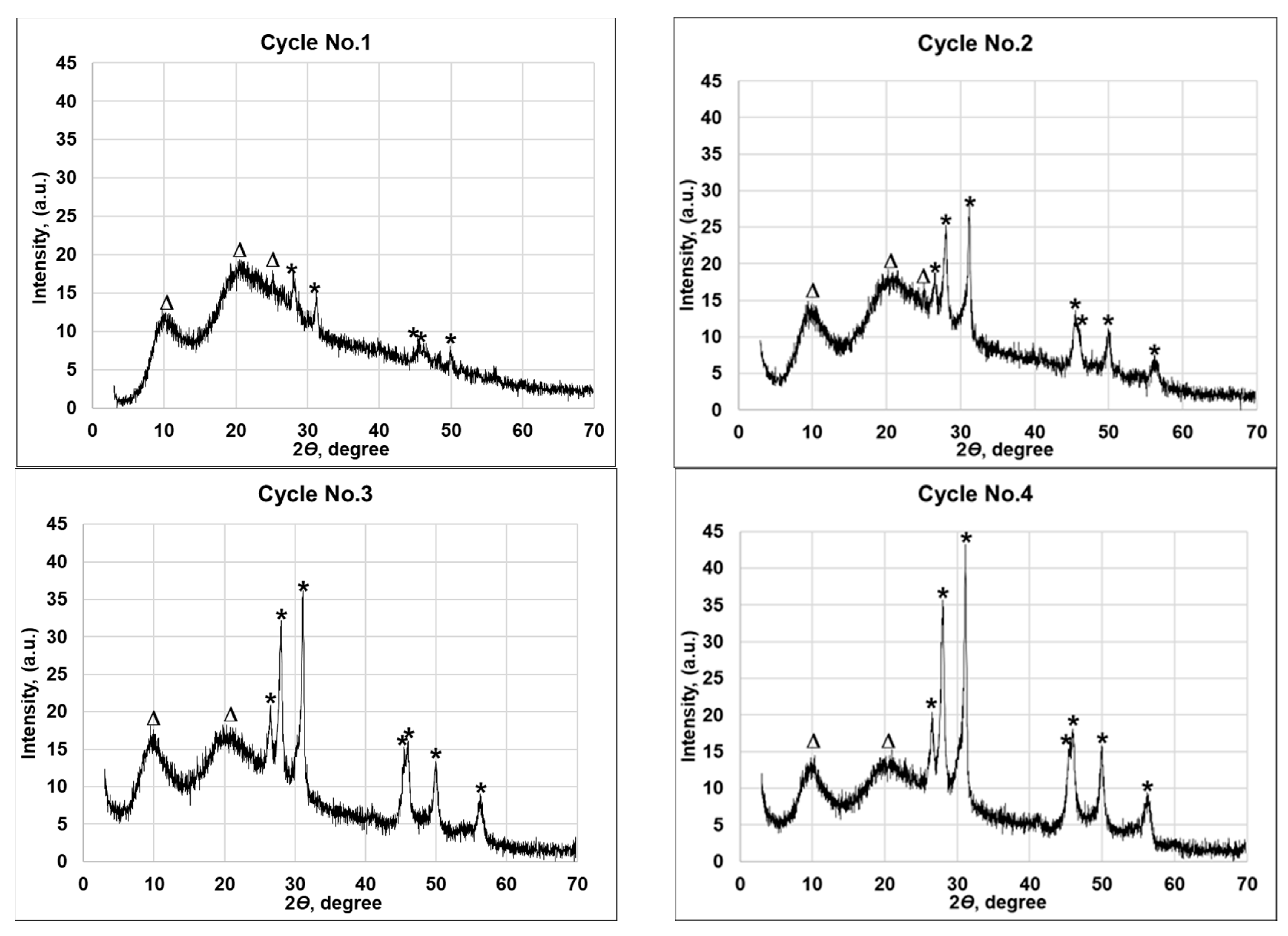
3.2. Bulk Chemical Composition and XRD Characterization of CuxSe Coatings
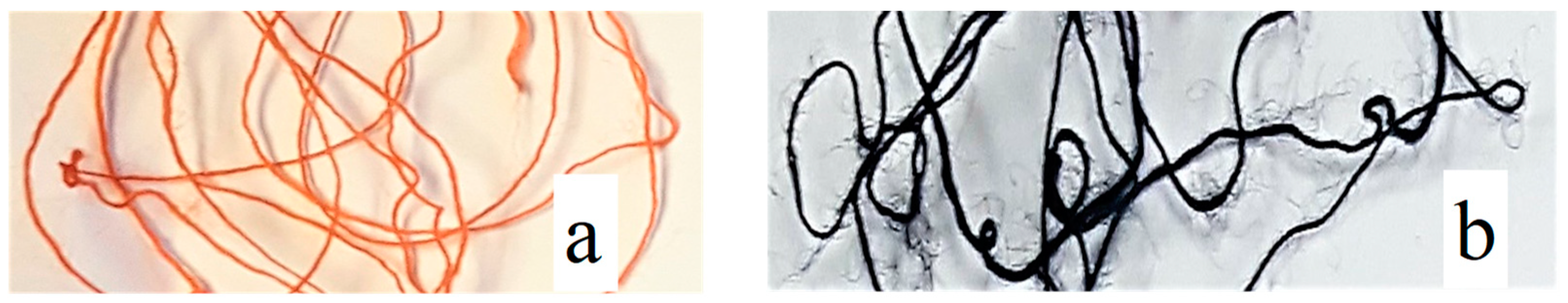
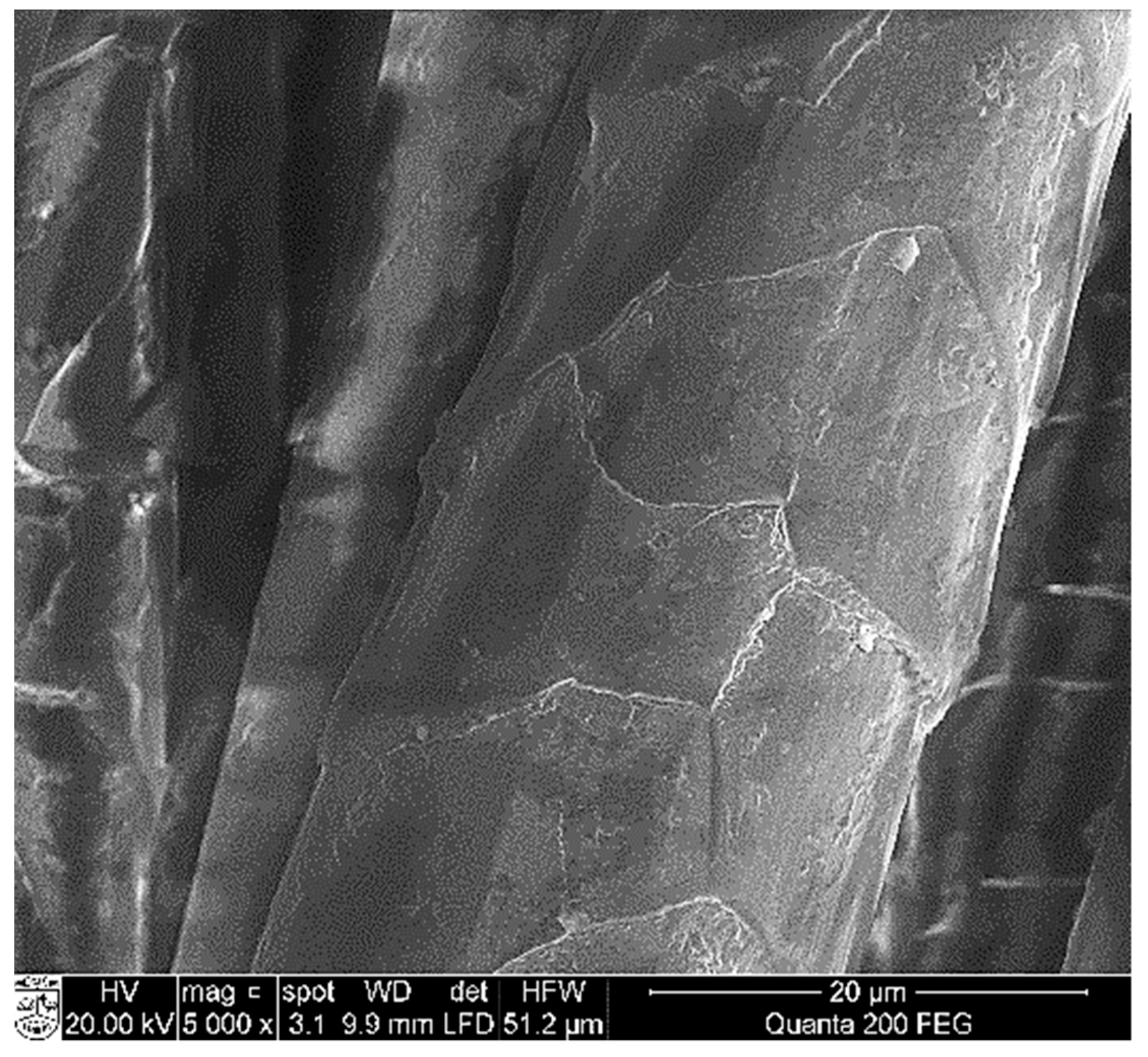
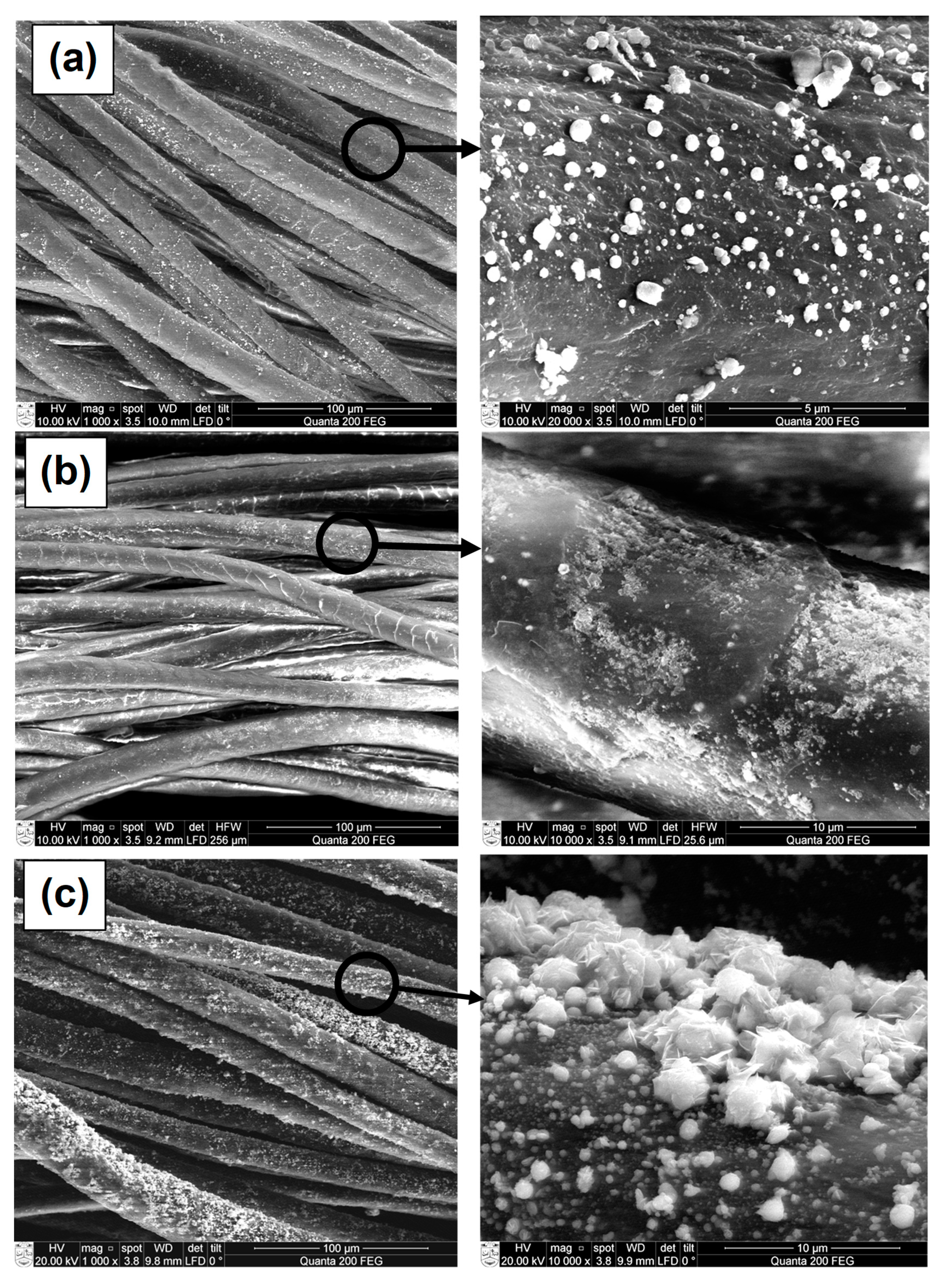
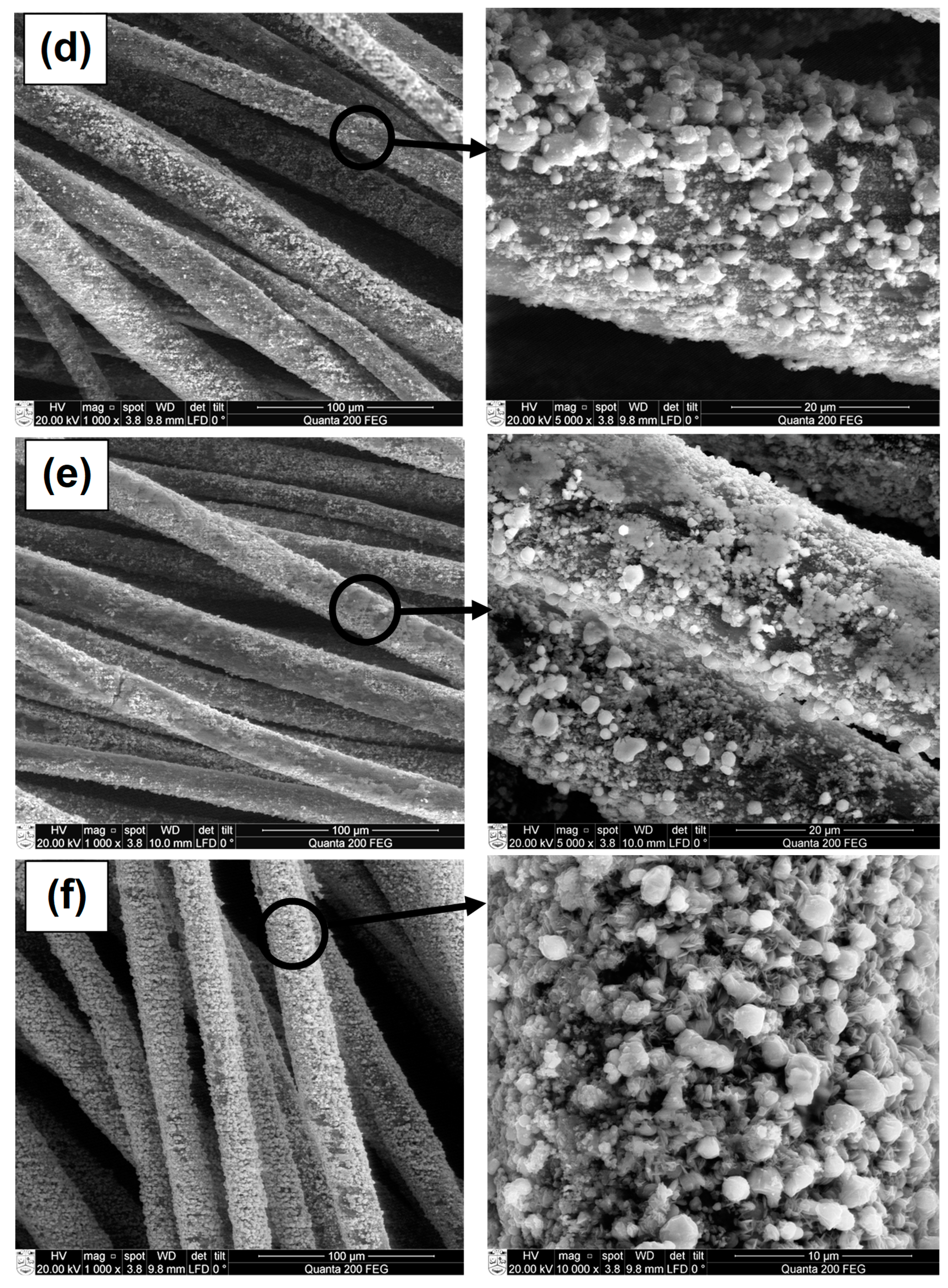
3.3. SEM and EDS Analysis of CuxSe Coatings on Wool Fibers
4. FTIR Analysis of CuxSe-Coated Wool Fibers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pritchard, C.; Silk, A.; Hansen, L. Are rises in Electro-Magnetic Field in the human environment, interacting with multiple environmental pollutions, the tripping point for increases in neurological deaths in the Western World? Med. Hypotheses 2019, 127, 76–83. [Google Scholar] [CrossRef]
- Habash, R.W.Y. Environmental and Human Impact of Electromagnetic Energy: Views toward Technology and Risk. Energy Environ. 2010, 21, 107–113. [Google Scholar] [CrossRef]
- Thirumavalavan, S.; Mani, K.; Sagadevan, S. Investigation of the structural, optical and electrical properties of copper selenide thin films. Mater. Res. 2015, 18, 1000–1007. [Google Scholar] [CrossRef]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Firat, Y.E.; Peksoz, A. Efficiently two-stage synthesis and characterization of CuSe/Polypyrrole composite thin films. J. Alloys Compd. 2017, 727, 177–184. [Google Scholar] [CrossRef]
- Manesh, F.Y.; Hasani, H.; Mortazavi, S.M. Analyzing the effect of yarn and fabrics parameters on electromagnetic shielding of metalized fabrics coated with polyaniline. J. Ind. Text. 2014, 44, 434–446. [Google Scholar] [CrossRef]
- Rybicki, T.; Karbownik, I. EMI shielding textile materials based on conducting polymers. In Materials for Potential EMI Shielding Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–285. [Google Scholar]
- Benetti, D.; Cui, D.; Zhao, H.; Rosei, F.; Vomiero, A. Direct Measurement of Electronic Band Structure in Single Quantum Dots of Metal Chalcogenide Composites. Small 2018, 14, 1801668. [Google Scholar] [CrossRef] [PubMed]
- Hazra, M.; Jana, A.; Datta, J. Self-controlled photo-degradation in coupled chalcogenide Bi-S-Se film for solar cell applications. Electrochim. Acta 2020, 329, 135168. [Google Scholar] [CrossRef]
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N.; Sharma, P.; Calle, F. A review on 2D transition metal di-chalcogenides and metal oxide nanostructures based NO2 gas sensors. Mater. Sci. Semicond. Process. 2020, 107, 104865. [Google Scholar] [CrossRef]
- Globe, M.K.; Hussain, S.A. Study of the structural and Optical Properties of Composite (PbS/CuS) Thin Films prepared by Thermal Evaporation Method. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 1664, p. 12012. [Google Scholar]
- Jalali, M.; Moakhar, R.S.; Abdelfattah, T.; Filine, E.; Mahshid, S.S.; Mahshid, S. Nanopattern-Assisted Direct Growth of Peony-like 3D MoS2/Au Composite for Nonenzymatic Photoelectrochemical Sensing. ACS Appl. Mater. Interfaces 2020, 12, 7411–7422. [Google Scholar] [CrossRef]
- Trifiletti, V.; Tseberlidis, G.; Colombo, M.; Spinardi, A.; Luong, S.; Danilson, M.; Grossberg, M.; Fenwick, O.; Binetti, S. Growth and Characterization of Cu2Zn1-xFexSnS4 Thin Films for Photovoltaic Applications. Materials 2020, 13, 1471. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yang, L.; Wei, J.; Yang, S.; Zhang, M.; Wang, X.; Yang, F. Doping Effect on Cu2Se Thermoelectric Performance: A Review. Materials 2020, 13, 5704. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, C.; Zhou, Y.; Zhao, L.-D. BiCuSeO Thermoelectrics: An Update on Recent Progress and Perspective. Materials 2017, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Hussain, I. Copper selenide thin films from growth to applications. Solid State Sci. 2020, 100, 106101. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Peng, W.; Yang, C.; Zhong, X. Quantum dot sensitized solar cells with efficiency up to 8.7% based on heavily copper-deficient copper selenide counter electrode. Nano Energy 2016, 23, 60–69. [Google Scholar] [CrossRef]
- Milašienė, D.; Ivanauskas, R. Obtaining of Multifuncional Materials with EMF Inhibition Properties by Modifying Soft Polymeric Materials with Silver Selenides (Ag2Se). Available online: https://worldwide.espacenet.com/publicationDetails/biblio?II=0&ND=4&adjacent=true&locale=en_EP&FT=D&date=20190110&CC=LT&NR=2017516A&KC=A (accessed on 23 February 2019).
- Güzeldir, B.; Saǧlam, M.; Ateş, A.; Türüt, A. Determination of the some electronic parameters of nanostructure copper selenide and Cu/Cu3Se2/n-GaAs/In structure. J. Alloys Compd. 2015, 627, 200–205. [Google Scholar] [CrossRef]
- Joshi, M.P.; Bhosale, P.N. Exploring the microstructural, optoelectronic properties of deposition time dependent Cu2Sn(S,Se)3 thin film synthesized by non-vacuum arrested precipitation technique. Superlattices Microstruct. 2021, 150, 106785. [Google Scholar] [CrossRef]
- Hua, X.; Li, J.; Liu, H.; Zhang, C.; Han, Y.; Gao, F.; Hodes, G.; Wang, P.; Yang, Z.; Liu, S. (Frank) Preparation of Cu2Se thin films by vacuum evaporation and hot-pressing. Vacuum 2021, 185, 109947. [Google Scholar] [CrossRef]
- Gong, J.; Tian, Y.; Yang, Z.; Wang, Q.; Hong, X.; Ding, Q. High-Performance Flexible All-Solid-State Asymmetric Supercapacitors Based on Vertically Aligned CuSe@Co(OH)2 Nanosheet Arrays. J. Phys. Chem. C 2018, 122, 2002–2011. [Google Scholar] [CrossRef]
- Henry, J.; Daniel, T.; Balasubramanian, V.; Mohanraj, K.; Sivakumar, G. Temperature dependent electrical and optical properties with higher photosensitivity of Cu2Se absorber thin films for photo voltaic application. Inorg. Nano Met. Chem. 2021, 51, 38–46. [Google Scholar] [CrossRef]
- Rubino, C.; Liuzzi, S.; Martellotta, F.; Stefanizzi, P.; Straziota, P. Nonwoven Textile Waste Added with PCM for Building Applications. Appl. Sci. 2021, 11, 1262. [Google Scholar] [CrossRef]
- Kawamura, K.; Sako, K.; Ogata, T.; Tanabe, K. Environmentally friendly, hydrothermal treatment of mixed fabric wastes containing polyester, cotton, and wool fibers: Application for HMF production. Bioresour. Technol. Rep. 2020, 11, 100478. [Google Scholar] [CrossRef]
- Shukla, V. Review of electromagnetic interference shielding materials fabricated by iron ingredients. Nanoscale Adv. 2019, 1, 1640–1671. [Google Scholar] [CrossRef]
- Jiang, D.; Murugadoss, V.; Wang, Y.; Lin, J.; Ding, T.; Wang, Z.; Shao, Q.; Wang, C.; Liu, H.; Lu, N.; et al. Electromagnetic Interference Shielding Polymers and Nanocomposites—A Review. Polym. Rev. 2019, 59, 280–337. [Google Scholar] [CrossRef]
- Zou, L.; Lan, C.; Yang, L.; Xu, Z.; Chu, C.; Liu, Y.; Qiu, Y. The optimization of nanocomposite coating with polyaniline coated carbon nanotubes on fabrics for exceptional electromagnetic interference shielding. Diam. Relat. Mater. 2020, 104, 107757. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, D.; Oh, I.K. Multilayered graphene-carbon nanotube-iron oxide three-dimensional heterostructure for flexible electromagnetic interference shielding film. Carbon 2017, 111, 248–257. [Google Scholar] [CrossRef]
- Thomassin, J.M.; Jérôme, C.; Pardoen, T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R Rep. 2013, 74, 211–232. [Google Scholar] [CrossRef]
- Ivanauskas, R.; Baltrusaitis, J. Synthesis and surface properties of polyamide-CuxSe composite thin films. Appl. Surf. Sci. 2013, 283. [Google Scholar] [CrossRef]
- Rathke, B. Mittheilungen aus dem Universitäts-Laboratorium zu Königsberg. XV. Beiträge zur Kenntniss der chemischen Aehnlichkeit von Schwefel und Selen. Selendithionige Säure. Selentrithionsäure. J. Pract. Chem. 1865, 95. [Google Scholar] [CrossRef]
- Agilent Technologies. Flame Atomic Absorption Spectrometry Analytical Methods, 14th ed.; Agilent Technologies Australia (M) Pty, Ltd.: Mulgrave, VIC, Australia, 2017. [Google Scholar]
- ISO 2062:2009. Textiles—Yarns from Packages—Determination of Single-End Breaking Force and Elongation at Break Using Constant Rate of Extension (CRE) Tester. Available online: https://www.iso.org/standard/45642.html (accessed on 20 March 2021).
- Jung, S.Y.; Kim, B.K.; Hirata, S.; Inada, M.; Oh, J.M. Particle size effect of layered double hydroxide on the porosity of calcined metal oxide. Appl. Clay Sci. 2020, 195. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles—A comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Espacenet-Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/064949493/publication/LT6597B?q=pn%3DLT6597B (accessed on 4 February 2021).
- Ivanauskas, R. Studies on application as precursor of selenotrithionate and the mechanism of seleniumization polyamide 6 films. Medziagotyra 2009, 15, 224–249. [Google Scholar]
- Ghermezgoli, Z.M.; Moezzi, M.; Yekrang, J.; Rafat, S.A.; Soltani, P.; Barez, F. Sound absorption and thermal insulation characteristics of fabrics made of pure and crossbred sheep waste wool. J. Build. Eng. 2021, 35, 102060. [Google Scholar] [CrossRef]
- Ragaišienė, A.; Rusinavičiūtė, J.; Milašienė, D.; Ivanauskas, R. Comparison of selected chemical properties of fibres from different breeds of dogs and german blackface sheep. Fibres Text. East. Eur. 2016, 24. [Google Scholar] [CrossRef][Green Version]
- Effenberger, H.; Pertlik, F. Ein Beitrag zur Kristallstruktur von alpha—CuSe (Klockmannit). New Yearb. Mineral. Mon. Books 1981, 197–205. [Google Scholar]
- Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 83-1814 (1997). Available online: https://www.icdd.com/ (accessed on 20 March 2021).
- Patil, P.A.; Wani, P.A. Growth and Morphology of CdS Crystals by Flux Method. Cryst. Res. Technol. 2001, 36, 371–378. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Y.; Wang, X. Effect of surface modification on the dynamic heat and mass transfer of wool fabrics. J. Therm. Biol. 2019, 85, 102416. [Google Scholar] [CrossRef]
- Chen, N.; Liu, C.K.; Brown, E.M.; Latona, N. Environment-friendly treatment to reduce photoyellowing and improve UV-blocking of wool. Polym. Degrad. Stab. 2020, 181, 109319. [Google Scholar] [CrossRef]
- Basak, S.; Samanta, K.K.; Chattopadhyay, S.K.; Pandit, P.; Maiti, S. Green fire retardant finishing and combined dyeing of proteinous wool fabric. Coloration Technol. 2016, 132, 135–143. [Google Scholar] [CrossRef]
- Yu, D.; Tian, W.; Sun, B.; Li, Y.; Wang, W.; Tian, W. Preparation of silver-plated wool fabric with antibacterial and anti-mould properties. Mater. Lett. 2015, 151. [Google Scholar] [CrossRef]
- Memiş, N.K.; Kaplan, S. Wool fabric having thermal comfort management function via shape memory polyurethane finishing. J. Text. Inst. 2020, 111, 734–744. [Google Scholar] [CrossRef]
- Chandwani, N.; Dave, P.; Jain, V.; Nema, S.; Mukherjee, S. Improving anti-felting characteristics of Merino wool fiber by 2.5 MHz atmosphere pressure air plasma. J. Phys. Conf. Ser. 2017, 823, 12010. [Google Scholar]
- Peets, P.; Kaupmees, K.; Vahur, S.; Leito, I. Reflectance FT-IR spectroscopy as a viable option for textile fiber identification. Herit. Sci. 2019, 7, 93. [Google Scholar] [CrossRef]
- Mirnezhad, S.; Safapour, S.; Sadeghi-Kiakhani, M. Dual-mode adsorption of cochineal natural dye on wool fibers: Kinetic, equilibrium, and thermodynamic studies. Fibers Polym. 2017, 18, 1134–1145. [Google Scholar] [CrossRef]

| Characteristics | Treatment Cycle Number | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Breaking tenacity, cN/tex | Min and max value, cN/tex | 1.23/2.70 | 1.82/3.58 | 1.67/3.91 | 1.17/3.15 | 1.58/4.13 | 1.16/3.24 | 0.92/2.86 |
| X *, cN/tex | 1.87 | 2.75 | 2.71 | 2.34 | 2.27 | 2.02 | 1.99 | |
| Relative error, % | 5.96 | 4.52 | 5.76 | 6.52 | 6.32 | 7.65 | 6.85 | |
| Elonga-tion at break, % | Min and max value, % | 0.89/10.77 | 5.77/29.41 | 6.56/30.86 | 2.69/30.22 | 3.24/26.76 | 1.74/23.31 | 1.31/16.49 |
| X, % | 5.47 | 17.23 | 17.80 | 14.31 | 12.94 | 9.67 | 7.38 | |
| Relative error, % | 14.04 | 9.37 | 10.58 | 13.09 | 12.94 | 17.02 | 13.33 | |
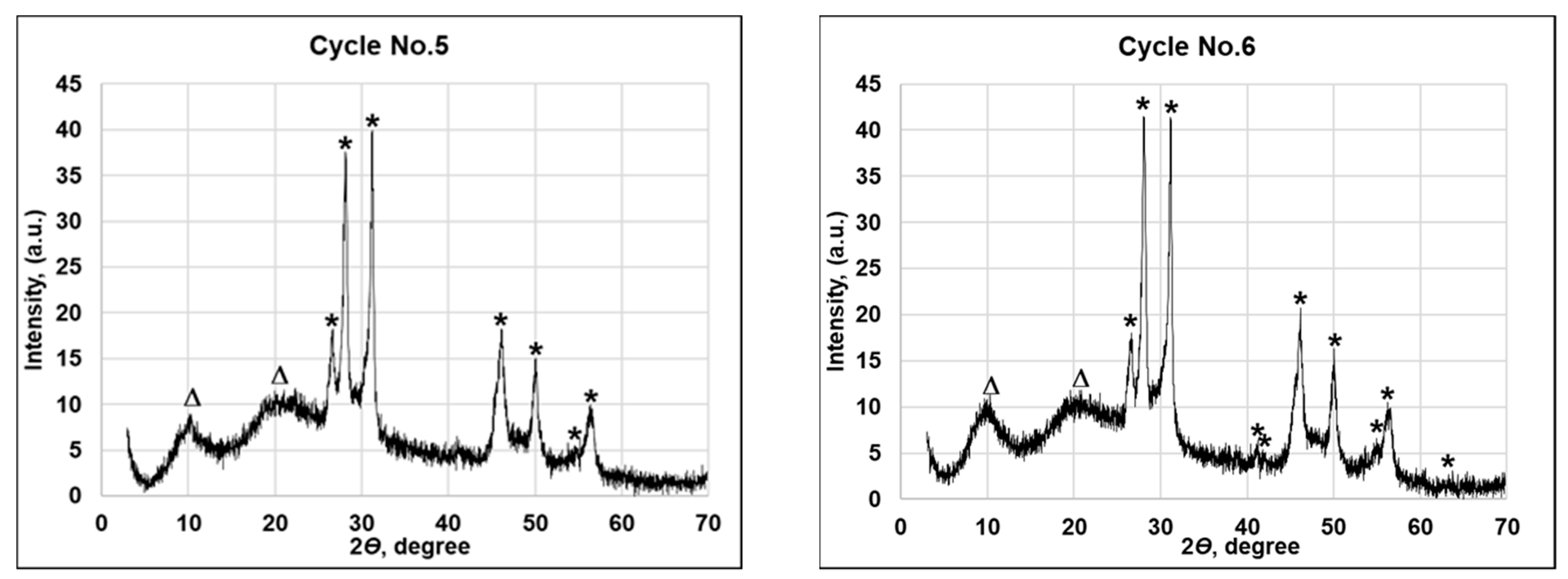
| Number of the Treatment Cycle | Symbol in Figure 4; File Number of the Crystallographic Phase of Joint Committee on Powder Diffraction; Peak Positions 2θ (Degrees) | Average Size of Crystallite, nm |
|---|---|---|
| No. 1 | (*) Cu0.87Se 83-1814 –28.09, 31.15, 45.41, 46.13, 49.98. (∆) wool fiber—10.24, 20.93, 25.19. | 358.83 |
| No. 2 | (*) Cu0.87Se 83-1814—26.58, 28.09, 31.15, 45.41, 46.13, 49.98, 56.51. (∆) wool fiber—10.24, 20.93, 25.19. | 327.31 |
| No. 3 | (*) Cu0.87Se 83-1814—26.58, 28.09, 31.15, 45.41, 46.13, 49.98, 56.51. (∆) wool fiber—10.24, 20.93, | 323.73 |
| No. 4 | (*) Cu0.87Se 83-1814—26.58, 28.09, 31.15, 45.41, 46.13, 49.98, 56.51. (∆) wool fiber—10.24, 20.93. | 318.39 |
| No. 5 | (*) Cu0.87Se 83-1814—26.58, 28.09, 31.15, 45.41, 46.13, 49.98, 56.23, 56.51. (∆) wool fiber—10.24, 20.93. | 318.26 |
| No. 6 | (*) Cu0.87Se 83-1814—26.58, 28.09, 31.15, 41.13, 41.92, 45.41, 46.13, 49.98, 56.23, 56.51, 63.27. (∆) wool fiber—10.24, 20.93. | 307.55 |
| Treatment Cycles Number | Molar Concentration of Elements, mmol/g | Molar Ratio of Cu/Se | |
|---|---|---|---|
| Cu | Se | ||
| 1 | 0.356 | 0.560 | 0.64 |
| 2 | 1.288 | 2.011 | 0.64 |
| 3 | 2.117 | 3.316 | 0.64 |
| 4 | 3.292 | 5.179 | 0.64 |
| 5 | 4.516 | 7.105 | 0.64 |
| 6 | 5.751 | 8.840 | 0.65 |
| Number of Treatment Cycle | Elemental Composition in Weight Percent [Wt%] | |||||
|---|---|---|---|---|---|---|
| C | O | N | S | Cu | Se | |
| 1 | 39.14 | 37.93 | 20.02 | 1.07 | 1.30 | 0.53 |
| 2 | 35.66 | 37.15 | 17.47 | 1.33 | 5.93 | 2.45 |
| 3 | 27.58 | 31.22 | 15.30 | 1.74 | 14.32 | 9.83 |
| 4 | 27.61 | 20.20 | 15.27 | 0.90 | 22.34 | 13.68 |
| 5 | 37.16 | 17.92 | 11.36 | 0.62 | 18.90 | 14.03 |
| 6 | 20.91 | 7.5 | 6.7 | 0.14 | 40.27 | 24.75 |
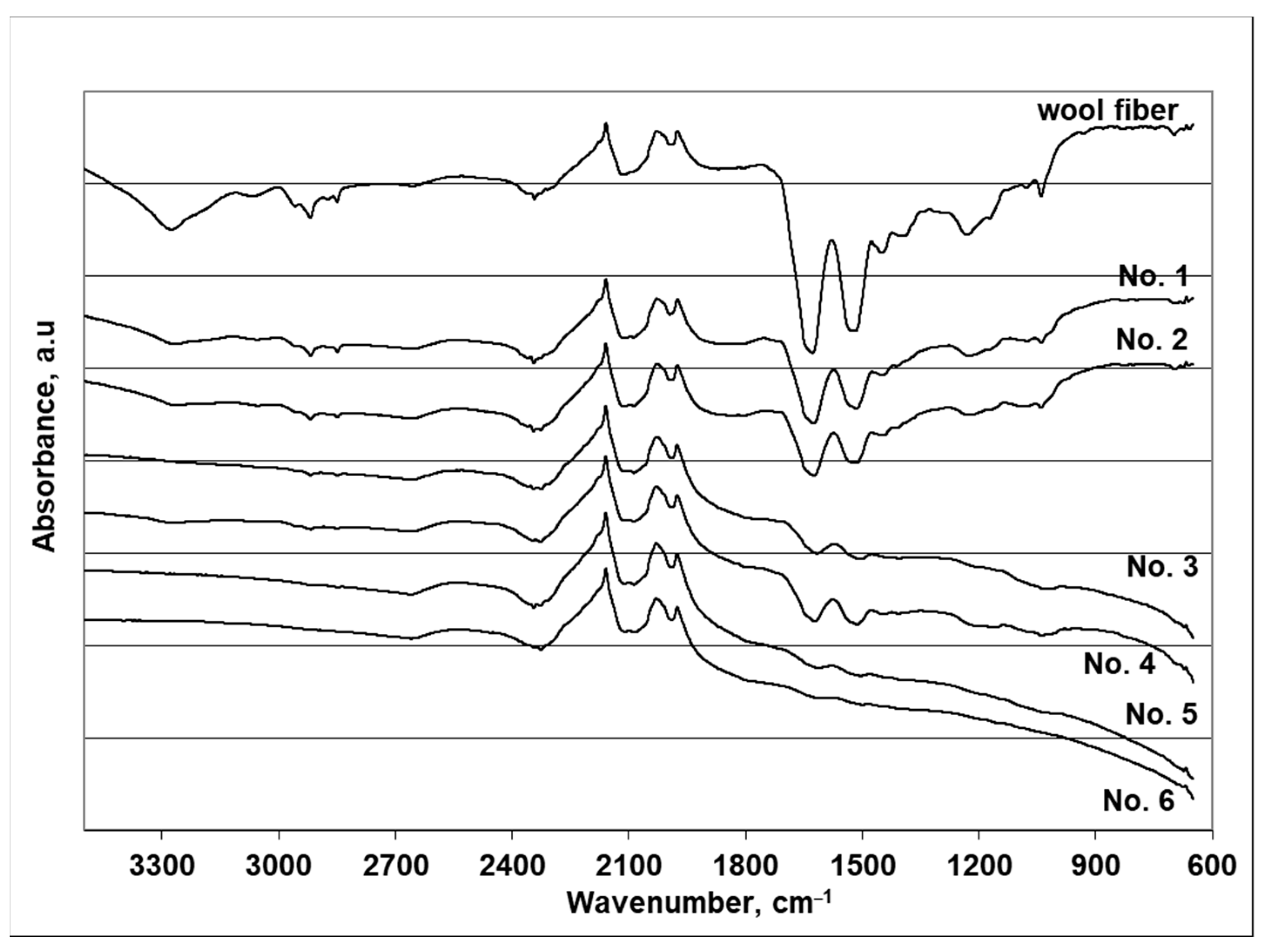
| Sample of Wool Fiber | Functional Group or Bond, to Which the Vibration Is Attributed and Band Position | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N–H, O–H | Secondary Amide, Amide II Overtone | Methylene Symmetric C–H Stretching | Methylene Asymmetric C–H Stretching | Amide I Band (=C=O) | Secondary Amide N–H Bending, C–N Wagging | Amide Band (III) | (SO) Cysteic Acid | (SO) Cysteic Monoxide | ||||
| Uncoated wool fiber | 3276 | 3068 | 2961 | 2919 | 2874 | 2850 | 1629 | 1517 | 1232 | 1172 | 1078 | 1040 |
| No. 1 | 3273 | - | 2959 | 2918 | 2874 | 2850 | 1625 | 1515 | 1224 | 1172 | 1076 | 1040 |
| No. 2 | - | - | 2961 | 2918 | 2874 | 2850 | 1625 | 1516 | 1226 | 1167 | 1080 | 1039 |
| No. 3 | - | - | 2921 | 2921 | - | 2850 | 1616 | 1508 | - | 1165 | - | 1039 |
| No. 4 | - | - | - | 2924 | - | 2848 | 1622 | 1513 | - | 1162 | - | 1043 |
| No. 5 | - | - | - | - | - | 1615 | 1508 | - | - | - | ||
| No. 6 | - | - | - | - | - | 1613 | - | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belukhina, O.; Milasiene, D.; Ivanauskas, R. Investigation of the Possibilities of Wool Fiber Surface Modification with Copper Selenide. Materials 2021, 14, 1648. https://doi.org/10.3390/ma14071648
Belukhina O, Milasiene D, Ivanauskas R. Investigation of the Possibilities of Wool Fiber Surface Modification with Copper Selenide. Materials. 2021; 14(7):1648. https://doi.org/10.3390/ma14071648
Chicago/Turabian StyleBelukhina, Olga, Daiva Milasiene, and Remigijus Ivanauskas. 2021. "Investigation of the Possibilities of Wool Fiber Surface Modification with Copper Selenide" Materials 14, no. 7: 1648. https://doi.org/10.3390/ma14071648
APA StyleBelukhina, O., Milasiene, D., & Ivanauskas, R. (2021). Investigation of the Possibilities of Wool Fiber Surface Modification with Copper Selenide. Materials, 14(7), 1648. https://doi.org/10.3390/ma14071648







