How to Improve Physico-Chemical Properties of Silk Fibroin Materials for Biomedical Applications?—Blending and Cross-Linking of Silk Fibroin—A Review
Abstract
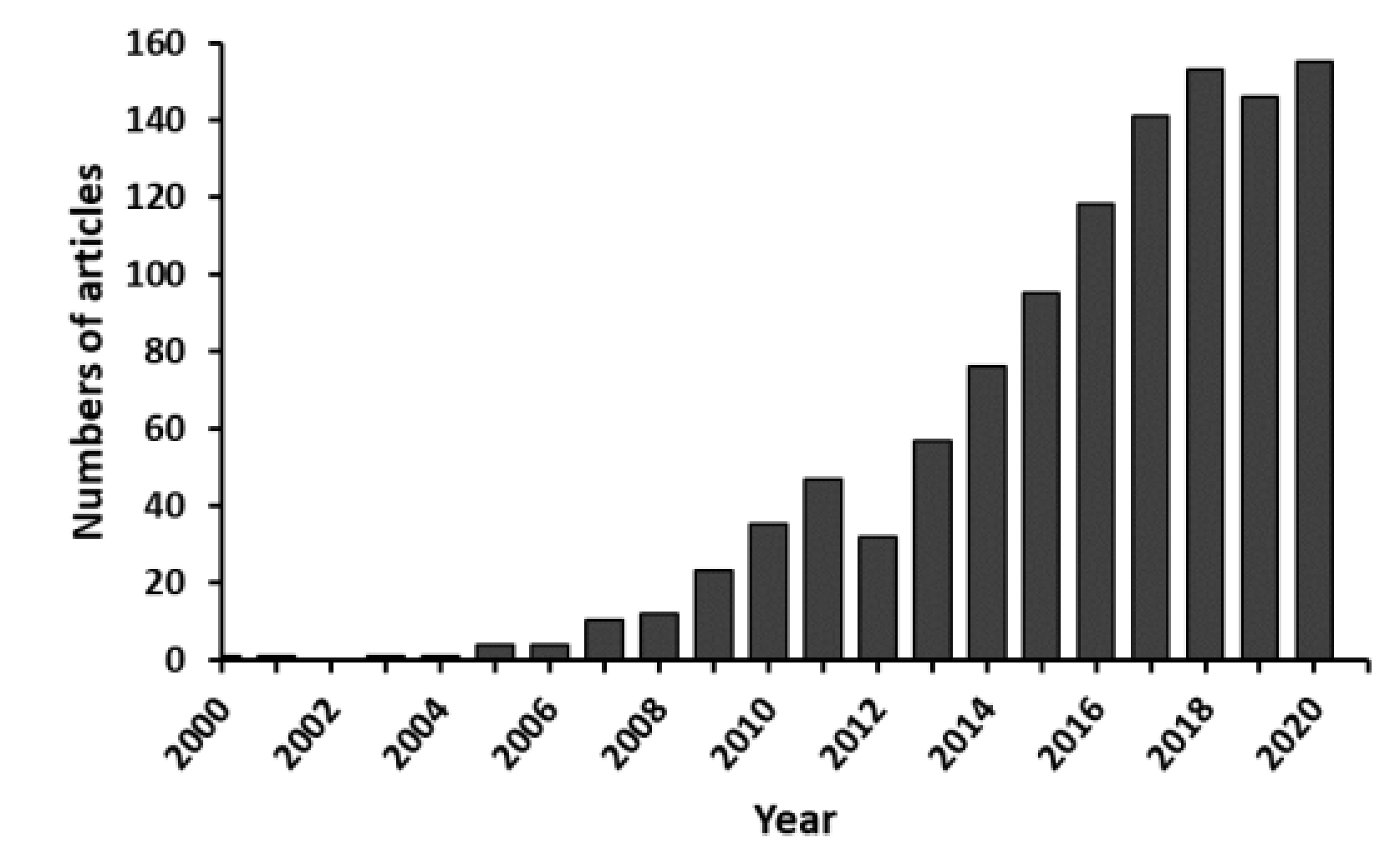
1. Introduction
- ✓
- formation and movement of cells,
- ✓
- having an influence on development and differentiation of cells,
- ✓
- merging the cells in the tissue,
- ✓
- mechanical support for proteins and cells.
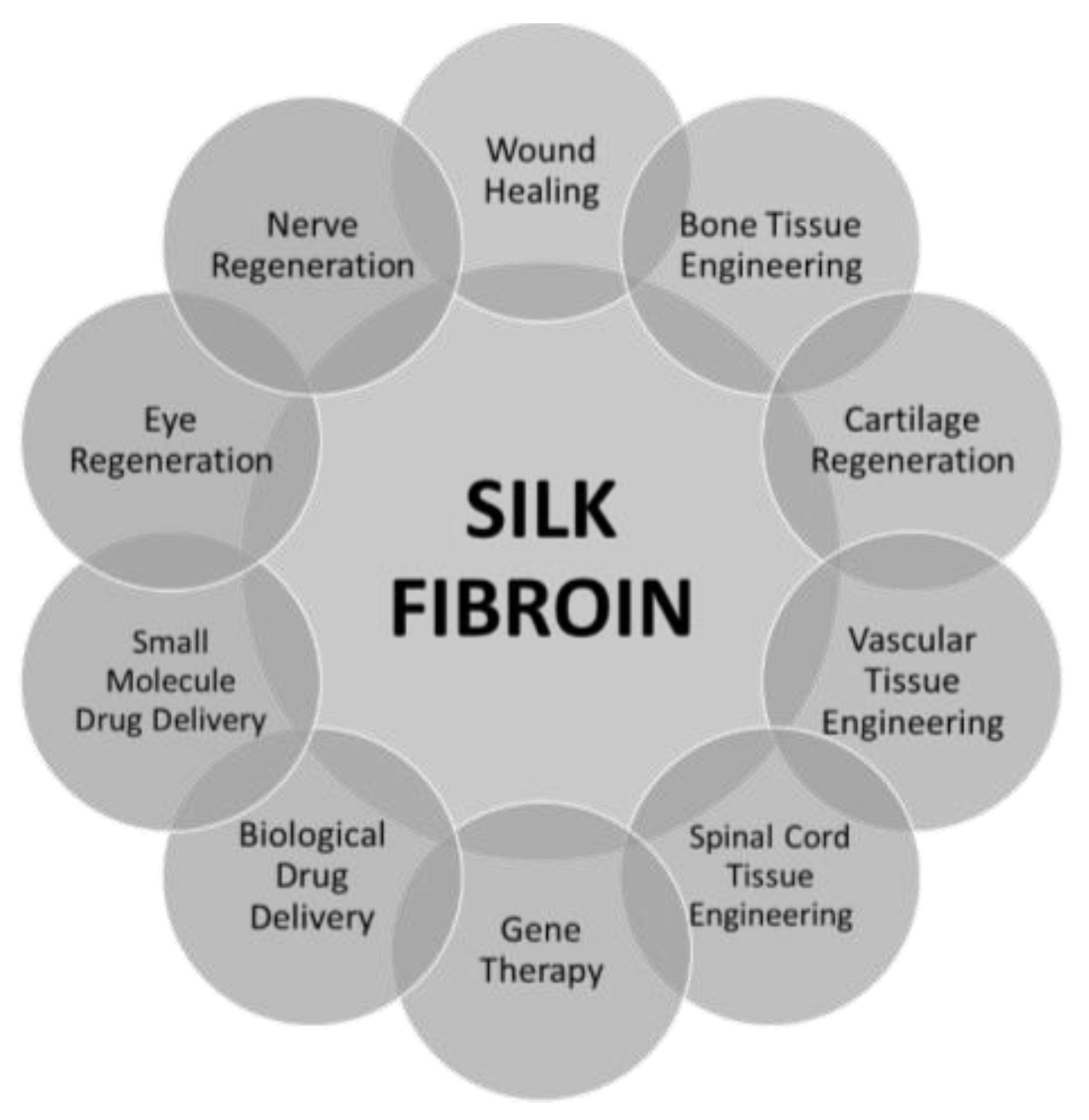
2. Silk Fibroin
3. Silk Fibroin Sources
3.1. Cocoon (Bombyx Mori) Silk
3.2. Spider (Nephila Clavipes) Silk
4. Types of Silk Fibroin Based Biomaterials
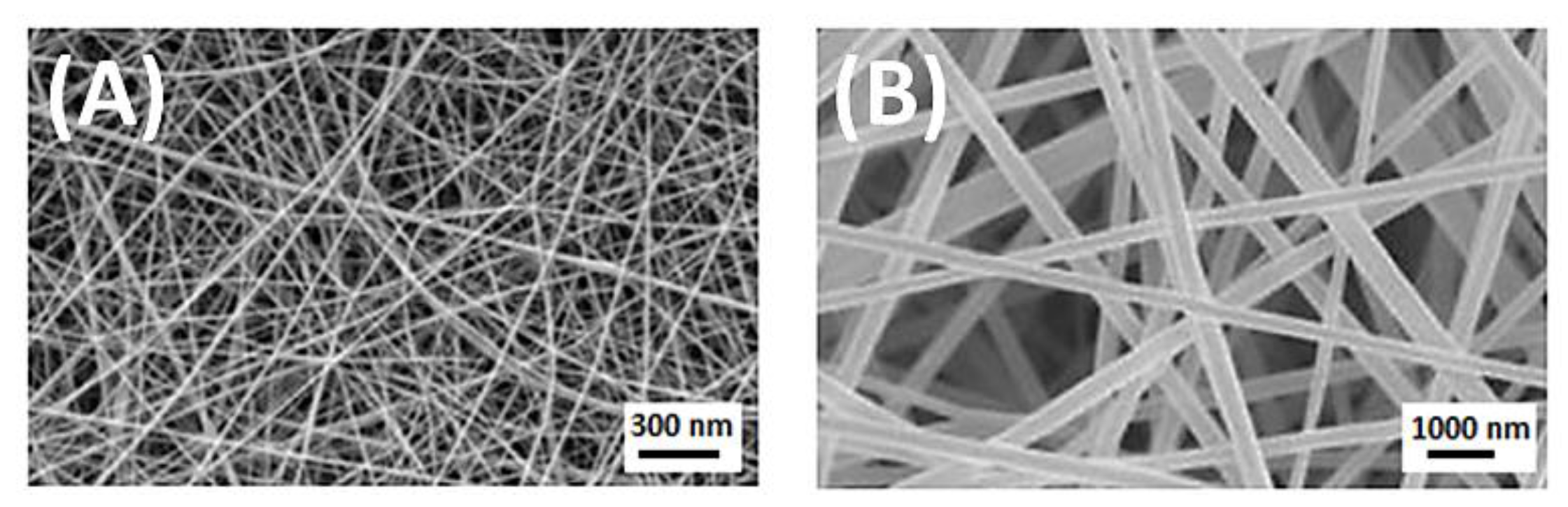
4.1. Fibres
4.2. 3D Porous Structures
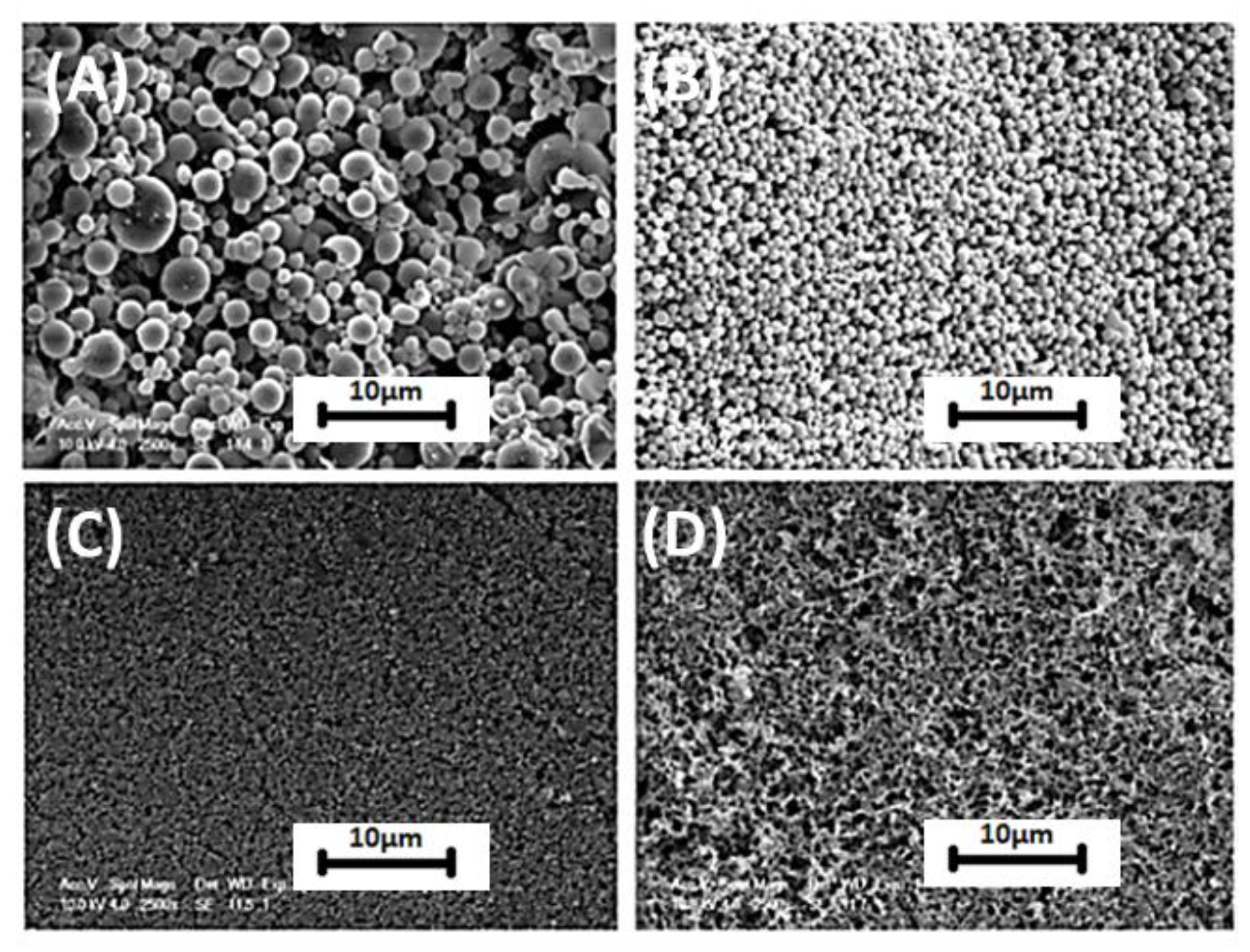
4.3. Particles
4.4. Films
4.5. Hydrogels
4.6. Aerogels
5. Blending
5.1. Collagen
5.2. Chitosan
5.3. Alginate
5.4. Hyaluronic Acid
5.5. Other Polymers
5.6. Three-Component Blends
5.7. Inorganic Additives
6. Cross-Linking
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| %SF | percentage fraction of silk fibroin in mixture |
| 3D | three dimensional |
| A | alanine |
| AFM | Atomic Force Microscopy |
| AL | alginate |
| ATDC-5 | chondrogenic cell line |
| b | polymer–polymer interactions term (at finite concentration related to the Huggins coefficient); bid*m: determined according to Krigbaum and Wall; bid**m: determined according to Garcia et al. |
| b | viscometry experimental parameter of the mixture |
| bid*m | polymer–polymer interactions term determined according to Krigbaum and Wall |
| bid**m | polymer–polymer interactions term determined according to Garcia et al. |
| Coll | collagen |
| CTS | chitosan |
| D | diiodomethane |
| DMTA | dynamic mechanical thermal analysis |
| DSC | differential scanning calorimetry |
| EDC | N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride |
| Emod | Young’s modulus |
| Fmax | tensile strength |
| FTIR | Fourier transform infrared spectroscopy |
| G | glycine |
| GL | glycerol |
| HA | hyaluronic acid |
| HAp | Hydroxyapatite |
| HFIP | hexafluoroisopropanol |
| hMSCs | human bone marrow mesenchymal stem cells |
| L | leucine |
| LB | Langmuir-Blodgett process |
| MaSp1 | major ampullate spidroin 1 |
| MaSp2 | major ampullate spidroin 2 |
| MG-63 | human osteosarcoma cell line |
| NHS | (N-hydroxysuccinimide) |
| P | pressure |
| PR | proline |
| PAM | Polyacrylamide |
| PBS | Phosphate Buffered Saline |
| PEG | Poly(ethylene Glycol) |
| PLA | Polylactide |
| PLGA | Poly(Lactic-co-glycolic Acid) |
| PUR | Polyurethane |
| PVA | Poly(vinyl Alcohol) |
| PVCL | Polycaprolactone |
| Q | glutamine |
| Rq | roughness parameter |
| S | serine |
| SA | sodium alginate |
| SEM | Scanning Electron Microscopy |
| SF | silk fibroin |
| T | temperature |
| TAE | transcatheter arterial embolization |
| Tg | glass transition temperature |
| TGF-β1 | transforming growth factor β1 |
| UV | ultraviolet |
| V | valine |
| w/o | water in oil emulsion |
| WColl | percentage fraction of collagen in mixture |
| WCTS | percentage fraction of chitosan in mixture |
| WSF | percentage fraction of silk fibroin in mixture |
| Y | tyrosine |
| γS | surface free energy |
| γSP/γSD | the ratio of polar to dispersive components of surface free energy |
| η | intrinsic viscosity |
References
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as Bone Substitutes: A Review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahi, T.; Gu, W.; Li, B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F. Bioinspired Materials for Wound Healing Application: The Potential of Silk Fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Pourmotabed, S.; Sharifi, E. A Review on Some Natural Biopolymers and Their Applications in Angiogenesis and Tissue Engineering. Appl. Biotechnol. Rep. 2018, 5, 81–91. [Google Scholar] [CrossRef][Green Version]
- Sionkowska, A. Current Research on the Blends of Natural and Synthetic Polymers as New Biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Agrawal, C.M. Reconstructing the Human Body Using Biomaterials. JOM 1998, 50, 31–35. [Google Scholar] [CrossRef]
- Clarke, B. Normal Bone Anatomy and Physiology. CJASN 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Kneser, U.; Schaefer, D.J.; Munder, B.; Klemt, C.; Andree, C.; Stark, G.B. Tissue Engineering of Bone. Minim. Invasive Ther. 2002, 11, 107–116. [Google Scholar] [CrossRef] [PubMed]
- McEntire, B.J.; Bal, B.S.; Rahaman, M.N.; Chevalier, J.; Pezzotti, G. Ceramics and Ceramic Coatings in Orthopaedics. J. Eur. Ceram. Soc. 2015, 35, 4327–4369. [Google Scholar] [CrossRef]
- Goriainov, V.; Cook, R.; Latham, J.M.; Dunlop, D.G.; Oreffo, R.O.C. Bone and Metal: An Orthopaedic Perspective on Osseointegration of Metals. Acta Biomater. 2014, 10, 4043–4057. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric Materials for Bone and Cartilage Repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Ma, L. Collagen/Chitosan Porous Scaffolds with Improved Biostability for Skin Tissue Engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, D.; Han, J.; Yuan, S.; Lin, H.; Zhao, C. Design and Fabrication of Porous Chitosan Scaffolds with Tunable Structures and Mechanical Properties. Carbohydr. Polym. 2017, 177, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Suesca, E.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C.; Fontanilla, M.R. Multifactor Analysis on the Effect of Collagen Concentration, Cross-Linking and Fiber/Pore Orientation on Chemical, Microstructural, Mechanical and Biological Properties of Collagen Type I Scaffolds. Mater. Sci. Eng. C 2017, 77, 333–341. [Google Scholar] [CrossRef]
- Ke, D.; Bose, S. Doped Tricalcium Phosphate Bone Tissue Engineering Scaffolds Using Sucrose as Template and Microwave Sintering: Enhancement of Mechanical and Biological Properties. Mater. Sci. Eng. C 2017, 78, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Nerem, R.M.; Sambanis, A. Tissue Engineering: From Biology to Biological Substitutes. Tissue Eng. 1995, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. Collagen-Based Scaffolds Enriched with Glycosaminoglycans Isolated from Skin of Salmo Salar Fish. Polym. Test. 2017, 62, 132–136. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk Biomaterials in Wound Healing and Skin Regeneration Therapeutics: From Bench to Bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Rasheed, S.; Yougen, C. Silk Fibroin/Hydroxyapatite Scaffold: A Highly Compatible Material for Bone Regeneration. Sci. Technol. Adv. Mater. 2020, 21, 242–266. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- Asakura, T.; Tanaka, T.; Tanaka, R. Advanced Silk Fibroin Biomaterials and Application to Small-Diameter Silk Vascular Grafts. ACS Biomater. Sci. Eng. 2019, 5, 5561–5577. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Chowdhury, S.K.; Dey, S.; Moses, J.C.; Mandal, B.B. Silk: A Promising Biomaterial Opening New Vistas Towards Affordable Healthcare Solutions. J. Indian Inst. Sci. 2019, 99, 445–487. [Google Scholar] [CrossRef]
- Meyer, M. Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical Applications of Collagen and Collagen-Based Materials. CMC 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Popescu, M.C.; Vasile, C.; Macocinschi, D.; Lungu, M.; Craciunescu, O. Biomaterials Based on New Polyurethane and Hydrolyzed Collagen, k-Elastin, Hyaluronic Acid and Chondroitin Sulfate. Int. J. Biol. Macromol. 2010, 47, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ng, S.C.; Yu, J.; Tsai, W.-B. Modification and Crosslinking of Gelatin-Based Biomaterials as Tissue Adhesives. Colloids Surf. B Biointerfaces 2019, 174, 316–323. [Google Scholar] [CrossRef]
- Seal, B. Polymeric Biomaterials for Tissue and Organ Regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites Containing Natural Polymers and Hydroxyapatite for Bone Tissue Engineering. Int. Journal of Biological Macromolecules 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Porter, B.D.; Oldham, J.B.; He, S.-L.; Zobitz, M.E.; Payne, R.G.; An, K.N.; Currier, B.L.; Mikos, A.G.; Yaszemski, M.J. Mechanical Properties of a Biodegradable Bone Regeneration Scaffold. J. Biomech. Eng. 2000, 122, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, K.P.; Metta, M.; Nagaraju, J. Molecular Phylogeny of Silkmoths Reveals the Origin of Domesticated Silkmoth, Bombyx Mori from Chinese Bombyx Mandarina and Paternal Inheritance of Antheraea Proylei Mitochondrial DNA. Mol. Phylogenet. Evol. 2006, 40, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Babb, P.L.; Lahens, N.F.; Correa-Garhwal, S.M.; Nicholson, D.N.; Kim, E.J.; Hogenesch, J.B.; Kuntner, M.; Higgins, L.; Hayashi, C.Y.; Agnarsson, I.; et al. The Nephila Clavipes Genome Highlights the Diversity of Spider Silk Genes and Their Complex Expression. Nat. Genet. 2017, 49, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Grabska, S.; Lewandowska, K.; Andrzejczyk, A. Polymer Films Based on Silk Fibroin and Collagen—The Physico-Chemical Properties. Mol. Cryst. Liq. Cryst. 2016, 640, 13–20. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Sangkert, S.; Meesane, J.; Kamonmattayakul, S.; Chai, W.L. Modified Silk Fibroin Scaffolds with Collagen/Decellularized Pulp for Bone Tissue Engineering in Cleft Palate: Morphological Structures and Biofunctionalities. Mater. Sci. Eng. C 2016, 58, 1138–1149. [Google Scholar] [CrossRef]
- Hashimoto, T.; Taniguchi, Y.; Kameda, T.; Tamada, Y.; Kurosu, H. Changes in the Properties and Protein Structure of Silk Fibroin Molecules in Autoclaved Fabrics. Polym. Degrad. Stab. 2015, 112, 20–26. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk Fibroin Biomaterials for Tissue Regenerations. Adv. Drug Deliver. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vongsanga, K.; Wang, X.; Byrne, N. What Happens during Natural Protein Fibre Dissolution in Ionic Liquids. Materials 2014, 7, 6158–6168. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Kurland, N.E.; Yadavalli, V.K.; Kundu, S.C. Isolation and Processing of Silk Proteins for Biomedical Applications. Int. J. Biol. Macromol. 2014, 70, 70–77. [Google Scholar] [CrossRef]
- Qiang, L.; Chuan-Bao, C.; Ying, Z.; He-Sun, Z. Preparation and Properties of Insoluble Fibroin Films by Novel Method. Chem. J. Chin. Univ. 2004, 25, 1752–1755. [Google Scholar]
- Huemmerich, D.; Helsen, C.W.; Quedzuweit, S.; Oschmann, J.; Rudolph, R.; Scheibel, T. Primary Structure Elements of Spider Dragline Silks and Their Contribution to Protein Solubility. Biochemistry 2004, 43, 13604–13612. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk Fibroin-Based Nanoparticles for Drug Delivery. IJMS 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Vasanthavada, K.; Kohler, K.; McNary, S.; Moore, A.M.F.; Vierra, C.A. Molecular Mechanisms of Spider Silk. Cell. Mol. Life Sci. CMLS 2006, 63, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.V. Spider Silk: Ancient Ideas for New Biomaterials. Chem. Rev. 2006, 106, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Humenik, M.; Smith, A.M.; Scheibel, T. Recombinant Spider Silks—Biopolymers with Potential for Future Applications. Polymers 2011, 3, 640. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk Fibroin in Tissue Engineering. Adv. Health Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Jao, D.; Mou, X.; Hu, X. Tissue Regeneration: A Silk Road. J. Funct. Biomater. 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, S.; Huang, J. Progress in Modification of Silk Fibroin Fiber. Sci. China Technol. Sci. 2019, 62, 919–930. [Google Scholar] [CrossRef]
- Babu, M.K. Developments in the processing and applications of silk. In Silk: Processing, Properties and Applications, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 129–142. ISBN 978-0-08-102540-6. [Google Scholar]
- Yukseloglu, S.M.; Sokmen, N.; Canoglu, S. Biomaterial Applications of Silk Fibroin Electrospun Nanofibres. Microelectron. Eng. 2015, 146, 43–47. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Silk-Based Delivery Systems of Bioactive Molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, O.J.; Lee, M.C.; Moon, B.M.; Ju, H.W.; Lee, J.M.; Kim, J.-H.; Kim, D.W.; Park, C.H. Fabrication of 3D Porous Silk Scaffolds by Particulate (Salt/Sucrose) Leaching for Bone Tissue Reconstruction. Int. J. Biol. Macromol. 2015, 78, 215–223. [Google Scholar] [CrossRef]
- Yu, Y.; Hua, S.; Yang, M.; Fu, Z.; Teng, S.; Niu, K.; Zhao, Q.; Yi, C. Fabrication and Characterization of Electrospinning/3D Printing Bone Tissue Engineering Scaffold. RSC Adv. 2016, 6, 110557–110565. [Google Scholar] [CrossRef]
- Mathur, A.B.; Gupta, V. Silk Fibroin-Derived Nanoparticles for Biomedical Applications. Nanomedicine 2010, 5, 807–820. [Google Scholar] [CrossRef]
- Imsombut, T.; Srisuwan, Y.; Srihanam, P.; Baimark, Y. Genipin-Cross-Linked Silk Fibroin Microspheres Prepared by the Simple Water-in-Oil Emulsion Solvent Diffusion Method. Powder Technol. 2010, 203, 603–608. [Google Scholar] [CrossRef]
- Yoshimizu, H.; Asakura, T. Preparation and Characterization of Silk Fibroin Powder and Its Application to Enzyme Immobilization. J. Appl. Polym. Sci. 1990, 40, 127–134. [Google Scholar] [CrossRef]
- Yeo, J.-H.; Lee, K.-G.; Lee, Y.-W.; Kim, S.Y. Simple Preparation and Characteristics of Silk Fibroin Microsphere. Eur. Polym. J. 2003, 39, 1195–1199. [Google Scholar] [CrossRef]
- Lammel, A.S.; Hu, X.; Park, S.-H.; Kaplan, D.L.; Scheibel, T.R. Controlling Silk Fibroin Particle Features for Drug Delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, X.; Yao, J.; Huang, L.; Shao, Z. The Preparation of Regenerated Silk Fibroin Microspheres. Soft Matter 2007, 3, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Wenk, E.; Wandrey, A.J.; Merkle, H.P.; Meinel, L. Silk Fibroin Spheres as a Platform for Controlled Drug Delivery. J. Control. Release 2008, 132, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wongpinyochit, T.; Uhlmann, P.; Urquhart, A.J.; Seib, F.P. PEGylated Silk Nanoparticles for Anticancer Drug Delivery. Biomacromolecules 2015, 16, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk Nanospheres and Microspheres from Silk/Pva Blend Films for Drug Delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Drug and Gene Delivery. Pharmaceutics 2019, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Goh, J.C.H. Self-Assembled Silk Fibroin Particles: Tunable Size and Appearance. Powder Technol. 2012, 215–216, 85–90. [Google Scholar] [CrossRef]
- Jin, H.-J.; Park, J.; Karageorgiou, V.; Kim, U.-J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Water-Stable Silk Films with Reduced β-Sheet Content. Adv. Funct. Mater. 2005, 15, 1241–1247. [Google Scholar] [CrossRef]
- Perry, H.; Gopinath, A.; Kaplan, D.L.; Dal Negro, L.; Omenetto, F.G. Nano- and Micropatterning of Optically Transparent, Mechanically Robust, Biocompatible Silk Fibroin Films. Adv. Mater. 2008, 20, 3070–3072. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Levin, B.; Redmond, S.L.; Li, L.H.; Wang, L.; Kanwar, J.R.; Atlas, M.D.; Wang, X. Structure and Properties of Biomedical Films Prepared from Aqueous and Acidic Silk Fibroin Solutions. J. Biomed. Mater. Res. Part A 2011, 97A, 37–45. [Google Scholar] [CrossRef]
- Kweon, H.; Um, I.C.; Park, Y.H. Structural and Thermal Characteristics of Antheraea Pernyi Silk Fibroin/Chitosan Blend Film. Polymer 2001, 42, 6651–6656. [Google Scholar] [CrossRef]
- Sionkowska, A.; Planecka, A. The Influence of UV Radiation on Silk Fibroin. Polym. Degrad. Stab. 2011, 96, 523–528. [Google Scholar] [CrossRef]
- Asakura, T.; Yoshimizu, H.; Kakizaki, M. An ESR Study of Spin-Labeled Silk Fibroin Membranes and Spin-Labeled Glucose Oxidase Immobilized in Silk Fibroin Membranes. Biotechnol. Bioeng. 1990, 35, 511–517. [Google Scholar] [CrossRef]
- Muller, W.S.; Samuelson, L.A.; Fossey, S.A.; Kaplan, D.L. Formation and Characterization of Langmuir Silk Films. Langmuir 1993, 9, 1857–1861. [Google Scholar] [CrossRef]
- Terada, D.; Yokoyama, Y.; Hattori, S.; Kobayashi, H.; Tamada, Y. The Outermost Surface Properties of Silk Fibroin Films Reflect Ethanol-Treatment Conditions Used in Biomaterial Preparation. Mater. Sci. Eng. C 2016, 58, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Sagnella, A.; Pistone, A.; Bonetti, S.; Donnadio, A.; Saracino, E.; Nocchetti, M.; Dionigi, C.; Ruani, G.; Muccini, M.; Posati, T.; et al. Effect of Different Fabrication Methods on the Chemo-Physical Properties of Silk Fibroin Films and on Their Interaction with Neural Cells. RSC Adv. 2016, 6, 9304–9314. [Google Scholar] [CrossRef]
- Wang, X.; Kim, H.J.; Xu, P.; Matsumoto, A.; Kaplan, D.L. Biomaterial Coatings by Stepwise Deposition of Silk Fibroin. Langmuir 2005, 21, 11335–11341. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Kim, D.-K.; Lee, O.J.; Kim, J.-H.; Ju, H.W.; Lee, J.M.; Moon, B.M.; Park, H.J.; Kim, D.W.; Kim, S.H.; et al. Fabrication of Silk Fibroin Film Using Centrifugal Casting Technique for Corneal Tissue Engineering. J. Biomed. Mater. Res. B 2016, 104, 508–514. [Google Scholar] [CrossRef]
- Chao, P.-H.G.; Yodmuang, S.; Wang, X.; Sun, L.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk Hydrogel for Cartilage Tissue Engineering. J. Biomed. Mater. Res. 2010, 95, 84–90. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem Cell-Based Tissue Engineering with Silk Biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel Production: Current Status, Research Directions, and Future Opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Scognamiglio, M.; Reverchon, E. A New Tool to Produce Alginate-Based Aerogels for Medical Applications, by Supercritical Gel Drying. J. Supercrit. Fluids 2019, 146, 152–158. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Natural Aerogels Production by Supercritical Gel Drying. Chem. Eng. Trans. 2015, 43, 739–744. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, L.; Dai, H.L.; Wang, X.Y.; Li, J.S.; Zhao, Z. Fabrication, characterization, and in vitro evaluation of biomimetic silk fibroin porous scaffolds via supercritical CO2 technology. J. Supercrit. Fluids 2019, 150, 86–93. [Google Scholar] [CrossRef]
- Baldino, L.; Concilio, S.; Cardea, S.; Reverchon, E. Interpenetration of Natural Polymer Aerogels by Supercritical Drying. Polymers 2016, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Cardea, S.; Reverchon, E. Loaded Silk Fibroin Aerogel Production by Supercritical Gel Drying Process for Nanomedicine Applications. Chem. Eng. Trans. 2016, 49, 343–348. [Google Scholar] [CrossRef]
- Najberg, M.; Haji Mansor, M.; Taillé, T.; Bouré, C.; Molina-Peña, R.; Boury, F.; Cenis, J.L.; Garcion, E.; Alvarez-Lorenzo, C. Aerogel Sponges of Silk Fibroin, Hyaluronic Acid and Heparin for Soft Tissue Engineering: Composition-Properties Relationship. Carbohydr. Polym. 2020, 237, 116107. [Google Scholar] [CrossRef] [PubMed]
- Goimil, L.; Santos-Rosales, V.; Delgado, A.; Évora, C.; Reyes, R.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; Gómez-Amoza, J.L.; Concheiro, A.; et al. ScCO2-Foamed Silk Fibroin Aerogel/Poly(ε-Caprolactone) Scaffolds Containing Dexamethasone for Bone Regeneration. J. CO2 Util. 2019, 31, 51–64. [Google Scholar] [CrossRef]
- Li, D.; Chen, K.; Duan, L.; Fu, T.; Li, J.; Mu, Z.; Wang, S.; Zou, Q.; Chen, L.; Feng, Y.; et al. Strontium Ranelate Incorporated Enzyme-Cross-Linked Gelatin Nanoparticle/Silk Fibroin Aerogel for Osteogenesis in OVX-Induced Osteoporosis. ACS Biomater. Sci. Eng. 2019, 5, 1440–1451. [Google Scholar] [CrossRef]
- Buckley, C.T.; Vinardell, T.; Thorpe, S.D.; Haugh, M.G.; Jones, E.; McGonagle, D.; Kelly, D.J. Functional Properties of Cartilaginous Tissues Engineered from Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells. J. Biomech. 2010, 43, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, L. Tissue Engineered Cartilage Generated from Human Trachea Using DegraPol® Scaffold. Eur. J. Cardio-Thorac. Surg. 2003, 24, 201–207. [Google Scholar] [CrossRef][Green Version]
- Sionkowska, A.; Michalska, M.; Walczak, M. Preparation and Characterization of Silk Fibroin/Collagen Sponge with Nanohydroxyapatite. Null 2016, 640, 106–112. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Vikhoreva, G.A. Polysaccharide-Based Polymer Blends: Methods of Their Production. Glycoconj. J. 2006, 23, 611. [Google Scholar] [CrossRef]
- Ghaeli, I.; de Moraes, M.; Beppu, M.; Lewandowska, K.; Sionkowska, A.; Ferreira-da-Silva, F.; Ferraz, M.; Monteiro, F. Phase Behaviour and Miscibility Studies of Collagen/Silk Fibroin Macromolecular System in Dilute Solutions and Solid State. Molecules 2017, 22, 1368. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B.; Michalska, M. The Miscibility of Collagen/Hyaluronic Acid/Chitosan Blends Investigated in Dilute Solutions and Solids. J. Mol. Liq. 2016, 220, 726–730. [Google Scholar] [CrossRef]
- Sionkowska, A.; Lewandowska, K.; Płanecka, A. Miscibility and Physical Properties of Chitosan and Silk Fibroin Mixtures. J. Mol. Liq. 2014, 198, 354–357. [Google Scholar] [CrossRef]
- Krigbaum, W.R.; Wall, F.T. Viscosities of Binary Polymeric Mixtures. J. Polym. Sci. 1950, 5, 505–514. [Google Scholar] [CrossRef]
- Garcίa, R.; Melad, O.; Gómez, C.M.; Figueruelo, J.E.; Campos, A. Viscometric Study on the Compatibility of Polymer–Polymer Mixtures in Solution. Eur. Polym. J. 1999, 35, 47–55. [Google Scholar] [CrossRef]
- Sionkowska, A.; Michalska, M.; Walczak, M.; Śmiechowski, K.; Grabska, S. Preparation and Characterization of Silk Fibroin/Collagen Sponge Modified by Chemical Cross-Linking. Mol. Cryst. Liq. Cryst. 2016, 640, 180–190. [Google Scholar] [CrossRef]
- Sionkowska, A.; Lewandowska, K.; Michalska, M.; Walczak, M. Characterization of Silk Fibroin 3D Composites Modified by Collagen. J. Mol. Liq. 2016, 215, 323–327. [Google Scholar] [CrossRef]
- Lin, X.-L.; Gao, L.-L.; Li, R.; Cheng, W.; Zhang, C.-Q.; Zhang, X. Mechanical Property and Biocompatibility of Silk Fibroin–Collagen Type II Composite Membrane. Mat. Sci. Eng. C 2019, 105, 110018. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ratanavaraporn, J.; Fauzi, M.B. Comprehensive Review of Hybrid Collagen and Silk Fibroin for Cutaneous Wound Healing. Materials 2020, 13, 3097. [Google Scholar] [CrossRef]
- Khalid, H.; Iqbal, H.; Zeeshan, R.; Nasir, M.; Sharif, F.; Akram, M.; Irfan, M.; Khan, F.A.; Chaudhry, A.A.; Khan, A.F. Silk Fibroin/Collagen 3D Scaffolds Loaded with TiO2 Nanoparticles for Skin Tissue Regeneration. Polym. Bull. 2020. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, N.; Yang, Q.; Zhang, Z.; Zhang, Q. Double-Layered Collagen/Silk Fibroin Composite Scaffold That Incorporates TGF-β1 Nanoparticles for Cartilage Tissue Engineering. J. Biomater. Tissue Eng. 2015, 5, 357–363. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-Chemical Characterization and Biological Tests of Collagen/Silk Fibroin/Chitosan Scaffolds Cross-Linked by Dialdehyde Starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Apinun, J.; Honsawek, S.; Kuptniratsaikul, S.; Jamkratoke, J.; Kanokpanont, S. Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells Encapsulated in Thai Silk Fibroin/Collagen Hydrogel: A Pilot Study in Vitro. Asian Biomed. 2018, 12, 273–279. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, L.; Shi, Y.; Qiu, J.; Fang, W.; Rong, M.; Guo, Z.; Gao, W. Characterization of Silk Fibroin/Chitosan 3D Porous Scaffold and In Vitro Cytology. PLoS ONE 2015, 10, e0128658. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A. Chitosan/Collagen Blends with Inorganic and Organic Additive-A Review. Adv. Polym. Technol. 2018, 37, 2367–2376. [Google Scholar] [CrossRef]
- Sionkowska, A.; Płanecka, A. Surface Properties of Thin Films Based on the Mixtures of Chitosan and Silk Fibroin. J. Mol. Liq. 2013, 186, 157–162. [Google Scholar] [CrossRef]
- Rujiravanit, R.; Kruaykitanon, S.; Jamieson, A.M.; Tokura, S. Preparation of Crosslinked Chitosan/Silk Fibroin Blend Films for Drug Delivery System. Macromol. Biosci. 2003, 3, 604–611. [Google Scholar] [CrossRef]
- Niamsa, N.; Srisuwan, Y.; Baimark, Y.; Phinyocheep, P.; Kittipoom, S. Preparation of Nanocomposite Chitosan/Silk Fibroin Blend Films Containing Nanopore Structures. Carbohydr. Polym. 2009, 78, 60–65. [Google Scholar] [CrossRef]
- Gobin, A.S.; Froude, V.E.; Mathur, A.B. Structural and Mechanical Characteristics of Silk Fibroin and Chitosan Blend Scaffolds for Tissue Regeneration. J. Biomed. Mater. Res. 2005, 74A, 465–473. [Google Scholar] [CrossRef]
- Ruan, Y.; Lin, H.; Yao, J.; Chen, Z.; Shao, Z. Preparation of 3D Fibroin/Chitosan Blend Porous Scaffold for Tissue Engineering Via a Simplified Method. Macromol. Biosci. 2011, 11, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-W.; Lei, X.; He, F.-L.; He, J.; Liu, Y.-L.; Ye, Y.-J.; Deng, X.; Duan, E.; Yin, D.-C. Silk Fibroin/Chitosan Scaffold with Tunable Properties and Low Inflammatory Response Assists the Differentiation of Bone Marrow Mesenchymal Stem Cells. Int. J. Biol. Macromol. 2017, 105, 584–597. [Google Scholar] [CrossRef]
- Sionkowska, A.; Płanecka, A. Preparation and Characterization of Silk Fibroin/Chitosan Composite Sponges for Tissue Engineering. J. Mol. Liq. 2013, 178, 5–14. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.Y.; Ha, W.S.; Park, S.Y. Structural Changes and Their Effect on Mechanical Properties of Silk Fibroin/Chitosan Blends. J. Appl. Polym. Sci. 1999, 74, 2571–2575. [Google Scholar] [CrossRef]
- Kweon, H.; Ha, H.C.; Um, I.C.; Park, Y.H. Physical Properties of Silk Fibroin/Chitosan Blend Films. J. Appl. Polym. Sci. 2001, 80, 928–934. [Google Scholar] [CrossRef]
- Lai, G.-J.; Shalumon, K.T.; Chen, S.-H.; Chen, J.-P. Composite Chitosan/Silk Fibroin Nanofibers for Modulation of Osteogenic Differentiation and Proliferation of Human Mesenchymal Stem Cells. Carbohydr. Polym. 2014, 111, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, H.; Li, R.; Nian, Z.; Li, D.; Xu, C. Silk Fibroin/Collagen and Silk Fibroin/Chitosan Blended Three-Dimensional Scaffolds for Tissue Engineering. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, M.A.; Silva, M.F.; Weska, R.F.; Beppu, M.M. Silk Fibroin and Sodium Alginate Blend: Miscibility and Physical Characteristics. Mater. Sci. Eng. C 2014, 40, 85–91. [Google Scholar] [CrossRef]
- de Moraes, M.A.; Beppu, M.M. Biocomposite Membranes of Sodium Alginate and Silk Fibroin Fibers for Biomedical Applications. J. Appl. Polym. Sci. 2013, 130, 3451–3457. [Google Scholar] [CrossRef]
- Ming, J.; Zuo, B. Crystal Growth of Calcium Carbonate in Silk Fibroin/Sodium Alginate Hydrogel. J. Cryst. Growth 2014, 386, 154–161. [Google Scholar] [CrossRef]
- Ming, J.; Zuo, B. A Novel Silk Fibroin/Sodium Alginate Hybrid Scaffolds. Polym. Eng. Sci. 2014, 54, 129–136. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Yang, M.; Zhu, L. Silk Fibroin/Sodium Alginate Composite Nano-Fibrous Scaffold Prepared through Thermally Induced Phase-Separation (TIPS) Method for Biomedical Applications. Mater. Sci. Eng. C 2015, 55, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.F.; Zuo, B.Q.; Jiang, Y.X. Calcium Ion Treatment Behavior of Silk Fibroin/Sodium Alginate Scaffolds. Adv. Mater. Res. 2012, 528, 107–112. [Google Scholar] [CrossRef]
- Lopes, L.M.; de Moraes, M.A.; Beppu, M.M. Study of Phase Separation in Blends of Silk Fibroin and Sodium Alginate in Solution and in Solid State. J. Polym. Res. 2018, 25, 198. [Google Scholar] [CrossRef]
- Chen, G.; Wei, R.; Huang, X.; Wang, F.; Chen, Z. Synthesis and Assessment of Sodium Alginate-Modified Silk Fibroin Microspheres as Potential Hepatic Arterial Embolization Agent. Int. J. Biol. Macromol. 2020, 155, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, S.; Li, Y.; Niu, C.; Li, X.; Guo, Y.; Zhang, J.; Shi, J.; Wang, X. Silk Fibroin/Sodium Alginate Composite Porous Materials with Controllable Degradation. Int. J. Biol. Macromol. 2020, 150, 1314–1322. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A Promising Wound Dressing Material with Excellent Cytocompatibility and Proangiogenesis Action for Wound Healing: Strontium Loaded Silk Fibroin/Sodium Alginate (SF/SA) Blend Films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Shi, J.; Zhu, R.; Zhang, J.; Zhang, Z.; Ma, D.; Hou, Y.; Lin, F.; Yang, J.; et al. A Biomimetic Silk Fibroin/Sodium Alginate Composite Scaffold for Soft Tissue Engineering. Sci. Rep. 2016, 6, 39477. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Pan, F.; Zuo, B. Structure and Properties of Protein-Based Fibrous Hydrogels Derived from Silk Fibroin and Sodium Alginate. J. Sol-Gel Sci. Technol. 2015, 74, 774–782. [Google Scholar] [CrossRef]
- Jaipaew, J.; Wangkulangkul, P.; Suwanrath, C.; Meesane, J.; Krivimol, S.; Puttawibul, P. Chondrogenesis of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Silk-Fibroin/Hyaluronic Acid Scaffolds for Cartilage Tissue Engineering. J. Biotechnol. 2014, 185, S125. [Google Scholar] [CrossRef]
- Yan, S.; Li, M.; Zhang, Q.; Wang, J. Blend Films Based on Silk Fibroin/Hyaluronic Acid. Fibers Polym. 2013, 14, 188–194. [Google Scholar] [CrossRef]
- Foss, C.; Merzari, E.; Migliaresi, C.; Motta, A. Silk Fibroin/Hyaluronic Acid 3D Matrices for Cartilage Tissue Engineering. Biomacromolecules 2013, 14, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, L.; Yan, Z.; Yu, Q.; Mo, X. Electrospun Biomimic Nanofibrous Scaffolds of Silk Fibroin/Hyaluronic Acid for Tissue Engineering. Null 2012, 23, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, M.; Giger, E.; Meinel, L.; Merkle, H.P. The Effect of Hyaluronic Acid on Silk Fibroin Conformation. Biomaterials 2008, 29, 633–642. [Google Scholar] [CrossRef]
- Yan, S.; Han, G.; Wang, Q.; Zhang, S.; You, R.; Luo, Z.; Xu, A.; Li, X.; Li, M.; Zhang, Q.; et al. Directed Assembly of Robust and Biocompatible Silk Fibroin/Hyaluronic Acid Composite Hydrogels. Compos. Part B Eng. 2019, 176, 107204. [Google Scholar] [CrossRef]
- Guan, Y.; You, H.; Cai, J.; Zhang, Q.; Yan, S.; You, R. Physically Crosslinked Silk Fibroin/Hyaluronic Acid Scaffolds. Carbohydr. Polym. 2020, 239, 116232. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, J.; Choi, J.; Ki, C.S. Effect of Ethanol Treatment on Physical Property of Photopolymerized Hyaluronic Acid/Silk Fibroin Hybrid Hydrogel. Polymer 2020, 202, 122733. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Q.; Tariq, Z.; You, R.; Li, X.; Li, M.; Zhang, Q. Facile Preparation of Bioactive Silk Fibroin/Hyaluronic Acid Hydrogels. Int. J. Biol. Macromol. 2018, 118, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Sungkhaphan, P.; Ratanavaraporn, J.; Damrongsukkul, S. Development of Thai Silk Fibroin/Hyaluronic Acid Microspheres and the Application on Controlled Release of Curcumin. Eng. J. 2017, 21, 139–153. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Chen, S.; You, R.; Tariq, Z.; Huang, J.; Li, M.; Yan, S. Silk Fibroin/Hyaluronic Acid Porous Scaffold for Dermal Wound Healing. Fibers Polym. 2017, 18, 1056–1063. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk Fibroin/Gelatin Microcarriers as Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, X.; Wang, Z.; Jin, D.; Tong, S.; Wang, X. Construction and Characterization of Three-Dimensional Silk Fibroin-Gelatin Scaffolds. J. Hard Tissue Biol. 2016, 25, 269–276. [Google Scholar] [CrossRef][Green Version]
- Safdari, M.; Shakiba, E.; Kiaie, S.H.; Fattahi, A. Preparation and Characterization of Ceftazidime Loaded Electrospun Silk Fibroin/Gelatin Mat for Wound Dressing. Fibers Polym. 2016, 17, 744–750. [Google Scholar] [CrossRef]
- Burger, D.; Beaumont, M.; Rosenau, T.; Tamada, Y. Porous Silk Fibroin/Cellulose Hydrogels for Bone Tissue Engineering via a Novel Combined Process Based on Sequential Regeneration and Porogen Leaching. Molecules 2020, 25, 5097. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kwon, G.-J.; Hwang, K.; Byeon, J.; Ghodake, G.S.; Shinde, S.K.; Hyun, S.; Kim, D.-Y. Influence of Silk Fibroin Content on Cellulose Blend Film Using LiBr Solution. BioResources 2020, 15, 2459–2470. [Google Scholar]
- Yang, M.; Wang, Y.; Tao, G.; Cai, R.; Wang, P.; Liu, L.; Ai, L.; Zuo, H.; Zhao, P.; Umar, A.; et al. Fabrication of Sericin/Agrose Gel Loaded Lysozyme and Its Potential in Wound Dressing Application. Nanomaterials 2018, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Sow, W.T.; Devi, D.; Ng, K.W.; Mandal, B.B.; Cho, N.-J. Silk Fibroin–Keratin Based 3D Scaffolds as a Dermal Substitute for Skin Tissue Engineering. Integr. Biol. 2015, 7, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Gomes, A.C.; Cavaco-Paulo, A. Novel Silk Fibroin/Elastin Wound Dressings. Acta Biomater. 2012, 8, 3049–3060. [Google Scholar] [CrossRef]
- Yoo, C.R.; Yeo, I.-S.; Park, K.E.; Park, J.H.; Lee, S.J.; Park, W.H.; Min, B.-M. Effect of Chitin/Silk Fibroin Nanofibrous Bicomponent Structures on Interaction with Human Epidermal Keratinocytes. Int. J. Biol. Macromol. 2008, 42, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Wang, H.; Dong, Z. Preparation, Characterization and Biocompatibility of Electrospinning Heparin-Modified Silk Fibroin Nanofibers. Int. J. Biol. Macromol. 2011, 48, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadi, J.; Roshanfar, F.; Farokhi, M.; Nazarpak, M.H. Silk Fibroin/Kappa-Carrageenan Composite Scaffolds with Enhanced Biomimetic Mineralization for Bone Regeneration Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 951–958. [Google Scholar] [CrossRef]
- Kweon, H.; Yeo, J.; Lee, K.; Lee, H.C.; Na, H.S.; Won, Y.H.; Cho, C.S. Semi-Interpenetrating Polymer Networks Composed of Silk Fibroin and Poly(Ethylene Glycol) for Wound Dressing. Biomed. Mater. 2008, 3, 034115. [Google Scholar] [CrossRef]
- Sayed, M.M.; Mousa, H.M.; El-Aassar, M.R.; El-Deeb, N.M.; Ghazaly, N.M.; Dewidar, M.M.; Abdal-hay, A. Enhancing Mechanical and Biodegradation Properties of Polyvinyl Alcohol/Silk Fibroin Nanofibers Composite Patches for Cardiac Tissue Engineering. Mater. Lett. 2019, 255, 126510. [Google Scholar] [CrossRef]
- Li, G.; Kong, Y.; Zhao, Y.; Zhao, Y.; Zhang, L.; Yang, Y. Fabrication and Characterization of Polyacrylamide/Silk Fibroin Hydrogels for Peripheral Nerve Regeneration. J. Biomat. Sci. Polym. E 2015, 26, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Chae, T.; Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Park, H.J.; Park, Y.R.; Park, C.H. Three Dimensional Poly(ε-Caprolactone) and Silk Fibroin Nanocomposite Fibrous Matrix for Artificial Dermis. Mater. Sci. Eng. C 2016, 68, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Lok Toh, S.; Hong Goh, J.C. PLGA Nanofiber-Coated Silk Microfibrous Scaffold for Connective Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Watcharajittanont, N.; Putson, C.; Pripatnanont, P.; Meesane, J. Layer-by-Layer Electrospun Membranes of Polyurethane/Silk Fibroin Based on Mimicking of Oral Soft Tissue for Guided Bone Regeneration. Biomed. Mater. 2019, 14, 055011. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Lv, Q.; Cui, F.Z.; Xu, L.; Jiao, Y.P.; Wang, Y.; Feng, Q.L.; Wang, H.L.; Huang, L.Y. A Novel Poly(L-Lactide) (PLLA)/Fibroin Hybrid Scaffold to Promote Hepatocyte Viability and Decrease Macrophage Responses. J. Bioact. Compat. Polym. 2007, 22, 395–410. [Google Scholar] [CrossRef]
- Li, J.; Yang, B.; Qian, Y.; Wang, Q.; Han, R.; Hao, T.; Shu, Y.; Zhang, Y.; Yao, F.; Wang, C. Iota-Carrageenan/Chitosan/Gelatin Scaffold for the Osteogenic Differentiation of Adipose-Derived MSCs in Vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Jayakumar, R.; Anil, S.; Chalisserry, E.P.; Pallela, R.; Kim, S.-K. Development of Alginate-Chitosan-Collagen Based Hydrogels for Tissue Engineering. J. Biomater. Tissue Eng. 2015, 5, 458–464. [Google Scholar] [CrossRef]
- Grabska, S.; Sionkowska, A.; Kaczmarek, B. The Physicochemical Properties of 3D Materials Based on Hyaluronic Acid Modified by Tannic Acid Addition. Mol. Cryst. Liq. Cryst. 2018, 670, 90–96. [Google Scholar] [CrossRef]
- Sionkowska, A.; Grabska, S. Incorporation of Magnetite Particles in 3D Matrices Made from the Blends of Collagen, Chitosan, and Hyaluronic Acid. Adv. Polym. Technol. 2018, 37, 2905–2914. [Google Scholar] [CrossRef]
- Gokila, S.; Gomathi, T.; Vijayalakshmi, K.; Alsharani, F.A.; Anil, S.; Sudha, P.N. Development of 3D Scaffolds Using Nanochitosan/Silk-Fibroin/Hyaluronic Acid Biomaterials for Tissue Engineering Applications. Int. J. Biol. Macromol. 2018, 120, 876–885. [Google Scholar] [CrossRef]
- Kwak, H.; Shin, S.; Lee, H.; Hyun, J. Formation of a Keratin Layer with Silk Fibroin-Polyethylene Glycol Composite Hydrogel Fabricated by Digital Light Processing 3D Printing. J. Ind. Eng. Chem. 2019, 72, 232–240. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H.; Lan, Y.; Zhu, Q.; Liu, Y.; Huang, S.; Shi, S.; Hancharou, A.; Tang, B.; Guo, R. Preparation and Characterisation of a Novel Silk Fibroin/Hyaluronic Acid/Sodium Alginate Scaffold for Skin Repair. Int. J. Biol. Macromol. 2019, 130, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Sionkowska, A.; Coelho, C.C.; Monteiro, F.J. Silk Fibroin/Collagen/Chitosan Scaffolds Cross-Linked by a Glyoxal Solution as Biomaterials toward Bone Tissue Regeneration. Materials 2020, 13, 3433. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, Â.; Monteiro, F.J. Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, S.; Hu, K.; Feng, Q.; Cao, C.; Cui, F. Cytocompatibility and Blood Compatibility of Multifunctional Fibroin/Collagen/Heparin Scaffolds. Biomaterials 2007, 28, 2306–2313. [Google Scholar] [CrossRef]
- Gu, Z.; Xie, H.; Huang, C.; Li, L.; Yu, X. Preparation of Chitosan/Silk Fibroin Blending Membrane Fixed with Alginate Dialdehyde for Wound Dressing. Int. J. Biol. Macromol. 2013, 58, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.L.; Motta, A.; Marshall, M.J.; El Haj, A.J.; Cartmell, S.H. Osteoblast: Osteoclast Co-Cultures on Silk Fibroin, Chitosan and PLLA Films. Biomaterials 2009, 30, 5376–5384. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Nakahira, T.; Zhang, M.; Nakagawa, M.; Yoshikawa, M.; Midorikawa, T. Wet-Spun Blend Biofibers of Cellulose–Silk Fibroin and Cellulose–Chitin–Silk Fibroin. Carbohydr. Polym. 2002, 47, 121–124. [Google Scholar] [CrossRef]
- Lamlerd, T.; Soontornvipart, K.; Kesdangsakonwut, S.; Kanokpanont, S.; Damrongsakkul, S. In Vivo Bone Regeneration of Thai Silk Fibroin Scaffolds with Gelatin, Hydroxyapatite and Hyaluronic. Thai J. Vet. Med. 2017, 47, 165–172. [Google Scholar]
- Mou, Z.-L.; Duan, L.-M.; Qi, X.-N.; Zhang, Z.-Q. Preparation of Silk Fibroin/Collagen/Hydroxyapatite Composite Scaffold by Particulate Leaching Method. Mater. Lett. 2013, 105, 189–191. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Z.; Cao, H.; Hu, H.; Luo, Z.; Yang, X.; Cui, M.; Zhou, L. Genipin-Crosslinked Polyvinyl Alcohol/Silk Fibroin/Nano-Hydroxyapatite Hydrogel for Fabrication of Artificial Cornea Scaffolds-a Novel Approach to Corneal Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 1604–1619. [Google Scholar] [CrossRef]
- Sionkowska, A.; Tuwalska, A. Preparation and Characterization of New Materials Based on Silk Fibroin, Chitosan and Nanohydroxyapatite. Int. J. Polym. Anal. Charact. 2020, 25, 315–333. [Google Scholar] [CrossRef]
- Kim, J.-H.; Sheikh, F.A.; Ju, H.W.; Park, H.J.; Moon, B.M.; Lee, O.J.; Park, C.H. 3D Silk Fibroin Scaffold Incorporating Titanium Dioxide (TiO2) Nanoparticle (NPs) for Tissue Engineering. Int. J. Biol. Macromol. 2014, 68, 158–168. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.-K.; Lee, O.J.; Ju, H.W.; Lee, J.M.; Moon, B.M.; Park, H.J.; Kim, D.W.; Lee, J.H.; Park, C.H. Osteoinductive Silk Fibroin/Titanium Dioxide/Hydroxyapatite Hybrid Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2016, 82, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Johari, N.; Madaah Hosseini, H.R.; Samadikuchaksaraei, A. Mechanical Modeling of Silk Fibroin/TiO2 and Silk Fibroin/Fluoridated TiO2 Nanocomposite Scaffolds for Bone Tissue Engineering. Iran. Polym. J. 2020, 29, 219–224. [Google Scholar] [CrossRef]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/Silk Fibroin/TiO2 Bio-Nanocomposite as a Biocompatible Wound Dressing Bandage with Strong Antimicrobial Activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Jao, W.-C.; Yang, M.-C.; Lin, C.-H.; Hsu, C.-C. Fabrication and Characterization of Electrospun Silk Fibroin/TiO2 Nanofibrous Mats for Wound Dressings: Electrospun Silk Fibroin/TiO2 Nanofibrus Mats. Polym. Adv. Technol. 2012, 23, 1066–1076. [Google Scholar] [CrossRef]
- Min, Q.; Yu, X.; Liu, J.; Zhang, Y.; Wan, Y.; Wu, J. Controlled Delivery of Insulin-like Growth Factor-1 from Bioactive Glass-Incorporated Alginate-Poloxamer/Silk Fibroin Hydrogels. Pharmaceutics 2020, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, K.; Huang, X.; Liu, J.; Liu, H.; Boccaccini, A.R.; Wan, Y.; Guo, X.; Shao, Z. Thermally Triggered Injectable Chitosan/Silk Fibroin/Bioactive Glass Nanoparticle Hydrogels for in-Situ Bone Formation in Rat Calvarial Bone Defects. Acta Biomater. 2019, 91, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Kumar, S.; Sharma, A.; Kaur, K.; Shi, X.; Naruphontjirakul, P.; Jones, J.R.; Ghosh, S. Silk Fibroin-Bioactive Glass Based Advanced Biomaterials: Towards Patient-Specific Bone Grafts. Biomed. Mater. 2018, 13, 055012. [Google Scholar] [CrossRef]
- Singh, B.N.; Pramanik, K. Generation of Bioactive Nano-Composite Scaffold of Nanobioglass/Silk Fibroin/Carboxymethyl Cellulose for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 2011–2034. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jiao, G.; Zhang, W.; Chen, X.; Ning, R.; Du, C. Hybrid Scaffolding Strategy for Dermal Tissue Reconstruction: A Bioactive Glass/Chitosan/Silk Fibroin Composite. RSC Adv. 2016, 6, 19887–19896. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, N.; Feng, X.; Chen, J. Fabrication and Characterization of Silk Fibroin/Bioactive Glass Composite Films. Mater. Sci. Eng. C 2012, 32, 822–829. [Google Scholar] [CrossRef]
- Salama, A. Chitosan/Silk Fibroin/Zinc Oxide Nanocomposite as a Sustainable and Antimicrobial Biomaterial. Cell. Chem. Technol. 2018, 52, 903–907. [Google Scholar]
- Zhao, Y.-Z.; Lin, M.-T.; Lan, Q.-H.; Zhai, Y.-Y.; Xu, H.-L.; Xiao, J.; Kou, L.; Yao, Q. Silk Fibroin-Modified Disulfiram/Zinc Oxide Nanocomposites for PH Triggered Release of Zn2+ and Synergistic Antitumor Efficacy. Mol. Pharm. 2020, 17, 3857–3869. [Google Scholar] [CrossRef] [PubMed]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core–Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20, 1900328. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Grabska, S. Preparation and Characterization of 3D Collagen Materials with Magnetic Properties. Polym. Test. 2017, 62, 382–391. [Google Scholar] [CrossRef]
- Lalegül-Ülker, Ö.; Vurat, M.T.; Elçin, A.E.; Elçin, Y.M. Magnetic Silk Fibroin Composite Nanofibers for Biomedical Applications: Fabrication and Evaluation of the Chemical, Thermal, Mechanical, and in Vitro Biological Properties. J. Appl. Polym. Sci. 2019, 136, 48040. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z.; Yalçın, E.; Demirbilek, M.; Denkbaş, E.B. Magnetic Silk Fibroin E-Gel Scaffolds for Bone Tissue Engineering Applications. J. Bioact. Compat. Polym. 2017, 32, 596–614. [Google Scholar] [CrossRef]
- Bunea, M.C.; Vasile, E.; Galateanu, B.; Hudita, A.; Serban, M.; Zaharia, C. Silk Fibroin Films Decorated with Magnetic Nanoparticles for Wound Healling Applications. Mater. Plast. 2017, 54, 83. [Google Scholar] [CrossRef]
- Aliramaji, S.; Zamanian, A.; Mozafari, M. Super-Paramagnetic Responsive Silk Fibroin/Chitosan/Magnetite Scaffolds with Tunable Pore Structures for Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 70, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, A.N.; Burpo, F.J.; Nguyen, C.K.; Nagelli, E.A.; Ryu, M.Y.; Wang, J.; Sims, R.K.; Woronowicz, K.; Wickiser, J.K. Noble Metal Composite Porous Silk Fibroin Aerogel Fibers. Materials 2019, 12, 894. [Google Scholar] [CrossRef]
- Chen, W.; Wu, W.; Chen, H.; Shen, Z. Preparation and Characterization of Noble Metal Nanocolloids by Silk Fibroin in Situ Reduction. Sci. China Ser. B 2003, 46, 330–338. [Google Scholar] [CrossRef]
- Li, X.; Ye, F.; Li, G.; Cui, J.; Liu, Y.; Yang, L.; Cong, L.; Li, B. 3D Printed Hydroxyapatite/Silk Fibroin/Polycaprolactone Artificial Bone Scaffold and Bone Tissue Engineering Materials Constructed with Double-Transfected Bone Morphogenetic Protein-2 and Vascular Endothelial Growth Factor Mesenchymal Stem Cells to Repair Rabbit Radial Bone Defects. Nanosci. Nanotechnol. Lett. 2020, 12, 368–375. [Google Scholar] [CrossRef]
- Qi, Q.; Yao, Y.; Jia, X.; Meng, Y.; Zhao, K.; Jian, Y. Effects of Polyethylene Glycol Content on the Properties of a Silk Fibroin/Nano-Hydroxyapatite/Polyethylene Glycol Electrospun Scaffold. RSC Adv. 2019, 9, 33941–33948. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Kim, S.-G.; Kwon, K.-J.; Kweon, H.; Chae, W.-S.; Yang, W.-G.; Lee, E.-Y.; Seok, H. Silk Fibroin-Alginate-Hydroxyapatite Composite Particles in Bone Tissue Engineering Applications In Vivo. IJMS 2017, 18, 858. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Wegrzynowska-Drzymalska, K.; Bajek, A.; Maj, M.; Sionkowska, A. Is Dialdehyde Starch a Valuable Cross-Linking Agent for Collagen/Elastin Based Materials? J. Mater. Sci. Mater. Med. 2016, 27, 67. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Thakur, G.; Rodrigues, F.C.; Singh, K. Crosslinking Biopolymers for Advanced Drug Delivery and Tissue Engineering Applications. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 213–231. ISBN 9789811309502. [Google Scholar]
- Johari, N.; Moroni, L.; Samadikuchaksaraei, A. Tuning the Conformation and Mechanical Properties of Silk Fibroin Hydrogels. Eur. Polym. J. 2020, 134, 109842. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical Crosslinking of Biopolymeric Scaffolds: Current Knowledge and Future Directions of Crosslinked Engineered Bone Scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Chirila, T.V.; Suzuki, S.; Papolla, C. A Comparative Investigation of Bombyx Mori Silk Fibroin Hydrogels Generated by Chemical and Enzymatic Cross-Linking: Bombyx Mori Silk Fibroin Hydrogels. Biotechnol. Appl. Biochem. 2017, 64, 771–781. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Pieróg, M.; Gierszewska, M. Modification of chitosan—A concise overview. Wiadomości Chem. 2016, 70, 9–10. [Google Scholar]
- Dmour, I.; Taha, M. Natural and semisynthetic polymers in pharmaceutical nanotechnology. In Organic Materials as Smart Nanocarriers for Drug Delivery. Elsevier Inc.: Oxford, UK, 2018; ISBN 978-0-12-813663-8. [Google Scholar]
- Ruijgrok, J.M.; De Wijn, J.R.; Boon, M.E. Optimizing Glutaraldehyde Crosslinking of Collagen: Effects of Time, Temperature and Concentration as Measured by Shrinkage Temperature. J. Mater. Sci. Mater. Med. 1994, 5, 80–87. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To Cross-Link or Not to Cross-Link? Cross-Linking Associated Foreign Body Response of Collagen-Based Devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.O.; St Clair, M.B.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Kari, F.W. A Critical Review of the Toxicology of Glutaraldehyde. Crit. Rev. Toxicol. 1992, 22, 143–174. [Google Scholar] [CrossRef] [PubMed]



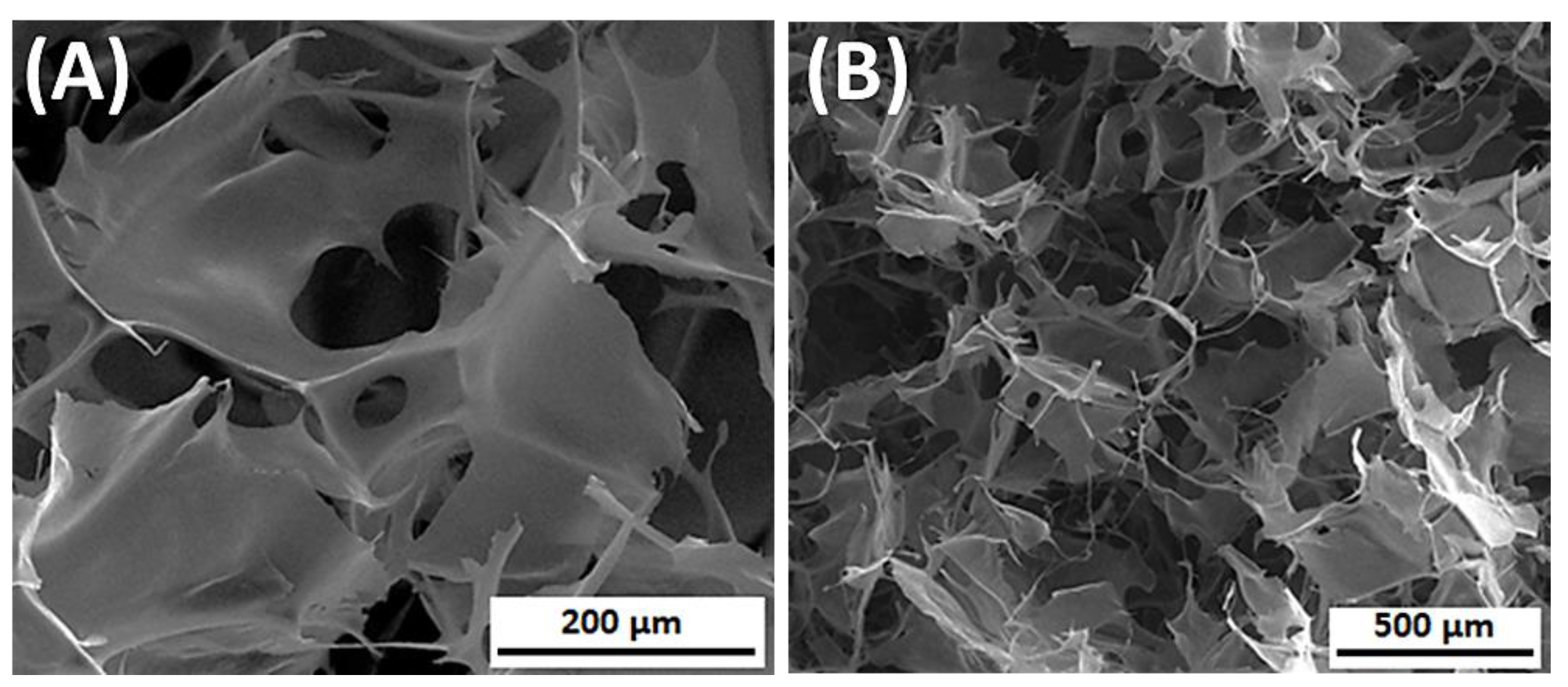
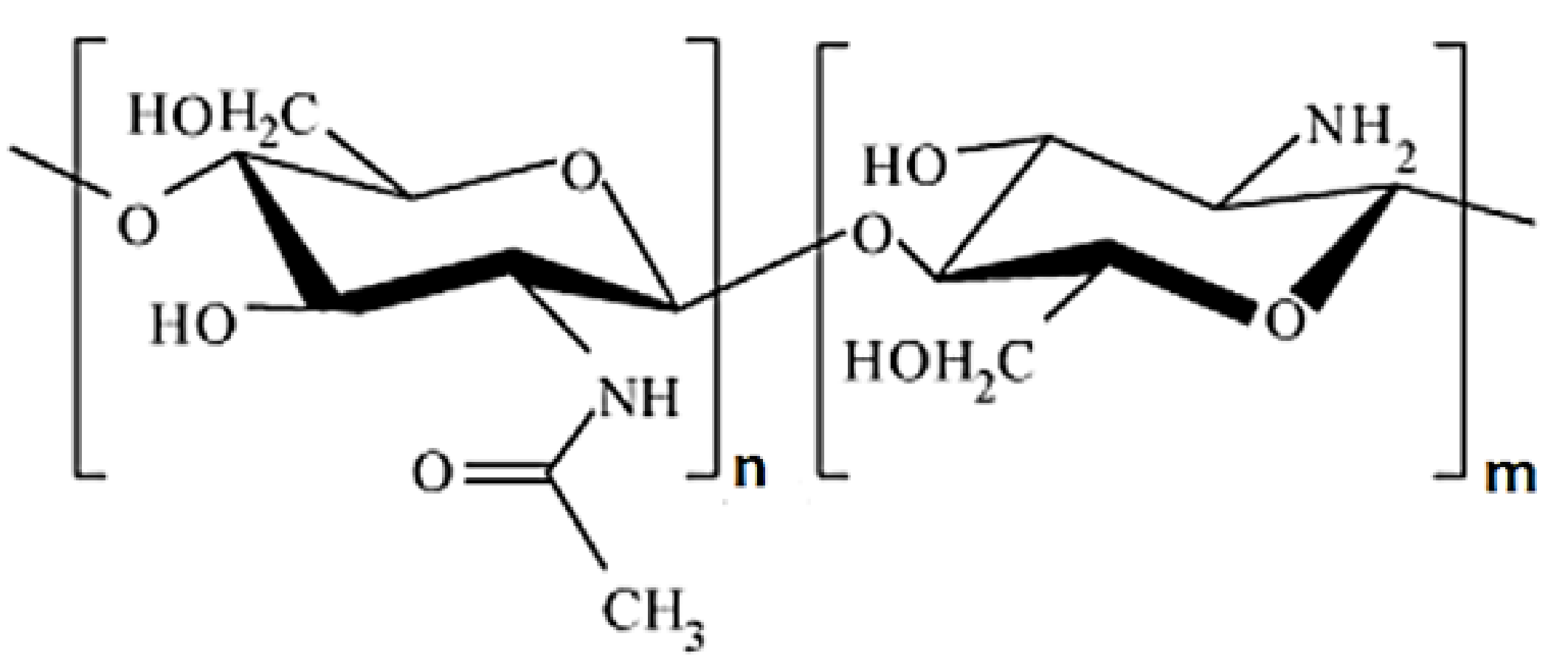
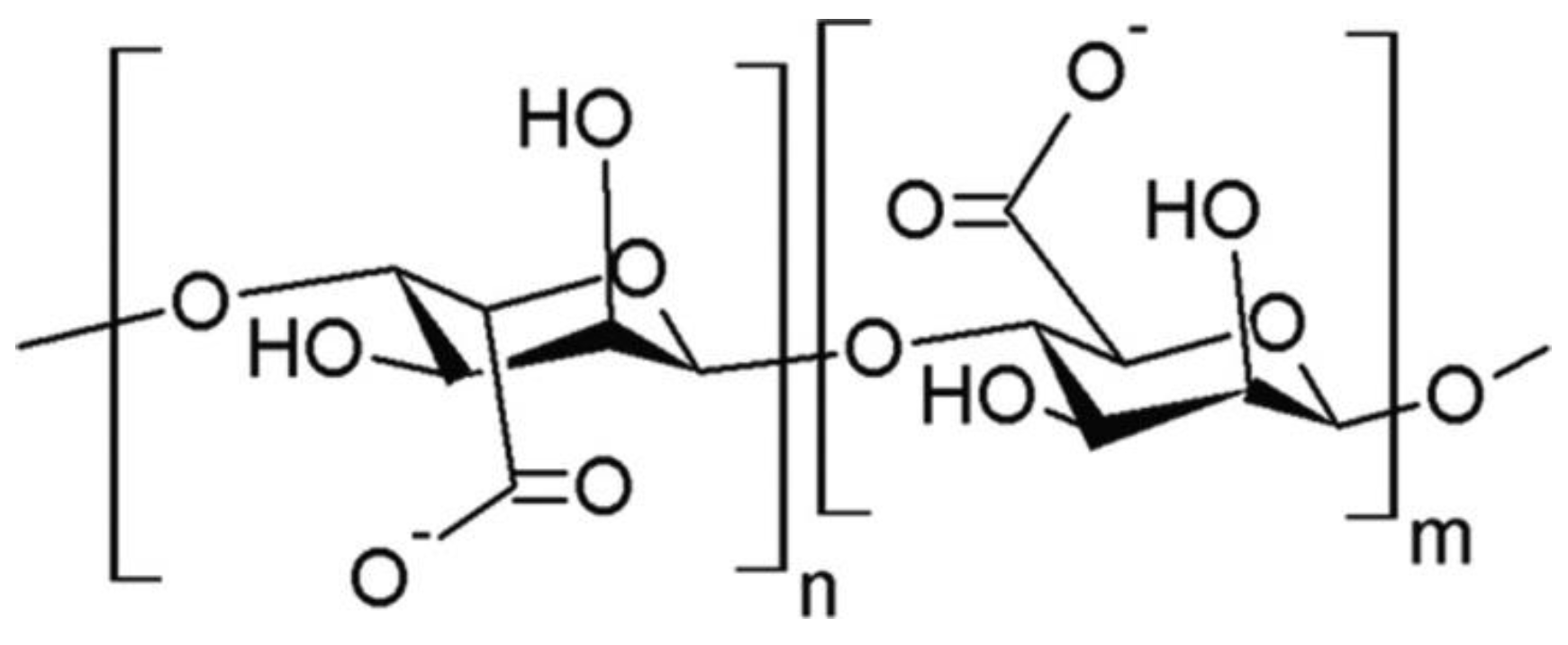
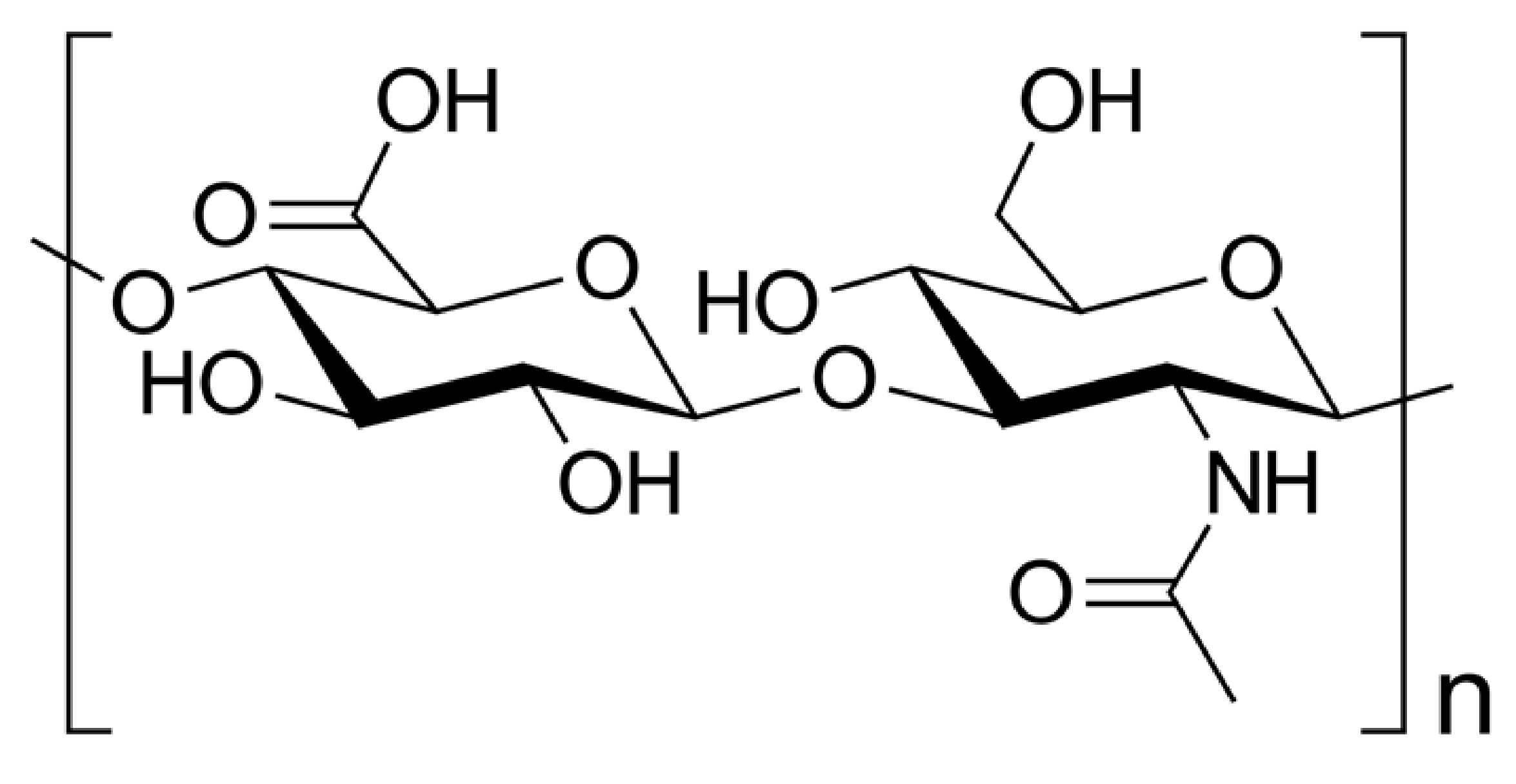
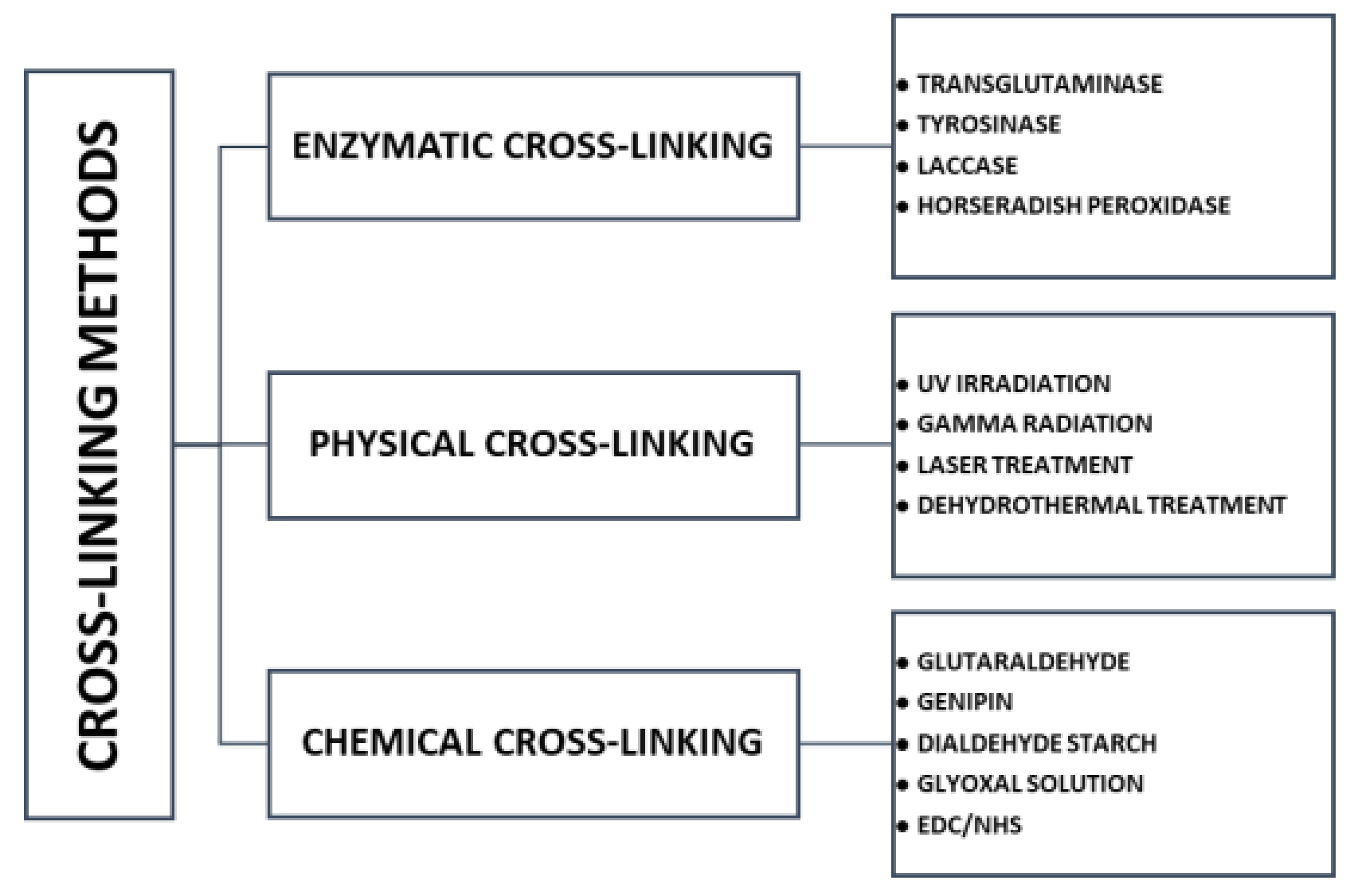
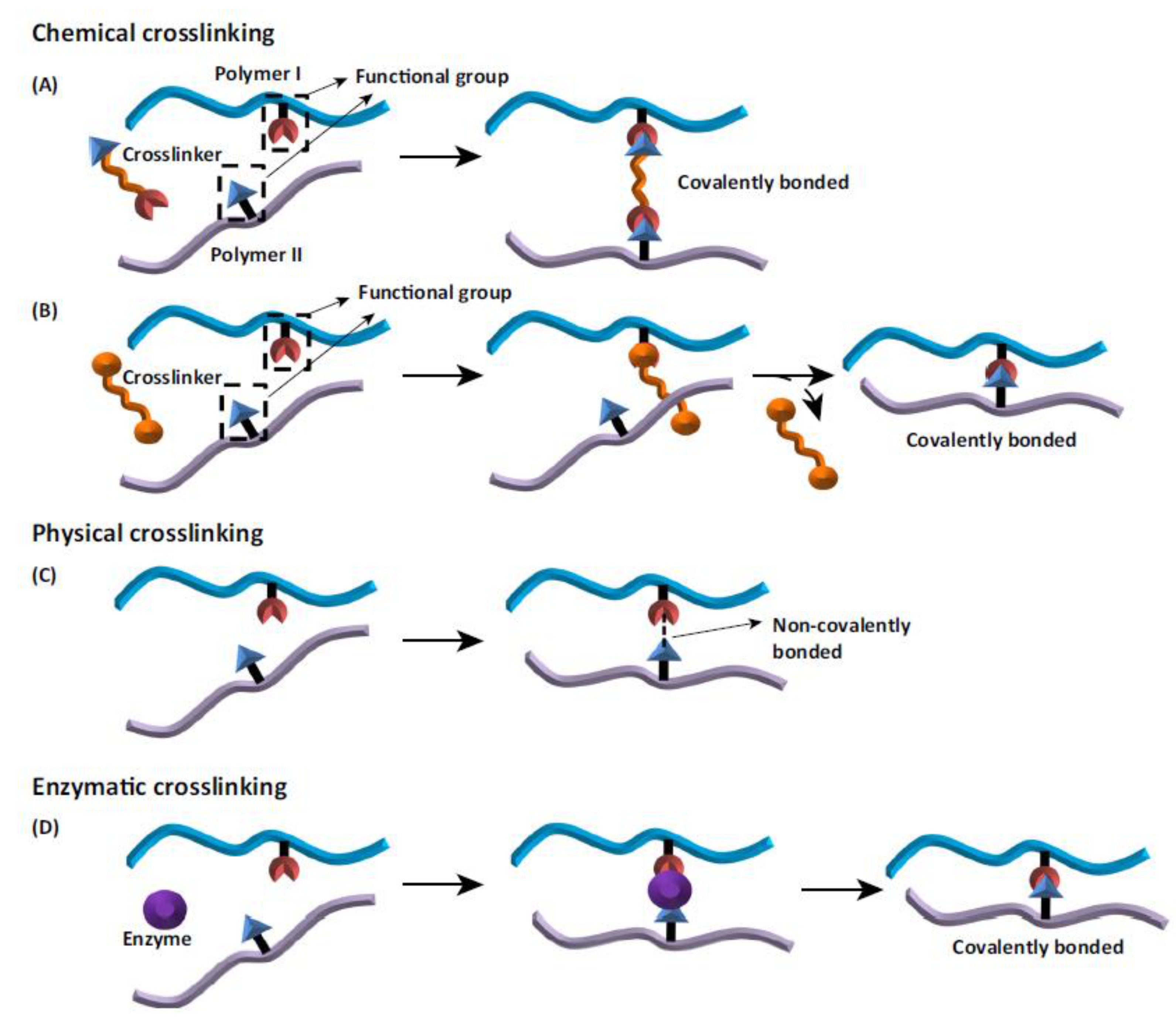
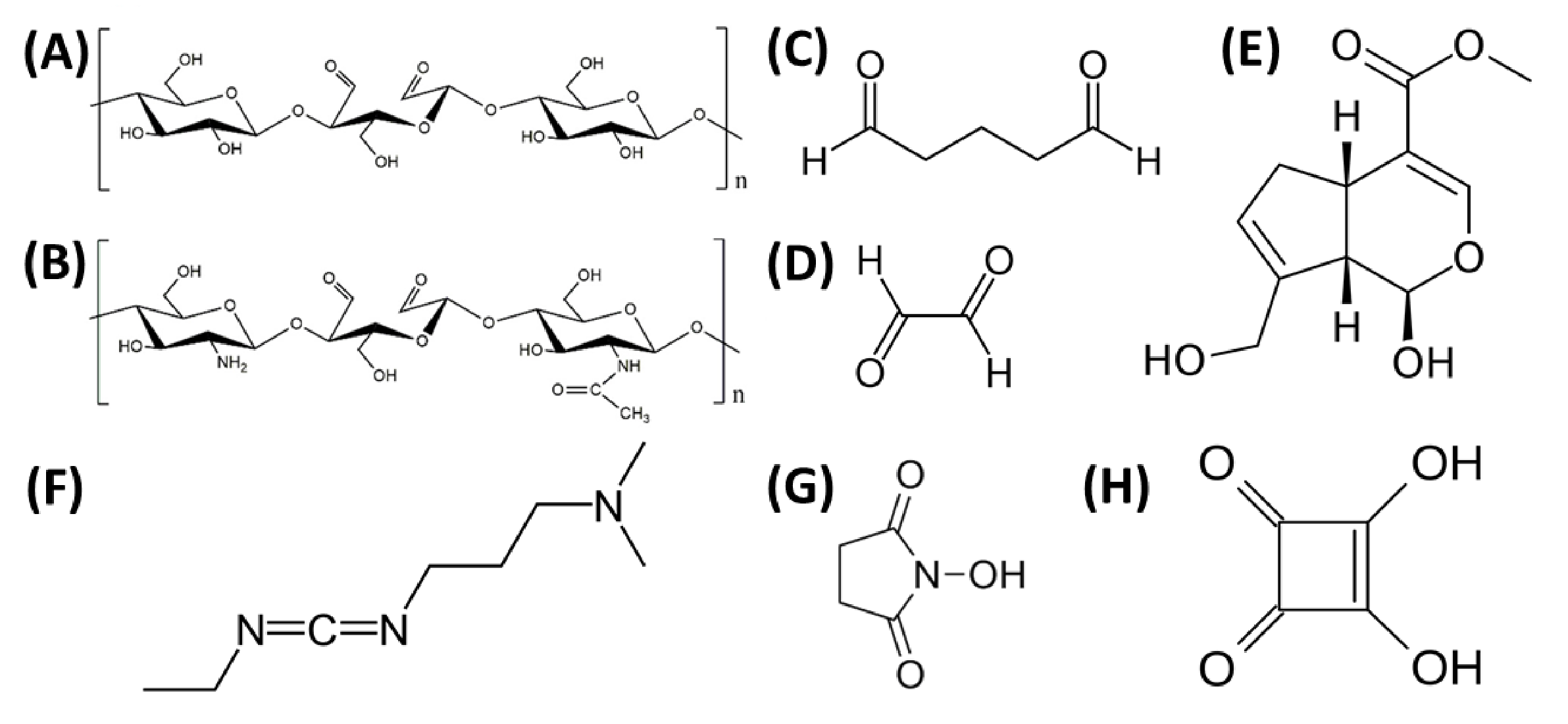
| Morphology Type | Processing Method | Applications | Source |
|---|---|---|---|
| fibers | reeling, spinning, electrospinning | tissue engineering, wound healing, nerve guides, stents and drug delivery systems | [34,52,53,54] |
| 3D structures (scaffolds, sponges, foams) | lyophilization, particulate-leaching, gas foaming, electrospinning, 3D printing | bone tissue engineering, cartilage tissue engineering, drug delivery, skin tissue engineering, intervertebral disc, meniscus liver | [24,54,55] |
| particles (microspheres, nanolayers, nanocoatings, nanoparticles, microcapsules) | spry drying, water in oil (W/O) emulsion solvent evaporation, water in oil emulsion solvent diffusion, ball-milling method, milling, freeze-drying and grinding, spray drying, self-assembly, freeze-thawing, jet breaking, desolvation, PVA blending | controlled-release drug delivery systems, bioactive molecule delivery, vaccine delivery, tissue engineering | [57,58,59,60,61,62,63,64,65] |
| films | casting, Langmuir-Blodgett (LB) process, spin coating, vertical deposition, manual or spin assisted layer by layer assembly, centrifugal casting | wound dressing/skin repair, biosensors, coating materials, bone, corneal, vascular tissue engineering | [53,64,74,75,76,77,78,79] |
| hydrogels | sol-gel transition in the presence of acid, ions or other additives, sonication, lyophilization | cartilage tissue engineering, guided bone repair, drug release/delivery | [41,53,65,80,81,82] |
| aerogels | ambient drying, freeze drying, direct supercritical drying, supercritical drying by CO2 extraction. | nerve regeneration, soft tissue engineering, bone tissue engineering, | [87,89,90,91,92] |
| WSF/WColl | bexpm [dL/g]2 | bid*m [dL/g]2 | ∆bm* | bid**m [dL/g]2 | ∆bm** | Miscibility |
|---|---|---|---|---|---|---|
| 25/75 | 72.41 | 30.04 | 42.37 | 21.64 | 50.77 | ✔ |
| 50/50 | 75.91 | 20.87 | 55.04 | 9.67 | 66.24 | ✔ |
| 75/25 | 39.54 | 10.94 | 28.60 | 2.54 | 37.00 | ✔ |
| %SF | Contact Angle [o] | γS [mJ/m2] | γSP/ γSD | Emod [GPa] | Fmax [MPa] | Rq [nm] | |
|---|---|---|---|---|---|---|---|
| D | GL | ||||||
| 100 | 51.4 ± 0.3 | 88.7 ± 0.4 | 33.13 | 0.026 | 0.43 ± 0.06 | 4.12 ± 0.94 | 5.10 |
| 90 | 50.8 ± 0.1 | 89.4 ± 0.2 | 33.55 | 0.020 | 0.92 ± 0.26 | 4.99 ± 1.34 | 5.90 |
| 70 | 49.6 ± 0.1 | 85.0 ± 0.3 | 33.97 | 0.046 | 1.02 ± 0.30 | 9.63 ± 2.51 | 7.60 |
| 50 | 49.9 ± 1.8 | 82.4 ± 0.7 | 33.82 | 0.071 | 0.82 ± 0.52 | 21.9 ± 10.7 | 6.70 |
| 30 | 48.0 ± 0.2 | 83.6 ± 0.3 | 34.83 | 0.050 | 0.79 ± 0.15 | 1.70 ± 1.06 | 7.40 |
| 10 | 44.3 ± 0.8 | 80.9 ± 0.3 | 36.80 | 0.058 | 2.55 ± 0.41 | 66.1 ± 4.93 | 33.00 |
| 0 | 44.4 ± 0.2 | 80.4 ± 0.4 | 36.76 | 0.063 | 2.41 ± 0.74 | 81.2 ± 1.87 | 26.60 |
| Sample | Porosity [%] | Density [mg/cm3] | Swelling Ratio [%] | Moisture Content in 100 g of Dry Sample [g] |
|---|---|---|---|---|
| SF | 95 ± 1.7 | 33.0 ± 0.4 | 1511 ± 147 | 7.09 ± 1.03 |
| Coll | 88 ± 0.5 | 16.9 ± 2.2 | Nd | 14.17 ± 1.36 |
| SF/Coll | 97 ± 1.0 | 14.8 ± 2.1 | 1891 ± 236 | 19.27 ± 0.92 |
| WSF/WCTS | bexpm [dL/g]2 | bid*m [dL/g]2 | ∆bm* | bid**m [dL/g]2 | ∆bm** | Miscibility |
|---|---|---|---|---|---|---|
| 20/80 | 115 | 147 | −32 | 147 | −32 | × |
| 50/50 | 58 | 58 | 0 | 57 | 1 | ✔ |
| 80/20 | 36 | 10 | 26 | 9 | 27 | ✔ |
| Scaffold Composition | Reference |
|---|---|
| Natural polymers | |
| Silk Fibroin + Gelatin | [146,147,148] |
| Silk Fibroin + Cellulose | [149,150] |
| Silk Fibroin + Agarose | [151] |
| Silk Fibroin + Keratin | [152] |
| Silk Fibroin + Elastin | [153] |
| Silk Fibroin + Chitin | [154] |
| Silk Fibroin + Heparin | [155] |
| Silk Fibroin + Carrageenan | [156] |
| Synthetic polymers | |
| Silk Fibroin + Poly(ethylene Glycol) (PEG) | [157] |
| Silk Fibroin + Poly(vinyl Alcohol) (PVA) | [158] |
| Silk Fibroin + Polyacrylamide (PAM) | [159] |
| Silk Fibroin + Polycaprolactone (PCL) | [91,160] |
| Silk Fibroin + Poly(Lactic-co-glycolic Acid) (PLGA) | [161] |
| Silk Fibroin + Polyurethane (PUR) | [162] |
| Silk Fibroin + Polylactide (PLA) | [163] |
| Composition | Morphology | Reference |
|---|---|---|
| Silk Fibroin + Chondroitin Sulphate + Hyaluronic Acid | 3D scaffold | [21] |
| Silk Fibroin + Polylactide + Gelatin | 3D scaffold | [50] |
| Silk Fibroin + Poly(lactic-co-glycolic Acid) + Collagen | 3D scaffold | [50] |
| Silk Fibroin + Nanochitosan + Hyaluronic Acid | 3D scaffold | [168] |
| Silk Fibroin + Polyethylene Glycol + Keratin | 3D scaffold | [169] |
| Silk Fibroin + Hyaluronic Acid + Sodium Alginate | 3D scaffold | [21,170] |
| Silk Fibroin + Hyaluronic Acid + Heparin | 3D scaffold | [90] |
| Silk Fibroin + Chitosan + Collagen | 3D scaffold | [108,171,172] |
| Silk Fibroin + Chitosan + Polylactide | 3D scaffold | [50] |
| Silk Fibroin + Chitosan + Heparin | 3D scaffold | [50] |
| Silk Fibroin + Collagen + Hyaluronan | 3D scaffold | [50] |
| Silk Fibroin + Collagen + Heparin | 3D scaffold | [173] |
| Silk Fibroin + Β-Cyclodextrin + Polyethyleneimine | hydrogel | [21] |
| Silk Fibroin + Calcium Alginate + Carboxymethyl Cellulose | hydrogel | [21] |
| Silk Fibroin + Chitosan + Alginate Dialdehyde | film | [174] |
| Silk Fibroin + Chitosan + Polylactide | film | [175] |
| Silk Fibroin + Cellulose + Chitin | fibers | [176] |
| Silk Fibroin + Poly(caprolactone) + Hyaluronic Acid | nanofibrous matrices | [21] |
| Silk Fibroin + Hyaluronic acid + Heparin | aerogel | [90] |
| Inorganic Additives | Material | Reference |
|---|---|---|
| Hydroxyapatite | Silk Fibroin/Hydroxyapatite Silk Fibroin/Nanohydroxyapatite Silk Fibroin/Collagen/Hydroxyapatite Silk Fibroin/Collagen/Nanohydroxyapatite Silk Fibroin/Chitosan/Nanohydroxyapatite Hydroxyapatite/Gelatin/Silk Fibroin Poly (vinyl Alcohol) /Silk Fibroin/Nano-Hydroxyapatite Silk Fibroin/Titanium Dioxide/Hydroxyapatite Hydroxyapatite/Silk Fibroin/Polycaprolactone Silk Fibroin/Nano-Hydroxyapatite/Polyethylene Glycol Silk Fibroin-Alginate-Hydroxyapatite | [50,95,177,178,179,180,202,203,204] |
| Titanium dioxide | Silk Fibroin/Titanium Dioxide Silk Fibroin/Titanium Dioxide/Hydroxyapatite Chitin/Silk Fibroin/Titanium Dioxide Silk Fibroin/Collagen/Titanium Dioxide | [106,181,182,183,184,185] |
| Bioactive glass | Silk Fibroin/Bioactive Glass Silk Fibroin/Poly (vinyl Alcohol) /Bioactive Glass Chitosan/Silk Fibroin/Bioactive Glass Bioactive Glass/Chitosan/Silk Fibroin Bioactive Glass/Alginate-Poloxamer/Silk Fibroin | [186,187,188,189,190,191] |
| Zinc oxide | Chitosan/Silk Fibroin/Zinc Oxide Hyaluronic Acid/Silk Fibroin/Zinc Oxide Silk Fibroin/Disulfiram/Zinc Oxide | [192,193,194] |
| Magnetic particles | Magnetic Silk Fibroin Composite Magnetic Silk Fibroin E-Gel Scaffolds Silk Fibroin Films Decorated With Magnetic Particles Silk Fibroin/Chitosan/Magnetite Scaffolds | [196,197,198,199] |
| Noble metals | Silk–Palladium Aerogel Fibers Silk-Platinum Aerogel Fibers Silk fibroin-Gold Colloid Silk fibroin-Silver Colloid | [200,201] |
| (+) | (−) |
| Enzymatic cross-linking | |
| Unlike many chemical agents, enzymes are most active under mild aqueous reaction conditions Crosslinking process can often be controlled by modifying temperatures, pH, or ionic strength | The most expensive crosslinker Substrate specificity |
| Physical cross-linking | |
| Safe Less toxic for cells than chemical agents Inexpensive Minimum tissue reaction after crosslinking process | Bonds are weaker than the chemical crosslinkers May alter the properties of the materials Needs more time for crosslinking Lack of control over the reaction kinetics of crosslinking Lower degrees of crosslinking |
| Chemical cross-linking | |
| + Forming very strong bonds | Cell toxicity remains to be tested Needs washing to remove the residual cross-linker More expensive than physical cross-linking |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabska-Zielińska, S.; Sionkowska, A. How to Improve Physico-Chemical Properties of Silk Fibroin Materials for Biomedical Applications?—Blending and Cross-Linking of Silk Fibroin—A Review. Materials 2021, 14, 1510. https://doi.org/10.3390/ma14061510
Grabska-Zielińska S, Sionkowska A. How to Improve Physico-Chemical Properties of Silk Fibroin Materials for Biomedical Applications?—Blending and Cross-Linking of Silk Fibroin—A Review. Materials. 2021; 14(6):1510. https://doi.org/10.3390/ma14061510
Chicago/Turabian StyleGrabska-Zielińska, Sylwia, and Alina Sionkowska. 2021. "How to Improve Physico-Chemical Properties of Silk Fibroin Materials for Biomedical Applications?—Blending and Cross-Linking of Silk Fibroin—A Review" Materials 14, no. 6: 1510. https://doi.org/10.3390/ma14061510
APA StyleGrabska-Zielińska, S., & Sionkowska, A. (2021). How to Improve Physico-Chemical Properties of Silk Fibroin Materials for Biomedical Applications?—Blending and Cross-Linking of Silk Fibroin—A Review. Materials, 14(6), 1510. https://doi.org/10.3390/ma14061510







