The Effect of SCMs in Blended Cements on Sorption Characteristics of Superabsorbent Polymers
Abstract
1. Introduction
2. Materials and Research Methodology
2.1. Cements
2.2. Superabsorbent Polymers (SAPs)
2.3. Experimental Method
3. Characterisation of SAPs
3.1. Physical Characteristics of SAPs
3.2. Chemical Characteristics of SAPs
3.3. pH of SAP Solutions
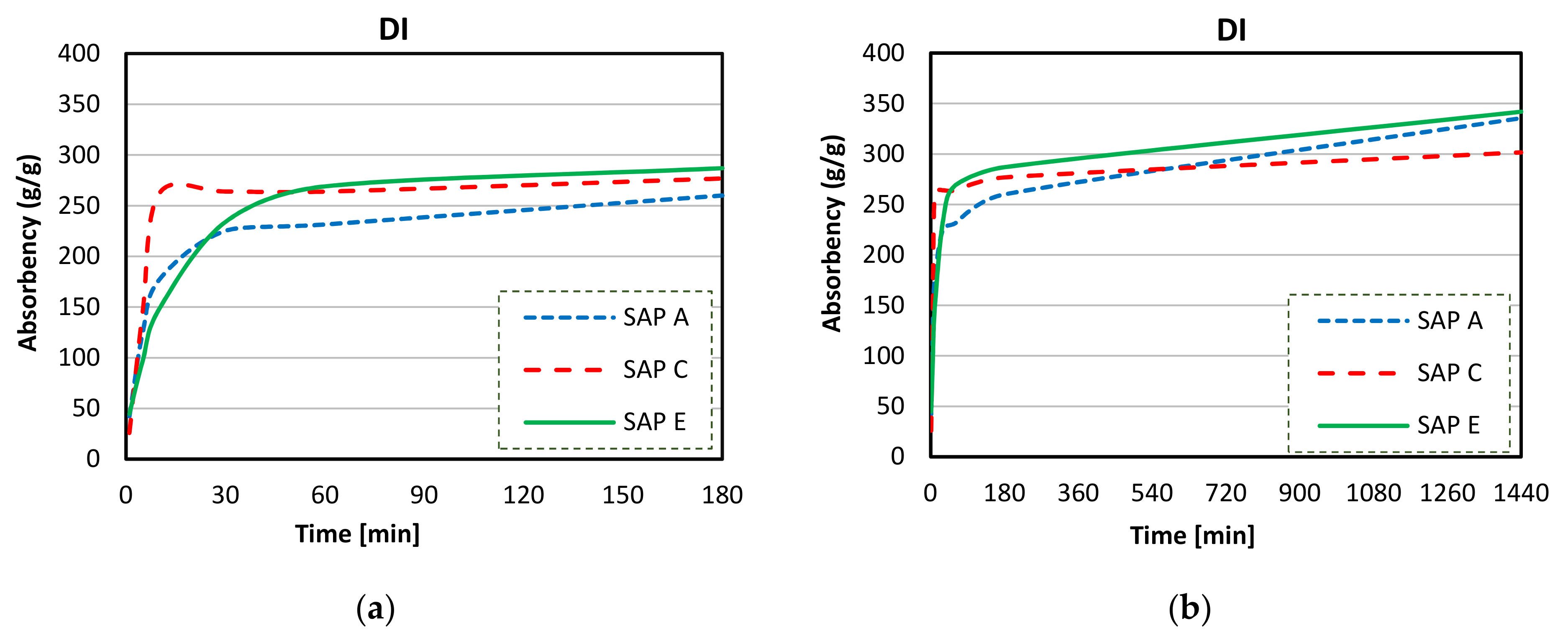
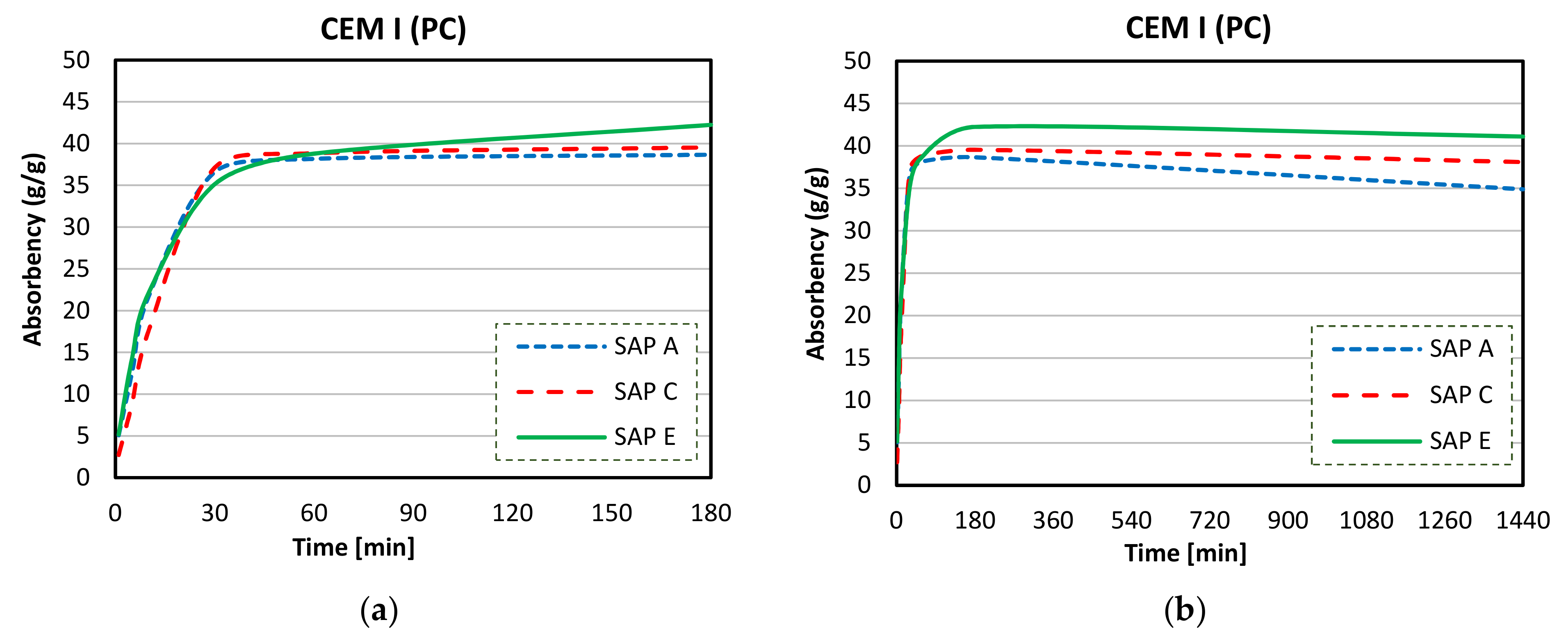
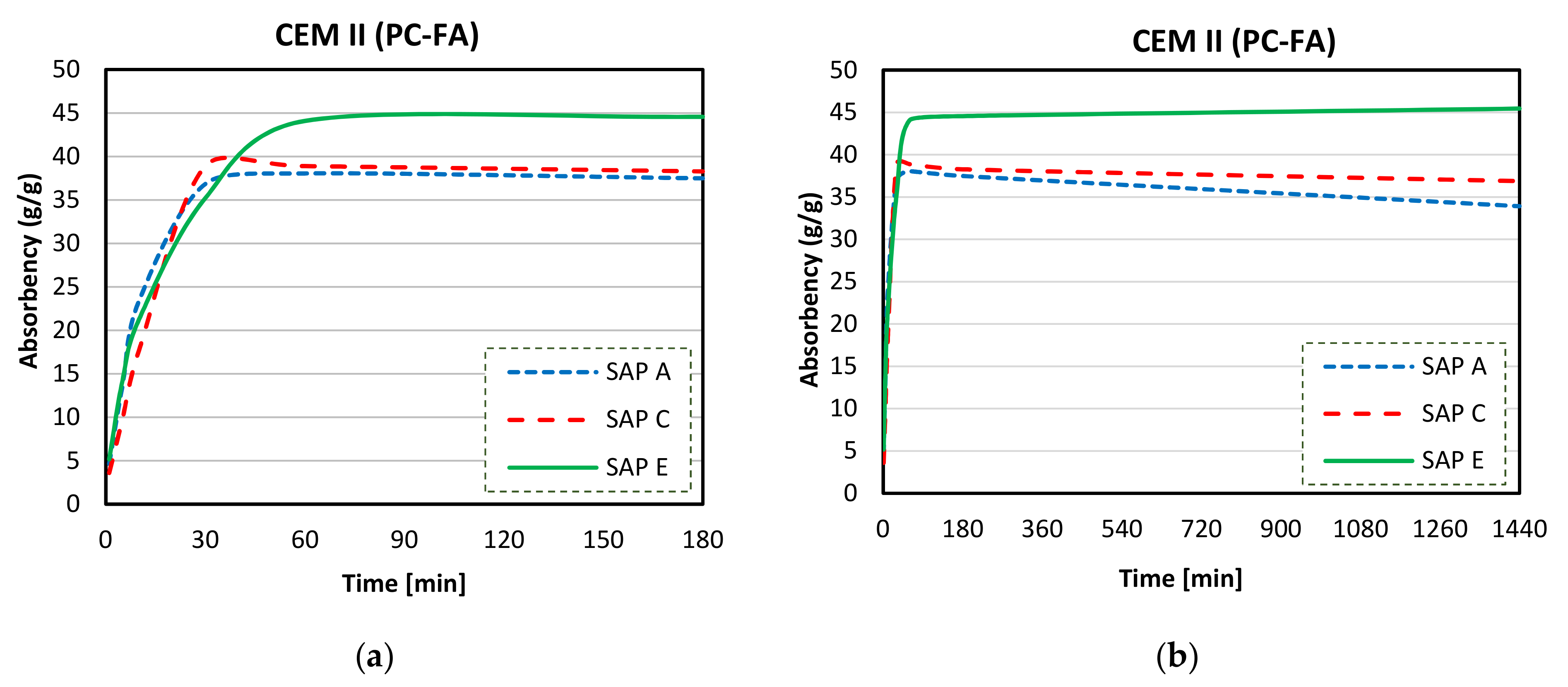
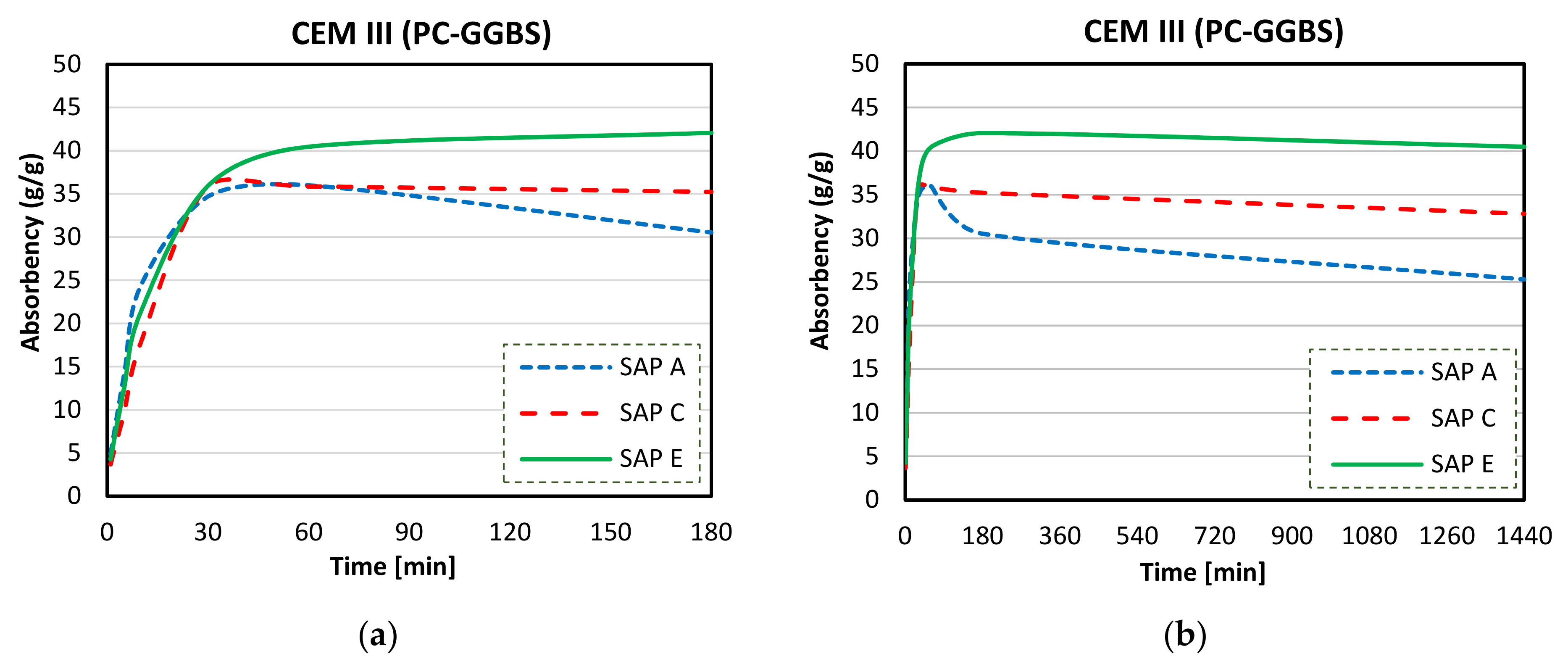
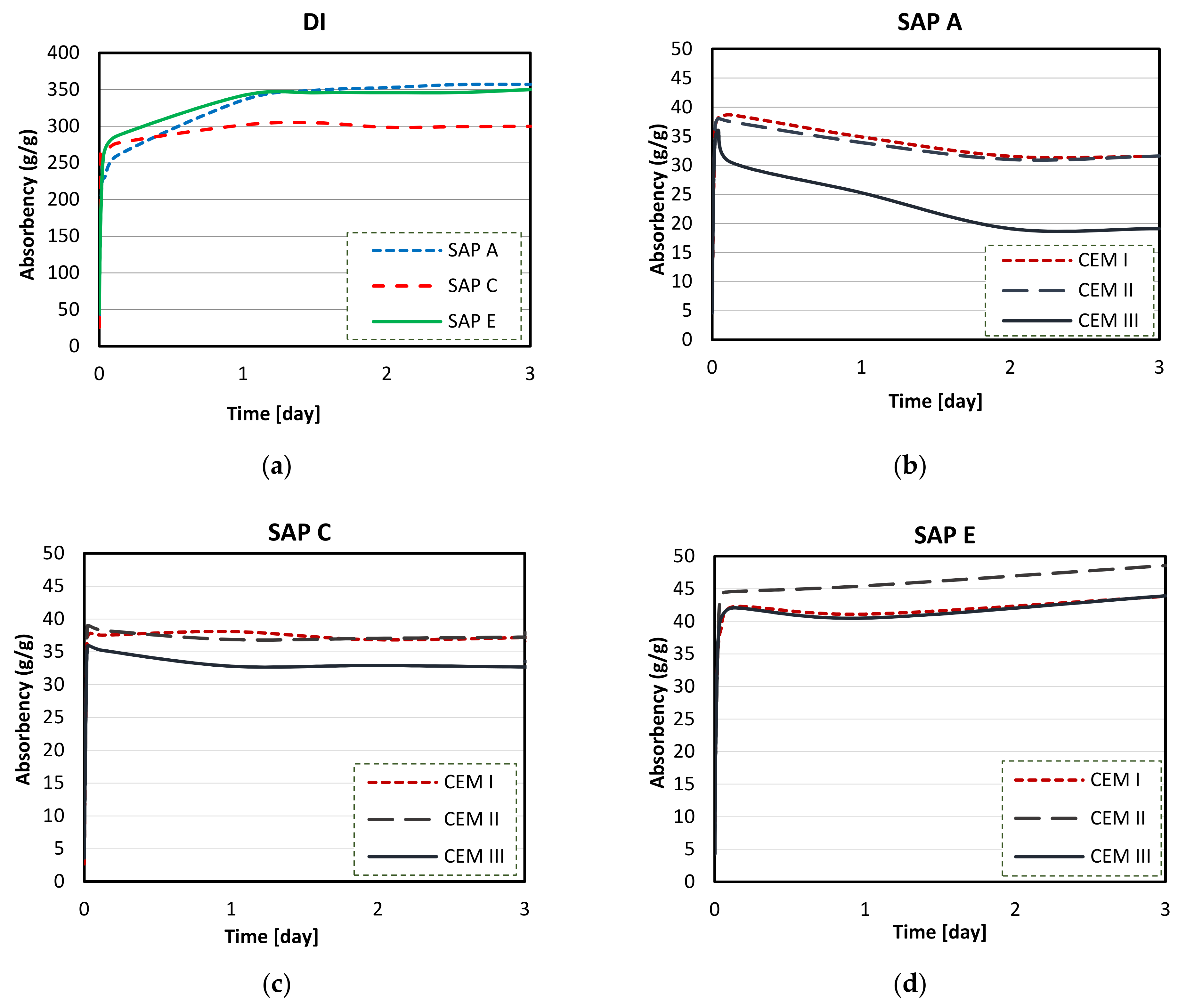
3.4. Absorption Capacity and Kinetics of SAPs
4. Conclusions
- CEM II (PC-FA) solution had the lowest pH due to lower alkalis concentration. Similar comments can be made for CEM III (PC-GGBS), which had lower pH than CEM I (PC) filtrate solution. This is due to the decreased alkali contents and hydroxide concentrations, which in turn are related to the pozzolanic reaction of FA; the pozzolanic reaction with portlandite leads to lower pH values of the pore solution. In the case of PC-GGBS, lower pH values could be associated with the formation of reduced sulphur species (HS−, SO32− and S2O32−). It was found that in all cementitious solutions, pH dropped considerably after the second day, which was most likely as a result of carbonation;
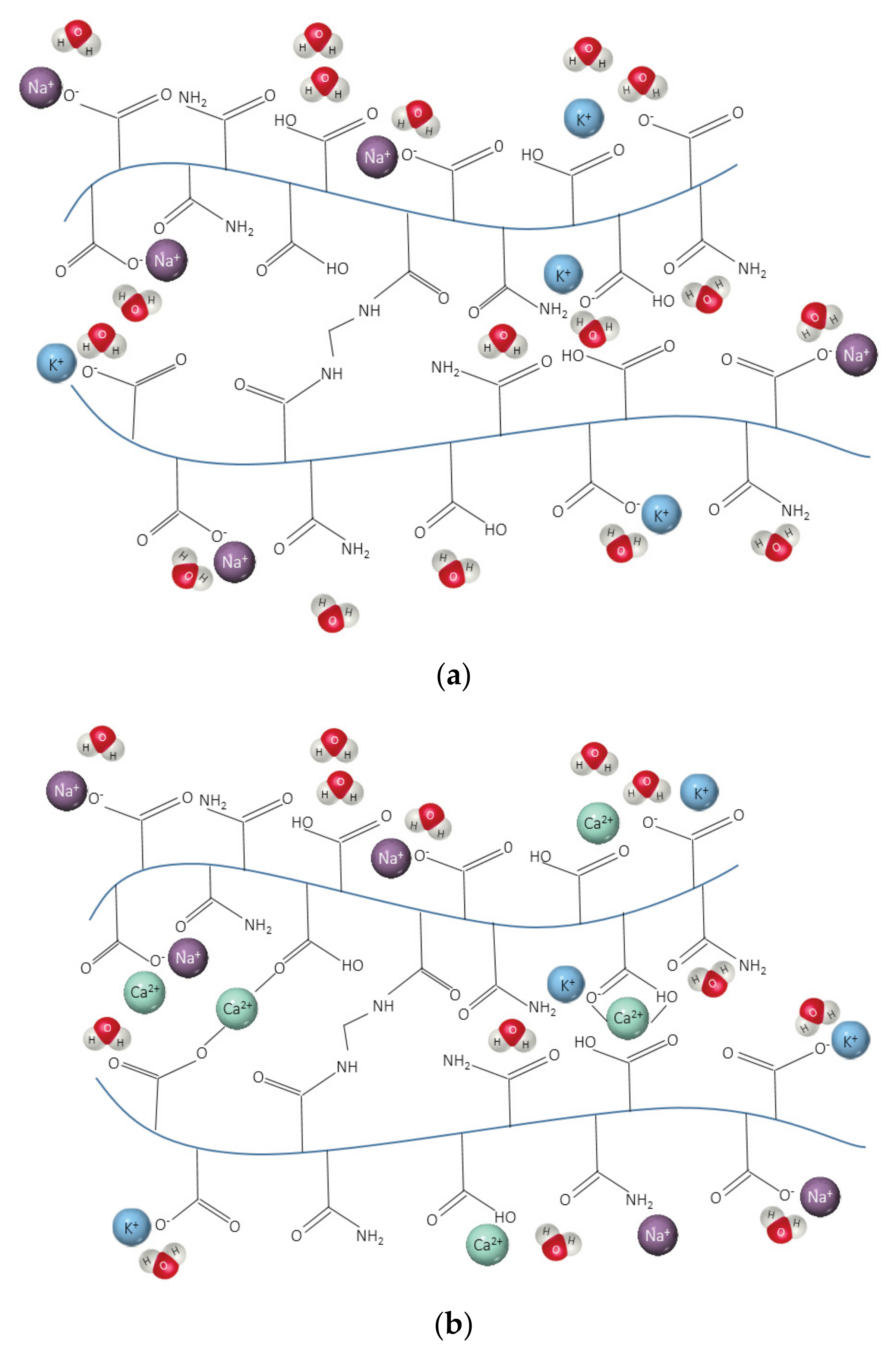
- SAPs in CEM I (PC) solution had a similar pH to their respective reference solution over time. However, in blended cements solutions, all SAPs displayed different behaviour depending on the type of polymer. This is the result of the complex formation between the anionic functional groups of the polymer and Ca2+, which decrease the efficient charge density of anionic groups. Overall, SAP E with a smaller particle size had the highest pH values, followed by SAP A and SAP C;
- Considerable reductions in swelling capacity were found for SAPs in cements solutions when compared to DI water. This could be attributed to the presence of dissolved ions in binder filtrates, especially K+, Na+, Mg2+, and Ca2+, which reduced the osmotic pressure and restricted swelling;
- SAP samples exhibited the highest absorption capacities in CEM II (PC-FA) solutions, and the lowest swelling capacities were found in CEM III (PC-GGBS) solutions. In particular, SAP E had the greatest WAC value for a PC-FA system due to smaller particle sizes, which resulted in an increased polymer network volume and specific surface area. Moreover, the lowest amount of K+ in SAP E increases osmotic pressure difference between the polymer and the cementitious aqueous solutions, affecting the diffusional processes involving the swelling behaviour of SAP;
- More stable swelling behaviour in cements filtrate solutions were observed for SAP C (specially for CEM I and CEM II systems). It stayed nearly unaltered over the time in both cements solutions, which may be related to its ideal alkali content, ability to form disulphide bonds, and potential influence of its molecular structure;
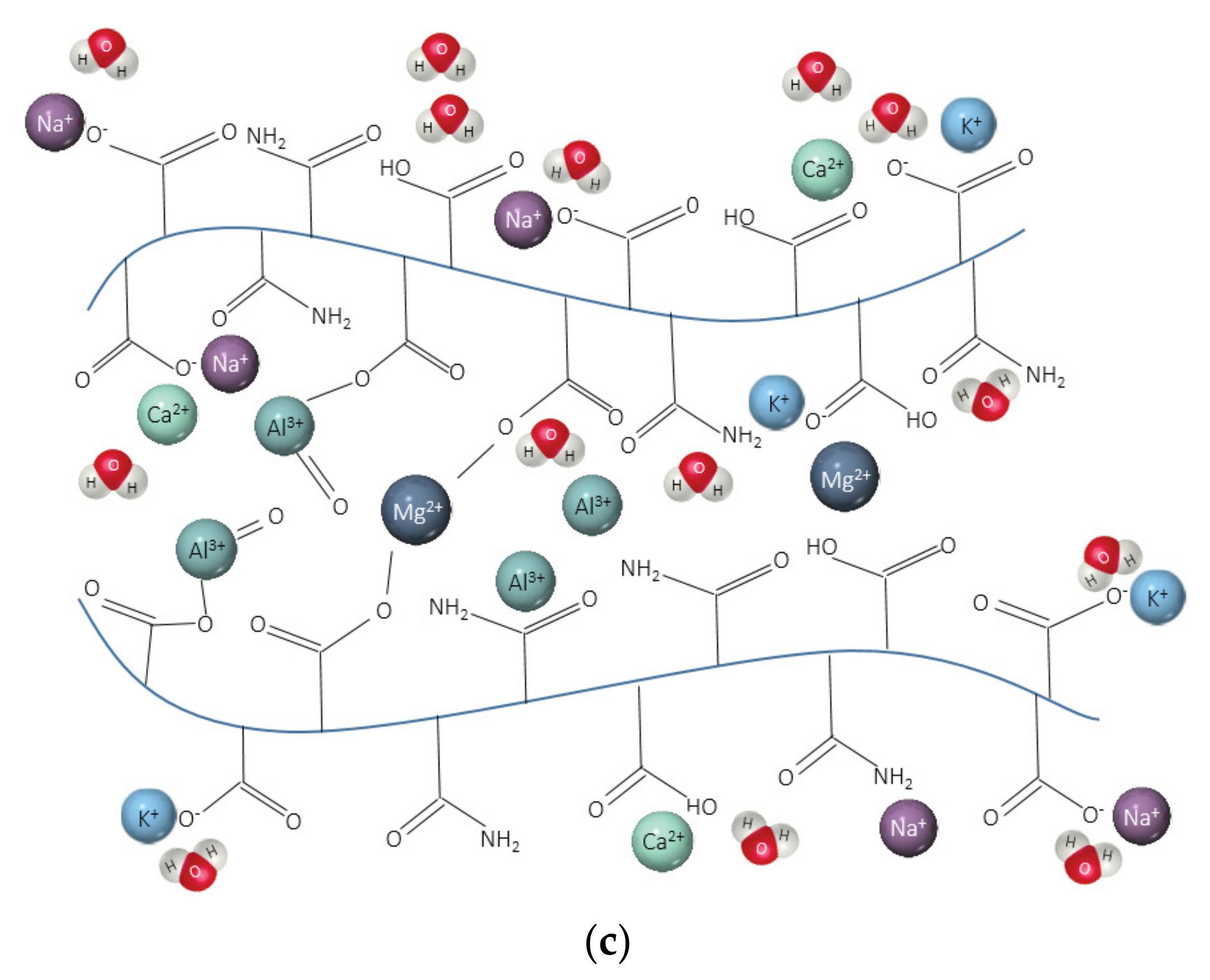
- The lower amount of divalent cations in CEM II solution compared to CEM III and CEM I led to the highest absorption capacities of SAPs. In blended cement solutions, these cations can form strong complexes with the polymer chain and consequently increase the crosslink density of the polymeric network. The chemical interaction of trivalent cations can also lead to the formation of complex bonds with those SAPs in blended cement environments, especially in CEM II (PC-FA), since amount of Al3+ concentration is higher than that of CEM I (PC).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Siddique, R.; Bennacer, R. Use of iron and steel industry by-product (GGBS) in cement paste and mortar. Resour. Conserv. Recycl. 2012, 69, 29–34. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Lothenbach, B.; De Belie, N.; Gruyaert, E.; Skibsted, J.; Snellings, R.; Vollpracht, A. TC 238-SCM: Hydration and microstructure of concrete with SCMs. Mater. Struct. 2015, 48, 835–862. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental impacts and decarbonization strategies in the cement and concrete industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Van den Heede, P.; De Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and ‘green’concretes: Literature review and theoretical calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Rostami, R.; Klemm, J.A. The influence of superabsorbent polymers on drying shrinkage of fibre reinforced mortars. In Proceedings of the 39th Cement and Concrete Science Conference, University of Bath, Bath, UK, 9–10 September 2019; pp. 54–57, ISBN 978-0-86197-201-2. [Google Scholar]
- Juenger, M.C.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Rostami, R.; Klemm, A.J.; Almeida, F.C.R. Combined effect of polymeric fibres and SAP on the performance of concrete and repair mortars: A review. In Long Abstracts, Proceedings of the Young Researchers’ Forum IV, Newcastle upon Tyne, UK, 9 April 2018; International Committee of the SCMT Conferences: Coventry, UK, 2013; p. 41. [Google Scholar]
- Almeida, F.C.R.; Klemm, A.J.; Rostami, R. Effect of aggregates on SAP performance in cementitious matrices with ground granulated blast-furnace slag. In Long Abstracts, Proceedings of the Young Researchers’ Forum IV, Newcastle upon Tyne, UK, 9 April 2018; International Committee of the SCMT Conferences: Coventry, UK, 2013; p. 97. [Google Scholar]
- Durdziński, P.T.; Haha, M.B.; Bernal, S.A.; De Belie, N.; Gruyaert, E.; Lothenbach, B.; Méndez, E.M.; Provis, J.L.; Schöler, A.; Stabler, C.; et al. Outcomes of the RILEM round robin on degree of reaction of slag and fly ash in blended cements. Mater. Struct. 2017, 50, 135. [Google Scholar] [CrossRef]
- Properties of Fresh and Hardened Concrete Containing Supplementary Cementitious Materials; De Belie, N., Soutsos, M., Gruyaert, E., Eds.; Springer: Berlin, Germany, 2018; Volume 25. [Google Scholar]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Xu, G.; Tian, Q.; Miao, J.; Liu, J. Early-age hydration and mechanical properties of high volume slag and fly ash concrete at different curing temperatures. Constr. Build. Mater. 2017, 149, 367–377. [Google Scholar] [CrossRef]
- Izquierdo, D.; Alonso, C.; Andrade, C.; Castellote, M. Potentiostatic determination of chloride threshold values for rebar depassivation: Experimental and statistical study. Electrochim. Acta 2004, 49, 2731–2739. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete: A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Teng, S.; Lim, T.Y.D.; Sabet Divsholi, B. Durability and mechanical properties of high strength concrete incorporating ultrafine ground granulated blast-furnace slag. Constr Build Mater 2013, 40, 875–881. [Google Scholar] [CrossRef]
- Gruyaert, E. Effect of Blast-Furnace Slag as Cement Replacement on Hydration, Microstructure, Strength and Durability of Concrete. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2011; p. 345. [Google Scholar]
- Tikalsky, P.J.; Carrasquillo, R.L.; Snow, P.G. Sulfate resistance of concrete containing fly ash. Spec. Publ. 1992, 131, 255–266. [Google Scholar]
- Yu, C.; Sun, W.; Scrivener, K. Degradation mechanism of slag blended mortars immersed in sodium sulfate solution. Cem. Concr. Res. 2015, 72, 37–47. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Moreira, M. Pore Structure in Blended Cement Pastes. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2011. [Google Scholar]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Aranda, M.A.; Santacruz, I. Hydration studies of calcium sulfoaluminate cements blended with fly ash. Cem. Concr. Res. 2013, 54, 12–20. [Google Scholar] [CrossRef]
- Vollpracht, A.; Lothenbach, B.; Snellings, R.; Haufe, J. The pore solution of blended cements: A review. Mater. Struct. 2016, 49, 3341–3367. [Google Scholar] [CrossRef]
- Ioannou, S.; Reig, L.; Paine, K.; Quillin, K. Properties of a ternary calcium sulfoaluminate–calcium sulfate–fly ash cement. Cem. Concr. Res. 2014, 56, 75–83. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, Y.; Wang, Y.; Zhou, Y.; Ma, C. Autogenous shrinkage of high-performance concrete containing mineral admixtures under different curing temperatures. Constr. Build. Mater. 2014, 61, 260–269. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, H.K.; Lee, S.H.; Kim, G.Y. Autogenous shrinkage of concrete containing granulated blast-furnace slag. Cem. Concr. Res. 2006, 36, 1279–1285. [Google Scholar] [CrossRef]
- Moghaddam, F.; Sirivivatnanon, V.; Vessalas, K. The effect of fly ash fineness on heat of hydration, microstructure, flow and compressive strength of blended cement pastes. Case Stud. Constr. Mater. 2019, 10, e00218. [Google Scholar] [CrossRef]
- Almeida, F.C.; Klemm, A.J. Efficiency of internal curing by Superabsorbent Polymers (SAP) in PC-GGBS mortars. Cem. Concr. Compos. 2018, 88, 41–51. [Google Scholar] [CrossRef]
- Rostami, R.; Klemm, A.J. Influence of superabsorbent polymers on properties of fibre-reinforced mortars containing flyashes. Roads Bridges 2020, 19, 149–163. [Google Scholar]
- Almeida, F.C.; Rostami, R.; Klemm, A.J. The Effect of SAP on Volumetric Changes and Microstructural Alterations in PC-GGBS Matrices. In 3rd International Conference on the Application of Superabsorbent Polymers (SAP) and Other New Admixtures Towards Smart Concrete, Proceedings of the International Conference on Application of Superabsorbent Polymers & Other New Admixtures Towards Smart Concrete, Skukuza, South Africa, 25–27 November 2019; Springer: Berlin/Heidelberg, Germany, 2019; pp. 97–105. [Google Scholar]
- Snoeck, D.; Jensen, O.M.; De Belie, N. The influence of superabsorbent polymers on the autogenous shrinkage properties of cement pastes with supplementary cementitious materials. Cem. Concr. Res. 2015, 74, 59–67. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Lura, P. September. Reduction of autogenous shrinkage in OPC and BFSC pastes with internal curing. In Proceedings of the XIII International Conference on Durability of Building Materials and Components, São Paulo, Brazil, 2–5 September 2014; pp. 2–5. [Google Scholar]
- Berodier, E.; Scrivener, K. Evolution of pore structure in blended systems. Cem. Concr. Res. 2015, 73, 25–35. [Google Scholar] [CrossRef]
- Application of Super Absorbent Polymers (SAP) in Concrete Construction: State-Of-The-Art Report Prepared by Technical Committee 225-SAP; Mechtcherine, V., Reinhardt, H.W., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Mechtcherine, V.; Gorges, M.; Schroefl, C.; Assmann, A.; Brameshuber, W.; Ribeiro, A.B.; Cusson, D.; Custˇdio, J.; da Silva, E.F.; Ichimiya, K.; et al. Effect of internal curing by using Superabsorbent Polymers (SAP) on autogenous shrinkage and other properties of a high-performance fine-grained concrete: Results of a RILEM round-robin test. Mater. Struct. 2014, 47, 541–562. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Igarashi, S.I.; Lura, P.; Mechtcherine, V. Recommendation of RILEM TC 260-RSC: Using superabsorbent polymers (SAP) to mitigate autogenous shrinkage. Mater. Struct. 2018, 51, 135. [Google Scholar] [CrossRef]
- Rostami, R.; Klemm, A.J. Effect of Superabsorbent Polymers on Plastic Shrinkage Cracking and Properties of Fresh State Mortars Reinforced by Polymeric Fibres. In Proceedings of the 2nd RILEM Spring Convention & International Conference on Sustainable Materials, New Generation of Construction Materials (SMSS2019), Rovinj, Croatia, 18–22 March 2019; pp. 606–613. [Google Scholar]
- Klemm, A.J.; Sikora, K.S. The effect of Superabsorbent Polymers (SAP) on microstructure and mechanical properties of fly ash cementitious mortars. Constr. Build. Mater. 2013, 49, 134–143. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Schroefl, C.; Wyrzykowski, M.; Gorges, M.; Lura, P.; Cusson, D.; Margeson, J.; De Belie, N.; Snoeck, D.; Ichimiya, K.; et al. Effect of Superabsorbent Polymers (SAP) on the freeze–thaw resistance of concrete: Results of a RILEM interlaboratory study. Mater. Struct. 2017, 50, 14. [Google Scholar] [CrossRef]
- Snoeck, D.; Steuperaert, S.; Van Tittelboom, K.; Dubruel, P.; De Belie, N. Visualization of water penetration in cementitious materials with superabsorbent polymers by means of neutron radiography. Cem. Concr. Res. 2012, 42, 1113–1121. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Potential of superabsorbent polymer for self-sealing cracks in concrete. Adv. Appl. Ceram. 2010, 109, 296–302. [Google Scholar] [CrossRef]
- Snoeck, D.; Dewanckele, J.; Cnudde, V.; De Belie, N. X-ray computed microtomography to study autogenous healing of cementitious materials promoted by superabsorbent polymers. Cem. Concr. Compos. 2016, 65, 8–93. [Google Scholar] [CrossRef]
- Snoeck, D.; Van Tittelboom, K.; Steuperaert, S.; Dubruel, P.; De Belie, N. Self-healing cementitious materials by the combination of microfibres and superabsorbent polymers. J. Intell. Mater. Syst. Struct. 2014, 25, 13–24. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Snoeck, D.; Schröfl, C.; De Belie, N.; Klemm, A.J.; Ichimiya, K.; Moon, J.; Wyrzykowski, M.; Lura, P.; Toropovs, N. Testing superabsorbent polymer (SAP) sorption properties prior to implementation in concrete: 642 results of a RILEM Round-Robin Test. Mater. Struct. 2018, 51, 28. [Google Scholar] [CrossRef]
- Almeida, F.C.; Klemm, A.J. Effect of GGBS on water absorption capacity and stability of superabsorbent polymers partially crosslinked with alkalis. J. Mater. Civ. Eng. 2018, 30, 04018315. [Google Scholar] [CrossRef]
- Schroefl, C.; Mechtcherine, V.; Vontobel, P.; Hovind, J.; Lehmann, E. Sorption kinetics of superabsorbent polymers (SAPs) in fresh Portland cement-based pastes visualized and quantified by neutron radiography and correlated to the progress of cement hydration. Cem. Concr. Res. 2015, 75, 1–13. [Google Scholar] [CrossRef]
- Schröfl, C.; Snoeck, D.; Mechtcherine, V. A review of characterisation methods for superabsorbent polymer (SAP) samples to be used in cement-based construction materials: Report of the RILEM TC 260-RSC. Mater. Struct. 2017, 50, 197. [Google Scholar] [CrossRef]
- Schröfl, C.; Mechtcherine, V.; Gorges, M. Relation between the molecular structure and the efficiency of superabsorbent polymers (SAP) as concrete admixture to mitigate autogenous shrinkage. Cem. Concr. Res. 2012, 42, 865–873. [Google Scholar] [CrossRef]
- Almeida, F.C.; Rostami, R.; Klemm, A.J. Characterization of Polyacrylamide based Superabsorbent Polymers for potential use in PC Matrices with Supplementary Cementitious Materials. In Proceedings of the MATEC Web of Conferences, Cape Town, South Africa, 19–21 November 2018; EDP Sciences: Les Ulis, France, 2018; Volume 199, p. 02023. [Google Scholar]
- Snoeck, D.; Schroefl, C.; Mechtcherine, V. Recommendation of RILEM TC 260-RSC: Testing sorption by superabsorbent polymers (SAP) prior to implementation in cement-based materials. Mater. Struct. 2018, 51, 116. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Secrieru, E.; Schröfl, C. Effect of superabsorbent polymers (SAPs) on rheological properties of fresh cement-based mortars—Development of yield stress and plastic viscosity over time. Cem. Concr. Res. 2015, 67, 52–65. [Google Scholar] [CrossRef]
- Mignon, A.; Graulus, G.J.; Snoeck, D.; Martins, J.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. pH-sensitive superabsorbent polymers: A potential candidate material for self-healing concrete. J. Mater. Sci. 2015, 50, 970–979. [Google Scholar] [CrossRef]
- Krafcik, M.J.; Erk, K.A. Characterization of superabsorbent poly (sodium-acrylate acrylamide) hydrogels and influence of chemical structure on internally cured mortar. Mater. Struct. 2016, 49, 4765–4778. [Google Scholar] [CrossRef]
- Mignon, A.; Vagenende, M.; Martins, J.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. Development of amine-based pH-responsive superabsorbent polymers for mortar applications. Constr. Build. Mater. 2017, 132, 556–564. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar] [CrossRef]
- Jensen, O.M. Water absorption of superabsorbent polymers in a cementitious environment. In Proc. Int. RILEM Conf. on Advances in Construction Materials through Science and Engineering; Leung, C., Wan, K.T., Eds.; RILEM Publications S.A.R.L.: Hong Kong, China, 2011; pp. 22–35. [Google Scholar]
- Tanaka, T.; Nishio, I.; Sun, S.T.; Ueno-Nishio, S. Collapse of gels in an electric field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Chu, M.; Zhu, S.Q.; Huang, Z.B.; Li, H.M. Influence of potassium humate on the swelling properties of a poly (acrylic acid-co-acrylamide)/potassium humate superabsorbent composite. J. Appl. Polym. Sci. 2008, 107, 3727–3733. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Wang, A. Synthesis, characterization and water absorbency properties of poly (acrylic acid)/sodium humate superabsorbent composite. Polym. Adv. Technol. 2005, 16, 675–680. [Google Scholar] [CrossRef]
- Brandt, N.N.; Chikishev, A.Y.; Kruzhilin, V.N. Raman study of the cleavage of disulphide bonds in albumin, chymotrypsin, and thrombin. Vib. Spectrosc. 2017, 89, 75–80. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Haha, M.B.; Figi, R.; Wieland, E. Hydration of a low-alkali CEM III/B–SiO2 cement (LAC). Cem. Concr. Res. 2012, 42, 410–423. [Google Scholar] [CrossRef]
- Chen, Y.W.; Binti Hassan, S.H.; Yahya, M.; Lee, H.V. Novel superabsorbent cellulose-based hydrogels: Present status, synthesis, characterization, and application prospects. Cellul. Based Superabsorbent Hydrogels 2019, 155–195. [Google Scholar] [CrossRef]
- Thomas, M. The effect of supplementary cementing materials on alkali-silica reaction: A review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Tachibana, Y.; Baba, T.; Kasuya, K.I. Environmental biodegradation control of polymers by cleavage of disulfide bonds. Polym. Degrad. Stab. 2017, 137, 67–74. [Google Scholar] [CrossRef]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Mignon, A.; Snoeck, D.; Schaubroeck, D.; Luickx, N.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. pH-responsive superabsorbent polymers: A pathway to self-healing of mortar. React. Funct. Polym. 2015, 93, 68–76. [Google Scholar] [CrossRef]
- Dho, S.K.; Choi, C.H. Effects of divalent cations on alkaline hydrolysis of poly (ethylene terephthalate) fabric. Text. Coloration Finish. 1995, 7, 61–73. [Google Scholar]
- Double, D.D. New developments in understanding the chemistry of cement hydration. Philos. Trans. R. Soc. A Lond. Ser. Math. Phys. Sci. 1983, 310, 53–66. [Google Scholar]
- Snoeck, D. Self-Healing and Microstructure of Cementitious Materials with Microfibres and Superabsorbent Polymers. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2015. [Google Scholar]
- Jar, P.B.; Wu, Y.S. Effect of counter-ions on swelling and shrinkage of polyacrylamide-based ionic gels. Polymer 1997, 38, 2557–2560. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Effect of alkalinity and calcium concentration of pore solution on the swelling and ionic exchange of superabsorbent polymers in cement paste. Cem. Concr. Compos. 2018, 88, 150–164. [Google Scholar] [CrossRef]
- Zhu, Q.; Barney, C.W.; Erk, K.A. Effect of ionic crosslinking on the swelling and mechanical response of model superabsorbent polymer hydrogels for internally cured concrete. Mater. Struct. 2015, 48, 2261–2276. [Google Scholar] [CrossRef]
- Bahaj, H.; Benaddi, R.; Bakass, M.; Bayane, C. Swelling of superabsorbents polymers in an aqueous medium. J. Appl. Polym. Sci. 2010, 115, 2479–2484. [Google Scholar] [CrossRef]

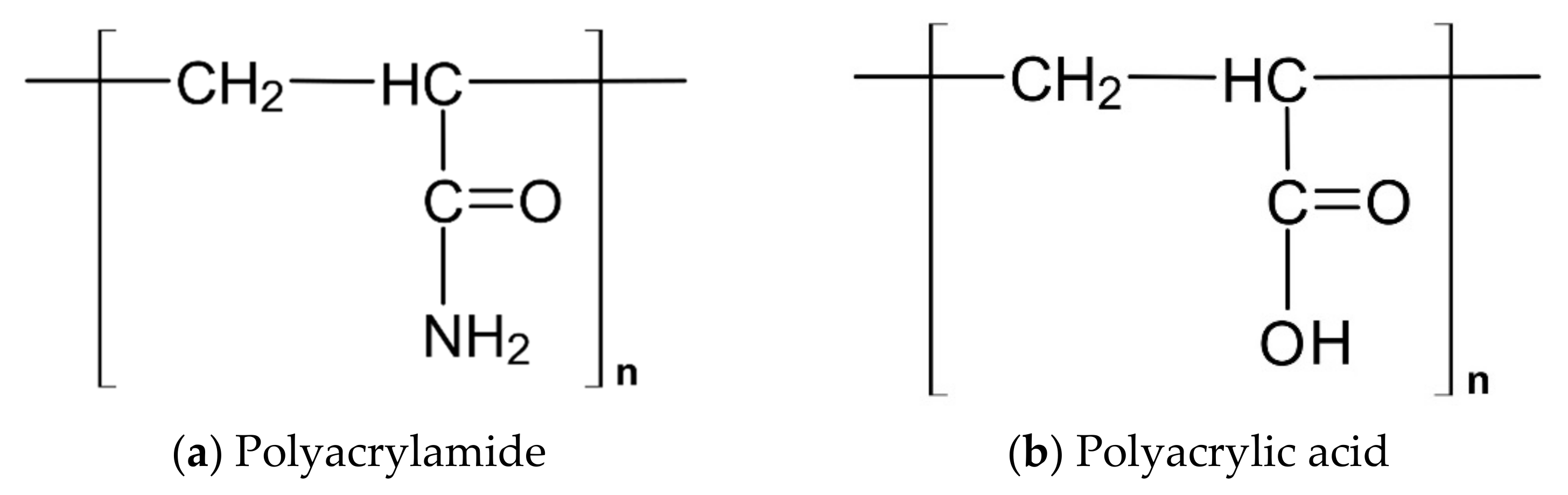
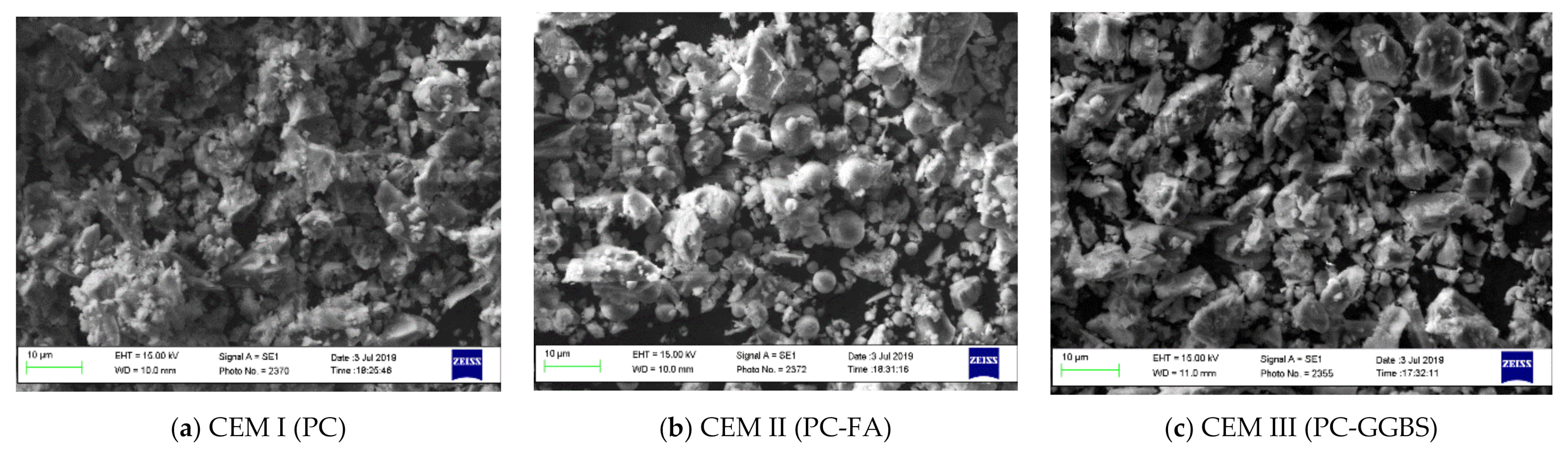
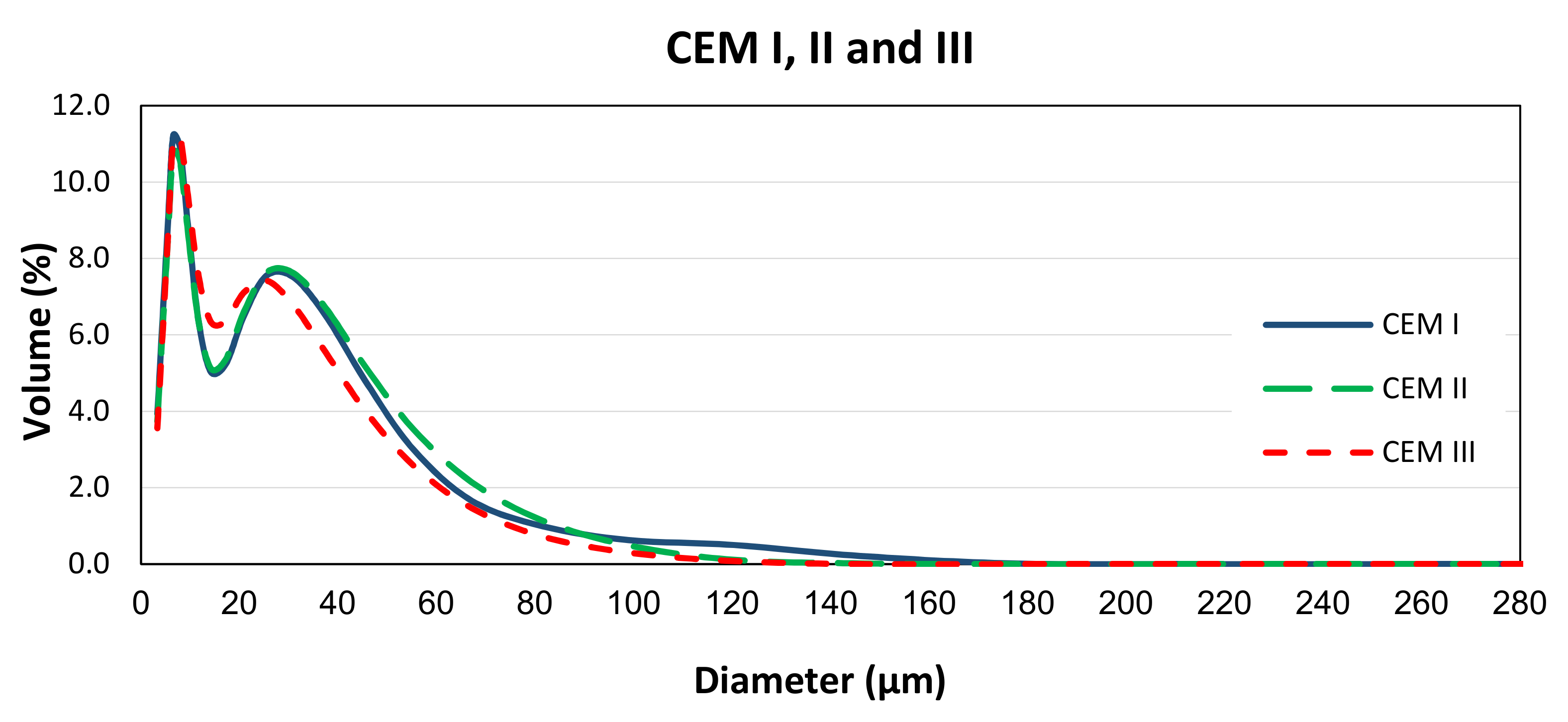
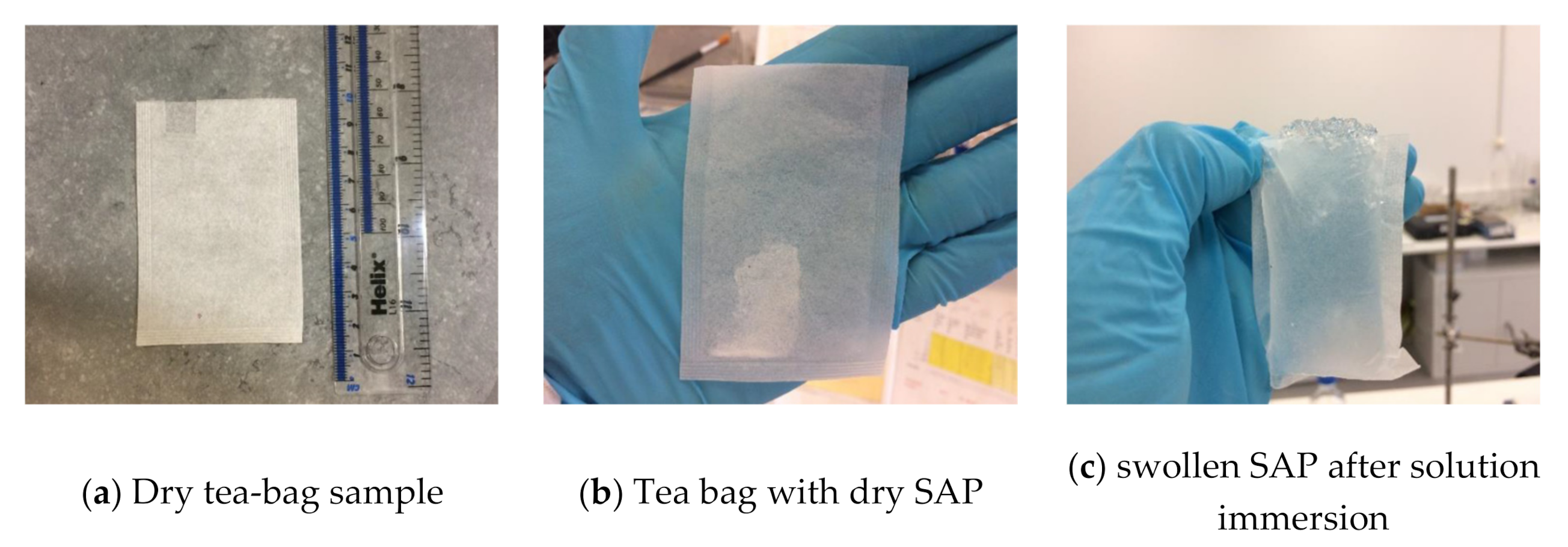
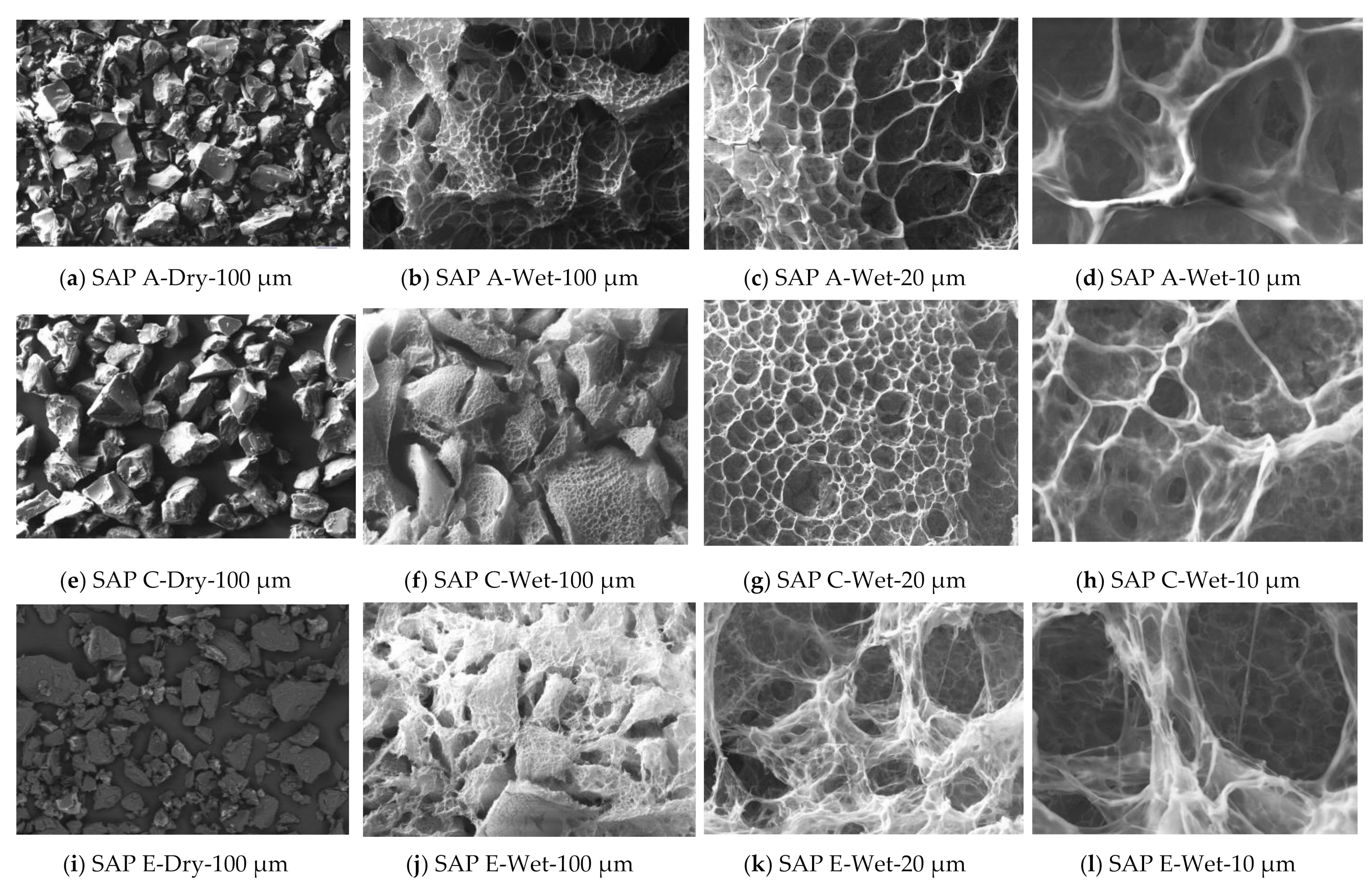
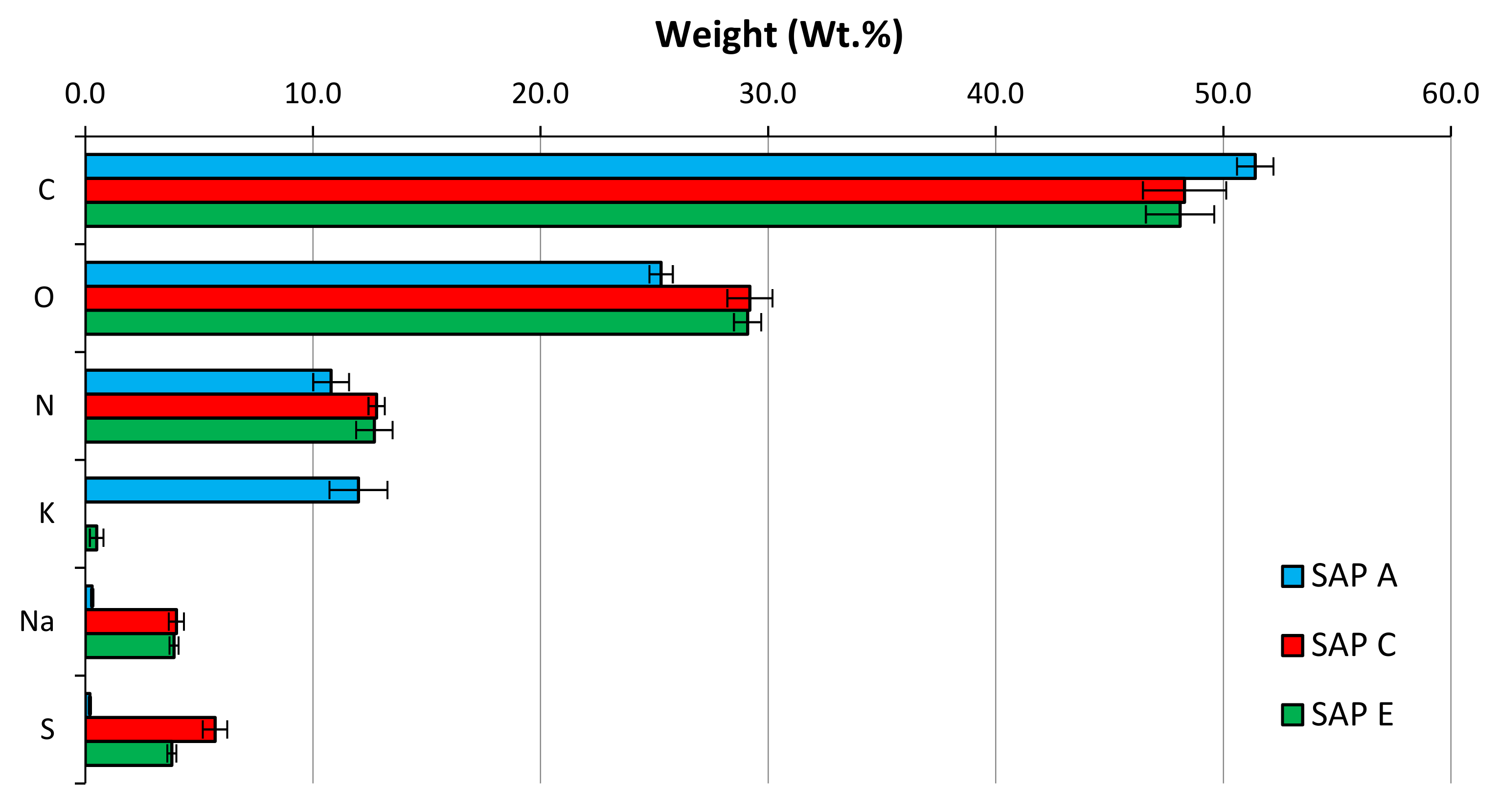
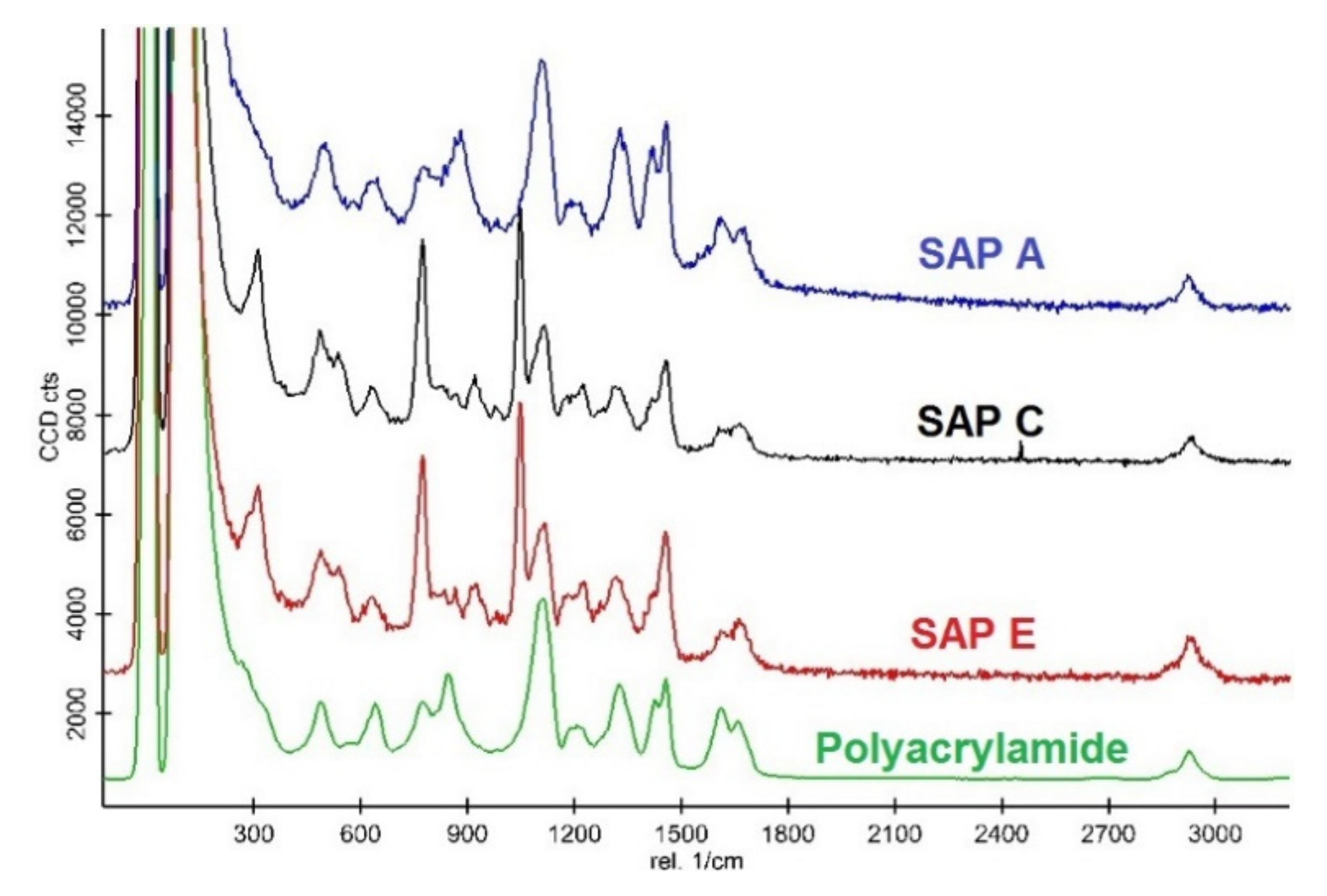


| Compound (%) | CEM I (PC) | CEM II/B-V (PC-FA) | CEM III/A (PC-GGBS) |
|---|---|---|---|
| SCM (%) | 0 | 30 | 50 |
| CaO | 64.3 | 43.48 | 57.13 |
| SiO2 | 20.76 | 32.69 | 24.50 |
| Al2O3 | 4.99 | 13.13 | 8.99 |
| MgO | 2.19 | 1.33 | 5.33 |
| Fe2O3 | 2.57 | 3.29 | 1.76 |
| K2O | 0.27 | 1.26 | - |
| Cl | 0.06 | - | 0.04 |
| MnO | - | 0.07 | 5.33 |
| TiO2 | - | 0.56 | 0.58 |
| ZnO | - | 0.02 | - |
| Mn3O4 | - | - | 0.16 |
| Loss on ignition | 2.39 | 0.16 | 1.19 |
| SAP Type | Elementary Molecular Structure | Particle Grading |
|---|---|---|
| SAP A | Copolymer of acrylamide and acrylic acid | Coarser |
| SAP C | Modified polyacrylamide | coarser |
| SAP E | Modified polyacrylamide | finer |
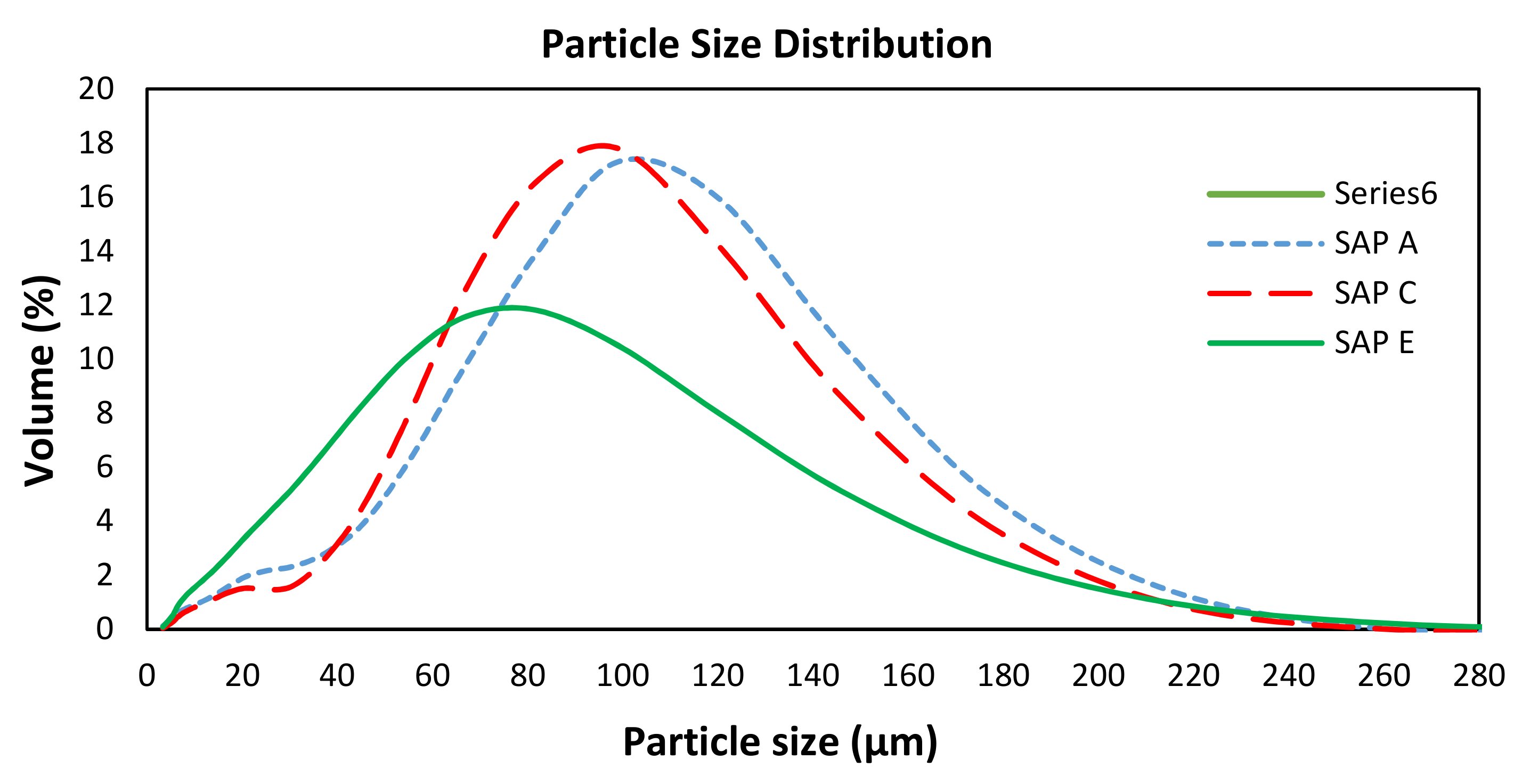
| Equivalent Diameter | SAP A | SAP C | SAP E | |||
|---|---|---|---|---|---|---|
| Average (µm) | SD (µm) | Average (µm) | SD (µm) | Average (µm) | SD (µm) | |
| d(v, 0.5) 1 | 89.55 | 0.36 | 84.88 | 0.53 | 61.45 | 0.66 |
| d(v, 0.1) 2 | 26.01 | 0.67 | 32.96 | 1.59 | 17.76 | 0.13 |
| Mode 3 | 102.51 | 0.43 | 95.19 | 0.44 | 76.72 | 0.22 |
| d(v, 0.9) 4 | 147.24 | 0.50 | 140.00 | 0.76 | 127.71 | 1.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostami, R.; Klemm, A.J.; Almeida, F.C.R. The Effect of SCMs in Blended Cements on Sorption Characteristics of Superabsorbent Polymers. Materials 2021, 14, 1609. https://doi.org/10.3390/ma14071609
Rostami R, Klemm AJ, Almeida FCR. The Effect of SCMs in Blended Cements on Sorption Characteristics of Superabsorbent Polymers. Materials. 2021; 14(7):1609. https://doi.org/10.3390/ma14071609
Chicago/Turabian StyleRostami, Rohollah, Agnieszka J. Klemm, and Fernando C. R. Almeida. 2021. "The Effect of SCMs in Blended Cements on Sorption Characteristics of Superabsorbent Polymers" Materials 14, no. 7: 1609. https://doi.org/10.3390/ma14071609
APA StyleRostami, R., Klemm, A. J., & Almeida, F. C. R. (2021). The Effect of SCMs in Blended Cements on Sorption Characteristics of Superabsorbent Polymers. Materials, 14(7), 1609. https://doi.org/10.3390/ma14071609








