Solution Blow Spinning of Polycaprolactone—Rheological Determination of Spinnability and the Effect of Processing Conditions on Fiber Diameter and Alignment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymer Solutions
2.3. Viscometry
2.4. Solution Blow Spinning
2.5. Sample Imaging
2.6. Image Analysis
3. Results and Discussion
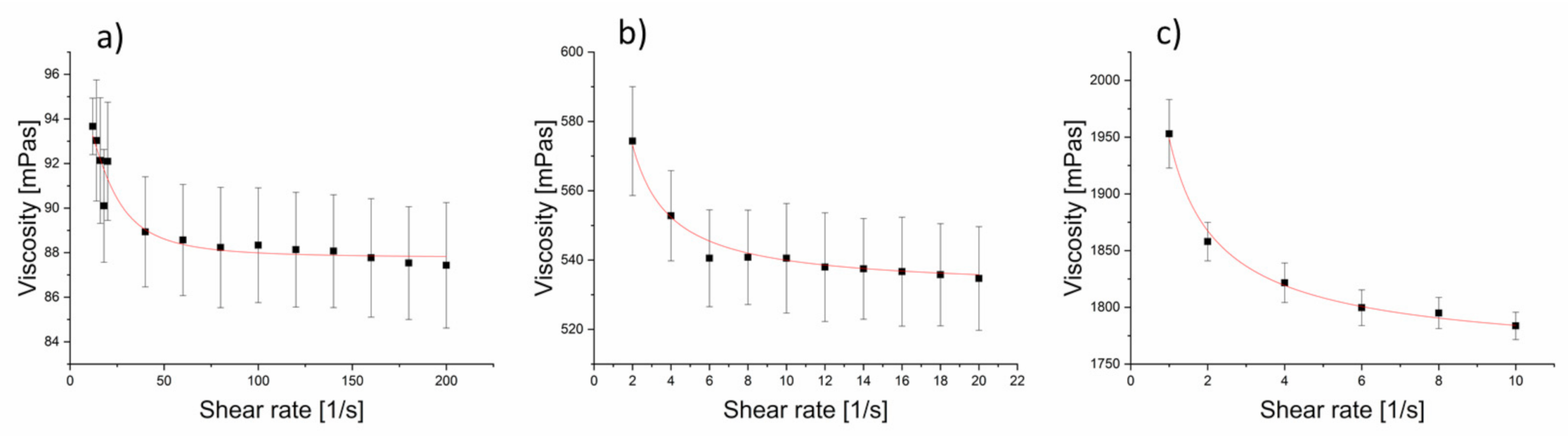
3.1. Viscometry
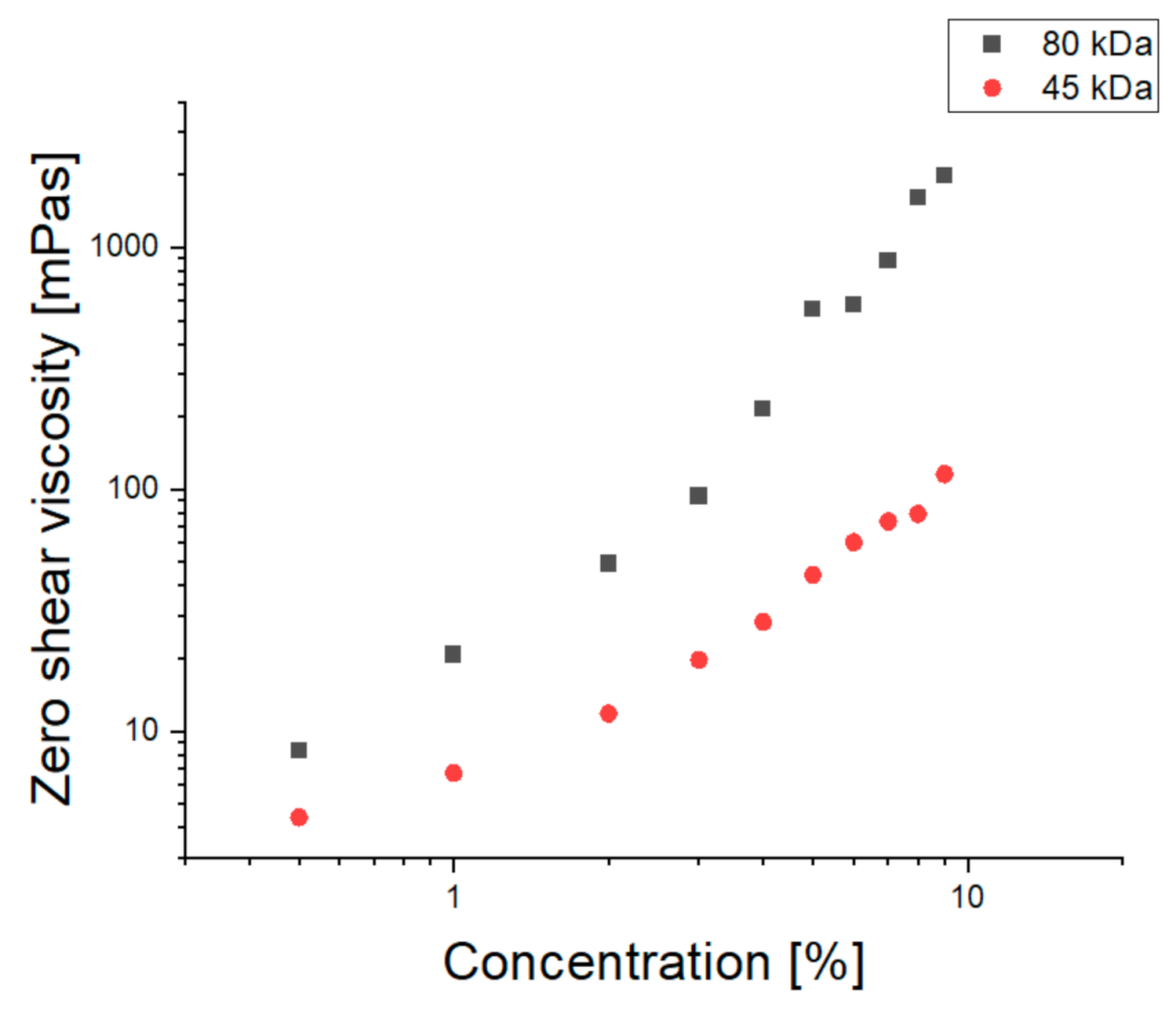
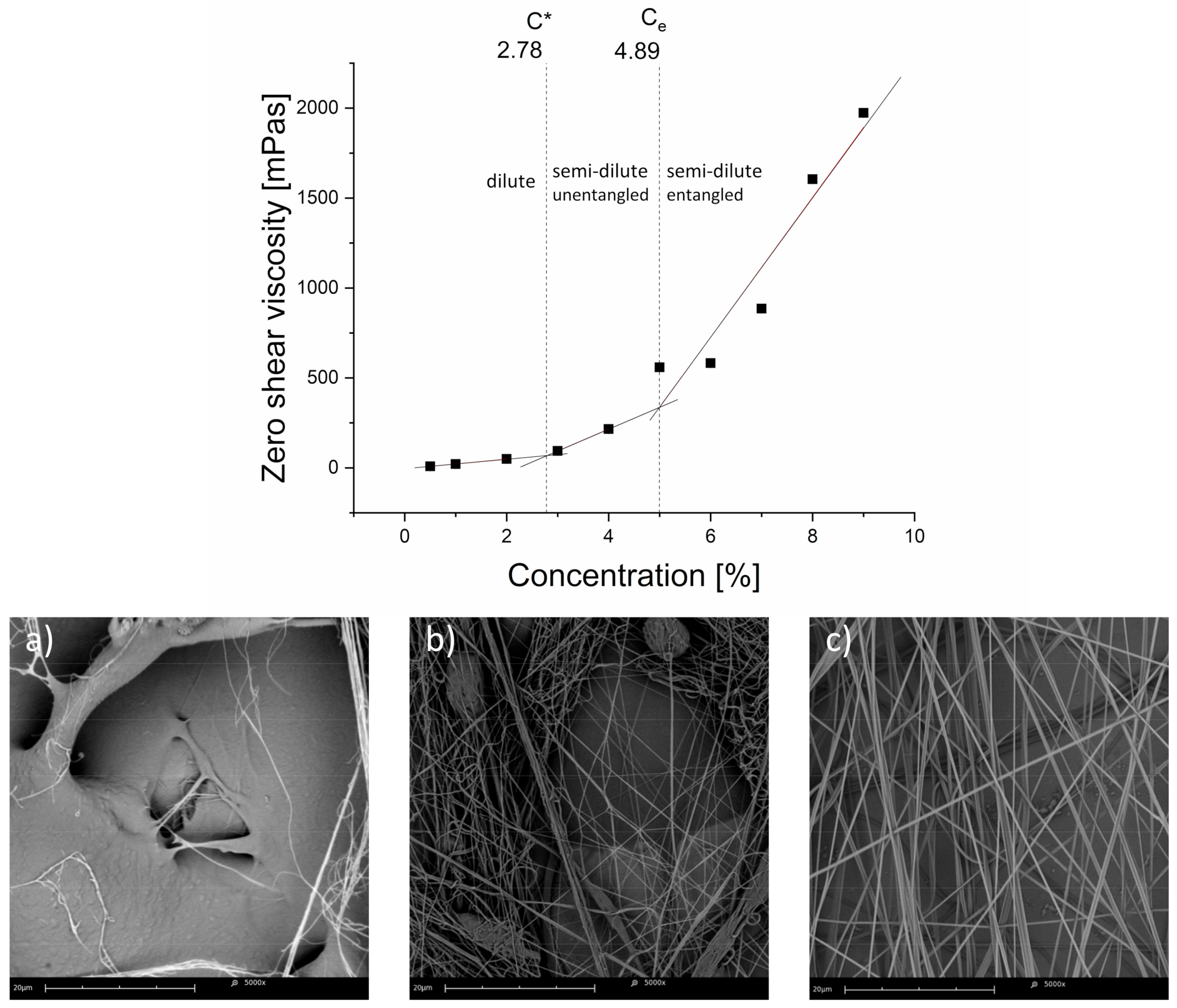
3.1.1. Spinnability Window
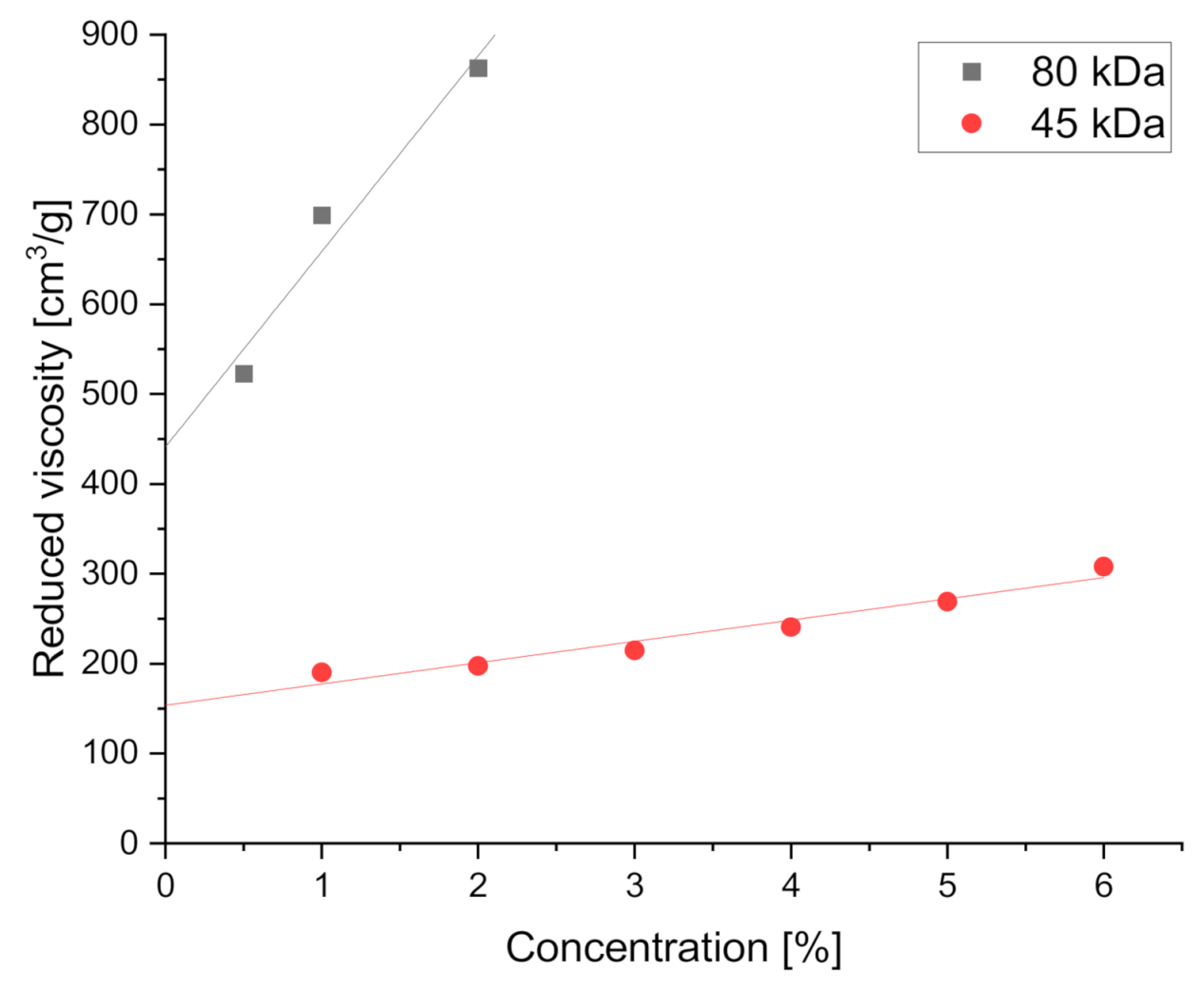
3.1.2. Macromolecular Conformation
3.2. Digital Image Analysis
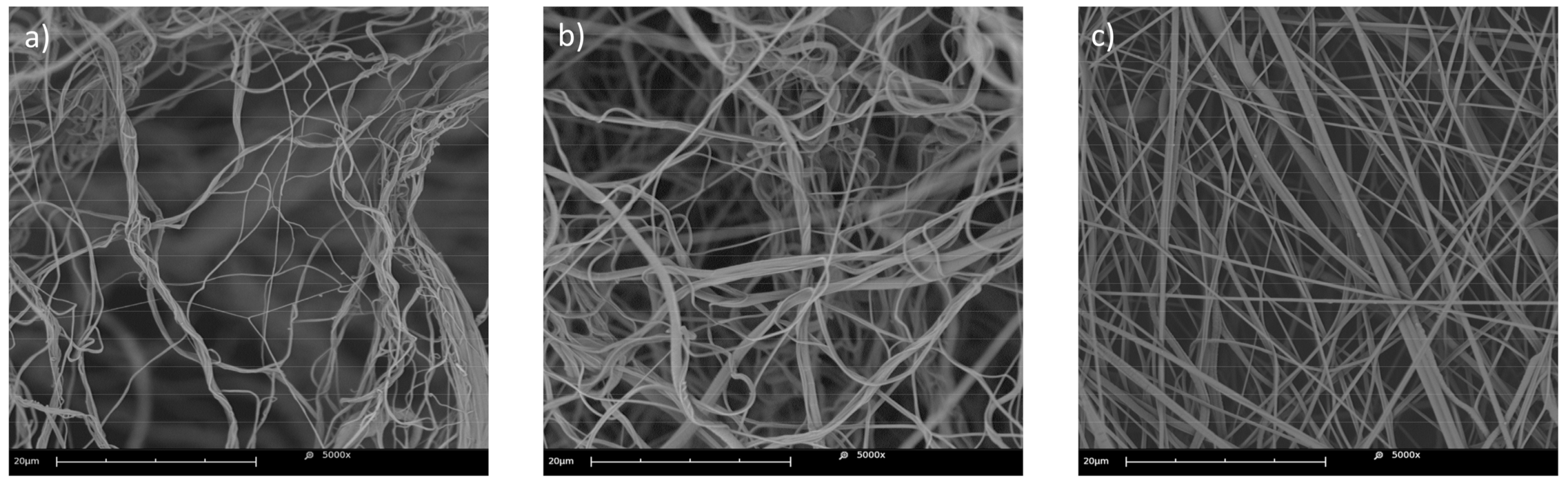
3.2.1. Qualitative Image Analysis
3.2.2. Fiber Diameter Measurements
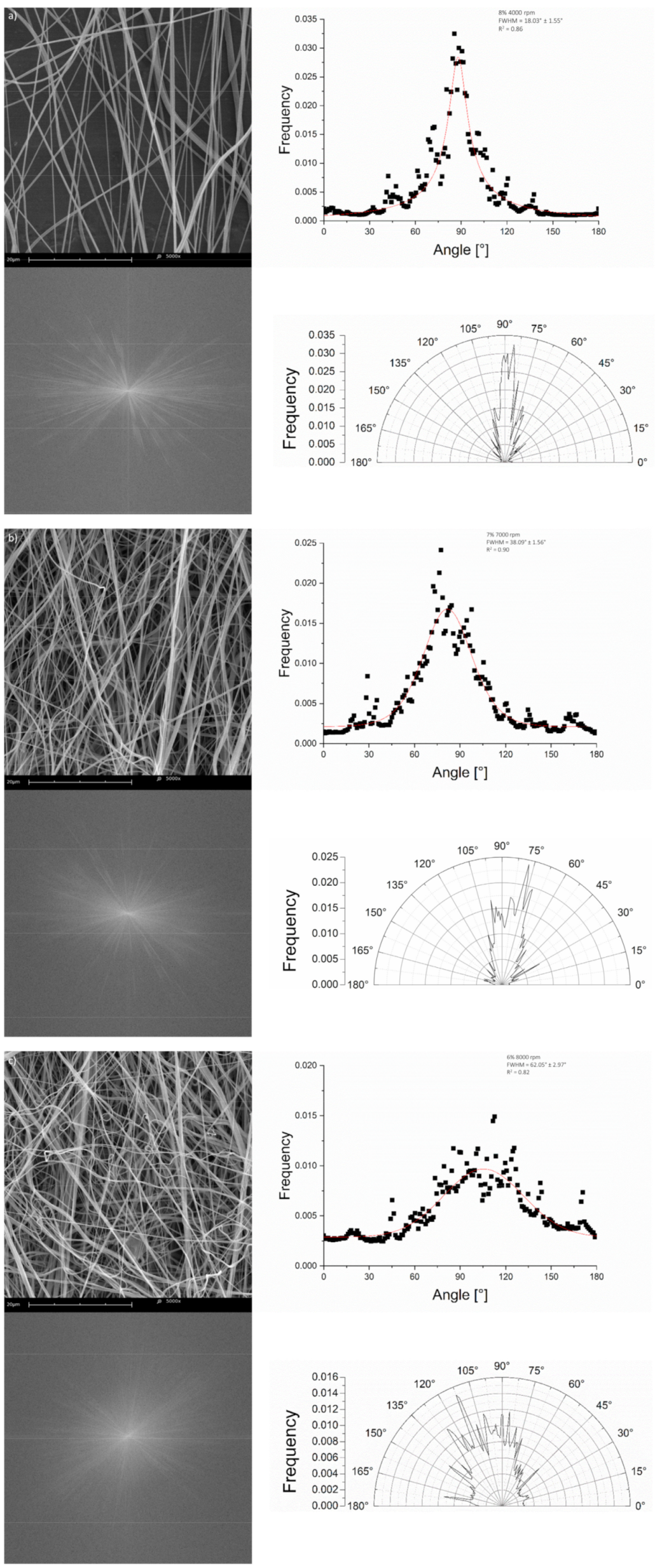
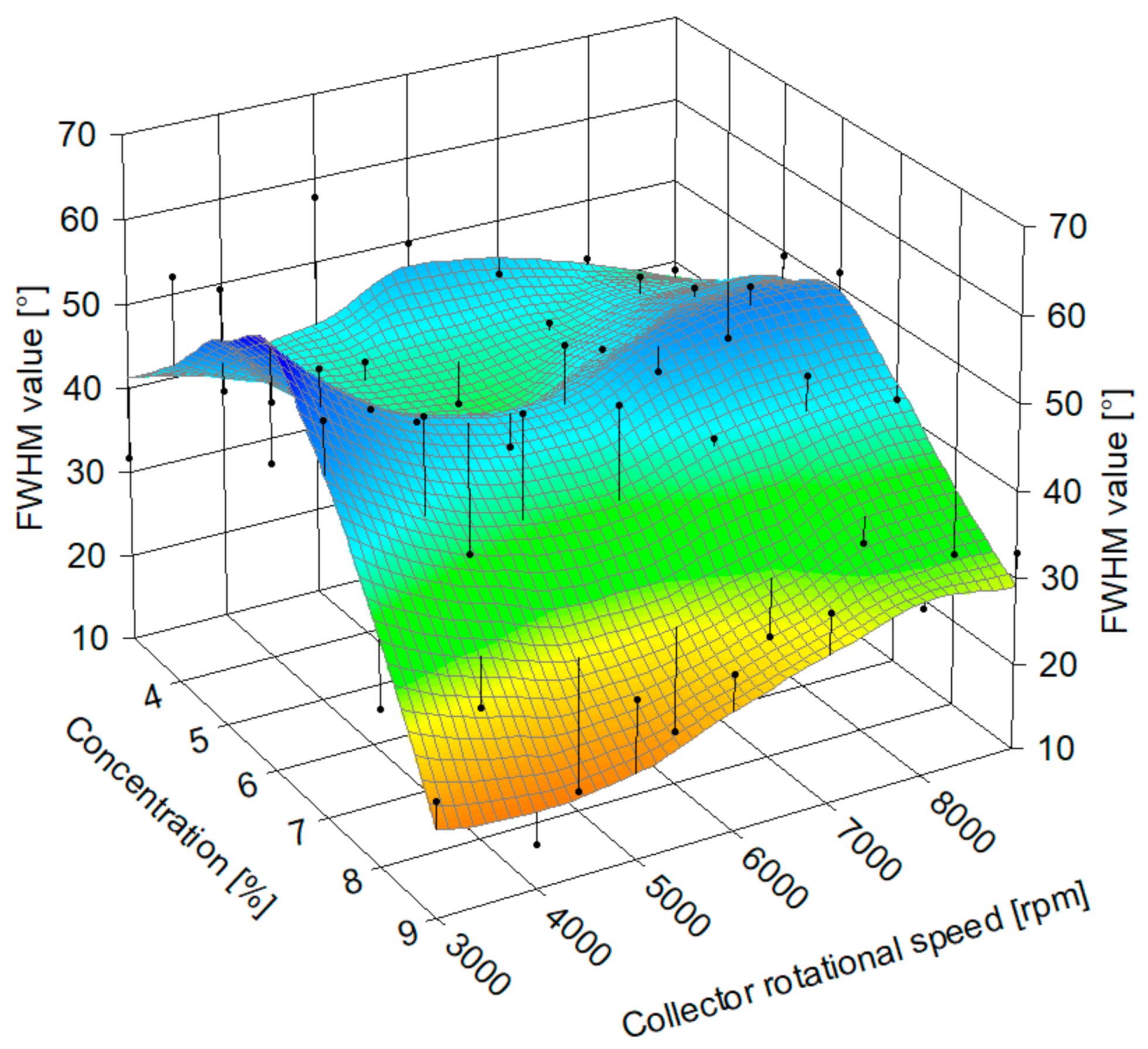
3.2.3. Fiber Alignment Assessment
3.2.4. Static Cylindrical Collector and Static Plate Collector
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surfaces B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, A.R.K.; Unnithan, A.R.; Park, C.H.; Kim, C.S. Nanofiber-based anticancer drug delivery platform. In Biomimetic Nanoengineered Materials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 11–36. ISBN 9780128149447. [Google Scholar]
- Nakielski, P.; Kowalczyk, T.; Zembrzycki, K.; Kowalewski, T.A. Experimental and numerical evaluation of drug release from nanofiber mats to brain tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 282–291. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Zhang, L.; Qiu, Y. Recent advances in energy materials by electrospinning. Renew. Sustain. Energy Rev. 2018, 81, 1825–1858. [Google Scholar] [CrossRef]
- Atif, R.; Khaliq, J.; Combrinck, M.; Hassanin, A.H.; Shehata, N.; Elnabawy, E.; Shyha, I. Solution Blow Spinning of Polyvinylidene Fluoride Based Fibers for Energy Harvesting Applications: A Review. Polymers 2020, 12, 1304. [Google Scholar] [CrossRef]
- Li, Y.; Abedalwafa, M.A.; Tang, L.; Li, D.; Wang, L. Electrospun nanofibers for sensors. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 571–601. ISBN 9780323512701. [Google Scholar]
- Aussawasathien, D.; Teerawattananon, C.; Vongachariya, A. Separation of micron to sub-micron particles from water: Electrospun nylon-6 nanofibrous membranes as pre-filters. J. Memb. Sci. 2008, 315, 11–19. [Google Scholar] [CrossRef]
- Faccini, M.; Borja, G.; Boerrigter, M.; Morillo Martín, D.; Martìnez Crespiera, S.; Vázquez-Campos, S.; Aubouy, L.; Amantia, D. Electrospun Carbon Nanofiber Membranes for Filtration of Nanoparticles from Water. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Huang, L.; Arena, J.T.; Manickam, S.S.; Jiang, X.; Willis, B.G.; McCutcheon, J.R. Improved mechanical properties and hydrophilicity of electrospun nanofiber membranes for filtration applications by dopamine modification. J. Memb. Sci. 2014, 460, 241–249. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Drew, N.K.; Eagleson, M.A.; Baldo, D.B., Jr.; Parker, K.K.; Grosberg, A. Metrics for Assessing Cytoskeletal Orientational Correlations and Consistency. PLoS Comput. Biol. 2015, 11, e1004190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Ghetti, M.; Topouzi, H.; Theocharidis, G.; Papa, V.; Williams, G.; Bondioli, E.; Cenacchi, G.; Connelly, J.T.; Higgins, C.A. Subpopulations of dermal skin fibroblasts secrete distinct extracellular matrix: Implications for using skin substitutes in the clinic. Br. J. Dermatol. 2018, 179, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Zaiss, S.; Brown, T.D.; Reichert, J.C.; Berner, A. Poly(ε-caprolactone) scaffolds fabricated by melt electrospinning for bone tissue engineering. Materials 2016, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Meng, H.Y.; Chang, B.; Liu, G.B.; Cheng, X.Q.; Tang, H.; Wang, Y.; Peng, J.; Zhao, Q.; Lu, S.B. Aligned fibers enhance nerve guide conduits when bridging peripheral nerve defects focused on early repair stage. Neural Regen. Res. 2019, 14, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cao, H.Q.; Shi, L.Y.; Ng, S.Y.; Stanton, L.W.; Chew, S.Y. Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment. Acta Biomater. 2012, 8, 1290–1302. [Google Scholar] [CrossRef]
- Zhu, L.; Jia, S.; Liu, T.; Yan, L.; Huang, D.; Wang, Z.; Chen, S.; Zhang, Z.; Zeng, W.; Zhang, Y.; et al. Aligned PCL Fiber Conduits Immobilized with Nerve Growth Factor Gradients Enhance and Direct Sciatic Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2002610. [Google Scholar] [CrossRef]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasché, P. Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98 B, 379–386. [Google Scholar] [CrossRef]
- Wojasiński, M.M.; Pilarek, M.; Ciach, T. Comparative Studies of Electrospinning and Solution Blow Spinning Processes for the Production of Nanofibrous Poly(L-Lactic Acid) Materials for Biomedical Engineering. Pol. J. Chem. Technol. 2014, 16, 43–50. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Vasireddi, R.; Kruse, J.; Vakili, M.; Kulkarni, S.; Keller, T.F.; Monteiro, D.C.F.; Trebbin, M. Solution blow spinning of polymer/nanocomposite micro-/nanofibers with tunable diameters and morphologies using a gas dynamic virtual nozzle. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution Blow Spinning; United States Patent and Trademark Office: Alexandria, VA, USA, 2014.
- Oliveira, J.E.; Mattoso, L.H.C.C.; Orts, W.J.; Medeiros, E.S.; Moraes, E.A.; Costa, R.G.F.F.; Afonso, A.S.; Mattoso, L.H.C.C.; Orts, W.J.; Medeiros, E.S. Nano and submicrometric fibers of poly(D,L-lactide) obtained by solution blow spinning: Process and solution variables. J. Appl. Polym. Sci. 2011, 122, 3396–3405. [Google Scholar] [CrossRef]
- Simbara, M.M.O.; Santos, A.R.; Andrade, A.J.P.; Malmonge, S.M. Comparative study of aligned and nonaligned poly(ε-caprolactone) fibrous scaffolds prepared by solution blow spinning. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Yoon, S.S.; Yarin, A.L. Solution-Blown Core-Shell Self-Healing Nano- and Microfibers. ACS Appl. Mater. Interfaces 2016, 8, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Parize, D.D.; Foschini, M.M.; de Oliveira, J.E.; Klamczynski, A.P.; Glenn, G.M.; Marconcini, J.M.; Mattoso, L.H.C. Solution blow spinning: Parameters optimization and effects on the properties of nanofibers from poly(lactic acid)/dimethyl carbonate solutions. J. Mater. Sci. 2016, 51, 4627–4638. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A Review of the Fundamental Principles and Applications of Solution Blow Spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution blow spinning: A new method to produce micro- and nanofibers from polymer solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Liu, F.; Avena-Bustillos, R.J.; Woods, R.; Chiou, B.S.; Williams, T.G.; Wood, D.F.; Bilbao-Sainz, C.; Yokoyama, W.; Glenn, G.M.; McHugh, T.H.; et al. Preparation of Zein Fibers Using Solution Blow Spinning Method. J. Food Sci. 2016, 81, N3015–N3025. [Google Scholar] [CrossRef] [PubMed]
- Hell, A.F.; Simbara, M.M.O.; Rodrigues, P.; Kakazu, D.A.; Malmonge, S.M. Production of fibrous polymer scaffolds for tissue engineering using an automated solution blow spinning system. Res. Biomed. Eng. 2018, 34, 273–278. [Google Scholar] [CrossRef]
- Sabbatier, G.; Abadie, P.; Dieval, F.; Durand, B.; Laroche, G. Evaluation of an air spinning process to produce tailored biosynthetic nanofibre scaffolds. Mater. Sci. Eng. C 2014, 35, 347–353. [Google Scholar] [CrossRef]
- Zhu, J.; Ge, Y.; Jasper, S.; Zhang, X. Physical characterization of electrospun nanofibers. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–238. ISBN 9780081009116. [Google Scholar]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Dadol, G.C.; Lim, K.J.A.; Cabatingan, L.K.; Tan, N.P.B. Solution blow spinning-polyacrylonitrile-assisted cellulose acetate nanofiber membrane. Nanotechnology 2020, 31, 345602. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.P.; Richardson, J.F. Non-Newtonian Flow and Applied Rheology; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780750685320. [Google Scholar]
- Kuang, Q.L.; Zhao, J.C.; Niu, Y.H.; Zhang, J.; Wang, Z.G. Celluloses in an ionic liquid: The rheological properties of the solutions spanning the dilute and semidilute regimes. J. Phys. Chem. B 2008, 112, 10234–10240. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, G.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Preparation of solution blown polyamic acid nanofibers and their imidization into polyimide nanofiber mats. Nanomaterials 2017, 7, 395. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Sunthar, P.; Deshpande, A.P.; Krishnan, J.M.; Kumar, P.B.S. Polymer Rheology BT—Rheology of Complex Fluids. In Rheology of Complex Fluids; Krishnan, J.M., Deshpande, A.P., Kumar, P.B.S., Eds.; Springer: New York, NY, USA, 2010; pp. 171–191. ISBN 978-1-4419-6494-6. [Google Scholar]
- Oliveira, J.E.; Mattoso, L.H.C.; Orts, W.J.; Medeiros, E.S. Structural and Morphological Characterization of Micro and Nanofibers Produced by Electrospinning and Solution Blow Spinning: A Comparative Study. Adv. Mater. Sci. Eng. 2013, 2013, 409572. [Google Scholar] [CrossRef]
- Kolbasov, A.; Sinha-Ray, S.; Joijode, A.; Hassan, M.A.; Brown, D.; Maze, B.; Pourdeyhimi, B.; Yarin, A.L. Industrial-Scale Solution Blowing of Soy Protein Nanofibers. Ind. Eng. Chem. Res. 2016, 55. [Google Scholar] [CrossRef]
- Carreau, P.J. Rheological Equations from Molecular Network Theories. Trans. Soc. Rheol. 1972, 16, 99–127. [Google Scholar] [CrossRef]
- Stadler, F.J.; Piel, C.; Kaschta, J.; Rulhoff, S.; Kaminsky, W.; Münstedt, H. Dependence of the zero shear-rate viscosity and the viscosity function of linear high-density polyethylenes on the mass-average molar mass and polydispersity. Rheol. Acta 2006, 45, 755–764. [Google Scholar] [CrossRef]
- Wang, Z.; Smith, D.E. Rheology Effects on Predicted Fiber Orientation and Elastic Properties in Large Scale Polymer Composite Additive Manufacturing. J. Compos. Sci. 2018, 2, 10. [Google Scholar] [CrossRef]
- Masuelli, M.A.; Sansone, M.G. Hydrodynamic Properties of Gelatin-Studies from Intrinsic Viscosity Measurements; InTech: London, UK, 2012. [Google Scholar]
- Khajavi, R.; Abbasipour, M. Controlling nanofiber morphology by the electrospinning process. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–123. ISBN 9780081009116. [Google Scholar]
- Masuelli, M.A. Viscometric study of pectin. Effect of temperature on the hydrodynamic properties. Int. J. Biol. Macromol. 2011, 48, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Chhatre, S.S.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. Solution spraying of poly(methyl methacrylate) blends to fabricate microtextured, superoleophobic surfaces. Polymer 2011, 52, 3209–3218. [Google Scholar] [CrossRef]
- Palangetic, L.; Reddy, N.K.; Srinivasan, S.; Cohen, R.E.; McKinley, G.H.; Clasen, C. Dispersity and spinnability: Why highly polydisperse polymer solutions are desirable for electrospinning. Polymer 2014, 55, 4920–4931. [Google Scholar] [CrossRef]
- Huggins, M.L. The Viscosity of Dilute Solutions of Long-Chain Molecules. IV. Dependence on Concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Dulnik, J.; Kołbuk, D.; Denis, P.; Sajkiewicz, P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nanofibres. Eur. Polym. J. 2018, 104, 147–156. [Google Scholar] [CrossRef]
- Tomecka, E.; Wojasinski, M.; Jastrzebska, E.; Chudy, M.; Ciach, T.; Brzozka, Z. Poly(L-lactic acid) and polyurethane nanofibers fabricated by solution blow spinning as potential substrates for cardiac cell culture. Mater. Sci. Eng. C 2017, 75, 305–316. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Liu, Z.-Q. Scale space approach to directional analysis of images. Appl. Opt. 1991, 30, 1369. [Google Scholar] [CrossRef] [PubMed]
- Ayres, C.E.; Jha, B.S.; Meredith, H.; Bowman, J.R.; Bowlin, G.L.; Henderson, S.C.; Simpson, D.G. Measuring fiber alignment in electrospun scaffolds: A user’s guide to the 2D fast Fourier transform approach. J. Biomater. Sci. Polym. Ed. 2008, 19, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Directionality—ImageJ. Available online: https://imagej.net/Directionality (accessed on 1 February 2021).
- Origin, Version 2019; OriginLab Corporation: Northampton, MA, USA, 2019.
- Chitra, R.; Smith, P.E. Properties of 2, 2, 2-trifluoroethanol and water mixtures. J. Chem. Phys. 2001, 114, 426–435. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Jin, X.; Ke, Q. An electrospun poly(ε-caprolactone) nanocomposite fibrous mat with a high content of hydroxyapatite to promote cell infiltration. RSC Adv. 2018, 8, 25228–25235. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Bertea, A.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2017; Volume 209, p. 012092. [Google Scholar]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin-formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Cleveland, W.S.; Devlin, S.J. Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- Tutak, W.; Sarkar, S.; Lin-Gibson, S.; Farooque, T.M.; Jyotsnendu, G.; Wang, D.; Kohn, J.; Bolikal, D.; Simon, C.G. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials 2013, 34, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Parize, D.D.; De Oliveira, J.E.; Foschini, M.M.; Marconcini, J.M.; Mattoso, L.H.C. Poly(lactic acid) fibers obtained by solution blow spinning: Effect of a greener solvent on the fiber diameter. J. Appl. Polym. Sci. 2016, 133, 43379. [Google Scholar] [CrossRef]
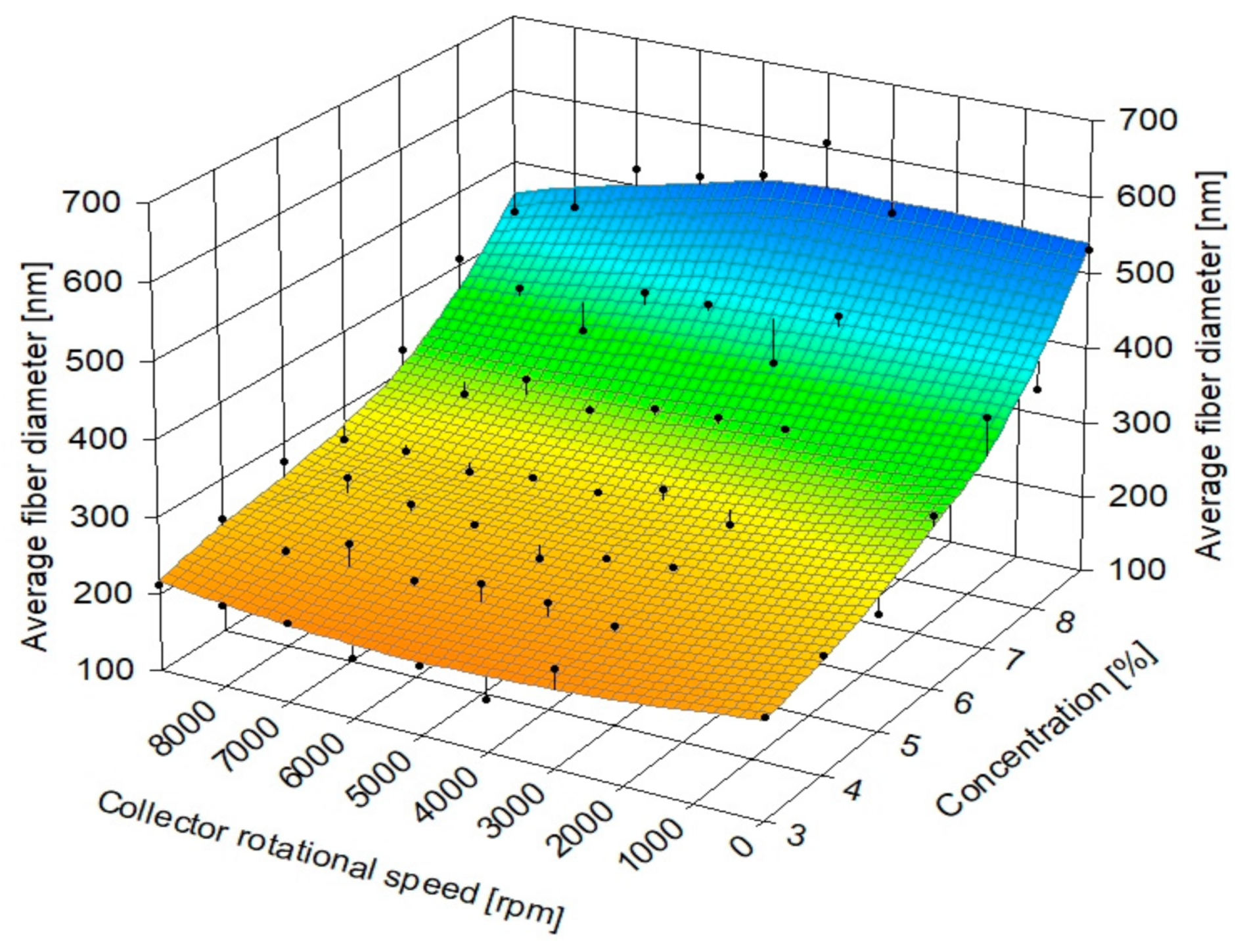
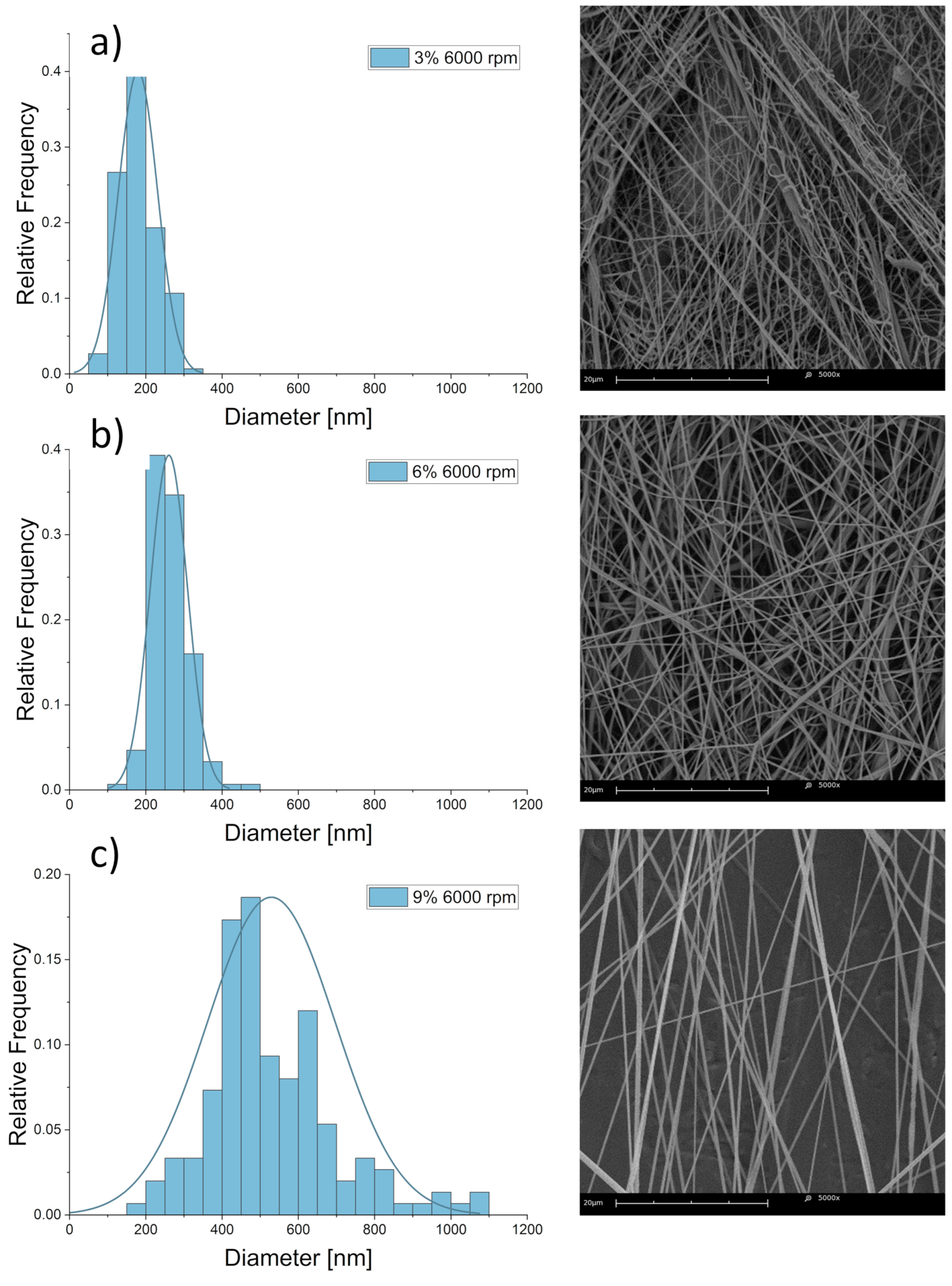
| Collector Rotational Speed [rpm] | Linear Speed [m/s] |
|---|---|
| 3000 | 3.93 |
| 4000 | 5.23 |
| 5000 | 6.54 |
| 6000 | 7.85 |
| 7000 | 9.16 |
| 8000 | 10.47 |
| 9000 | 11.78 |
| Mn (kDa) | Intrinsic Viscosity [η] (cm3/g) | a |
|---|---|---|
| 45 | 153.8 ± 10.4 | 1.83 ± 0.16 |
| 80 | 441.2 ± 61.8 |
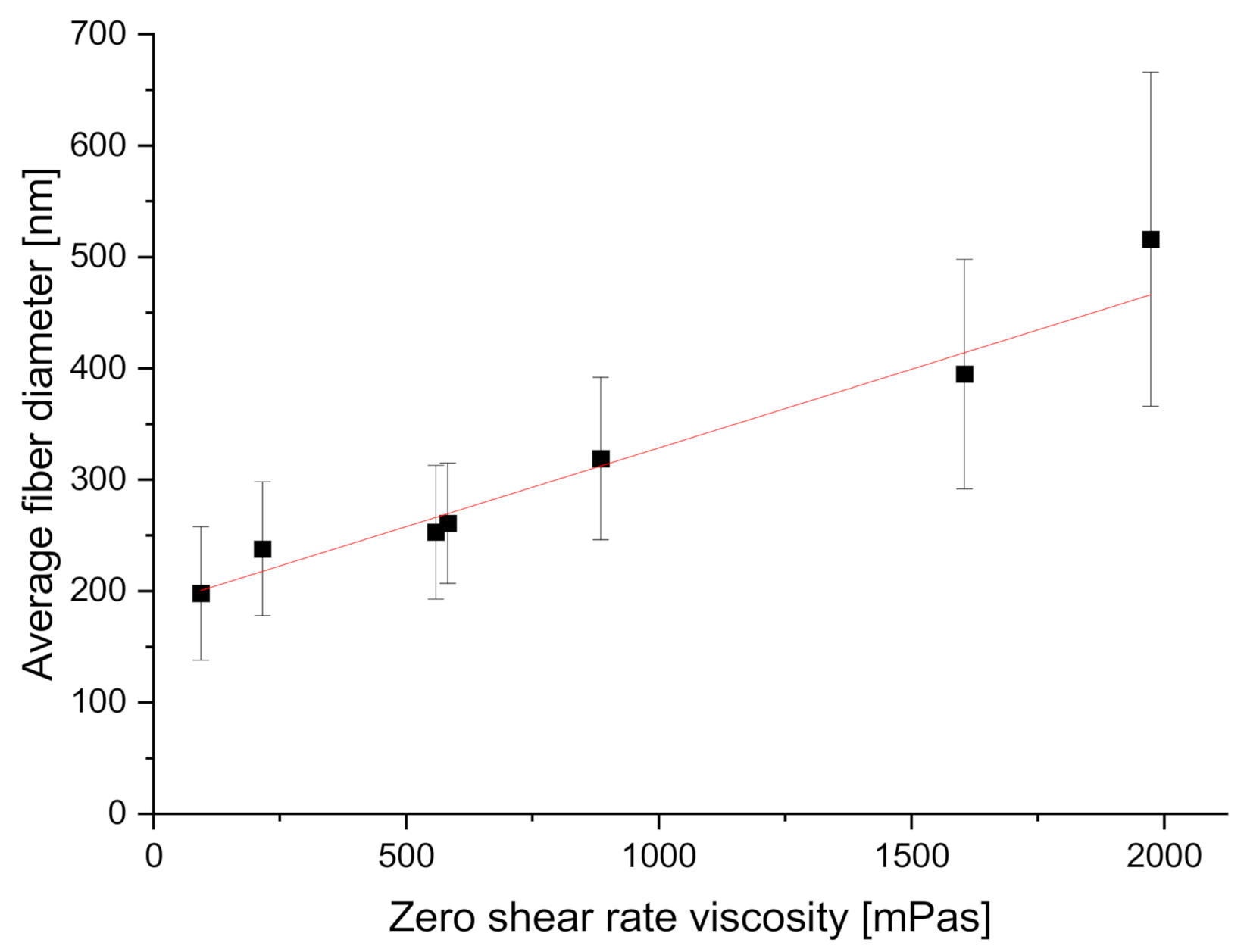
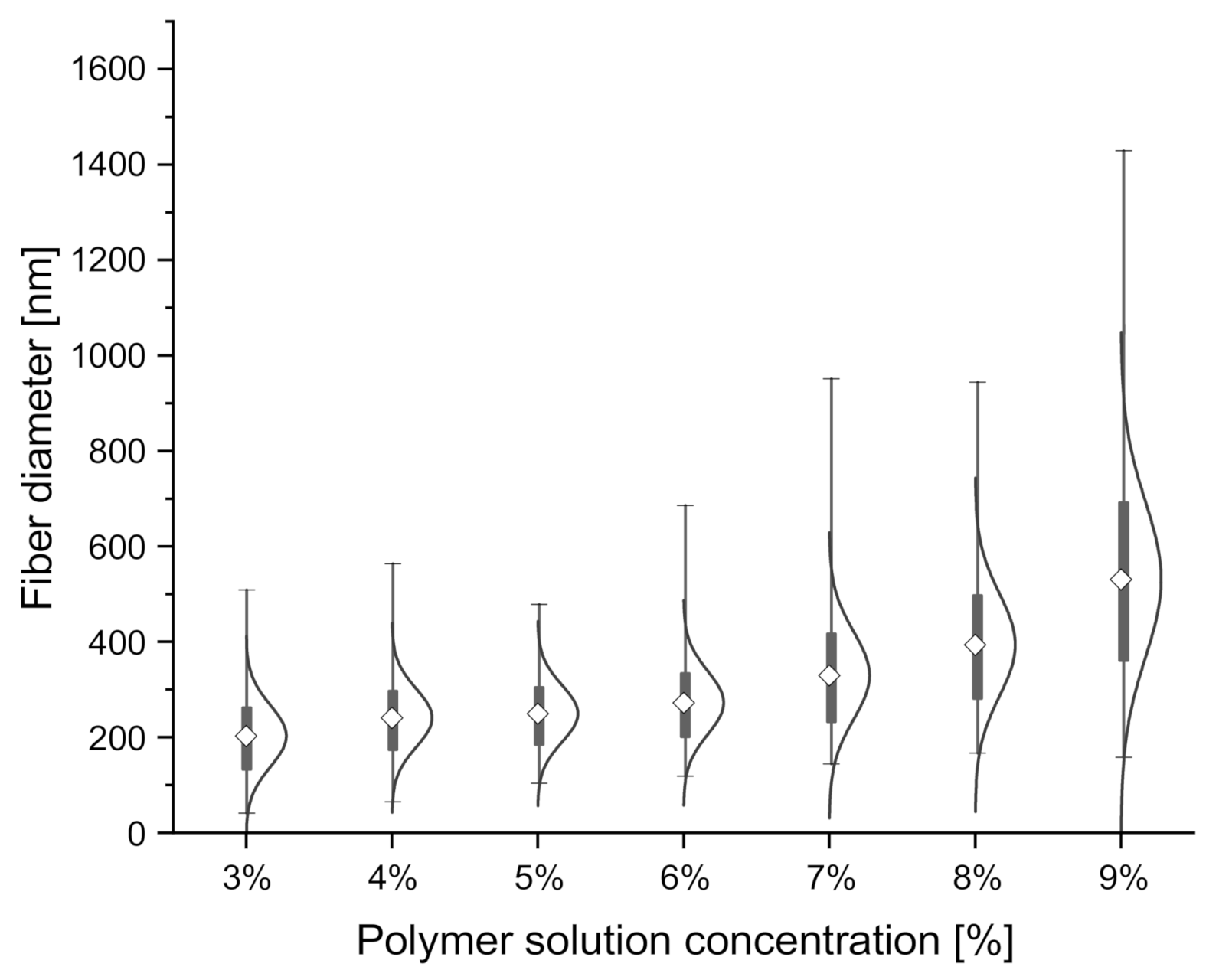
| Polymer Solution Concentration [%] | Zero Shear Rate Viscosity of the Solution, [mPa·s] | Average Fiber Diameter [nm] | Coefficient of Variation for Fiber Diameter Distribution |
|---|---|---|---|
| 3 | 93.7 ± 0.2 | 198 ± 60 | 30.3% |
| 4 | 215.5 ± 0.9 | 238 ± 60 | 25.21% |
| 5 | 558.8 ± 4.9 | 253 ± 60 | 25.53% |
| 6 | 582.4 ± 3.7 | 261 ± 54 | 20.69% |
| 7 | 885.1 ± 7.3 | 319 ± 73 | 22.88% |
| 8 | 1604.5 ± 5.3 | 395 ± 103 | 25.08% |
| 9 | 1973.4 ± 6.2 | 516 ± 150 | 29.07% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecka, K.; Wojasiński, M.; Ciach, T.; Sajkiewicz, P. Solution Blow Spinning of Polycaprolactone—Rheological Determination of Spinnability and the Effect of Processing Conditions on Fiber Diameter and Alignment. Materials 2021, 14, 1463. https://doi.org/10.3390/ma14061463
Czarnecka K, Wojasiński M, Ciach T, Sajkiewicz P. Solution Blow Spinning of Polycaprolactone—Rheological Determination of Spinnability and the Effect of Processing Conditions on Fiber Diameter and Alignment. Materials. 2021; 14(6):1463. https://doi.org/10.3390/ma14061463
Chicago/Turabian StyleCzarnecka, Katarzyna, Michał Wojasiński, Tomasz Ciach, and Pawel Sajkiewicz. 2021. "Solution Blow Spinning of Polycaprolactone—Rheological Determination of Spinnability and the Effect of Processing Conditions on Fiber Diameter and Alignment" Materials 14, no. 6: 1463. https://doi.org/10.3390/ma14061463
APA StyleCzarnecka, K., Wojasiński, M., Ciach, T., & Sajkiewicz, P. (2021). Solution Blow Spinning of Polycaprolactone—Rheological Determination of Spinnability and the Effect of Processing Conditions on Fiber Diameter and Alignment. Materials, 14(6), 1463. https://doi.org/10.3390/ma14061463








