Ce/Sm/Sr-Incorporating Ceramic Scaffolds Obtained via Sol-Gel Route
Abstract
1. Introduction
2. Experimental
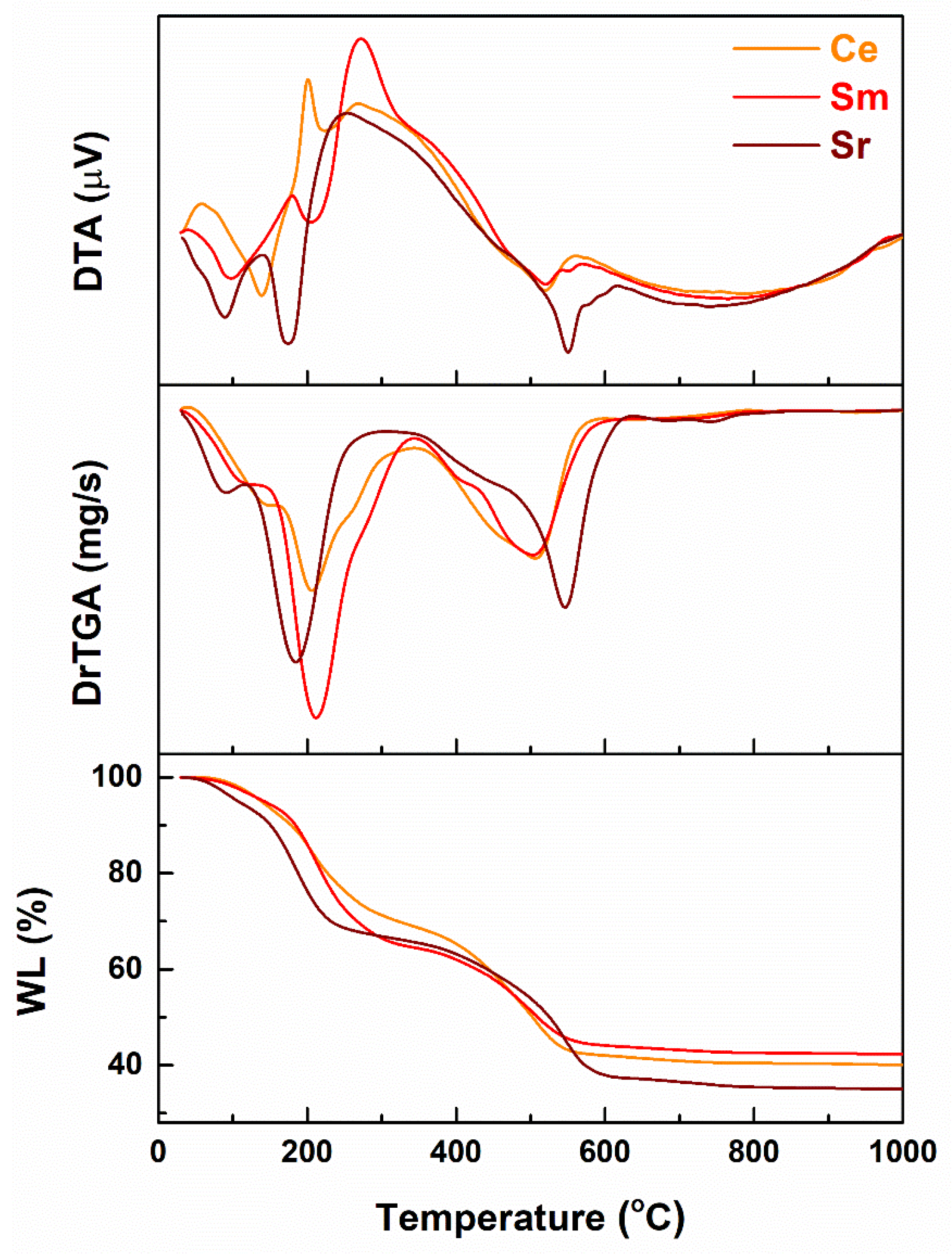
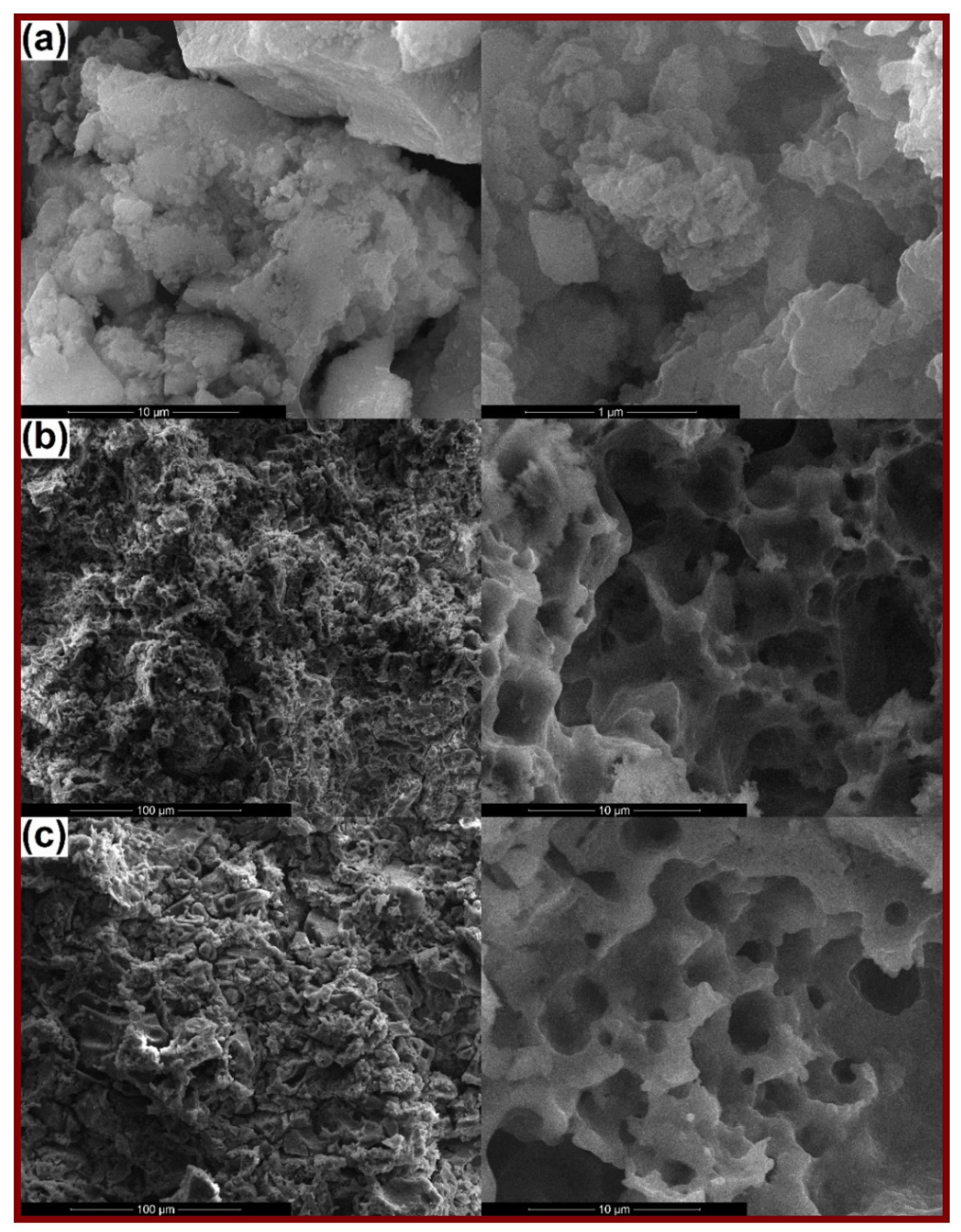
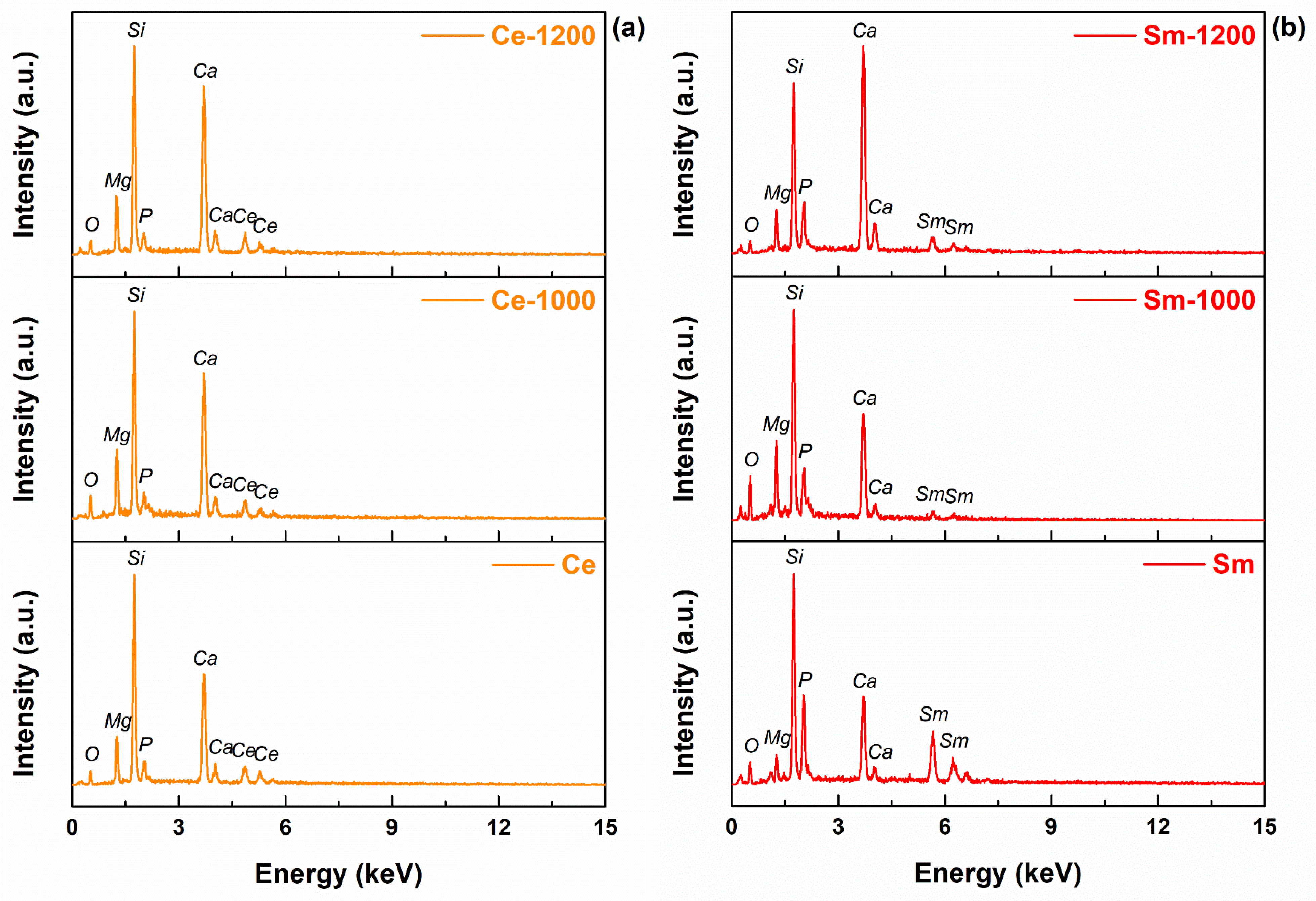
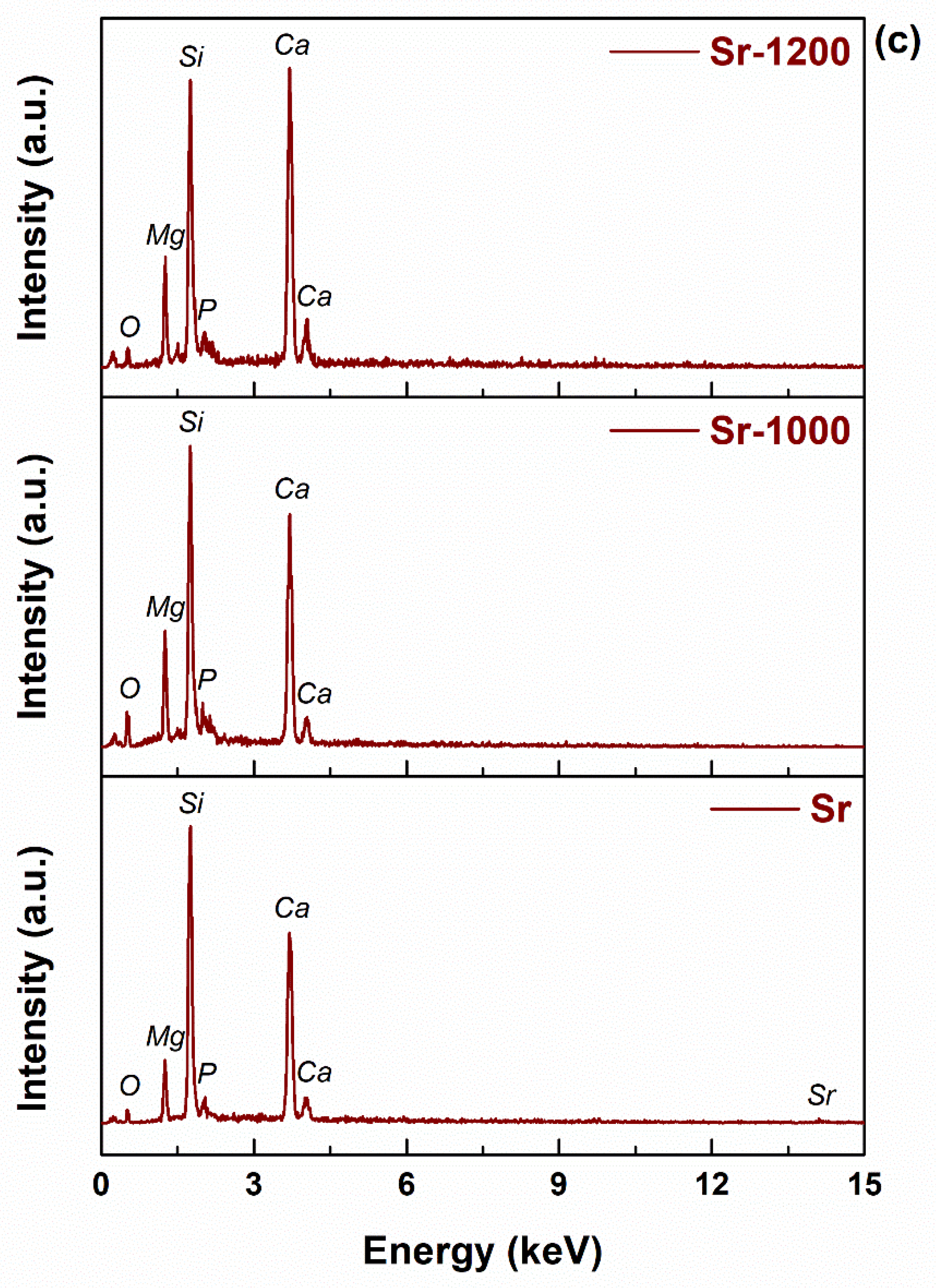
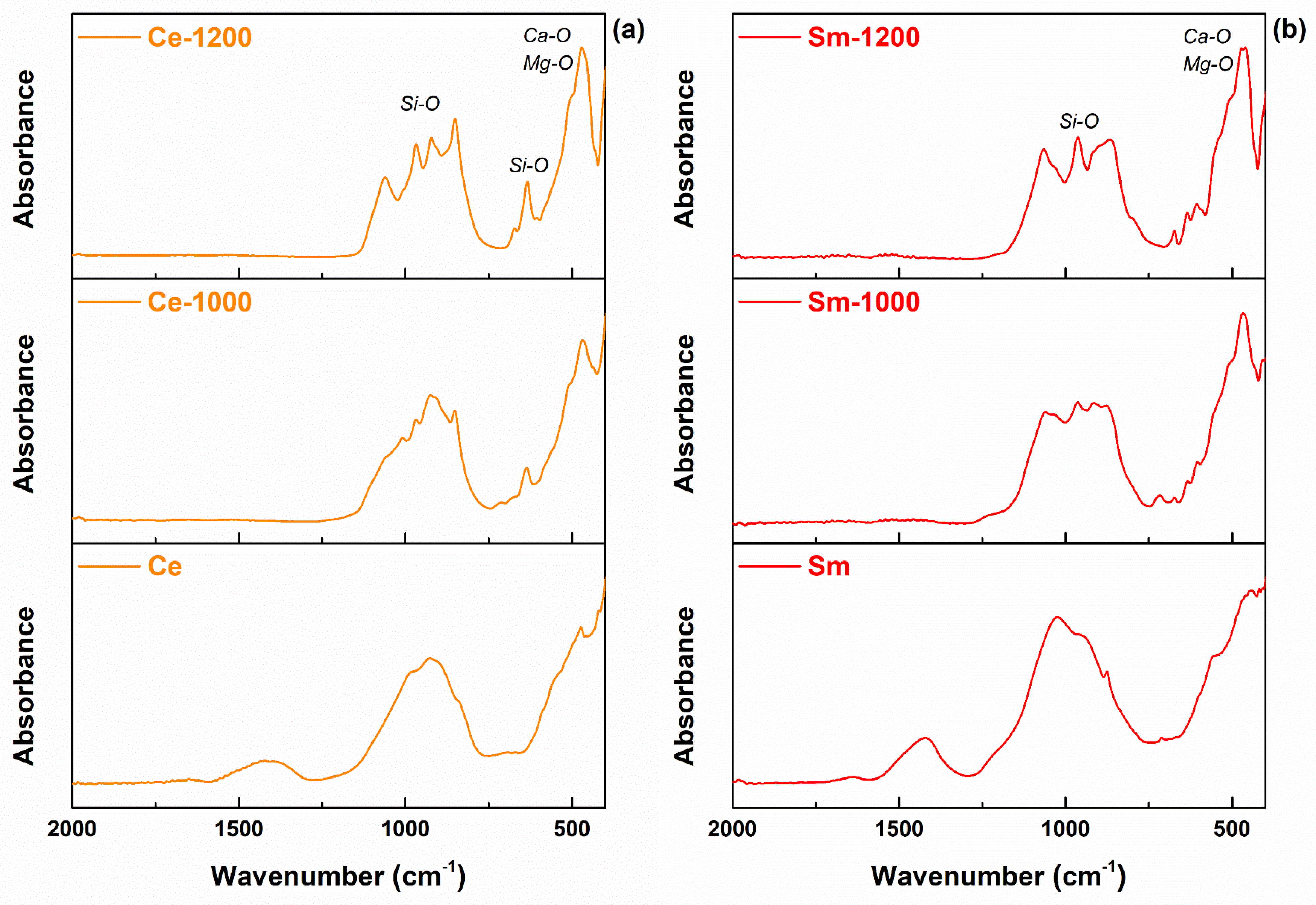
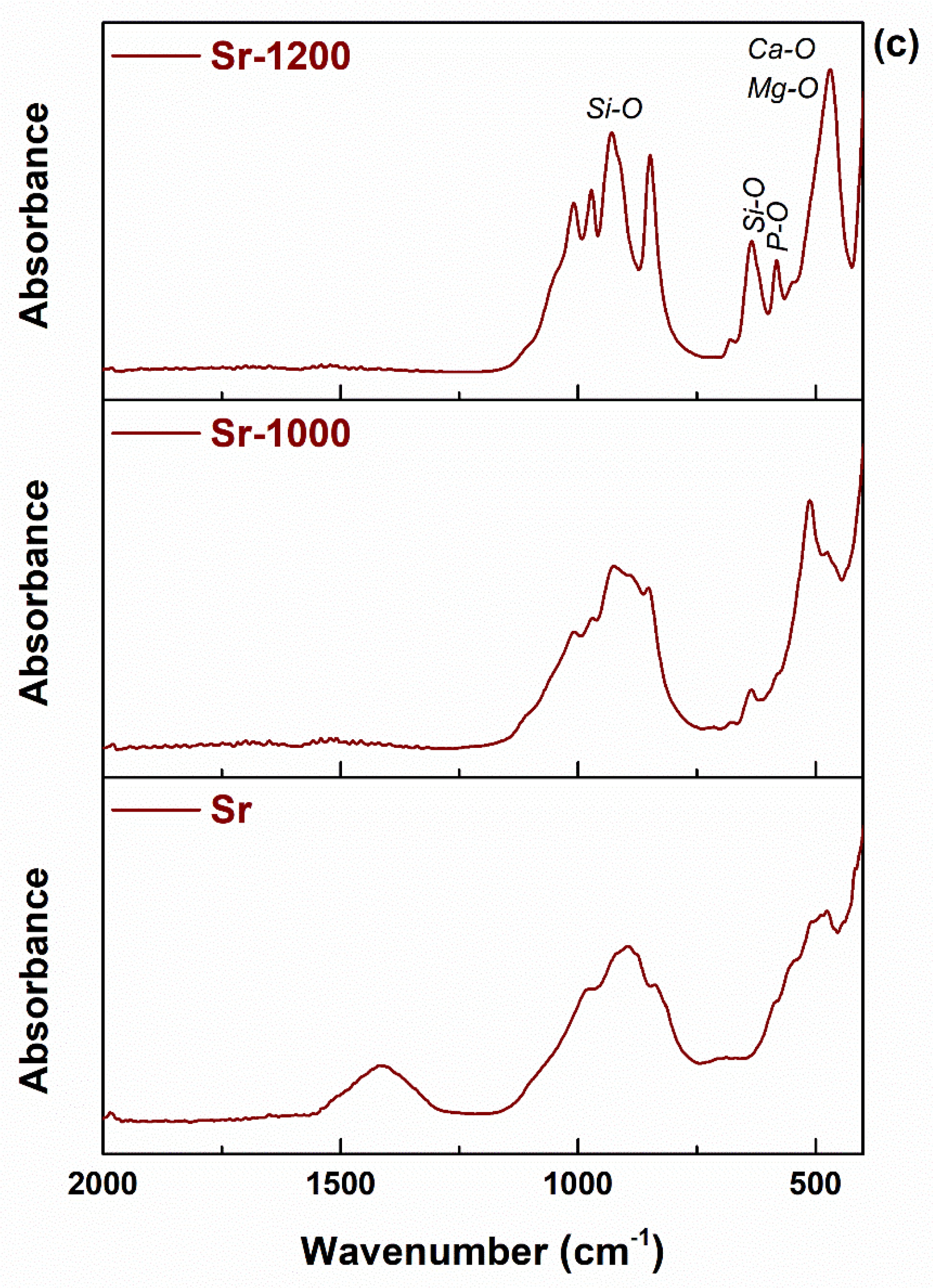
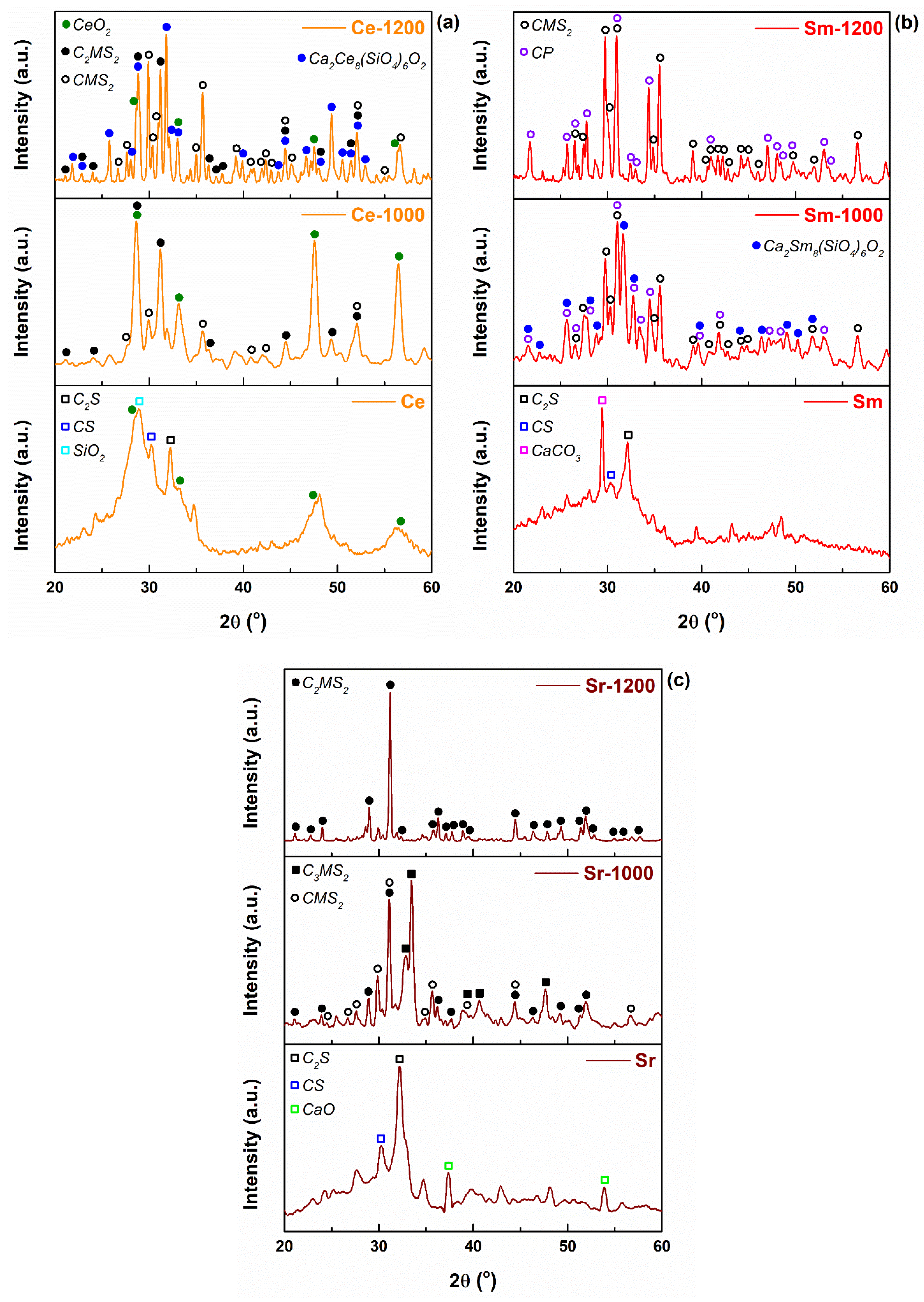
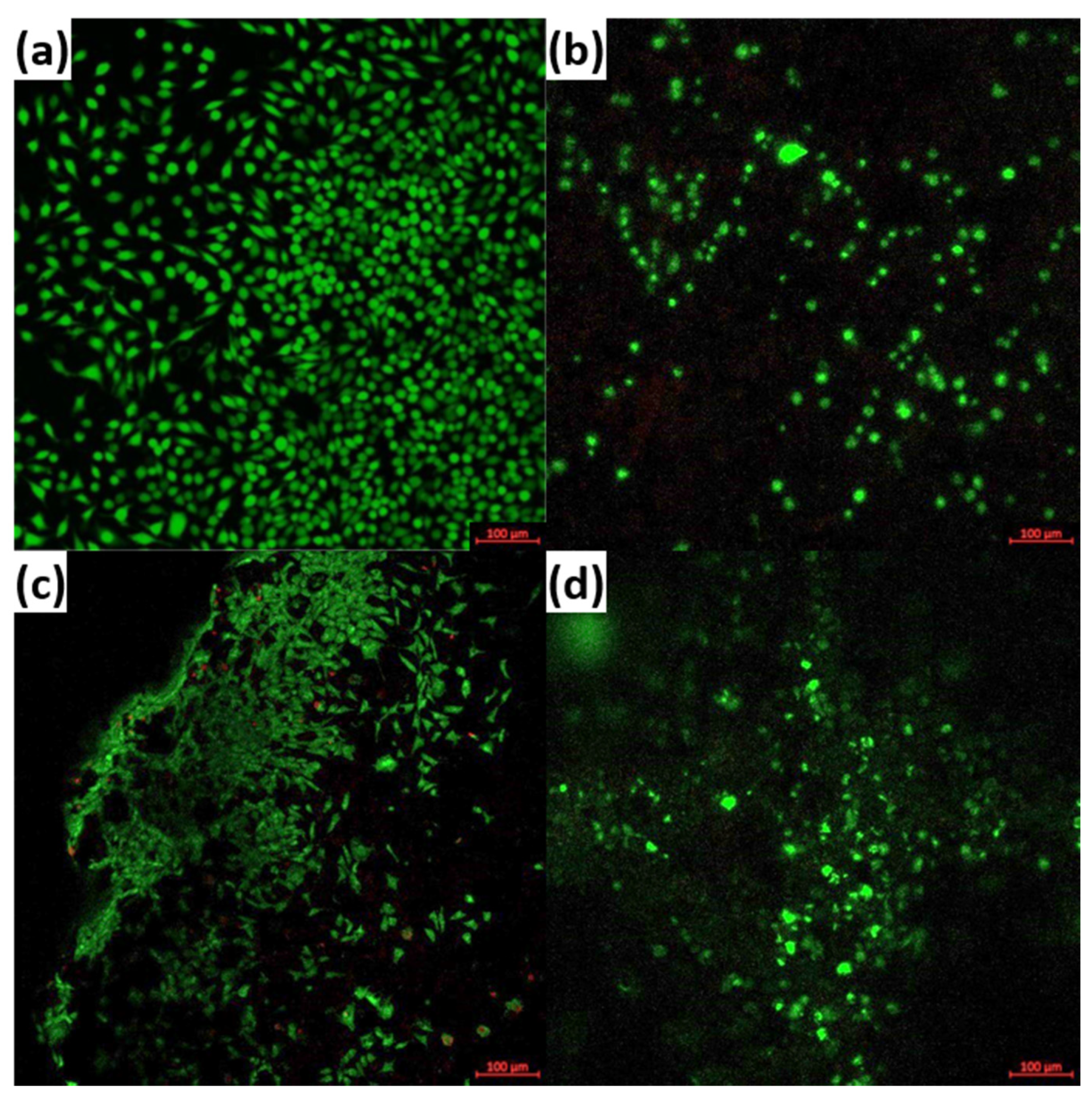
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draghici, A.D.; Busuioc, C.; Mocanu, A.; Nicoara, A.I.; Iordache, F.; Jinga, S.I. Composite scaffolds based on calcium phosphates and barium titanate obtained through bacterial cellulose templated synthesis. Mater. Sci. Eng. C 2020, 110, 110704. [Google Scholar] [CrossRef]
- Jinga, S.I.; Constantinoiu, I.; Surdu, V.A.; Iordache, F.; Busuioc, C. Sol-gel-derived mineral scaffolds within SiO2–P2O5–CaO–MgO–ZnO–CaF2 system. J. Sol-Gel Sci. Technol. 2019, 90, 411–421. [Google Scholar] [CrossRef]
- Jinga, S.I.; Toma, V.T.; Constantinoiu, I.; Banciu, A.; Banciu, D.D.; Busuioc, C. Development of new Mg- or Sr-containing bioactive interfaces to stimulate osseointegration of metallic implants. Appl. Sci. 2020, 10, 6647. [Google Scholar] [CrossRef]
- Jinga, S.I.; Draghici, A.D.; Mocanu, A.; Nicoara, A.I.; Iordache, F.; Busuioc, C. Bacterial cellulose-assisted synthesis of glass-ceramic scaffolds with TiO2 crystalline domains. Int. J. Appl. Ceram. Technol. 2020, 17, 2017–20124. [Google Scholar] [CrossRef]
- Busuioc, C.; Voicu, G.; Jinga, S.I.; Mitran, V.; Cimpean, A. The influence of barium titanate on the biological properties of collagen-hydroxyapatite composite scaffolds. Mater. Lett. 2019, 253, 317–322. [Google Scholar] [CrossRef]
- Skripka, A.; Karabanovas, V.; Jarockyte, G.; Marin, R.; Tam, V.; Cerruti, M.; Rotomskis, R.; Vetrone, F. Decoupling theranostics with rare earth doped nanoparticles. Adv. Funct. Mater. 2019, 29, 1807105. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, F.; Zhou, X.; Chen, W.; Li, Y. Novel Eu2+-doped 3Y–TZP bioceramics with fluorescence similar to natural teeth. Ceram. Int. 2014, 40, 13729–13733. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Saranya, S.; Prema Rani, M. Fine structure analysis and antibacterial property of strontium doped hydroxyapatite. Asian J. Adv. Nanomater. 2020, 1, 28–35. [Google Scholar]
- Zhu, H.; Guo, D.; Sun, L.; Li, H.; Hanaor, D.A.H.; Schmidt, F.; Xu, K. Nanostructural insights into the dissolution behavior of Sr-doped hydroxyapatite. J. Eur. Ceram. Soc. 2018, 38, 5554–5562. [Google Scholar] [CrossRef]
- Amudha, S.; Ramana Ramya, J.; Thanigai Arul, K.; Deepika, A.; Sathiamurthi, P.; Mohana, B.; Asokan, K.; Dong, C.L.; Narayana Kalkura, S. Enhanced mechanical and biocompatible properties of strontium ions doped mesoporous bioactive glass. Compos. Part B-Eng. 2020, 196, 108099. [Google Scholar] [CrossRef]
- Maciel, P.P.; Pessoa, J.A.M.; de Medeiros, E.L.G.; Batista, A.U.D.; Figueiredo, L.R.F.; de Medeiros, E.S.; de Oliveira Duarte, D.F.; Alves, A.F.; de Sousa, F.B.; Vieira, B.R.; et al. Use of strontium doping glass-ceramic material for bone regeneration in critical defect: In vitro and In vivo analyses. Ceram. Int. 2020, 46, 24940–24954. [Google Scholar] [CrossRef]
- Sabareeswaran, A.; Basu, B.; Shenoy, S.J.; Jaffer, Z.; Saha, N.; Stamboulis, A. Early osseointegration of a strontium containing glass ceramic in a rabbit model. Biomaterials 2013, 34, 9278–9286. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, A.D.; Nerantzaki, M.; Gounari, E.; Duggal, M.S.; Giannoudis, P.V.; Jha, A.; Bikiaris, D. Antibacterial properties and regenerative potential of Sr2+ and Ce3+ doped fluorapatites: A potential solution for peri-implantitis. Sci. Rep. 2019, 9, 14469. [Google Scholar] [CrossRef] [PubMed]
- Ranga, N.; Poonia, E.; Jakhar, S.; Sharma, A.K.; Kumar, A.; Devi, S.; Duhan, S. Enhanced antimicrobial properties of bioactive glass using strontium and silver oxide nanocomposites. J. Asian Ceram. Soc. 2019, 7, 75–81. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Jiang, F.; Zhu, Z.; Wen, J.; Jiang, X. Study of Sr–Ca–Si-based scaffolds for bone regeneration in osteoporotic models. Int. J. Oral Sci. 2020, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Yu, W.X.; Hwang, P.A.; Huang, S.S.; Lin, H.M.; Hsu, Y.W.; Hsu, F.Y. Fabrication and characterization of strontium-substituted hydroxyapatite–CaO–CaCO3 nanofibers with a mesoporous structure as drug delivery carriers. Pharmaceutics 2018, 10, 179. [Google Scholar] [CrossRef]
- Draghici, D.A.; Mihai, A.A.; Aioanei, M.O.; Negru, N.E.; Nicoara, A.I.; Jinga, S.I.; Miu, D.; Bacalum, M.; Busuioc, C. Strontium-substituted bioactive glass-ceramic films for tissue engineering. Bol. Soc. Esp. Ceram. Vidrio 2020, in press. [Google Scholar] [CrossRef]
- Mao, Z.; Li, Y.; Yang, Y.; Fang, Z.; Chen, X.; Wang, Y.; Kang, J.; Qu, X.; Yuan, W.; Dai, K.; et al. Osteoinductivity and antibacterial properties of strontium ranelate-loaded poly (lactic-co-glycolic acid) microspheres with assembled silver and hydroxyapatite nanoparticles. Front. Pharmacol. 2018, 9, 368. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Santos, M.V.B.; Oliveira, A.L.; Osajima, J.A.; Silva-Filho, E.C. Development of composites scaffolds with calcium and cerium-hydroxyapatite and gellan gum. Ceram. Int. 2020, 46, 3811–3817. [Google Scholar] [CrossRef]
- Xiang, J.; Li, J.; He, J.; Tang, X.; Dou, C.; Cao, Z.; Yu, B.; Zhao, C.; Kang, F.; Yang, L.; et al. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl. Mater. Interfaces 2016, 8, 4489–4499. [Google Scholar] [CrossRef]
- Prefac, G.A.; Milea, M.L.; Vadureanu, A.M.; Muraru, S.; Dobrin, D.I.; Isopencu, G.O.; Jinga, S.I.; Raileanu, M.; Bacalum, M.; Busuioc, C. CeO2 containing thin films as bioactive coatings for orthopaedic implants. Coatings 2020, 10, 642. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, J.; Zhao, S.; Zhu, Y. Three-dimensional printing of cerium-incorporated mesoporous calcium-silicate scaffolds for bone repair. J. Mater. Sci. 2016, 51, 836–844. [Google Scholar] [CrossRef]
- Mandoli, C.; Pagliari, F.; Pagliari, S.; Forte, G.; Di Nardo, P.; Licoccia, S.; Traversa, E. Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv. Funct. Mater. 2010, 20, 1617–1624. [Google Scholar] [CrossRef]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Chou, C.F.; Gupta, M.K.; Mishra, N.C. Gelatin-alginate-cerium oxide nanocomposite scaffold for bone regeneration. Mater. Sci. Eng. C 2020, 116, 111111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, C.; Zhai, X.; Luo, F.; Du, Y.; Yan, C. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci. China Mater. 2019, 62, 1727–1739. [Google Scholar] [CrossRef]
- Marino, A.; Turo, C.T.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G.; et al. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Biophys. Acta 2017, 1861, 386–395. [Google Scholar] [CrossRef]
- Augustine, R.; Dalvi, Y.B.; Dan, P.; George, N.; Helle, D.; Varghese, R.; Thomas, S.; Menu, P.; Sandhyarani, N. Nanoceria can act as the cues for angiogenesis in tissue-engineering scaffolds: Toward next-generation In situ tissue engineering. ACS Biomater. Sci. Eng. 2018, 4, 4338–4353. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.T.U.; Di Silvio, L.; Evans, J.R.G. In vitro evaluation of samarium (III) oxide as a bone substituting material. J. Biomed. Mater. Res. 2010, 94, 130–136. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Motelica-Heino, M.; Predoi, D. Evaluation of samarium doped hydroxyapatite, ceramics for medical application: Antimicrobial activity. J. Nanomater. 2015, 2015, 849216. [Google Scholar] [CrossRef]
- Morais, D.S.; Coelho, J.; Ferraz, M.P.; Gomes, P.S.; Fernandes, M.H.; Hussain, N.S.; Santos, J.D.; Lopes, M.A. Samarium doped glass-reinforced hydroxyapatite with enhanced osteoblastic performance and antibacterial properties for bone tissue regeneration. J. Mater. Chem. B 2014, 2, 5872–5881. [Google Scholar] [CrossRef]
- Ershad, M.; Vyas, V.K.; Prasad, S.; Ali, A.; Pyare, R. Effect of Sm2O3 substitution on mechanical and biological properties of 45S5 bioactive glass. J. Aust. Ceram. Soc. 2018, 54, 621–630. [Google Scholar] [CrossRef]
- Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Prodan, A.M.; Predoi, M.V. Physico-chemical properties and In vitro antifungal evaluation of samarium doped hydroxyapatite coatings. Coatings 2020, 10, 827. [Google Scholar] [CrossRef]
- Turculet, C.S.; Prodan, A.M.; Negoi, I.; Teleanu, G.; Popa, M.; Andronescu, E.; Beuran, M.; Stanciu, G.A.; Hristu, R.; Badea, M.L.; et al. Preliminary evaluation of the antifungal activity of samarium doped hydroxyapatite thin films. Rom. Biotechnol. Lett. 2018, 23, 13927–13932. [Google Scholar]
- Mandiwana, V.; Kalombo, L.; Venter, K.; Sathekge, M.; Grobler, A.; Zeevaart, J.R. Samarium oxide as a radiotracer to evaluate the In vivo biodistribution of PLGA nanoparticles. J. Nanopart. Res. 2015, 17, 375. [Google Scholar] [CrossRef][Green Version]
- Popova-Kuznetsova, E.; Tikhonowski, G.; Popov, A.A.; Duflot, V.; Deyev, S.; Klimentov, S.; Zavestovskaya, I.; Prasad, P.N.; Kabashin, A.V. Laser-ablative synthesis of isotope-enriched samarium oxide nanoparticles for nuclear nanomedicine. Nanomaterials 2020, 10, 69. [Google Scholar] [CrossRef]
- Donanzam, B.A.; Campos, T.P.R.; Dalmazio, I.; Valente, E.S. Synthesis and characterization of calcium phosphate loaded with Ho-166 and Sm-153: A novel biomaterial for treatment of spine metastases. J. Mater. Sci. Mater. Med. 2013, 24, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Q. The bioactivity of gradient composite bioceramic coating with different contents of multiple rare earth deoxide fabricated by wide band laser cladding. Adv. Mater. Res. 2013, 706‒708, 318–322. [Google Scholar] [CrossRef]
- Voicu, G.; Ene, V.L.; Sava, D.F.; Surdu, V.A.; Busuioc, C. Sol-gel derived vitroceramic materials for biomedical applications. J. Non-Cryst. Solids 2016, 449, 75–82. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. LIVE/DEAD™ Viability/Cytotoxicity Kit for Mammalian Cells; Thermo Fisher Scientific: Waltham, MA, USA, 2005. [Google Scholar]
- Negrea, R.; Busuioc, C.; Constantinoiu, I.; Miu, D.; Enache, C.; Iordache, F.; Jinga, S.I. Akermanite based coatings grown by pulsed laser deposition for metallic implants employed in orthopaedics. Surf. Coat. Technol. 2019, 357, 1015–1026. [Google Scholar] [CrossRef]
- Jinga, S.I.; Costea, C.C.; Zamfirescu, A.I.; Banciu, A.; Banciu, D.D.; Busuioc, C. Composite fibre networks based on polycaprolactone and bioactive glass-ceramics for tissue engineering applications. Polymers 2020, 12, 1806. [Google Scholar] [CrossRef]
- Karacaoglu, E.; Karasu, B. Effect of activators and calcination on luminescence properties of akermanite type phosphors. Indian J. Chem. 2015, 54A, 1394–1401. [Google Scholar]
- Ochi, Y. Crystal structure of Sr-akermanite glass-ceramics. Mater. Res. Bull. 2006, 41, 1825–1834. [Google Scholar] [CrossRef]
- Han, Z.; Feng, P.; Gao, C.; Shen, Y.; Shuai, C.; Peng, S. Microstructure, mechanical properties and In vitro bioactivity of akermanite scaffolds fabricated by laser sintering. Biomed. Mater. Eng. 2014, 24, 2073–2080. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ismail, Y.M.B.; Shariff, K.A.B.; Noor, A.F.M. Synthesis and characterization of akermanite by mechanical milling and subsequent heat treatment. J. Phys. Conf. Ser. 2018, 1082, 012021. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. A novel akermanite bioceramic: Preparation and characteristics. J. Biomater. Appl. 2006, 21, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.M.G.; Tulyaganov, D.U.; Agathopoulos, S.; Ferreira, J.M.F. Sintering and crystallization of akermanite-based glass-ceramics. Mater. Lett. 2006, 60, 1488–1491. [Google Scholar] [CrossRef]
- Martin, R.B.; Burr, D.B.; Sharkey, N.A. Mechanical properties of bone. In Skeletal Tissue Mechanics; Springer: New York, NY, USA, 1998; pp. 127–180. [Google Scholar]
- Lu, Y.; Cheng, L.L.; Yang, Z.; Li, J.; Zhu, H. Relationship between the morphological, mechanical and permeability properties of porous bone scaffolds and the underlying microstructure. PLoS ONE 2020, 15, e0238471. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Valainis, D.; Bhattacharya, K.; van Griensven, M.; Dondl, P. Optimization of bone scaffold porosity distributions. Sci. Rep. 2019, 9, 9170. [Google Scholar] [CrossRef] [PubMed]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Rajagopal, R.R.; Ferreira, J.M.F. Influence of strontium on structure, sintering and biodegradation behaviour of CaO–MgO–SrO–SiO2–P2O5–CaF2 glasses. Acta. Biomater. 2011, 7, 4071–4080. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Singh, K.J.; Yadav, A.K.; Kaur, S.; Kaur, R.; Kaur, S. Growth of bone like hydroxyapatite and cell viability studies on CeO2 doped CaO–P2O5–MgO–SiO2 bioceramics. Mater. Chem. Phys. 2020, 243, 122352. [Google Scholar] [CrossRef]



| Code | SiO2 | P2O5 | CaO | MgO | CeO2 | Sm2O3 | SrO |
|---|---|---|---|---|---|---|---|
| (mol%) | |||||||
| Ce | 38 | 4 | 36 | 18 | 4 | 0 | 0 |
| Sm | 0 | 4 | 0 | ||||
| Sr | 0 | 0 | 4 | ||||
| Sample | Si | P | Ca | Mg | Ce/Sm/Sr | O |
|---|---|---|---|---|---|---|
| Ce | 32.39 | 6.12 | 24.42 | 10.61 | 11.48 | 14.97 |
| Ce-1000 | 23.82 | 3.95 | 34.27 | 10.36 | 13.34 | 14.26 |
| Ce-1200 | 27.98 | 3.88 | 29.19 | 11.03 | 9.15 | 18.77 |
| Sm | 27.25 | 17.89 | 13.82 | 3.60 | 24.51 | 12.92 |
| Sm-1000 | 25.46 | 9.15 | 26.8 | 9.10 | 6.92 | 22.57 |
| Sm-1200 | 23.03 | 9.90 | 36.54 | 7.29 | 9.85 | 13.39 |
| Sr | 32.16 | 4.77 | 35.39 | 7.88 | 5.77 | 14.03 |
| Sr-1000 | 20.00 | 4.13 | 38.00 | 9.04 | 11.41 | 17.43 |
| Sr-1200 | 18.26 | 3.71 | 48.15 | 7.39 | 11.04 | 11.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jinga, S.-I.; Anghel, A.-M.; Brincoveanu, S.-F.; Bucur, R.-M.; Florea, A.-D.; Saftau, B.-I.; Stroe, S.-C.; Zamfirescu, A.-I.; Busuioc, C. Ce/Sm/Sr-Incorporating Ceramic Scaffolds Obtained via Sol-Gel Route. Materials 2021, 14, 1532. https://doi.org/10.3390/ma14061532
Jinga S-I, Anghel A-M, Brincoveanu S-F, Bucur R-M, Florea A-D, Saftau B-I, Stroe S-C, Zamfirescu A-I, Busuioc C. Ce/Sm/Sr-Incorporating Ceramic Scaffolds Obtained via Sol-Gel Route. Materials. 2021; 14(6):1532. https://doi.org/10.3390/ma14061532
Chicago/Turabian StyleJinga, Sorin-Ion, Ana-Maria Anghel, Silvia-Florena Brincoveanu, Raluca-Maria Bucur, Andrei-Dan Florea, Bianca-Irina Saftau, Stefania-Cristina Stroe, Andreea-Ioana Zamfirescu, and Cristina Busuioc. 2021. "Ce/Sm/Sr-Incorporating Ceramic Scaffolds Obtained via Sol-Gel Route" Materials 14, no. 6: 1532. https://doi.org/10.3390/ma14061532
APA StyleJinga, S.-I., Anghel, A.-M., Brincoveanu, S.-F., Bucur, R.-M., Florea, A.-D., Saftau, B.-I., Stroe, S.-C., Zamfirescu, A.-I., & Busuioc, C. (2021). Ce/Sm/Sr-Incorporating Ceramic Scaffolds Obtained via Sol-Gel Route. Materials, 14(6), 1532. https://doi.org/10.3390/ma14061532




.JPG)


