In Vitro Studies on Mg-Zn-Sn-Based Alloys Developed as a New Kind of Biodegradable Metal
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloys Preparation
2.2. Microstructure Analysis and Mechanical Testing
2.3. In Vitro Corrosion Evaluation
2.4. In Vitro Cell Test
2.4.1. Cell Culture and Preparation of Extraction
2.4.2. Cytocompatibility and Cell Morphology
2.4.3. In Vitro Osteogenic Differentiation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microstructures and Electrochemical Evaluations
3.2. Mechanical Properties
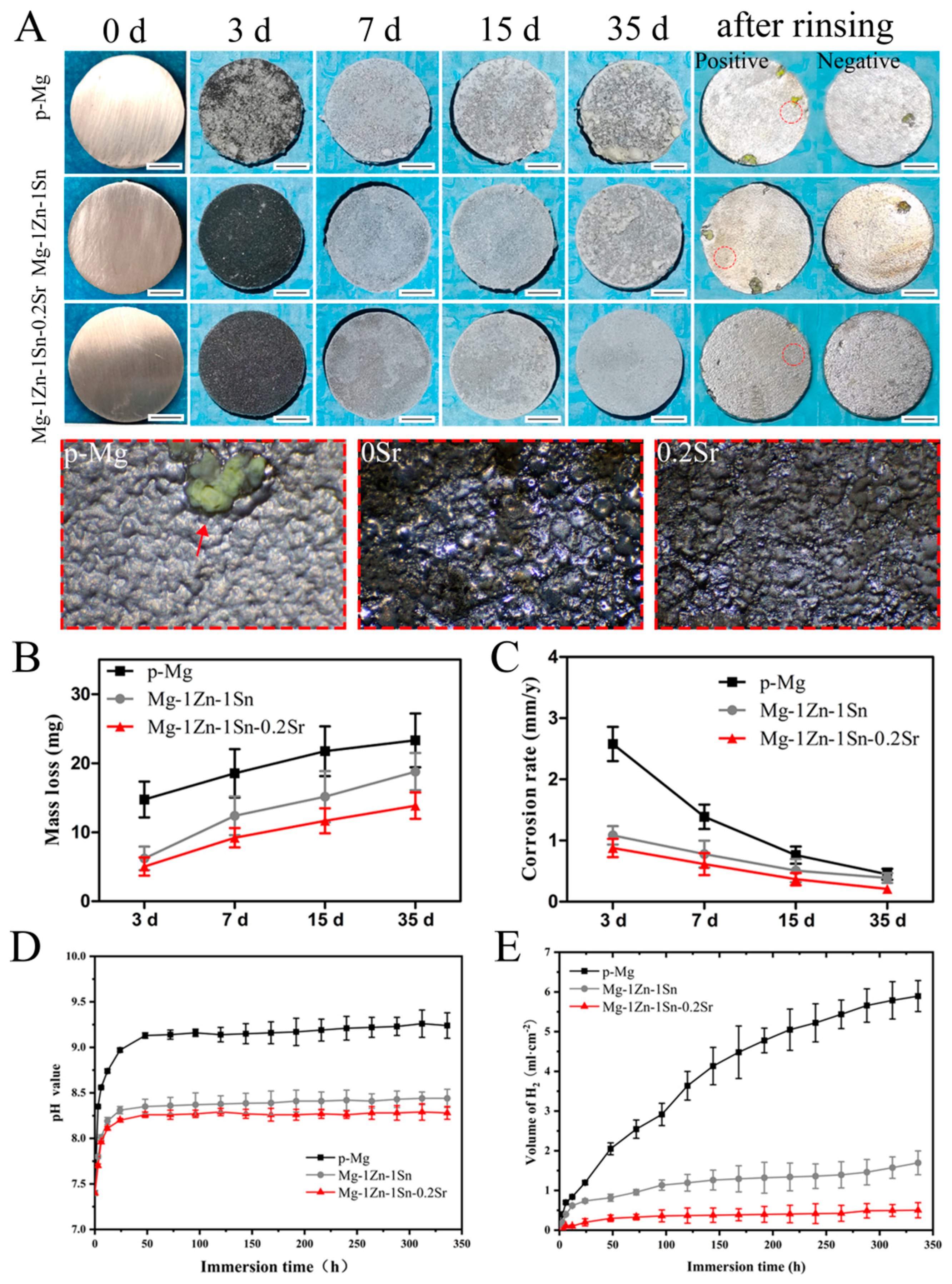
3.3. Immersion Tests
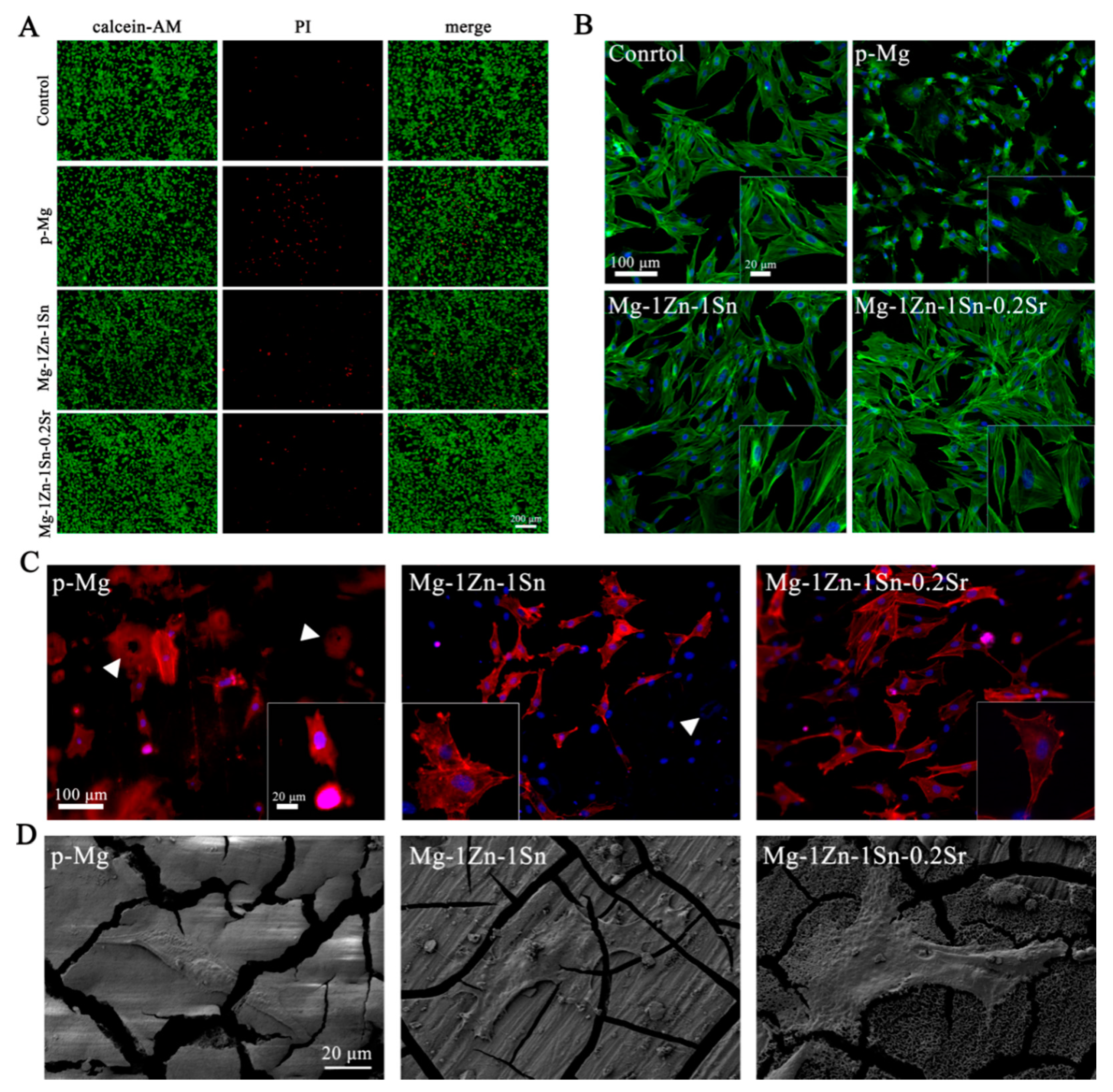
3.4. Cell Viability, Cytocompatibility and Cell Morphology
3.5. In Vitro Osteogenesis Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Moravej, M.; Mantovani, D. Biodegradable Metals for Cardiovascular Stent Application: Interests and New Opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corro-sion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Ding, W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.; Wen, C.; Wright, P.F.A.; Li, Y. Mechanical, corrosion, and biocompatibility properties of Mg-Zr-Sr-Sc alloys for biodegradable implant applications. Acta Biomater. 2020, 102, 493–507. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable Orthopedic Magnesium-Calcium (MgCa) Alloys, Processing, and Corrosion Performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef]
- Pande, G.; Ragamouni, S.; Li, Y.; Kumar, M.J.; Harishankar, N.; Hodgson, P.D.; Wen, C.; Mushahary, D.; Sravanthi, R. Zirconium, calcium, and strontium contents in magnesium based biodegradable alloys modulate the efficiency of implant-induced osseointegration. Int. J. Nanomed. 2013, 8, 2887–2902. [Google Scholar] [CrossRef][Green Version]
- Bornapour, M.; Muja, N.; Shum-Tim, D.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg–Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef]
- Kubasek, J.; Vojtech, D.; Lipov, J.; Ruml, T. Structure, mechanical properties, corrosion behavior and cytotoxicity of bio-degradable Mg–X (X=Sn, Ga, In) alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2421–2432. [Google Scholar] [CrossRef]
- Liu, X.; Shan, D.; Song, Y.; Chen, R.; Han, E. Influences of the quantity ofMg2Sn phase on the corrosion behavior of Mg–7Sn magnesium alloy. Electrochimica Acta. 2011, 56, 2582–2590. [Google Scholar] [CrossRef]
- Marie, P.J.; Felsenberg, D.; Brandi, M.L. How strontium ranelate, via opposite effects on bone resorption and formation, prevents osteoporosis. Osteoporos. Int. 2011, 22, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.F.; Lin, J.; Lin, A.; Sallee, A.; Celene, C.A.M.; Alcaraz, M.C.C.; Guan, R.-G.; Botimer, G.; Inceoğlu, S.; Liu, H. Degradation of Bioresorbable Mg–4Zn–1Sr Intramedullary Pins and Associated Biological Responses in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 44332–44355. [Google Scholar] [CrossRef]
- ASTM International. A Standard, E9-09, Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM International. A Standard, ASTM E8/E8M-16a, Standard Test Methods for Tension Testing of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Cipriano, A.F.; Sallee, A.; Guan, R.-G.; Zhao, Z.-Y.; Tayoba, M.; Sanchez, J.; Liu, H. Investigation of magnesium–zinc–calcium alloys and bone marrow derived mesenchymal stem cell response in direct culture. Acta Biomater. 2015, 12, 298–321. [Google Scholar] [CrossRef]
- ASTM International. A Standard, G102−89 (Reapproved 2015) e1 Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Lai, H.; Li, J.; Li, J.; Zhang, Y.; Xu, Y. Effects of Sr on the microstructure, mechanical properties and corrosion behavior of Mg-2Zn-xSr alloys. J. Mater. Sci. Mater. Med. 2018, 29, 87. [Google Scholar] [CrossRef]
- Guan, R.G.; Cipriano, A.F.; Zhao, Z.Y.; Lock, J.; Tie, D.; Zhao, T.; Cui, T.; Liu, H. Development and evaluation of a magnesium–zinc–strontium alloy for biomedical applications—Alloy processing, microstructure, mechanical properties, and biodegradation. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3661–3669. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Wen, C. Effects of Mg17Sr2Phase on the Bio-Corrosion Behavior of Mg-Zr-Sr Alloys. Adv. Eng. Mater. 2015, 18, 259–268. [Google Scholar] [CrossRef]
- Li, H.; Peng, Q.; Li, X.; Li, K.; Han, Z.; Fang, D. Microstructures, mechanical and cytocompatibility of degradable Mg–Zn based orthopedic biomaterials. Mater. Des. 2014, 58, 43–51. [Google Scholar] [CrossRef]
- Chu, P.-W.; Le Mire, E.; Marquis, E.A. Microstructure of localized corrosion front on Mg alloys and the relationship with hydrogen evolution. Corros. Sci. 2017, 128, 253–264. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, J.; Liu, Q.; Zhao, W.; Jiang, D.; Guo, S. Low hydrogen release behavior and antibacterial property of Mg-4Zn-xSn alloys. Mater. Lett. 2019, 241, 88–91. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, F.; Zhao, S.; Pan, H.; Song, K.; Tang, A. Microstructure, corrosion behavior and cytotoxicity of biodegradable Mg–Sn implant alloys prepared by sub-rapid solidification. Mater. Sci. Eng. C 2015, 54, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.-Y.; Kang, J.-Y.; Yang, J.; Yim, C.D.; You, B.S. Role of Sn in corrosion and passive behavior of extruded Mg-5 wt%Sn alloy. Corros. Sci. 2016, 102, 355–362. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, J.; Yan, H.; Su, B.; Yu, Z.; Xia, W.; Gong, X. Effects of minor Sr addition on microstructure, mechanical and bio-corrosion properties of the Mg-5Zn based alloy system. J. Alloy. Compd. 2017, 691, 95–102. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Wu, J.; Yang, Q.; Jiang, D.; Qiao, B. Polydopamine-induced hydroxyapatite coating facilitates hydroxyap-atite/polyamide 66 implant osteogenesis: An in vitro and in vivo evaluation. Int. J. Nanomed. 2018, 13, 8179–8193. [Google Scholar] [CrossRef]
- Cipriano, A.F.; Sallee, A.; Tayoba, M.; Alcaraz, M.C.C.; Lin, A.; Guan, R.-G.; Zhao, Z.-Y.; Liu, H. Cytocompatibility and early inflammatory response of human endothelial cells in direct culture with Mg-Zn-Sr alloys. Acta Biomater. 2017, 48, 499–520. [Google Scholar] [CrossRef]
- Bian, D.; Zhou, W.; Deng, J.; Liu, Y.; Li, W.; Chu, X.; Xiu, P.; Cai, H.; Kou, Y.; Jiang, B.; et al. Development of magnesium-based biodegradable metals with dietary trace element germanium as orthopaedic implant applications. Acta Biomater. 2017, 64, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.F.; Zhao, T.; Johnson, I.; Guan, R.-G.; Garcia, S.; Liu, H. In vitro degradation of four magnesium–zinc–strontium alloys and their cytocompatibility with human embryonic stem cells. J. Mater. Sci. Mater. Med. 2013, 24, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Györgyey, Á.; Ungvári, K.; Kecskeméti, G.; Kopniczky, J.; Hopp, B.; Oszkó, A.; Pelsöczi, I.; Rakonczay, Z.; Nagy, K.; Turzó, K. Attachment and proliferation of human osteoblast-like cells (MG-63) on laser-ablated titanium implant material. Mater. Sci. Eng. C 2013, 33, 4251–4259. [Google Scholar] [CrossRef] [PubMed]
- Romani, A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch. Biochem. Biophys. 2007, 458, 90–102. [Google Scholar] [CrossRef]
- Marie, P. Strontium ranelate: A physiological approach for optimizing bone formation and resorption. Bone 2006, 38, 10–14. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Stronti-um-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef]
- Mao, L.; Liu, J.; Zhao, J.; Chang, J.; Xia, L.; Jiang, L.; Wang, X.; Lin, K.; Fang, B. Effect of micro-nano-hybrid structured hydrox-yapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt sig-naling pathway. Int. J. Nanomed. 2015, 10, 7031–7044. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Gan, J.; Zhang, Z.; Chen, H.; Jiang, X.; Chang, J. Tailoring the nanostructured surfaces of hydroxyapatite bi-oceramics to promote protein adsorption, osteoblast growth, and osteogenic differentiation. ACS Appl. Mater. Interfaces 2013, 5, 8008–8017. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: New insights into its dual mode of action. Bone 2007, 40, S5–S8. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef]
- Bakker, A.D.; Zandieh-Doulabi, B.; Klein-Nulend, J. Strontium Ranelate affects signaling from mechanically-stimulated osteocytes towards osteoclasts and osteoblasts. Bone 2013, 53, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, V.; Chappard, D.; Marty, C.; Libouban, H.; Ostertag, A.; Lalande, A.; de Vernejoul, M.C. Strontium ranelate de-creases the incidence of new caudal vertebral fractures in a growing mouse model with spontaneous fractures by improving bone microarchitecture. Osteoporos Int. 2011, 22, 289–297. [Google Scholar] [CrossRef][Green Version]
- Grynpas, M.; Hamilton, E.; Cheung, R.; Tsouderos, Y.; Deloffre, P.; Hott, M.; Marie, P. Strontium increases vertebral bone volume in rats at a low dose that does not induce detectable mineralization defect. Bone 1996, 18, 253–259. [Google Scholar] [CrossRef]
- Grynpas, M.; Marie, P. Effects of low doses of strontium on bone quality and quantity in rats. Bone 1990, 11, 313–319. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, H.K.; Kim, Y.J.; Jang, J.H.; Song, H.; Hanawa, T. Osteoblast response and osseointegration of a Ti-6Al-4V al-loy implant incorporating strontium. Acta Biomater. 2010, 6, 2843–2851. [Google Scholar] [CrossRef]







| Nominal Composition | Actual Composition | ||||||
|---|---|---|---|---|---|---|---|
| Zn | Sn | Sr | Fe | Si | Ni | Mg | |
| Mg-1Zn-1Sn | 1.04 | 1.13 | 0 | <0.01 | <0.01 | <0.001 | Balance |
| Mg-1Zn-1Sn-0.2Sr | 1.02 | 1.12 | 0.21 | <0.01 | <0.01 | <0.001 | Balance |
| Components | Concentration |
|---|---|
| NaCl | 7.996 g/L |
| KCl | 0.224 g/L |
| CaCl2 | 0.278 g/L |
| NaHCO3 | 0.350 g/L |
| MgCl·6H2O | 0.305 g/L |
| K2HPO4·3H2O | 0.228 g/L |
| Na2SO4 | 0.071 g/L |
| HCl (1 mol/L) | 40 mL |
| (CH2OH)2CNH2 | 6.051 g/L |
| Gene | Primers (F = Forward; R = Reverse) |
|---|---|
| Runx2 | F: 5′-ATCCAGCCACCTTCACTTACAAA-3′ |
| R: 5′-GGGACCATTGGGAACTGATAGG-3′ | |
| OPN | F: 5′-CCAAGCGTGGAAACACACAGCC-3′ |
| R: 5′-GGCTTTGGAACTCGCCTGACTG-3′ | |
| Bglap | F: 5′-GAGCTGCCCTGCACTGGGTG-3′ |
| R: 5′-TGGCCCCAGACCTCTTCCCG-3′ | |
| β-actin | F: 5′-ATCGTGGGCCGCCCTAGGCA-3′ |
| R: 5′-TGGCCTTAGGGTTCAGAGGGG-3′ |
| Materials | Corrosion Rate (mm/y) Calculated by H2 | Corrosion Rate (mm/y) Calculated by Mass Loss | Corrosion Rate (mm/y) Calculated by Icorr |
|---|---|---|---|
| Mg | 0.72 ± 0.28 | 0.44 ± 0.09 | 0.61 ± 0.16 |
| Mg-1Zn-1Sn | 0.24 ± 0.09 | 0.31 ± 0.05 | 0.29 ± 0.08 |
| Mg-1Zn-1Sn-0.2Sr | 0.18 ± 0.02 | 0.20 ± 0.03 | 0.23 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Liu, Q.; Zhao, W.; Yang, Q.; Wang, J.; Jiang, D. In Vitro Studies on Mg-Zn-Sn-Based Alloys Developed as a New Kind of Biodegradable Metal. Materials 2021, 14, 1606. https://doi.org/10.3390/ma14071606
Wen Y, Liu Q, Zhao W, Yang Q, Wang J, Jiang D. In Vitro Studies on Mg-Zn-Sn-Based Alloys Developed as a New Kind of Biodegradable Metal. Materials. 2021; 14(7):1606. https://doi.org/10.3390/ma14071606
Chicago/Turabian StyleWen, Yafeng, Qingshan Liu, Weikang Zhao, Qiming Yang, Jingfeng Wang, and Dianming Jiang. 2021. "In Vitro Studies on Mg-Zn-Sn-Based Alloys Developed as a New Kind of Biodegradable Metal" Materials 14, no. 7: 1606. https://doi.org/10.3390/ma14071606
APA StyleWen, Y., Liu, Q., Zhao, W., Yang, Q., Wang, J., & Jiang, D. (2021). In Vitro Studies on Mg-Zn-Sn-Based Alloys Developed as a New Kind of Biodegradable Metal. Materials, 14(7), 1606. https://doi.org/10.3390/ma14071606





