The Effect of Electrochemical Composite Coatings with LaF3-LaB6 Particles in Nickel–Copper Matrix on the Metallurgical Processes in Arc Welding of Low Alloy Ferrite-Pearlite Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- A model of the metallurgical processes for arc welding of low alloy pearlitic steels was proposed. It considers the formation of refractory sulfides and oxides of REMs as the crystallization nuclei in the weld pool and centers of acicular ferrite nucleation. Refractory non-metallic inclusions of oxides and sulfides of type REM2O3, REM2S3, and nitride BN can be formed in the weld pool due to a mechanism of the exchange reactions of FeO, MnO, SiO2 deoxidation and FeS, MnS desulfurization as they interact with rare earth elements La, Y, Ce, Th, and boron B. During the heating of the composite coating, the fluorides and borides of REMs dissociate and the micro-alloying elements are adsorbed on the surface of the molten droplets and weld pool. As a result, the weld pool is saturated with REMs and boron and active metallurgical treatment becomes possible. Due to the formation refractory sulfides and oxides of REMs, the growth of crystallites and austenite grains is limited, the microstructure is refined, and the strength and impact toughness of the welds at low temperatures increases.
- Improving weldability and microstructure of pearlitic steel welds can be achieved thanks to the progressive design of the composite wire and the technology of its manufacturing, which imply that the composite coating is formed from copper and nickel matrix with nano-dispersed particles of fluorides and borides of REMs. A model is suggested for electrochemical adsorption of Ni2+ and Cu2+ cations with electrostatic deposition of nano-dispersed insoluble particles on the wire surface and subsequent formation of composite coatings in colloidal electrolytes based on Ni(BF4)2 and CuSO4 in ethanol C2H6O and distilled water H2O.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villalobos, J.C.; Del-Pozo, A.; Campillo, B.; Mayén, J.; Serna, S. Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service. Metals 2018, 8, 351. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Liang, Y.; Liu, P.; Yang, L. The microstructure and mechanical properties of multi-strand, composite welding-wire welded joints of high nitrogen austenitic stainless steel. Materials 2019, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Schaupp, T.; Rhode, M.; Yahyaoui, H.; Kannengiesser, T. Influence of heat control on hydrogen distribution in high-strength multi-layer welds with narrow groove. Weld. World 2019, 63, 607–616. [Google Scholar] [CrossRef]
- Viyanit, E.; Keawkumsai, S.; Wongpinkeaw, K.; Bunchoo, N.; Khonraeng, W.; Trachoo, T.; Boellinghaus, T. Hydrogen assisted cracking of an AISI 321 stainless steel seamless pipe exposed to hydrogen-containing hot gas at high pressure. Eng. Fail. Anal. 2019, 100, 288–299. [Google Scholar] [CrossRef]
- Artola, G.; Arredondo, A.; Fernández-Calvo, A.I.; Aldazabal, J. Hydrogen Embrittlement Susceptibility of R4 and R5 High-Strength Mooring Steels in Cold and Warm Seawater. Metals 2018, 8, 700. [Google Scholar] [CrossRef]
- Miletić, I.; Ilić, A.; Nikolić, R.R.; Ulewicz, R.; Ivanović, L.; Sczygiol, N. Analysis of Selected Properties of Welded Joints of the HSLA Steels. Materials 2020, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Królicka, A.; Ambroziak, A.; Żak, A. Welding Capabilities of Nanostructured Carbide-Free Bainite: Review of Welding Methods, Materials, Problems, and Perspectives. Appl. Sci. 2019, 9, 3798. [Google Scholar] [CrossRef]
- Królicka, A.; Radwański, K.; Janik, A.; Kustroń, P.; Ambroziak, A. Metallurgical Characterization of Welded Joint of Nanostructured Bainite: Regeneration Technique versus Post Welding Heat Treatment. Materials 2020, 13, 4841. [Google Scholar] [CrossRef]
- Szymczak, T.; Makowska, K.; Kowalewski, Z.L. Influence of the Welding Process on the Mechanical Characteristics and Fracture of the S700MC High Strength Steel under Various Types of Loading. Materials 2020, 13, 5249. [Google Scholar] [CrossRef]
- Nunes, A.R.V.; Zeemann, A.; De Almeida, L.H. The contribution of impurities to unexpected cold cracks in a thick C-Mn steel plate. J. Mater. Res. Technol. 2019, 8, 4364–4373. [Google Scholar] [CrossRef]
- Yi, H.-J.; Lee, Y.-J.; Kim, J.-Y.; Kang, S.-S. Effect of microstructure and chemical composition on cold crack susceptibility of high-strength weld metal. J. Mech. Sci. Technol. 2011, 25, 2185–2193. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, J.; Chen, H.-L.; Su, Y.-H.; Kuo, C.-L.; Chen, S.-H.; Lin, K.-J.; Hsieh, P.-H.; Hwang, W.-S. Effects of Rare Earth Metals on Steel Microstructures. Materials 2016, 9, 417. [Google Scholar] [CrossRef]
- Shang, Z.; Li, T.; Yang, S.; Yan, J.; Guo, H. Three-dimensional characterization of typical inclusions in steel by X-ray Micro-CT. J. Mater. Res. Technol. 2020, 9, 3686–3698. [Google Scholar] [CrossRef]
- Seo, K.; Kim, Y.-M.; Evans, G.M.; Kim, H.J.; Lee, C. Formation of Mn-depleted zone in Ti-containing weld metals. Weld. World 2015, 59, 373–380. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boulder, CO, USA, 2017. [Google Scholar]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of Adding Cerium on Microstructure and Morphology of Ce-Based Inclusions Formed in Low-Carbon Steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef]
- Nako, H.; Okazaki, Y.; Speer, J.G. Acicular Ferrite Formation on Ti-Rare Earth Metal-Zr Complex Oxides. ISIJ Int. 2015, 55, 250–256. [Google Scholar] [CrossRef]
- Milani, J.M.; Saeid, T. Acicular ferrite nucleation and growth in API5L-X65 steel submerged arc welded joints. Mater. Sci. Technol. 2020, 36, 1398–1406. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Wang, G. Inclusion characteristics and acicular ferrite nucleation in Ti-containing weld metals of X70 pipeline steel. Steel Res. Int. 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Cai, Z.; Kong, H. Inclusion and Microstructure Characteristics in a Steel Sample with TiO2 Nanoparticle Addition and Mg Treatment. Metals 2019, 9, 171. [Google Scholar] [CrossRef]
- Evans, G.M. Microstructure and properties of ferritic steel welds containing Ti and B. Weld. J. 1996, 75, 251–260. [Google Scholar]
- Blais, C.; L’Esperance, G.; Evans, G.M. Characterization of inclusions found in C-Mn steel welds containing titanium. Sci. Technol. Weld. Join. 1999, 4, 143–150. [Google Scholar] [CrossRef]
- Drápala, J.; Brožová, S.; Szurman, I.; Konečná, K.; Kostiuková, G.; Vontorová, J.; Jonšta, P.; Sobotková, K. Influence of selected rare earth metals on structural characteristics of 42CrMo4 steel. Metalurgija 2016, 55, 757–760. [Google Scholar]
- Luo, S.; Shen, Z.; Yu, Z.; Wang, W.; Zhu, M. Effect of Ce Addition on Inclusions and Grain Structure in Gear Steel 20CrNiMo. Steel Res. Int. 2021, 92, 1–20. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Q. Microstructure and strengthening mechanism of grain boundary strengthened W-ZrB2 alloy. J Mater. Res. Technol. 2020, 9, 4007–4015. [Google Scholar] [CrossRef]
- Bai, J.; Cui, Y.; Wang, J.; Dong, N.; Qurashi, M.S.; Wei, H.; Yang, Y.; Han, P. Effect of Boron Addition on the Precipitation Behavior of S31254. Metals 2018, 8, 497. [Google Scholar] [CrossRef]
- Kil, W.; Shin, M.-J.; Bang, K.-S. Effects of Fluoride in the Flux on Hydrogen Content in Weld Metal and Operating Behavior in FCAW-S. J. Weld. Join. 2017, 35, 65–70. [Google Scholar] [CrossRef]
- Coetsee, T. Phase chemistry of Submerged Arc Welding (SAW) fluoride based slags. J. Mater. Res. Technol. 2020, 9, 9766–9776. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF Thermochemical Tables, 4th ed.; NIST: New York, NY, USA, 1998.
- Gurvich, L.V.; Veits, I.V.; Medvedev, V.A.; Bergman, G.A.; Yungman, V.S.; Khachkuruzov, G.A.; Iorish, V.S.; Dorofeeva, O.V.; Osina, E.A.; Tolmach, P.I.; et al. Thermodynamic Properties of Individual Substances. In Handbook. Tables of Thermodynamic Properties, 1st ed.; Nauka: Moscow, Russia, 1982; Volume 4, Part 2. (In Russian) [Google Scholar]
- Boniardi, M.; Casaroli, A. Steel Metallurgy; Politecnico: Milan, Italy, 2017; Volume I, p. 51. [Google Scholar]
- Campbell, J. Castings; Butterworth-Heinemann: Oxford, UK, 2017; p. 135. [Google Scholar]
- You, D.; Michelic, S.K.; Presoly, P.; Liu, J.; Bernhard, C. Modeling inclusion formation during solidification of steel: A review. Metals 2017, 7, 460. [Google Scholar] [CrossRef]
- Terazawa, Y.; Ichimiya, K.; Hase, K. Nucleation Effect of Ca-Oxysulfide Inclusions of Low Carbon Steel in Heat Affected Zone by Welding. Mater. Sci. Forum 2018, 941, 130–134. [Google Scholar] [CrossRef]
- Li, Y.-W.; Liu, H.-T.; Wang, Z.-J.; Zhang, Z.-H.; Li, W.-T.; Shen, H.-Y.; Zhang, X.-M.; Wang, G.-D. Solidification microstructure of high borated stainless steels with rare earth and titanium additions. Rare Met. 2019, 39, 1483–1491. [Google Scholar] [CrossRef]
- Salas-Reyes, A.E.; Altamirano-Guerrero, G.; Chávez-Alcalá, J.F.; Barba-Pingarrón, A.; Figueroa, I.A.; Bolarín-Miró, A.M.; Jesús, F.S.-D.; Deaquino-Lara, R.; Salinas, A. Influence of Boron Content on the Solidification Structure, Magnetic Properties and Hot Mechanical Behavior in an Advanced As-Cast TWIP Steel. Metals 2020, 10, 1230. [Google Scholar] [CrossRef]
- Xie, Y.u.-M.; Song, M.-M.; Wang, F.-F.; Xue, Z.-L.; Xu, R.-S.; Wang, W. Influence of Al deoxidation on the formation of acicular ferrite in steel containing La. High Temp. Mater. Process. 2020, 39, 236–246. [Google Scholar] [CrossRef]
- Loder, D.; Michelic, S.K.; Mayerhofer, A.; Bernhard, C. On the Capability of Nonmetallic Inclusions to Act as Nuclei for Acicular Ferrite in Different Steel Grades. Metall. Mater. Trans. B 2017, 48, 1992–2006. [Google Scholar] [CrossRef]
- Yamada, T.; Terasaki, H.; Komizo, Y.-I. Relation between Inclusion Surface and Acicular Ferrite in Low Carbon Low Alloy Steel Weld. ISIJ Int. 2009, 49, 1059–1062. [Google Scholar] [CrossRef]
- Parshin, S.G. Metallurgy of Welding; Politech-Press: Saint-Petersburg, Russia, 2020; pp. 344–376. [Google Scholar]
- Radke, C. Gibbs adsorption equation for planar fluid–fluid interfaces: Invariant formalism. Adv. Colloid Interface Sci. 2015, 222, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Lapshin, D.N.; Jorge, M.; Campbell, E.E.B.; Sarkisov, L. On competitive gas adsorption and absorption phenomena in thin films of ionic liquids. J. Mater. Chem. A 2020, 8, 11781–11799. [Google Scholar] [CrossRef]
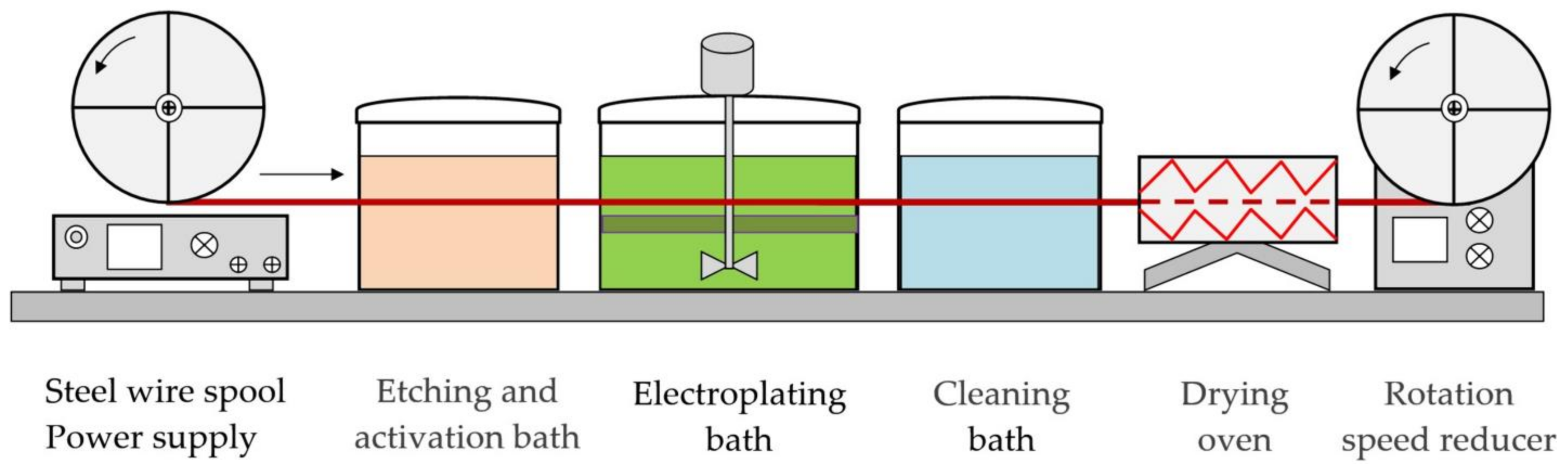
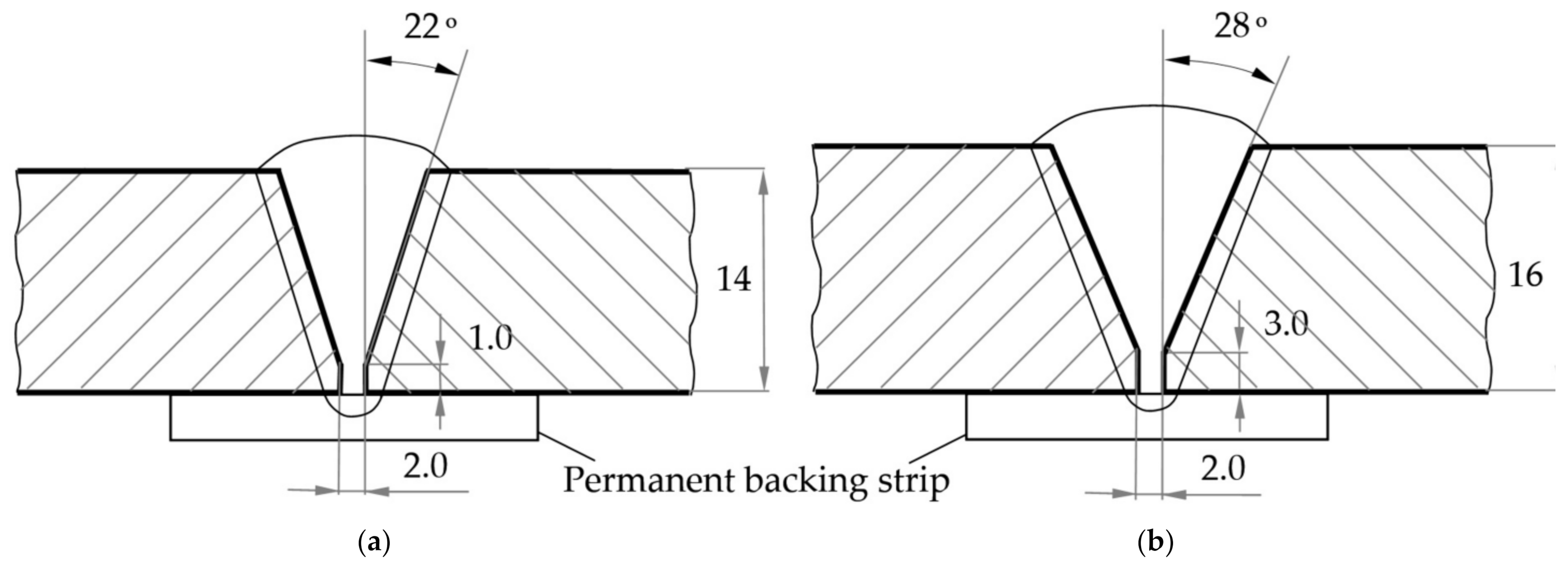
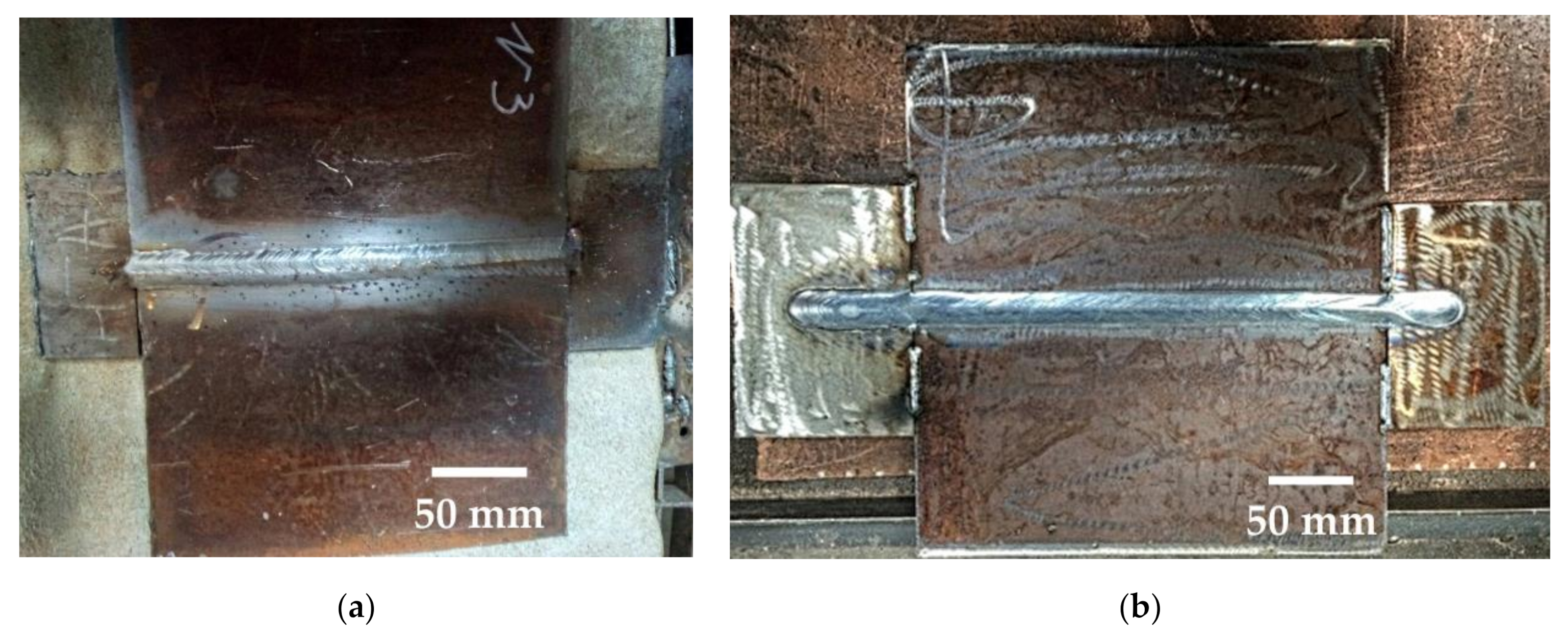
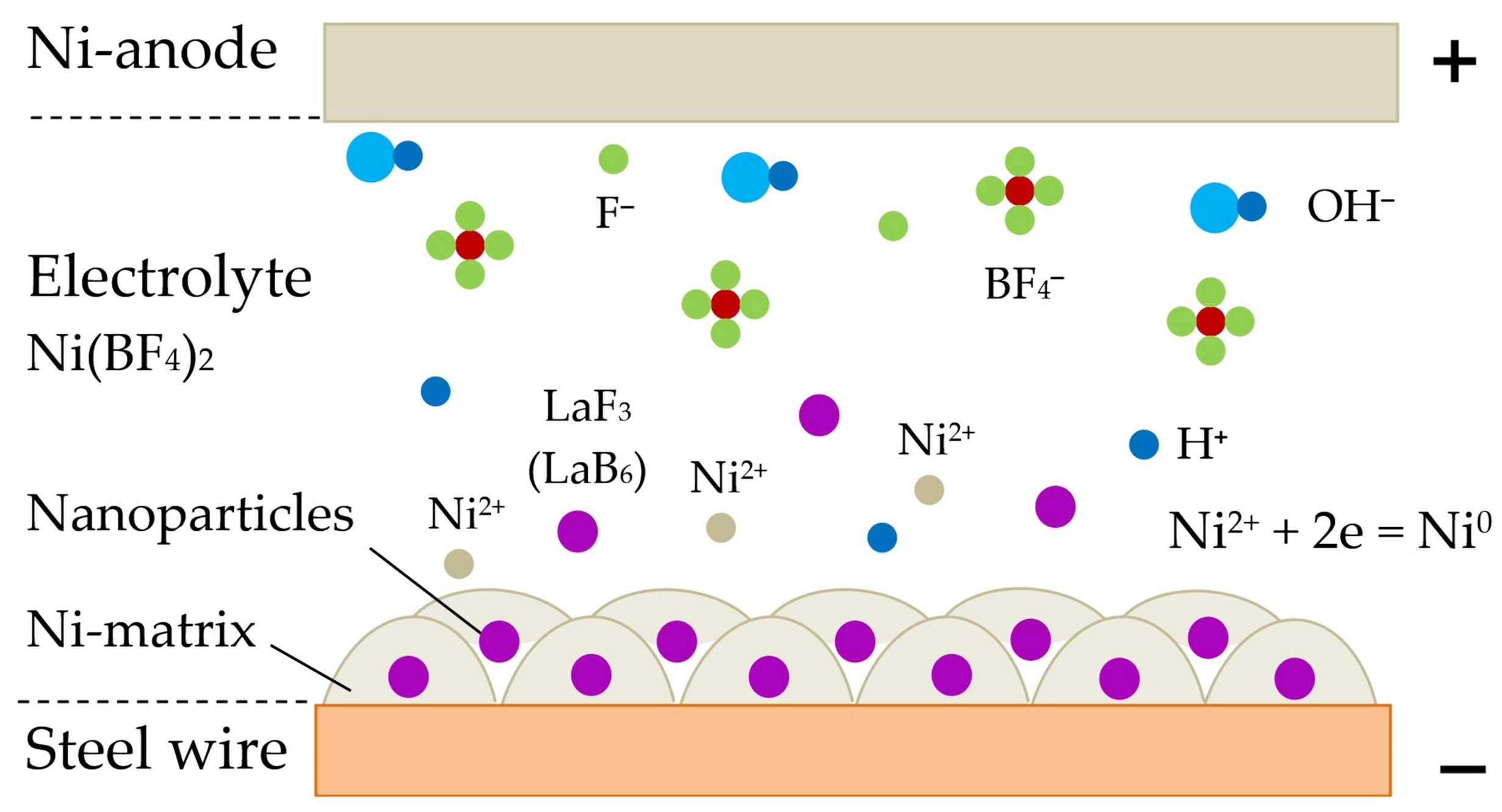
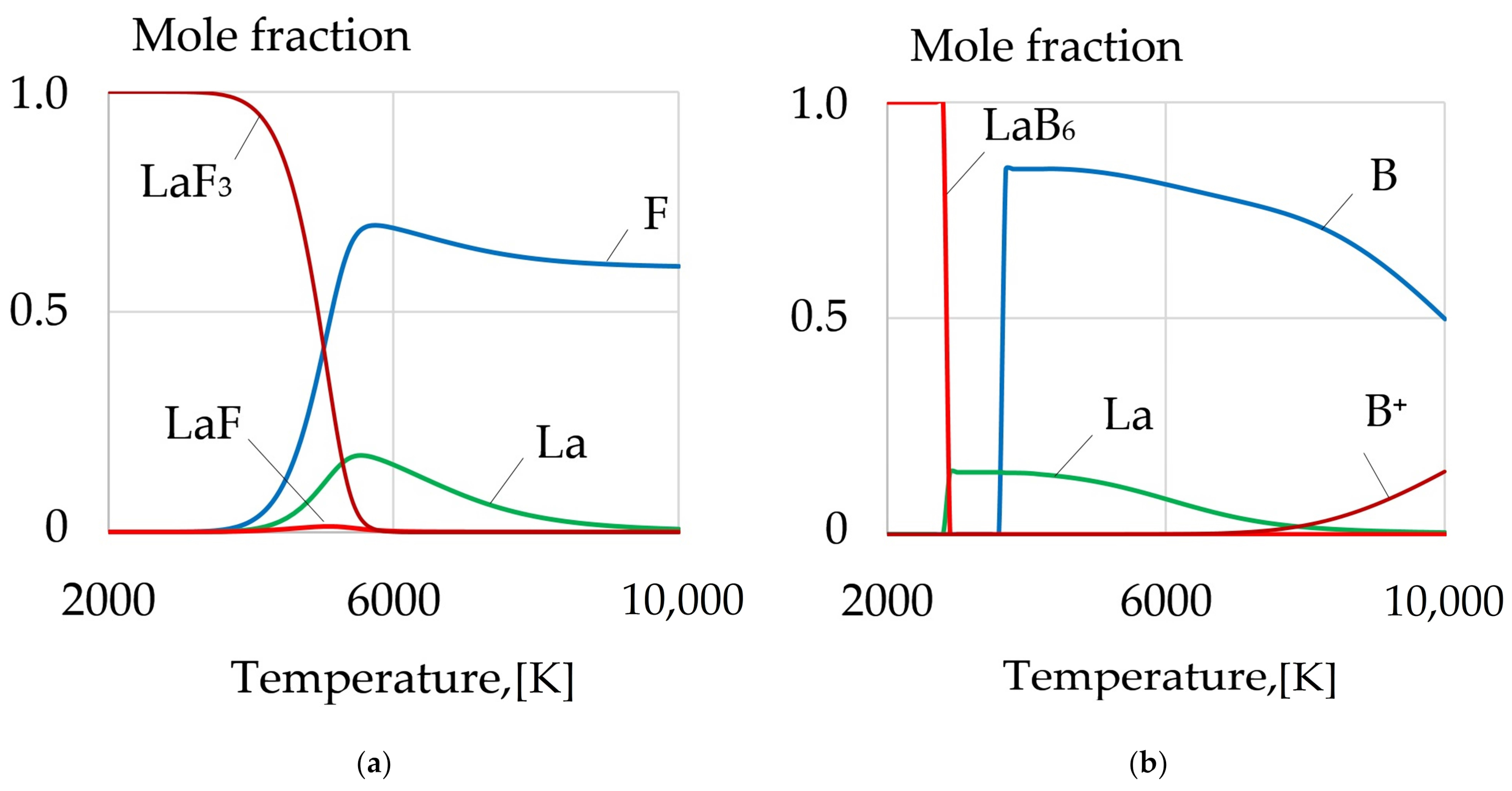
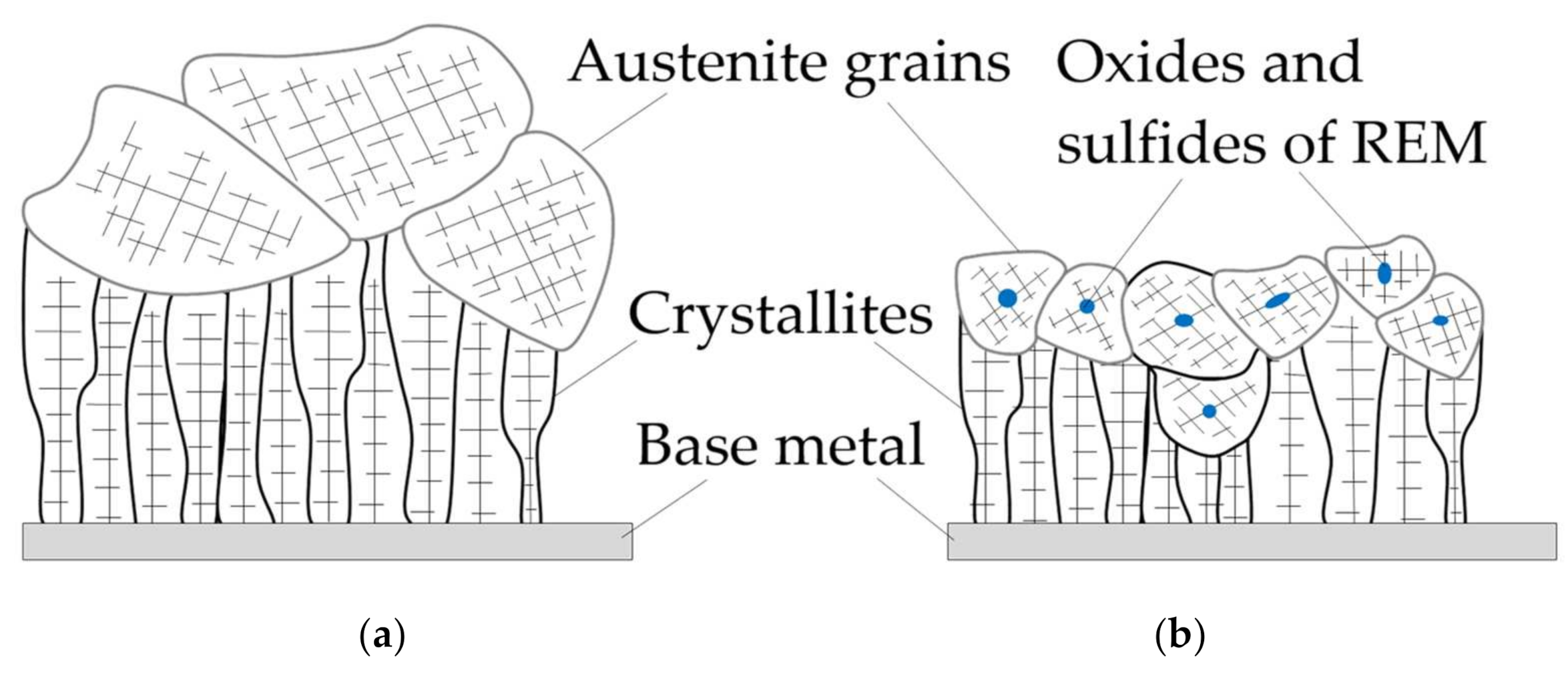

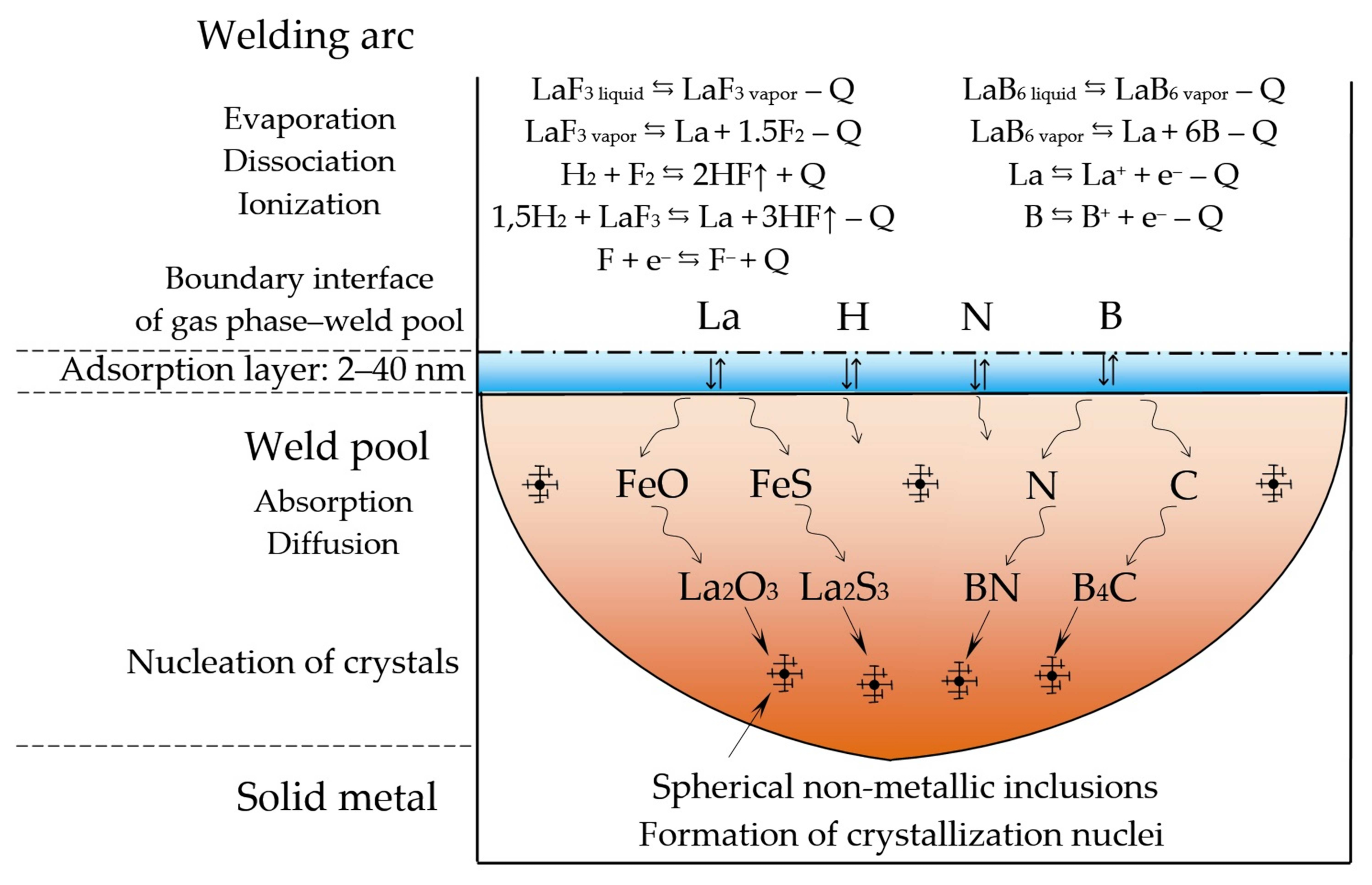
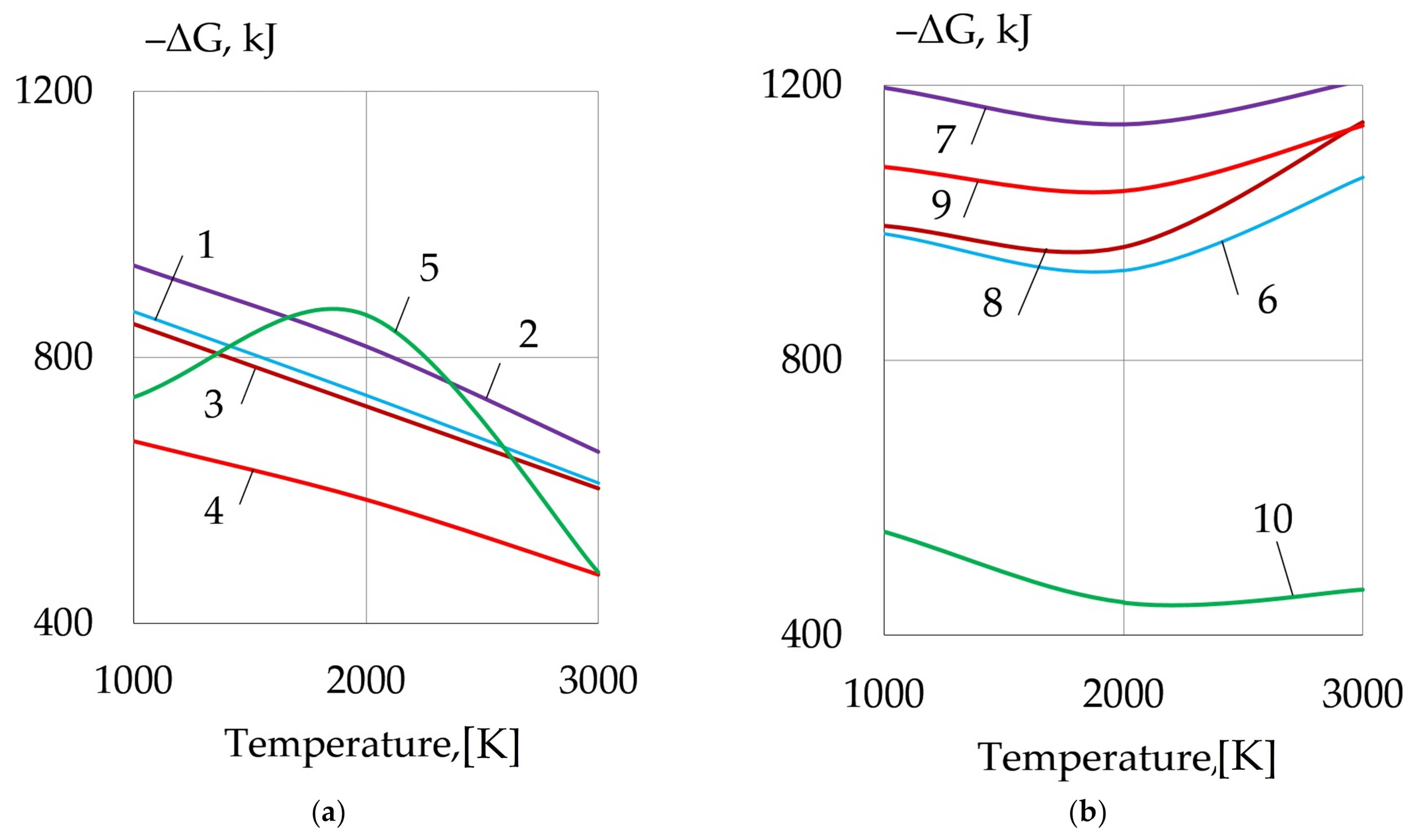
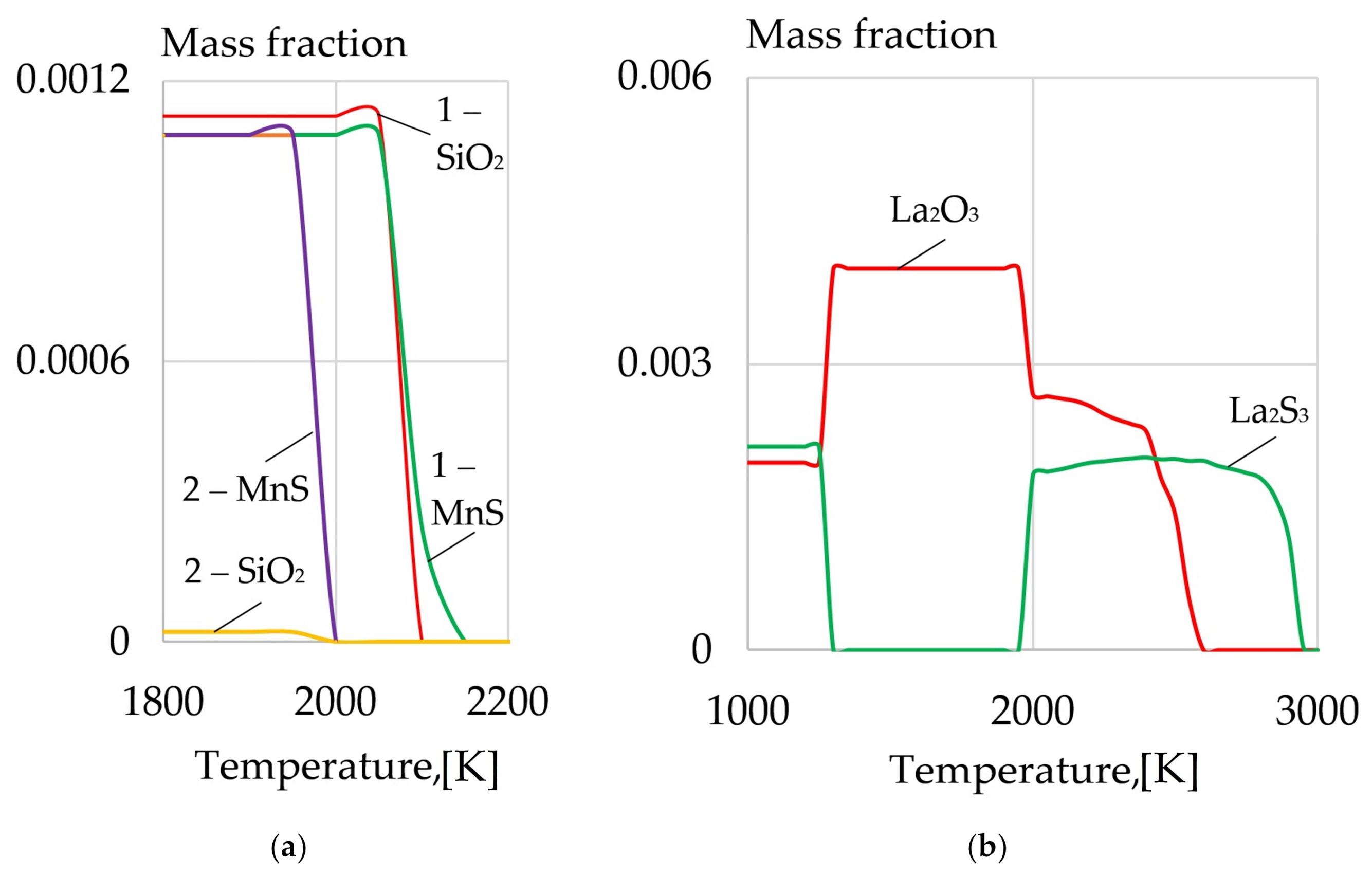
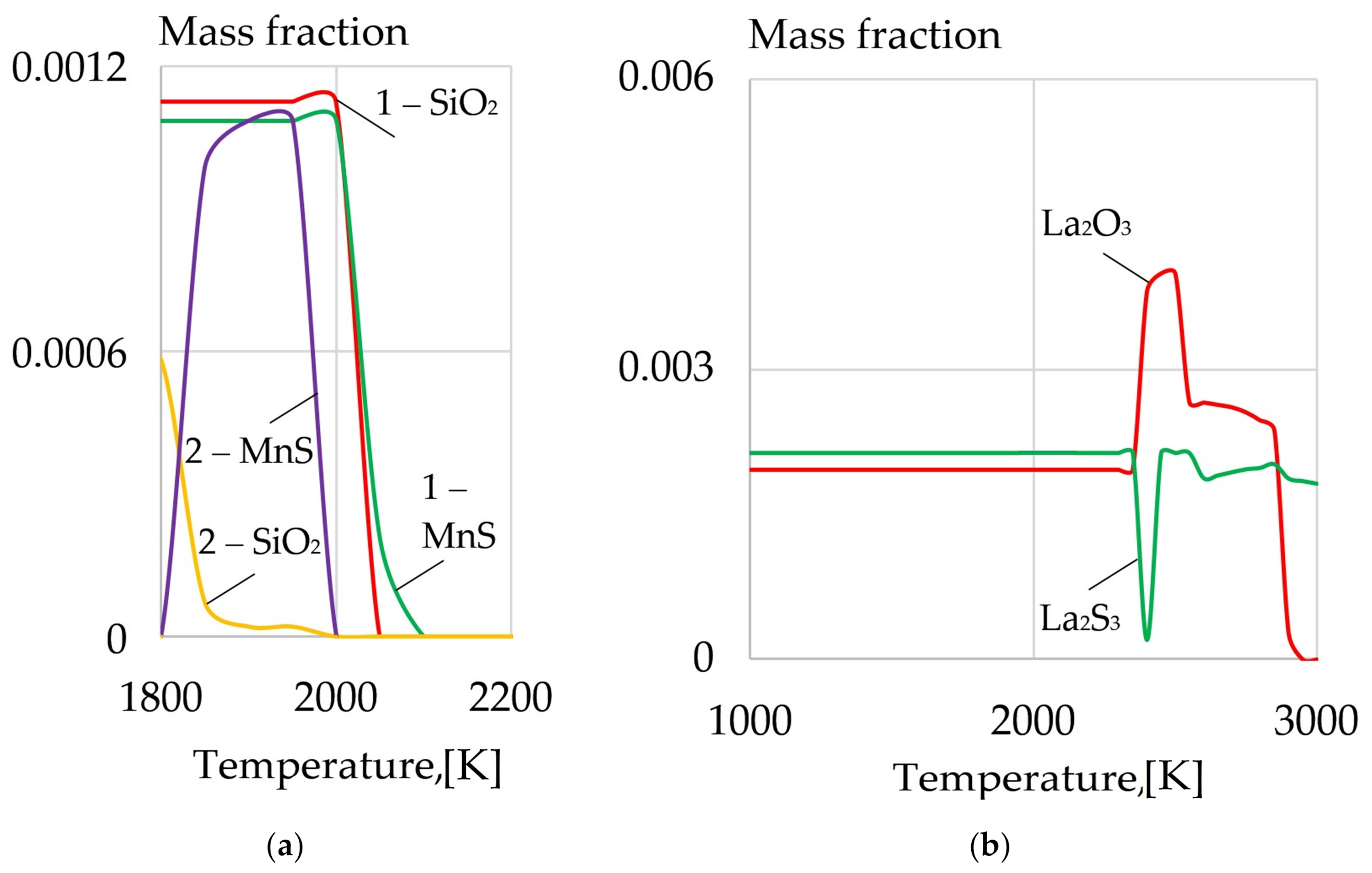
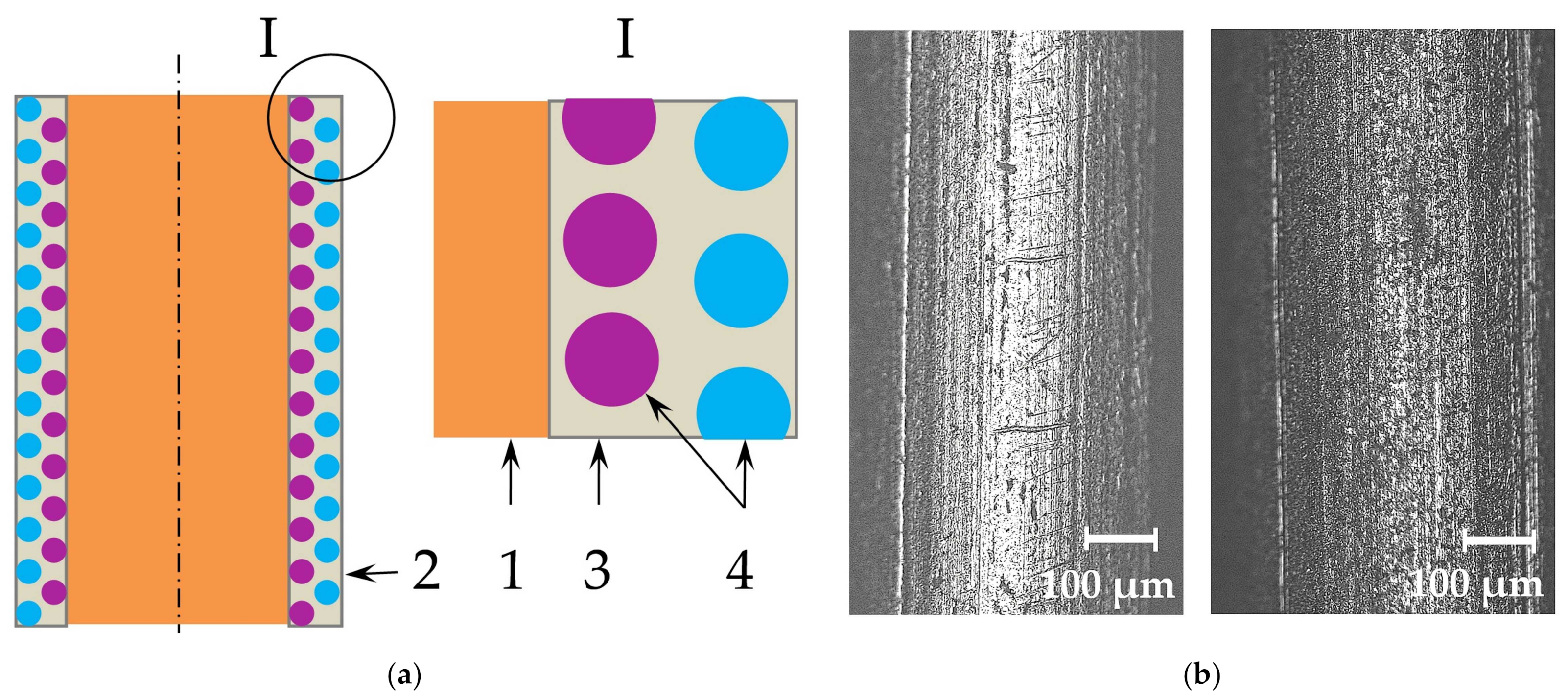
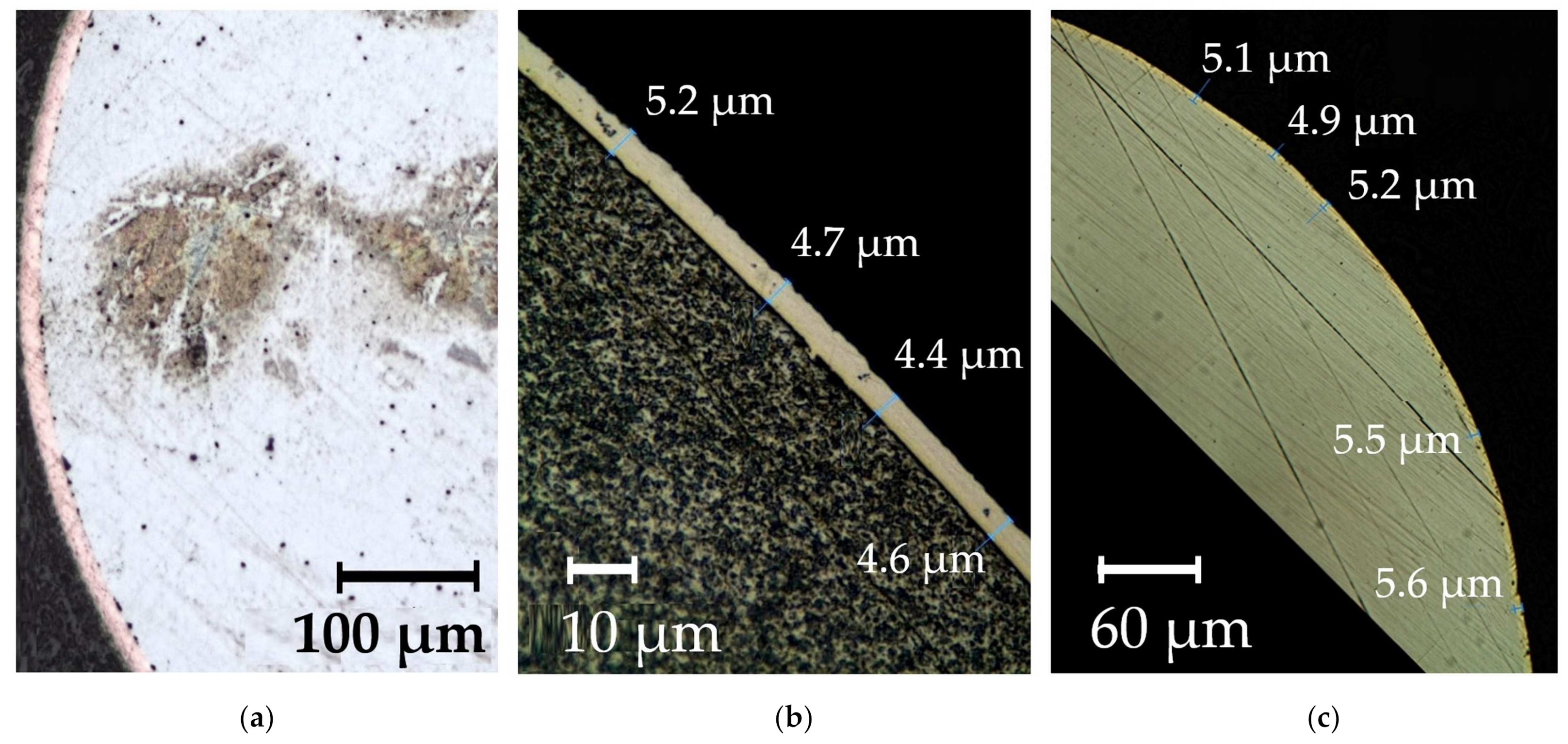
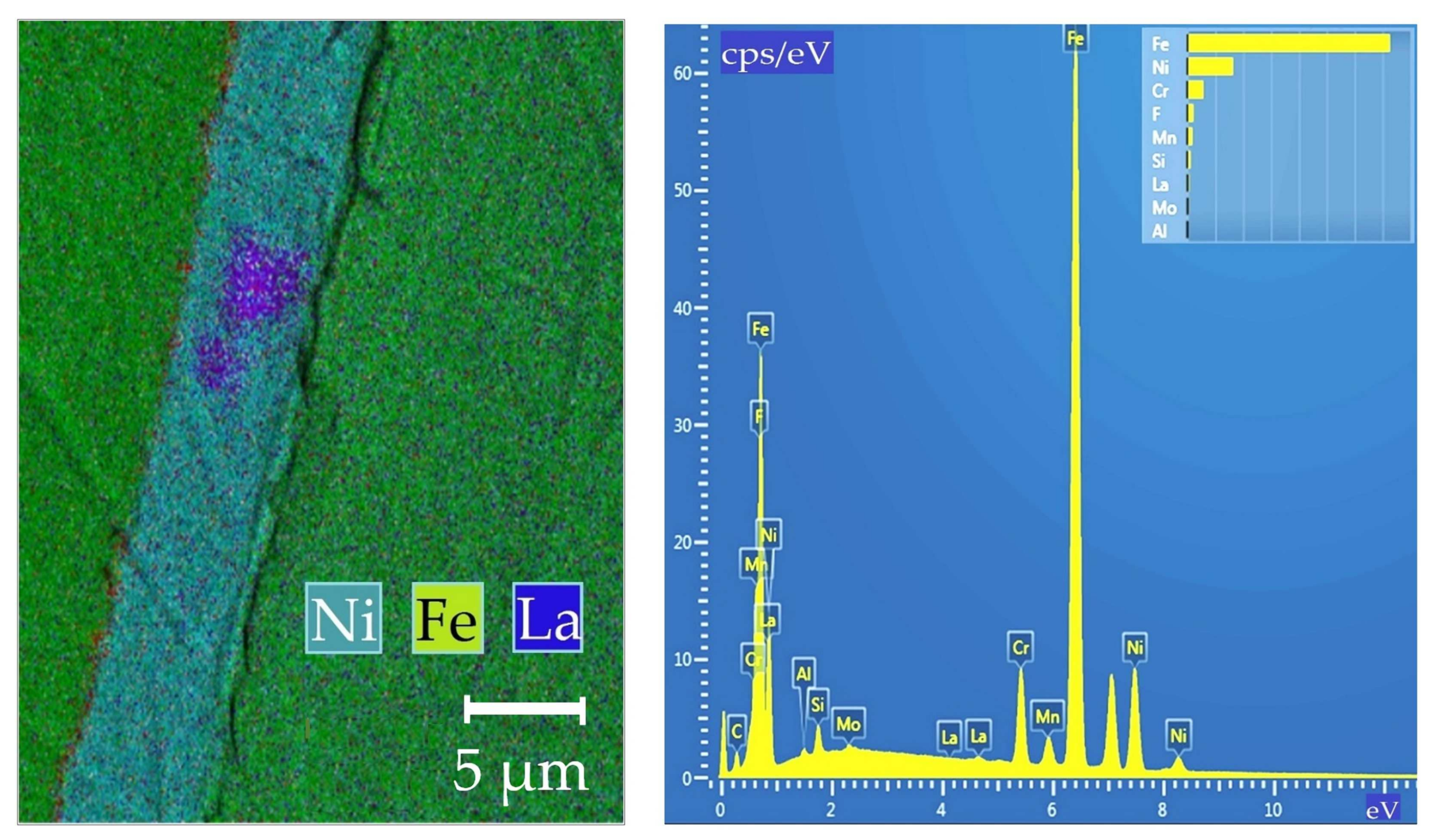
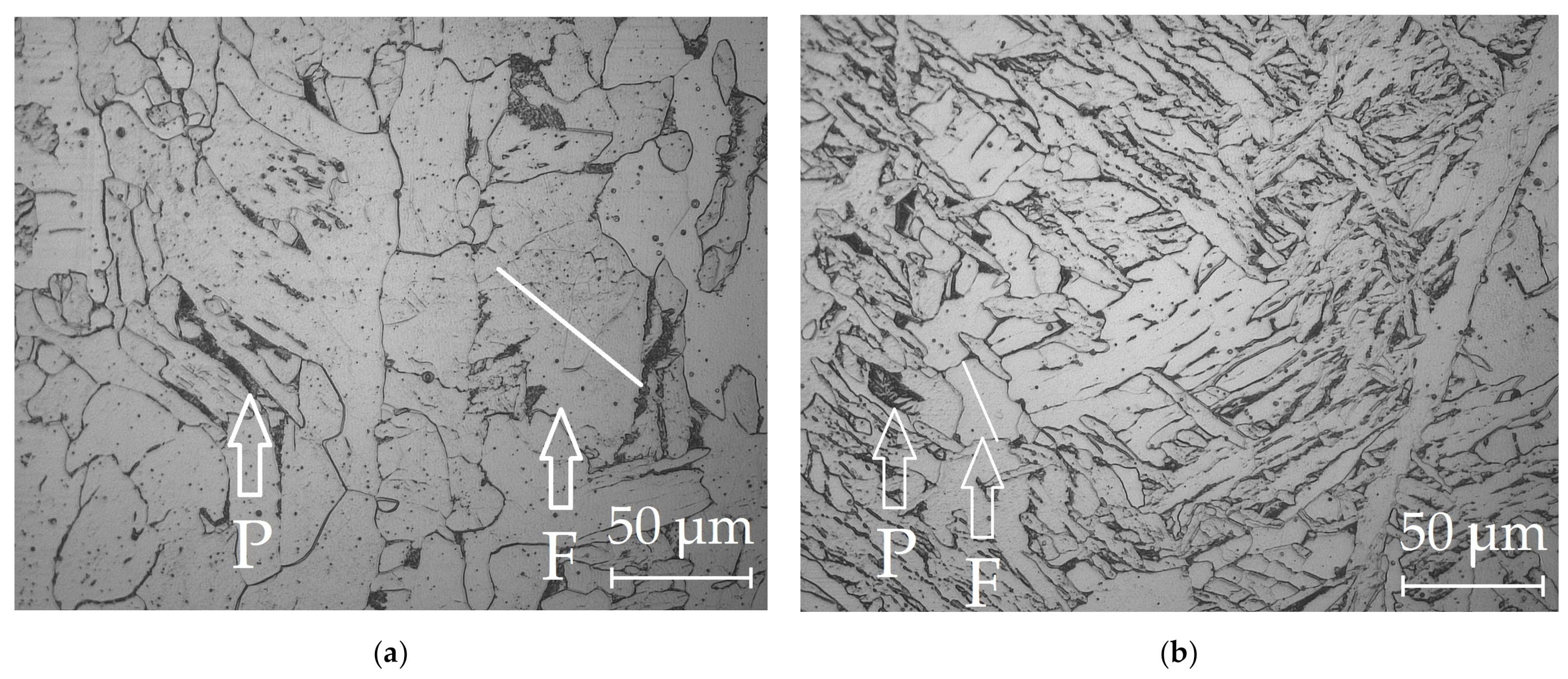
| REM | Melting Temperature, °C | Density, g/cm3 | REM | Melting Temperature, °C | Density, g/cm3 |
|---|---|---|---|---|---|
| LaF3 | 1493 | 5.9 | LaB6 | 2715 | 4.76 |
| CeF3 | 1430 | 6.157 | CeB6 | 2550 | 4.87 |
| YF3 | 1155 | 4.01 | YB6 | 2600 | 3.72 |
| NdF3 | 1377 | 6.51 | NdB6 | 2610 | 4.93 |
| ThF4 | 1110 | 6.1 | ThB6 | 2450 | 6.99 |
| Mass Concentration, kg/m3 | Temperature, °C | Electrolyte pH | Current Density, A/dm2 | Current, A | Voltage, V | Wire Feed Rate, m/min |
|---|---|---|---|---|---|---|
| Ni(BF4)2·6H2O—500; NiCl2—70; LaF3—50 or LaB6—50; Ethanol·C2H6O—solvent | 60–70 | 0.5–1 | 20–50 | 0.9–1.5 | 8–12 | 1.0–2.5 |
| CuSO4·5H2O—250; H2SO4—70; LaF3—50 or LaB6—50; Distilled water H2O—solvent | 25–30 | 1–1.5 | 5–8 | 1–2 | 10–14 | 0.5–1.5 |
| Weld Passes | 10HSND with G3Si1 1.2 mm Mechanized Welding | 09G2S with S2Mo 3.0 mm Automatic Welding | ||||
|---|---|---|---|---|---|---|
| Current, A | Voltage, V | Wire Feed Rate, m/min | Current, A | Voltage, V | Travel Speed, m/min | |
| First Root Pass | 135 | 19 | 3.6 | – | – | – |
| Filling Passes | 215 | 23 | 5.8 | 420–430 | 33 | 0.35 |
| Base Metal | C | Si | Mn | Cr | Mo | Ni | Al |
| 0.08–0.09 | 0.95–0.98 | 0.55–0.56 | 0.82–0.84 | – | 0.53–0.55 | – | |
| V | Cu | Ti | La | B | P | S | |
| – | 0.45–0.47 | – | – | – | <0.006 | <0.006 | |
| Weld Metal G3Si1 Wire | C | Si | Mn | Cr | Mo | Ni | Al |
| 0.05–0.06 | 0.79–0.86 | 1.07–1.08 | 0.14–0.15 | – | 0.09–0.11 | – | |
| V | Cu | Ti | La | B | P | S | |
| – | 0.09–0.1 | – | – | – | <0.01 | <0.008 | |
| Weld Metal G3Si1 Wire with Cu-LaF3 Coating | C | Si | Mn | Cr | Mo | Ni | Al |
| 0.09–0.1 | 0.8–0.95 | 1.13–1.16 | 0.2–0.23 | – | 0.12–0.17 | – | |
| V | Cu | Ti | La | B | P | S | |
| – | 1.2–1.7 | – | 0.003–0.004 | – | <0.01 | <0.006 | |
| Weld Metal G3Si1 Wire with Ni-LaF3 Coating | C | Si | Mn | Cr | Mo | Ni | Al |
| 0.06–0.07 | 0.81–0.85 | 1.09–1.11 | 0.1–0.12 | – | 0.25–0.28 | – | |
| V | Cu | Ti | La | B | P | S | |
| – | 0.08–0.10 | – | 0.002–0.004 | – | <0.01 | <0.008 | |
| Weld Metal G3Si1 Wire with Ni-LaB6 Coating | C | Si | Mn | Cr | Mo | Ni | Al |
| 0.05–0.06 | 0.83–0.86 | 1.14–1.15 | 0.09–0.1 | – | 0.3–0.32 | – | |
| V | Cu | Ti | La | B | P | S | |
| – | 0.07–0.08 | – | 0.001–0.003 | <0.001 | <0.01 | <0.007 |
| Wire | Yield Strength, MPa | Tensile Strength, MPa | Elongation, % | Impact Toughness, KCV+20, J | Hardness, HV10 | |
|---|---|---|---|---|---|---|
| Weld Metal | Weld Metal | HAZ | ||||
| G3Si1 Wire | 420–430 425 | 575–580 577.5 | 22–24 23 | 186–188 187 | 186–208 197 | 203–236 219.5 |
| G3Si1 Wire with Cu-LaF3 Coating | 420–430 425 | 580–590 585 | 23–24 23.5 | 208–218 213 | 203–216 209.5 | 239–256 247.5 |
| G3Si1 Wire with Ni-LaF3 Coating | 425–435 430 | 597–602 599.5 | 23–25 24 | 183–211 197 | 199–210 204.5 | 196–228 212 |
| G3Si1 Wire with Ni-LaB6 Coating | 415–425 420 | 600–602 601 | 24–26 25 | 192–218 205 | 222–232 227 | 217–229 223 |
| Wire | Yield Strength, MPa | Tensile Strength, MPa | Elongation, % | Impact Toughness, KCV−40, J | Hardness, HV10 | ||
|---|---|---|---|---|---|---|---|
| Weld Metal | HAZ | Weld Metal | HAZ | ||||
| S2Mo Wire | 405–415 410 | 512–515 513.5 | 19.5–20.7 20.1 | 55–68 61.5 | 203–287 245 | 203–208 205.5 | 188–212 200 |
| S2Mo Wire with Ni-LaF3 Coating | 418–420 419 | 580–590 519.5 | 19.4–20.2 19.8 | 66–82 74 | 256–290 273 | 218–232 225 | 209–219 214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshin, S.G.; Karkhin, V.A.; Mayr, P.; Maystro, A.S. The Effect of Electrochemical Composite Coatings with LaF3-LaB6 Particles in Nickel–Copper Matrix on the Metallurgical Processes in Arc Welding of Low Alloy Ferrite-Pearlite Steels. Materials 2021, 14, 1509. https://doi.org/10.3390/ma14061509
Parshin SG, Karkhin VA, Mayr P, Maystro AS. The Effect of Electrochemical Composite Coatings with LaF3-LaB6 Particles in Nickel–Copper Matrix on the Metallurgical Processes in Arc Welding of Low Alloy Ferrite-Pearlite Steels. Materials. 2021; 14(6):1509. https://doi.org/10.3390/ma14061509
Chicago/Turabian StyleParshin, Sergey G., Victor A. Karkhin, Peter Mayr, and Alexey S. Maystro. 2021. "The Effect of Electrochemical Composite Coatings with LaF3-LaB6 Particles in Nickel–Copper Matrix on the Metallurgical Processes in Arc Welding of Low Alloy Ferrite-Pearlite Steels" Materials 14, no. 6: 1509. https://doi.org/10.3390/ma14061509
APA StyleParshin, S. G., Karkhin, V. A., Mayr, P., & Maystro, A. S. (2021). The Effect of Electrochemical Composite Coatings with LaF3-LaB6 Particles in Nickel–Copper Matrix on the Metallurgical Processes in Arc Welding of Low Alloy Ferrite-Pearlite Steels. Materials, 14(6), 1509. https://doi.org/10.3390/ma14061509








