Analysis of the Effect of Conditions of Preparation of Nitrogen-Doped Activated Carbons Derived from Lotus Leaves by Activation with Sodium Amide on the Formation of Their Porous Structure
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion of the Obtained Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vunain, E.; Houndedjihou, D.; Monjerezi, M.; Muleja, A.A.; Kodom, B.T. Adsorption, kinetics and equilibrium studies on removal of catechol and resorcinol from aqueous solution using low-cost activated carbon prepared from sunflower (helianthus annuus) seed hull residues. Water Air Soil Pollut. 2018, 229, 366. [Google Scholar] [CrossRef]
- Guo, J.; Song, Y.; Ji, X.; Ji, L.; Cai, L.; Wang, Y.; Zhang, H.; Song, W. Preparation and characterization of nanoporous activated carbon derived from prawn shell and its application for removal of heavy metal ions. Materials 2019, 12, 241. [Google Scholar] [CrossRef]
- Wu, H.; Chen, R.; Du, H.; Zhang, J.; Shi, L.; Qin, Y.; Yue, L.; Wang, J. Synthesis of activated carbon from peanut shell as dye adsorbents for wastewater treatment. Adsorp. Sci. Technol. 2019, 37, 34–48. [Google Scholar] [CrossRef]
- Bernal, V.A.; Giraldo, L.; Moreno-Piraján, J.; Balsamo, M.; Erto, A. Mechanisms of methylparaben adsorption onto activated carbons: Removal tests supported by a calorimetric study of the adsorbent–adsorbate interactions. Molecules 2019, 24, 413. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Liu, S.; Wang, L.; Wu, J.; Wu, Z.; Ma, C.; Yu, Q.; Hu, X. Novel nitrogen-doped porous carbons derived from graphene for effective CO2 capture. Ind. Eng. Chem. Res. 2019, 58, 3349–3358. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E. An analysis of the porous structure of activated carbons obtained from hazelnut shells by various physical and chemical methods of activation. Coll. Surf. A 2017, 529, 443–453. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Larbia, K.; Benderdouche, N.; Reinert, L.; Lévéque, J.M.; Delpeux-Ouldriane, S.; Benadjemia, M.; Bestani, B.; Duclaux, L. Tailored activated carbons prepared by phosphoric activation of apricot, date and loquat stones and their mixtures; relation between the pore size and the composition in biopolymer. Desalin. Water Treat. 2018, 120, 217–227. [Google Scholar] [CrossRef]
- Veksha, A.; Bhuiyan, T.I.; Hill, J.M. Activation of aspen wood with carbon dioxide and phosphoric acid for removal of total organic carbon from oil sands produced water: Increasing the yield with bio-oil recycling. Materials 2016, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Rao, L.; Yang, P.; Wang, X.; Wang, L.; Ma, R.; Yue, L.; Hu, X. Superior CO2 uptake on nitrogen doped carbonaceous adsorbents from commercial phenolic resin. J. Environ. Sci. 2020, 93, 109–116. [Google Scholar] [CrossRef]
- Ma, R.; Qin, X.; Liu, Z.; Fu, Y. Adsorption property, kinetic and equilibrium studies of activated carbon fiber prepared from liquefied wood by ZnCl2 activation. Materials 2019, 12, 1377. [Google Scholar] [CrossRef]
- Mamyrbekova, A.; Mamitova, A.D.; Kassymova, M.K.; Mamyrbekova, A.; Daribayev, Z.E.; Pralieva, R.E.; Yermakhanov, M.N.; Mutasheva, G.S. Kinetics of sulfur dioxide adsorption on modified activated coal produced from peach shells. Rasayan J. Chem. 2020, 13, 2332–2339. [Google Scholar] [CrossRef]
- Rao, L.; Yue, L.; Wang, L.; Wu, Z.; Ma, C.; An, L.; Hu, X. Low-temperature and single-step synthesis of N-doped porous carbons with a high CO2 adsorption performance by sodium amide activation. Energy Fuels 2018, 32, 10830–10837. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.-L.; Zhang, J.-Y.; Tao, D.-J.; Liu, F.; Dai, S. Simultaneous activation and N-doping of hydrothermal carbons by NaNH2: An effective approach to CO2 adsorbents. J. CO2 Util. 2019, 33, 405–412. [Google Scholar] [CrossRef]
- Liu, S.; Ma, R.; Hu, X.; Wang, L.; Wang, X.; Radosz, M.; Fan, M. CO2 adsorption on hazelnut-shell-derived nitrogen-doped porous carbons synthesized by single-step sodium amide activation. Ind. Eng. Chem. Res. 2020, 59, 7046–7053. [Google Scholar] [CrossRef]
- Moura, F.C.C.; Rios, R.D.F.; Galvão, B.R.L. Emerging contaminants removal by granular activated carbon obtained from residual Macauba biomass. Environ. Sci. Pollut. Res. 2018, 25, 26482–26492. [Google Scholar] [CrossRef]
- Yan, S.; Lin, J.; Liu, P.; Zhao, Z.; Lian, J.; Chang, W.; Yao, L.; Liu, Y.; Lin, H.; Han, S. Preparation of nitrogen-doped porous carbons for high-performance supercapacitor using biomass of waste lotus stems. RSC Adv. 2018, 8, 6806–6813. [Google Scholar] [CrossRef]
- Ren, S.; Deng, L.; Zhang, B.; Lei, Y.; Ren, H.; Lv, J.; Zhao, R.; Chen, X. Effect of air oxidation on texture, surface properties and dye adsorption of wood-derived porous carbon materials. Materials 2019, 12, 1675. [Google Scholar] [CrossRef]
- Yokoyama, J.T.C.; Cazetta, A.L.; Bedin, K.C.; Spessato, L.; Fonseca, J.M.; Carraro, P.S.; Ronix, A.; Silva, M.C.; Silva, T.L.; Almeida, V.C. Stevia residue as new precursor of CO2-activated carbon: Optimization of preparation condition and adsorption study of triclosan. Ecotoxicol. Environ. Saf. 2019, 172, 403–410. [Google Scholar] [CrossRef]
- Kosheleva, R.I.; Mitropoulos, A.C.; Kyzas, G.Z. Synthesis of activated carbon from food waste. Environ. Chem. Lett. 2019, 17, 429–438. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefinery 2019, 9, 75–802. [Google Scholar] [CrossRef]
- Machrouhi, A.; Alilou, H.; Farnane, M.; El Hamidi, S.; Sadiq, M.; Abdennouri, M.; Tounsadi, H.; Barka, N. Statistical optimization of activated carbon from Thapsia transtagana stems and dyes removal efficiency using central composite design. J. Sci. Adv. Mater. Devices 2019, 4, 544–553. [Google Scholar] [CrossRef]
- Serafin, J.; Baca, M.; Biegun, M.; Mijowska, E.; Kalenczuk, R.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Direct conversion of biomass to nanoporous activated biocarbons for high CO2 adsorption and supercapacitor applications. Appl. Surf. Sci. 2019, 497, 143722. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Ma, M.-G.; Ji, X.-X. Preparation of lignin-based carbon materials and its application as a sorbent. Materials 2019, 12, 1111. [Google Scholar] [CrossRef]
- Yang, P.; Rao, L.; Zhu, W.; Wang, L.; Ma, R.; Chen, F.; Lin, G.; Hu, X. Porous carbons derived from sustainable biomass via a facile one-step synthesis strategy as efficient CO2 adsorbents. Ind. Eng. Chem. Res. 2020, 59, 6194–6201. [Google Scholar] [CrossRef]
- Waribam, P.; Ngo, S.D.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Chanlek, N.; Wei, L.; Zhang, H.; Guan, G.; Samart, C. Waste biomass valorization through production of xylose-based porous carbon microspheres for supercapacitor applications. Waste Manag. 2020, 105, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Barreto, D.; Rodriguez Estupiñan, P.; Moreno-Piraján, J.; Ramírez, R.; Giraldo, L. Adsorption and photocatalytic study of phenol using composites of activated carbon prepared from onion leaves (allium fistulosum) and metallic oxides (ZnO and TiO2). Catalysts 2020, 10, 574. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Ariga, K.; Shrestha, L.K. Nanoporous carbon materials derived from washnut seed with enhanced supercapacitance. Materials 2020, 13, 2371. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, S.; Wang, L.; Chen, F.; Shao, J.; Hu, X. Efficient nitrogen doped porous carbonaceous CO2 adsorbents based on lotus leaf. J. Environ. Sci. 2021, 103, 268–278. [Google Scholar] [CrossRef]
- Liu, S.; Yang, P.; Wang, L.; Li, Y.; Wu, Z.; Ma, R.; Wu, J.; Hu, X. Nitrogen-doped porous carbons from lotus leaf for CO2 capture and supercapacitor electrodes. Energy Fuels 2019, 33, 6568–6576. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Evans, R.; Tarazona, P. Theory of condensation in narrow capillaries. Phys. Rev. Lett. 1984, 52, 557–560. [Google Scholar] [CrossRef]
- Seaton, N.A.; Walton, J.P.R.B.; Quirke, N. A new analysis method for the determination of the pore size distribution of porous carbons from nitrogen adsorption measurements. Carbon 1989, 27, 853–861. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Caguiat, J.; Kirk, D.; Jia, C. Uncertainties in characterization of nanoporous carbons using density functional theory-based gas physisorption. Carbon 2014, 72, 47–56. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Policicchio, A.; Seredych, M.; Bandosz, T.J. Evaluation of CO2 interactions with S-doped nanoporous carbon and its composites with a reduced GO: Effect of surface features on an apparent physical adsorption mechanism. Carbon 2016, 98, 250–258. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Hameed, B.H. An evaluation of the reliability of the characterization of the porous structure of activated carbons based on incomplete nitrogen adsorption isotherms. J. Mol. Model. 2017, 23, 238. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Kalderis, D.; Diamadopoulos, E. Numerical analysis of the influence of the impregnation ratio on the microporous structure formation of activated carbons, prepared by chemical activation of waste biomass with phosphoric acid. J. Phys. Chem. Solids 2017, 105, 81–85. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Delgadillo, D.P.V. Computer analysis of the effect of the type of activating agent on the formation of the porous structure of activated carbon monoliths. J. Mater. Res. Technol. 2019, 8, 4457–4463. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Fierro, V.; Celzard, A. Confrontation of various adsorption models for assessing the porous structure of activated carbons. Adsorption 2019, 25, 1673–1682. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E. An evaluation of the reliability of the results obtained by the LBET, QSDFT, BET, and DR methods for the analysis of the porous structure of activated carbons. Materials 2020, 13, 3929. [Google Scholar] [CrossRef] [PubMed]
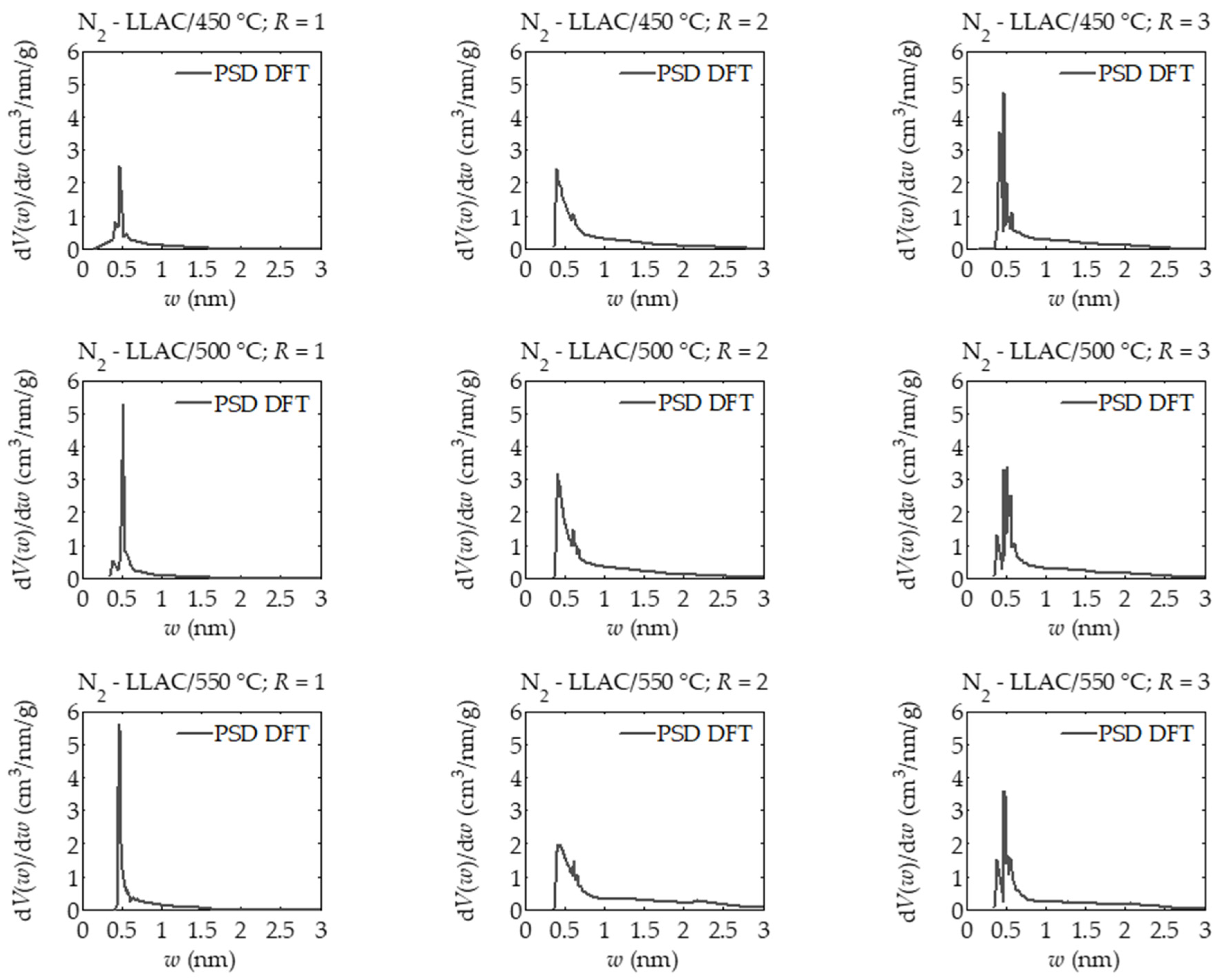
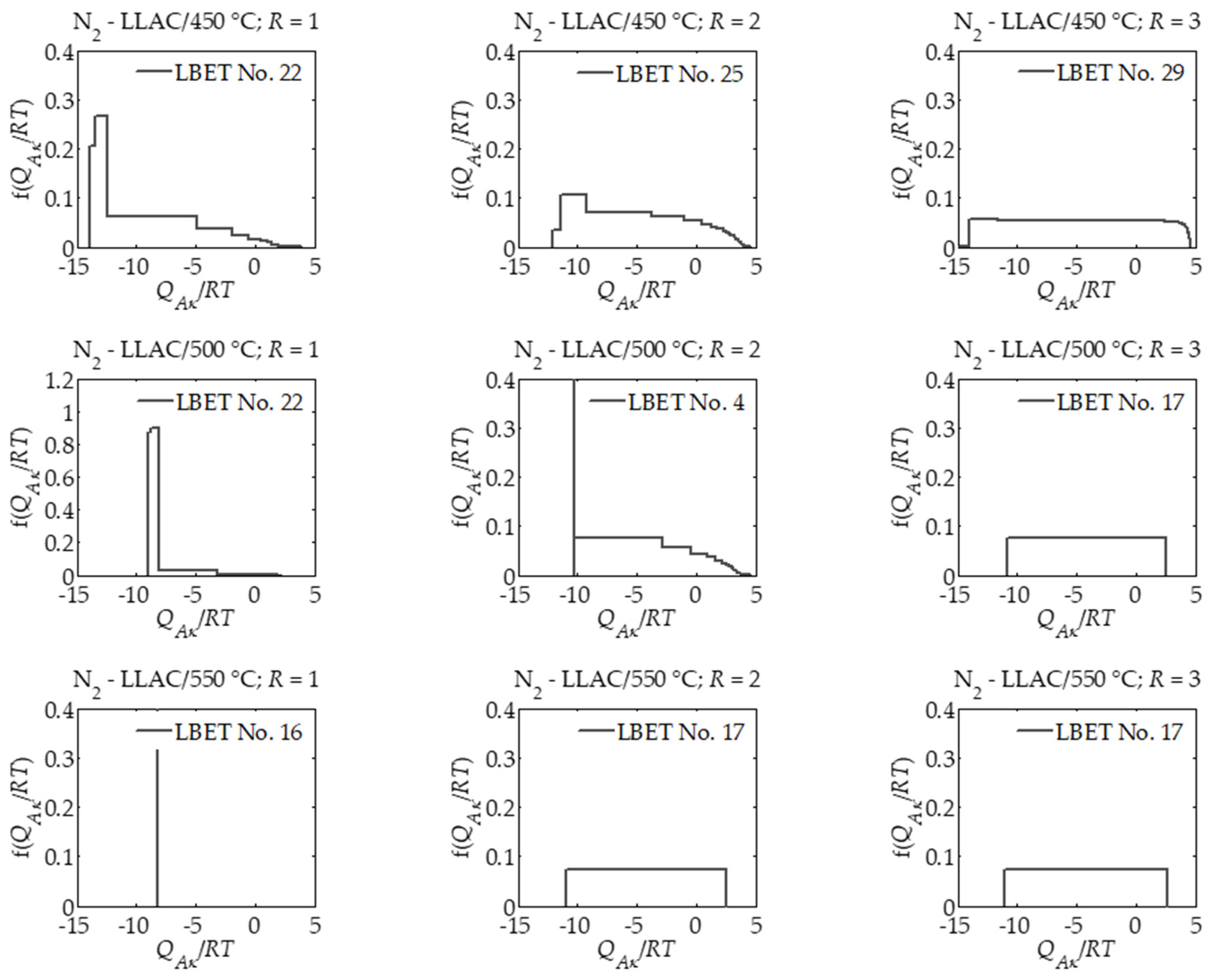
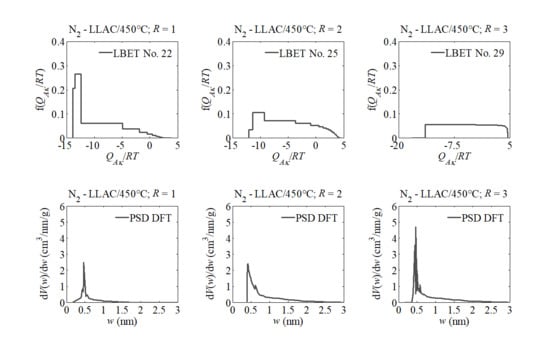
| T (°C) | 450 | 500 | 550 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| SBET (m2) | 792 | 1650 | 1557 | 833 | 1923 | 1666 | 1086 | 1883 | 1310 |
| Smicro, T-Plot (m2) | 710 | 1520 | 1481 | 766 | 1772 | 1526 | 1086 | 1673 | 1099 |
| Sext. T-Plot (m2) | 82.14 | 130 | 75 | 67 | 151 | 140 | 0.00 | 210 | 201 |
| Vmicro T-Plot (cm3/g) | 0.287 | 0.671 | 0.637 | 0.312 | 0.794 | 0.719 | 0.445 | 0.897 | 0.556 |
| Vmicro DR (cm3/g) | 0.327 | 0.710 | 0.658 | 0.345 | 0.788 | 0.715 | 0.463 | 0.818 | 0.558 |
| Vmicro DFT (cm3/g) | 0.319 | 0.707 | 0.655 | 0.333 | 0.828 | 0.722 | 0.444 | 0.861 | 0.580 |
| Vtotal DFT (cm3/g) | 0.397 | 0.819 | 0.778 | 0.406 | 0.974 | 0.887 | 0.493 | 1.197 | 0.812 |
| T (°C) | 450 | 500 | 550 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| LBET No. | 22 | 25 | 29 | 22 | 4 | 17 | 16 | 17 | 17 |
| VhA (cm3/g) | 0.293 | 0.764 | 0.822 | 0.298 | 0.875 | 0.946 | 0.350 | 1.060 | 0.705 |
| −QA/RT | 13.85 | 12.07 | 17.10 | 9.02 | 10.27 | 10.81 | 8.26 | 10.93 | 11.12 |
| BC | 4.16 | 7.67 | 7.84 | 1.14 | 7.03 | 1.01 | 1.00 | 1.00 | 1.00 |
| ZA | 0.582 | 0.534 | 0.670 | 0.451 | 0.485 | 0.500 | 0.431 | 0.503 | 0.508 |
| h | 5 | 7 | 9 | 5 | 3 | 1 | 0 | 1 | 1 |
| α | 0.70 | 0.90 | 1.00 | 0.16 | 0.83 | 0.09 | 0.92 | 0.33 | 0.31 |
| β | 1.00 | 1.00 | 1.00 | 1.41 | 1.00 | 1.00 | 1.09 | 1.00 | 1.00 |
| σe | 0.51 | 0.59 | 0.84 | 0.64 | 0.47 | 0.61 | 0.67 | 0.73 | 0.55 |
| wid | 0.06 | 0.09 | 0.01 | 0.07 | 0.07 | 0.09 | 0.02 | 0.09 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, M.; Hu, X. Analysis of the Effect of Conditions of Preparation of Nitrogen-Doped Activated Carbons Derived from Lotus Leaves by Activation with Sodium Amide on the Formation of Their Porous Structure. Materials 2021, 14, 1540. https://doi.org/10.3390/ma14061540
Kwiatkowski M, Hu X. Analysis of the Effect of Conditions of Preparation of Nitrogen-Doped Activated Carbons Derived from Lotus Leaves by Activation with Sodium Amide on the Formation of Their Porous Structure. Materials. 2021; 14(6):1540. https://doi.org/10.3390/ma14061540
Chicago/Turabian StyleKwiatkowski, Mirosław, and Xin Hu. 2021. "Analysis of the Effect of Conditions of Preparation of Nitrogen-Doped Activated Carbons Derived from Lotus Leaves by Activation with Sodium Amide on the Formation of Their Porous Structure" Materials 14, no. 6: 1540. https://doi.org/10.3390/ma14061540
APA StyleKwiatkowski, M., & Hu, X. (2021). Analysis of the Effect of Conditions of Preparation of Nitrogen-Doped Activated Carbons Derived from Lotus Leaves by Activation with Sodium Amide on the Formation of Their Porous Structure. Materials, 14(6), 1540. https://doi.org/10.3390/ma14061540